Doppler Peripheral Venous Duplex Assessment, Protocols, and Interpretation
Doppler Peripheral Venous Duplex Assessment, Protocols, and Interpretation
Introduction
Numerous imaging modalities are used in medicine to evaluate a wide range of benign and malignant conditions, and their use has increased significantly over recent decades.[1] Despite the availability of advanced technologies, handheld manual ultrasound machines remain crucial in radiology and vascular surgery departments across the country.[2] Ultrasound plays a critical role in diagnosing and monitoring deep venous thrombosis (DVT), evaluating vascular malformations, and supporting the long-term management of select intravascular devices (see Image. Acute Deep Venous Thrombosis on Brightness Mode). This activity focuses on the anatomic appearance of veins on ultrasound, the benefits and limitations of this imaging modality, the basic physics underlying image formation, Doppler techniques, and widely accepted standard protocols for peripheral venous duplex assessment.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Peripheral veins fall into 3 anatomical categories: deep, superficial, and perforating. Deep veins course within the muscular fascia. Superficial veins lie external to the fascia and drain the cutaneous microcirculation. Perforating veins connect the superficial and deep systems. From superficial to deep, venous walls consist of an outer collagen-rich layer (tunica adventitia), a smooth muscle layer (tunica media), and an innermost endothelial lining (tunica intima). On ultrasound examination, arteries and veins can be distinguished by the visible pulsations of arteries and the compressibility of veins under mild probe pressure.
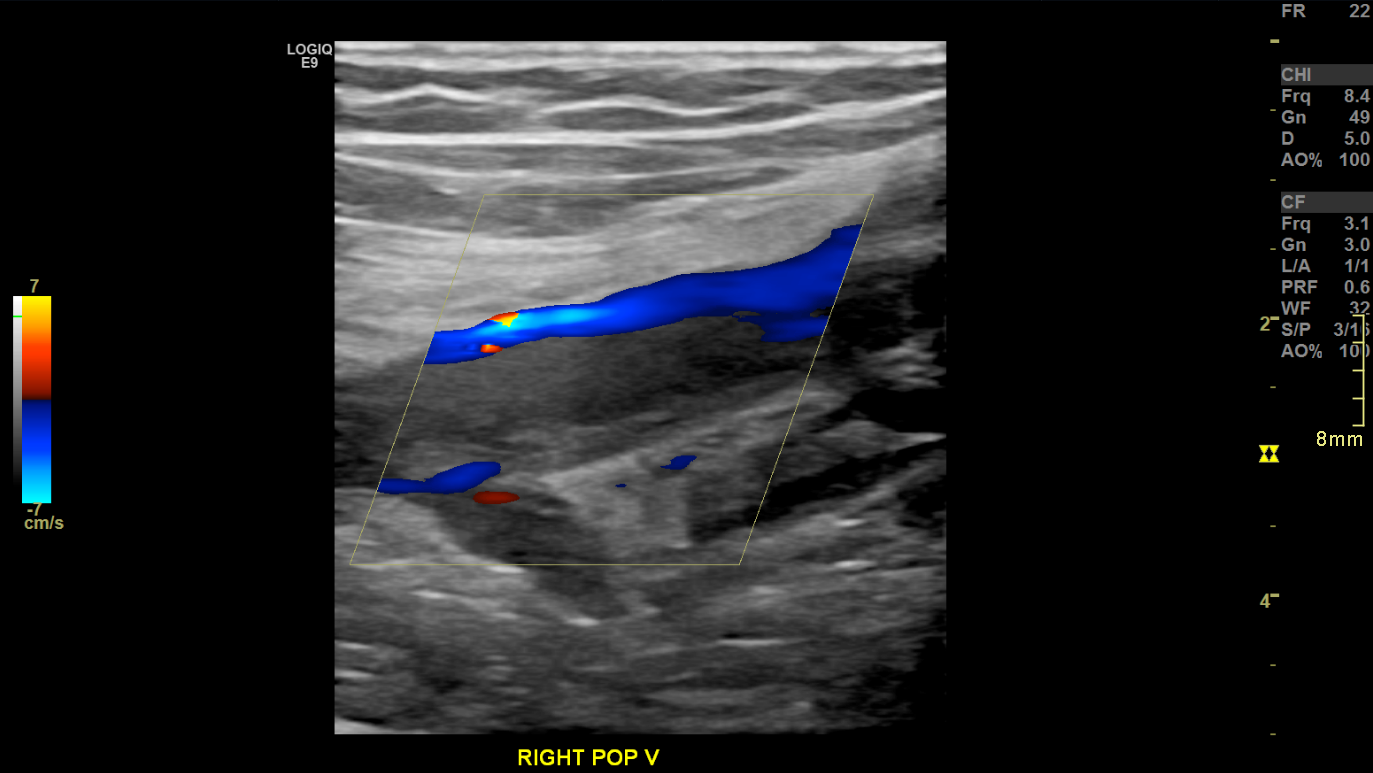
Peripheral veins return blood from the extremities against gravity through a combination of pressure gradients, cardiac pulsation, skeletal muscle contraction, and the action of 1-way bicuspid valves.[3] Blood typically flows in a laminar pattern, characterized by unidirectional movement in parallel layers with minimal intermixing. The central layer exhibits the highest velocity, while adjacent layers flow at slightly reduced speeds. Laminar flow produces a narrow spectral waveform or uniform color distribution on spectral (SDI) and color Doppler imaging (CDI), respectively. In contrast, diseased, obstructed, or anatomically abnormal vessels exhibit turbulent flow, marked by disorganized mixing of velocities. Turbulence produces spectral broadening and may result in aliasing artifacts on Doppler evaluation.[4][5]
Turbulent flow can arise from various pathologic processes, including stenosis, pseudoaneurysm, and arteriovenous fistulas.[6] Certain anatomic structures, such as the carotid bulb, may also produce physiologic turbulence. Artifactual turbulence may result from improper Doppler sampling, particularly when the sample gate is placed too close to the vessel wall, generating localized turbulent signals rather than reflecting true intraluminal flow disturbance.[7] Proper attention to Doppler settings and transducer positioning can help minimize this effect.
Variant anatomy poses diagnostic challenges in any imaging modality, particularly in the peripheral venous system, which demonstrates considerable variability. In the upper extremity, common variants include unpaired brachial veins and low-lying basilic veins.[8] In the lower extremities, the great saphenous vein (GSV) may present as a single trunk, duplicated segments, or a vessel with a hypoplastic middle portion. Accessory saphenous veins and large tributaries may course parallel to the GSV, and the small saphenous vein may or may not join the popliteal vein. Certain variants may contribute to pathologic conditions, as in the case of a persistent sciatic vein, an embryologic remnant associated with vascular malformations and chronic venous insufficiency.
Common Pathologies
The range of pathologies that may be identified or evaluated using peripheral venous duplex assessment is extensive and exceeds the scope of this article. However, familiarity with the more frequently encountered conditions targeted by ordering clinicians remains essential.
Venous malformations are congenital vascular anomalies characterized by a propensity for blood stasis. Thrombosis within these slow-flow lesions may result in pain, swelling, and overlying skin changes. On duplex evaluation, venous malformations often appear as heterogeneous, hypoechoic to anechoic, compressible vascular spaces, though their imaging characteristics can vary significantly.[9] Magnetic resonance imaging is generally preferred for definitive characterization.
Venous insufficiency is a prevalent disorder that can significantly impair quality of life. The condition commonly presents as peripheral vein dilation, particularly in the lower extremities. Venous insufficiency begins with loss of smooth muscle and elastin within the venous wall, leading to progressive dilatation. Valve incompetence follows, permitting reflux and eventually resulting in multilevel valve failure and blood stasis. Duplex imaging typically reveals reversed flow on both spectral and color Doppler interrogation.[10]
DVTs are intraluminal clots that develop within deep veins. DVTs may produce pain and swelling due to partial or complete obstruction of venous flow, though asymptomatic presentations are also common. These clots are clinically significant because they can dislodge, forming thromboemboli that travel to the lungs and occlude pulmonary vessels, resulting in pulmonary embolism.[11] Restoration of venous flow through recanalization or thrombolysis may resolve symptoms, although recurrence or development of postthrombotic syndrome can occur.
Imaging features of DVTs on peripheral venous duplex assessment vary with thrombus age. Acute thrombi often produce complete luminal obstruction, loss of venous compressibility, smooth surface contour, and a mildly hyperechoic, homogeneous intraluminal appearance (see Image. Acute Deep Vein Thrombosis on Doppler Ultrasound, Longitudinal View).
Chronic thrombi typically present as partially occlusive filling defects with irregular surfaces and heteroechoic texture, reflecting fibrotic remodeling and possible calcification (see Image. Chronic Deep Venous Thrombosis on Brightness Mode). Variability in these imaging characteristics often complicates efforts to determine thrombus chronicity.
A pseudoaneurysm is an extraluminal collection of blood that forms a false cavity while retaining a patent connection to the injured vessel. This pathology often arises as a complication of vascular access procedures, such as central venous catheter placement. Duplex interrogation typically reveals a swirling flow pattern at the neck of the pseudoaneurysm, known as the yin-yang sign, along with turbulent, pulsatile flow within the false lumen (see Image. Common Femoral Artery Pseudoaneurysm on Doppler Ultrasound, Long View). The turbulent flow pattern frequently produces color aliasing. Targeted compression at the pseudoaneurysm neck, performed under Doppler guidance, may promote thrombosis and closure of the cavity, thereby reducing the need for surgical management.
Indications
Peripheral venous duplex assessment is performed for a variety of clinical indications. The most common is evaluation for venous thrombotic disease or venous obstruction, either prompted by clinical suspicion or required for follow-up in patients with a known history of DVT. Additional indications include assessment of venous insufficiency, reflux, or varicosities; preprocedural vein mapping for anticipated vascular access, such as arteriovenous graft or fistula placement for hemodialysis; evaluation of suspected or confirmed vascular anomalies; postinterventional assessment of treated peripheral vessels; and preprocedural evaluation of venous patency prior to cannulation. Peripheral venous duplex imaging offers several advantages, including noninvasiveness, portability, high patient tolerability, and the absence of ionizing radiation.[12]
Contraindications
Doppler peripheral venous duplex assessment has no absolute contraindications. However, several factors may limit effective evaluation of the peripheral venous system. Obesity, overlying casts, and dressings can obstruct or attenuate acoustic transmission, reducing image quality. Limited access or patient intolerance may preclude adequate examination of the target vessels in patients with recent trauma, burns, surgical wounds, or significant pain.
Equipment
Doppler peripheral venous duplex assessment requires an imaging system equipped with grayscale brightness mode (B-mode), color-flow Doppler, and cinematic recording capabilities to document vessel compressibility, distinguish laminar from turbulent flow, and identify pathologic findings in peripheral veins. A 5-MHz linear or curved-linear transducer is generally appropriate for most examinations. In select cases, a 2.5-MHz transducer or phased array probe may be necessary to evaluate deeper structures. The display monitor must accurately depict both the direction and relative amplitude of blood flow signals.[13]
Personnel
Since duplex imaging is highly operator-dependent, the examination should be performed by a credentialed and accredited sonographer, vascular technologist, or physician with appropriate training. The interpreting physician must meet specialty-specific training requirements in accordance with established accreditation standards in radiology, vascular surgery, or related fields. Both the performing and interpreting providers must demonstrate proficiency in imaging physics, artifact recognition, technical limitations of the modality, normal and variant anatomy, and the pathological processes commonly affecting the peripheral venous system.
Preparation
Doppler peripheral venous duplex assessment requires minimal patient preparation. Optimal visualization and patient comfort are achieved by positioning the patient in Trendelenburg, prone, or lateral decubitus orientation, depending on the area of interest. Clothing and overlying medical support devices should be removed when safe to do so, with continuous attention to patient privacy. The imaging system should be placed within reach to allow the operator to adjust the technique efficiently throughout the examination.
Technique or Treatment
This section focuses on handheld manual imaging systems utilizing pulse wave Doppler mode. A foundational understanding of key terminology and physics principles is necessary to perform and interpret duplex imaging effectively. B-mode is the most widely used and versatile imaging mode. Images are generated by transmitting and receiving a spectrum of ultrasound frequencies along a defined path, with returning echoes aggregated and weighted based on time and amplitude. The resulting image is displayed as a function of brightness, where structures that are closer to the transducer or produce stronger reflections appear more echogenic.[14]
The Doppler effect describes acoustic frequency changes produced by a moving object relative to a stationary receiver. Wavelengths in front of the object compress as it moves toward the receiver. As the object moves away, wavelengths behind the object elongate. These wavelength changes result in a corresponding variation in frequency, known as the Doppler shift. The magnitude of this shift is directly proportional to the velocity of the moving object.
In clinical imaging, Doppler interrogation does not produce a single frequency shift but rather a spectrum of frequencies. This spectrum is influenced by anatomic factors, such as intraluminal filling defects, and physiologic factors, such as cardiac output. The presence of multiple frequency components gives rise to the term "spectral Doppler imaging" (SDI). The data are typically displayed as a graph of frequency or velocity versus time, creating a waveform (see Image. Chronic Deep Vein Thrombosis on Brightness Mode with Spectral Doppler Imaging, Long View).
Color flow imaging, also known as color Doppler imaging (CDI), enhances this technique. CDI applies a mean Doppler shift across a selected region to generate a 2-dimensional color map overlaid onto a B-mode image. This method provides a real-time representation of flow direction and relative velocity within the vessel lumen.
Further discussion of Doppler physics, SDI, and CDI lies beyond the scope of this manuscript. However, filters warrant mention. Doppler systems commonly use adjustable filters to suppress low-frequency noise and aberrant signals generated by patient motion or extraneous sources. These filters, while useful, may occasionally suppress true vascular signals and should be applied with caution.
Pulsed wave Doppler, often referred to as "duplex Doppler," is the most commonly employed technique for evaluating vascular structures. This modality combines grayscale B-mode imaging with Doppler frequency analysis. B-mode generates the structural image, while Doppler interrogation samples a defined depth during an intermittent (pulsed) time interval. Together, these components provide both anatomic and hemodynamic information regarding the target vessel.
Image optimization is essential, particularly when assessing vascular structures. The performing provider must appropriately configure technical parameters such as wall filter settings, gain, power output, and transducer selection. Focal zones should be positioned within 1 to 2 cm of the vessel of interest to maximize resolution. During Doppler interrogation, the insonation angle must be maintained at less than 60°, consistent with the cosine dependency of the Doppler equation. Strict angle correction is less critical in peripheral venous assessment than in arterial imaging. Angle accuracy becomes more relevant when evaluating for venous insufficiency or performing postsurgical vascular graft surveillance. In most clinical contexts, duplex evaluation of the peripheral venous system is focused on identifying venous obstruction or thrombosis.
Various publications from the American Institute of Ultrasound in Medicine (AIUM) and other national organizations recommend evaluating peripheral veins for DVT, thromboembolic disease, and venous insufficiency using a lateral approach, aligned with the expected vascular course. For upper extremity examinations, the symptomatic arm is typically positioned above the patient’s head while the patient lies in the supine position. All accessible segments of the innominate, internal jugular, subclavian, and axillary veins should be assessed using grayscale imaging, CDI, and SDI.
In addition, grayscale compression of the basilic, brachial, and cephalic veins should be performed down to the level of the elbow. Compression is applied in the transverse plane using sufficient pressure to completely collapse the venous lumen, typically up to a depth of 2 cm.
Grayscale still or cine images should be archived at all evaluated levels. Color and spectral Doppler interrogation of the internal jugular, subclavian, and axillary veins should be acquired in the longitudinal axis. For comparison, the contralateral axillary vein should also be evaluated using grayscale imaging, CDI, and SDI. A comparable protocol is applied during lower extremity venous assessment.
The AIUM recommends evaluation of the lower extremity venous system from the level of the inguinal ligament to the ankle when assessing for DVT. However, this comprehensive approach is not uniformly adopted across medical institutions. Regardless of institutional variation, standard examination includes grayscale imaging, CDI, SDI, and compression of the common femoral, femoral, deep femoral, and popliteal veins.
Given the prevalence of pathology and the frequency of anatomic variants, imaging must include the confluence of the common femoral vein and GSV, as well as the proximal deep femoral vein adjacent to the proximal femoral vein. When indicated, further evaluation of the posterior tibial, peroneal, and anterior tibial veins should be performed. Images obtained at baseline and during provocative maneuvers should be recorded when venous reflux is suspected. Reflux duration should be measured and reported in seconds or milliseconds.
Venous mapping is most frequently performed in preparation for surgical intervention. Coordination with the ordering provider, vascular surgery, or interventional radiology may be necessary to tailor the examination to the clinical objective. This assessment is less commonly conducted in smaller institutions and is not routinely performed in general clinical practice. Venous mapping differs from standard peripheral venous duplex evaluation. This assessment is typically limited to superficial veins within a defined anatomical region of interest. Augmentation techniques, such as tourniquet application, may be employed to enhance visualization of superficial venous structures.
According to published standards from the AIUM, all acquired images must include clear demographic information and anatomical site labels. Vessels should be labeled by region, and an indication of whether compression was applied during evaluation should be included. When Doppler interrogation is used, the insonation angle must be documented on the image. Targeted evaluation of the specified region should be performed if the examination extends beyond standard protocol. In the presence of pathology, including thrombus, vascular malformations, nonvascular abnormalities, or anatomic variants, further imaging is warranted to assess the size and extent of the abnormality.
Complications
Peripheral vascular ultrasound is not associated with acute or long-term complications. As mentioned, the procedure is noninvasive, well-tolerated, and does not involve ionizing radiation.
Clinical Significance
The clinical significance of the examination depends primarily on the underlying pathology.[15] DVT often necessitates long-term medical therapy and, in select cases, surgical intervention.[16] Management of vascular anomalies varies based on the lesion’s size and characteristics and may involve complex endovascular or surgical procedures.
Enhancing Healthcare Team Outcomes
Peripheral venous duplex assessment serves as a noninvasive, widely used modality for diagnosing DVT, venous insufficiency, vascular malformations, and pseudoaneurysms. This technique remains integral to radiology, vascular surgery, emergency medicine, and other clinical specialties. Accurate interpretation depends on detailed knowledge of venous anatomy, Doppler principles, and standardized scanning protocols. Proper image acquisition and optimization are essential for identifying pathology and guiding clinical decision-making.
Interprofessional collaboration strengthens the diagnostic utility of peripheral venous duplex assessment. Physicians and advanced practitioners determine indications, interpret findings, and initiate appropriate management. Sonographers and nurses support image acquisition and patient positioning, while pharmacists contribute to anticoagulation strategies in patients with DVT. Effective communication among healthcare team members enhances care coordination, minimizes diagnostic error, and reinforces patient-centered care. Integration of shared expertise and defined responsibilities improves safety, treatment outcomes, and overall team performance across clinical settings.
Media
(Click Image to Enlarge)
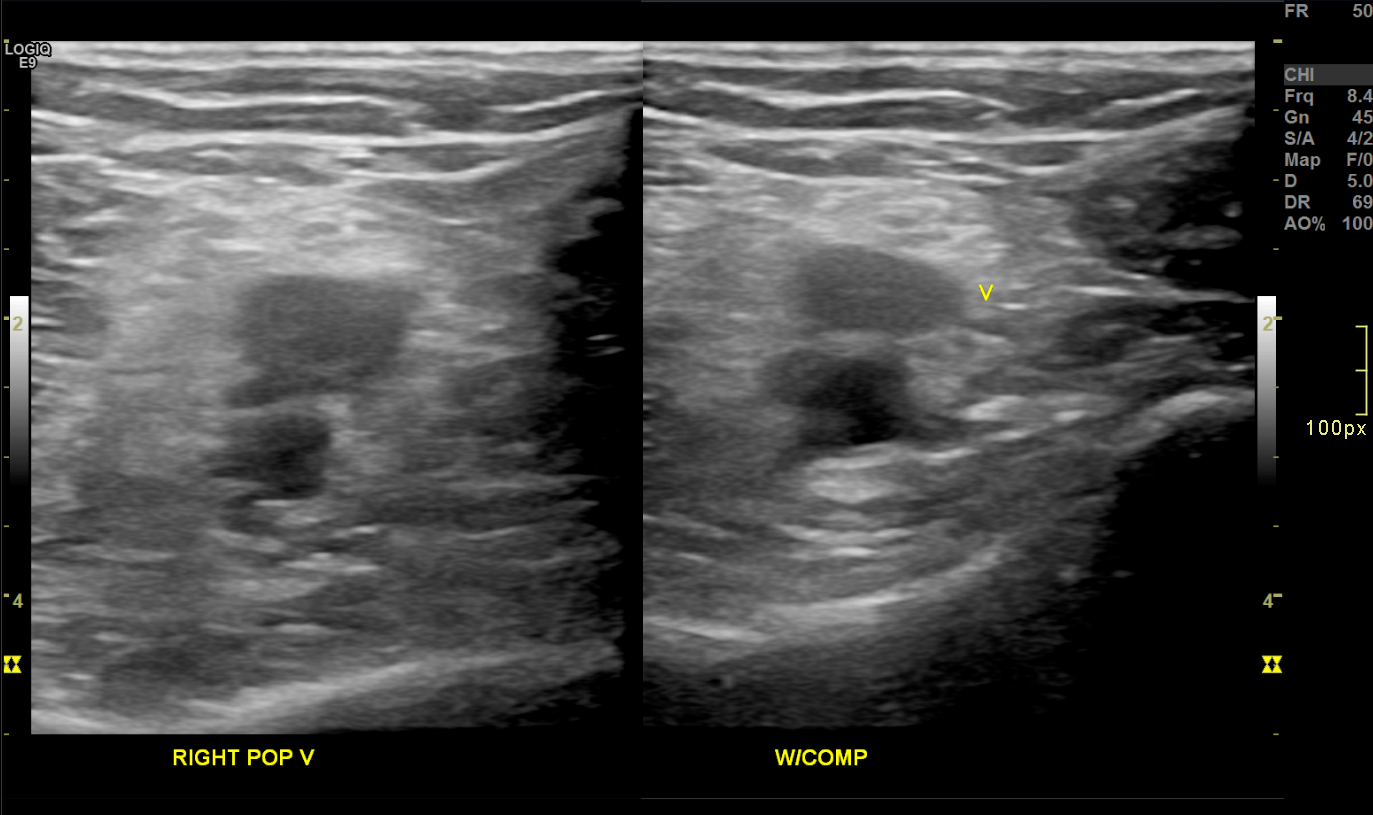
Acute Deep Venous Thrombosis on Brightness Mode. Transverse brightness mode images of the right popliteal vein before (left) and during (right) compression demonstrate noncompressibility and intraluminal echogenic material, consistent with acute deep vein thrombosis.
Contributed by Nathaniel Shapiro, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
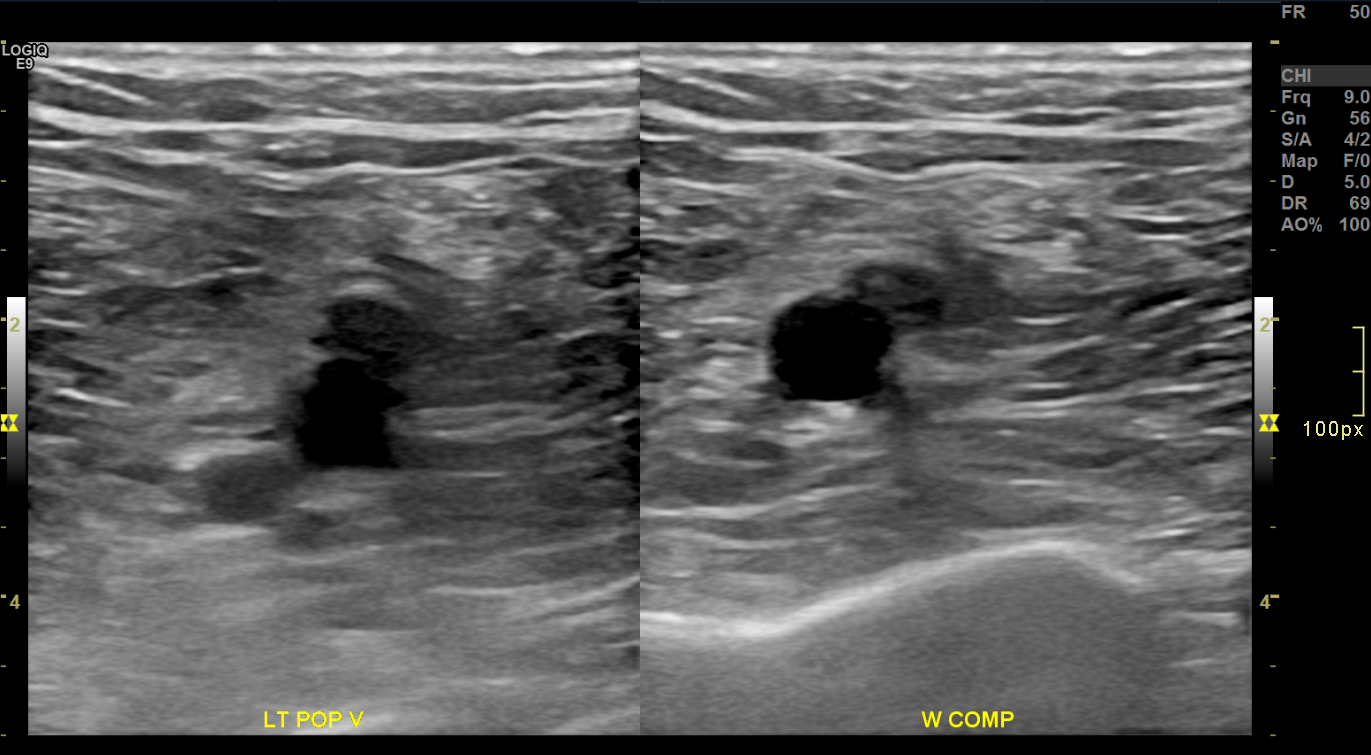
Chronic Deep Venous Thrombosis on Brightness Mode. Transverse brightness mode images of the left popliteal vein before (left) and during (right) compression demonstrate a partially compressible, eccentrically thickened venous segment. The lumen appears contracted and surrounded by echogenic material, consistent with chronic thrombus and vein wall fibrosis.
Contributed by Nathaniel Shapiro, MD
(Click Image to Enlarge)
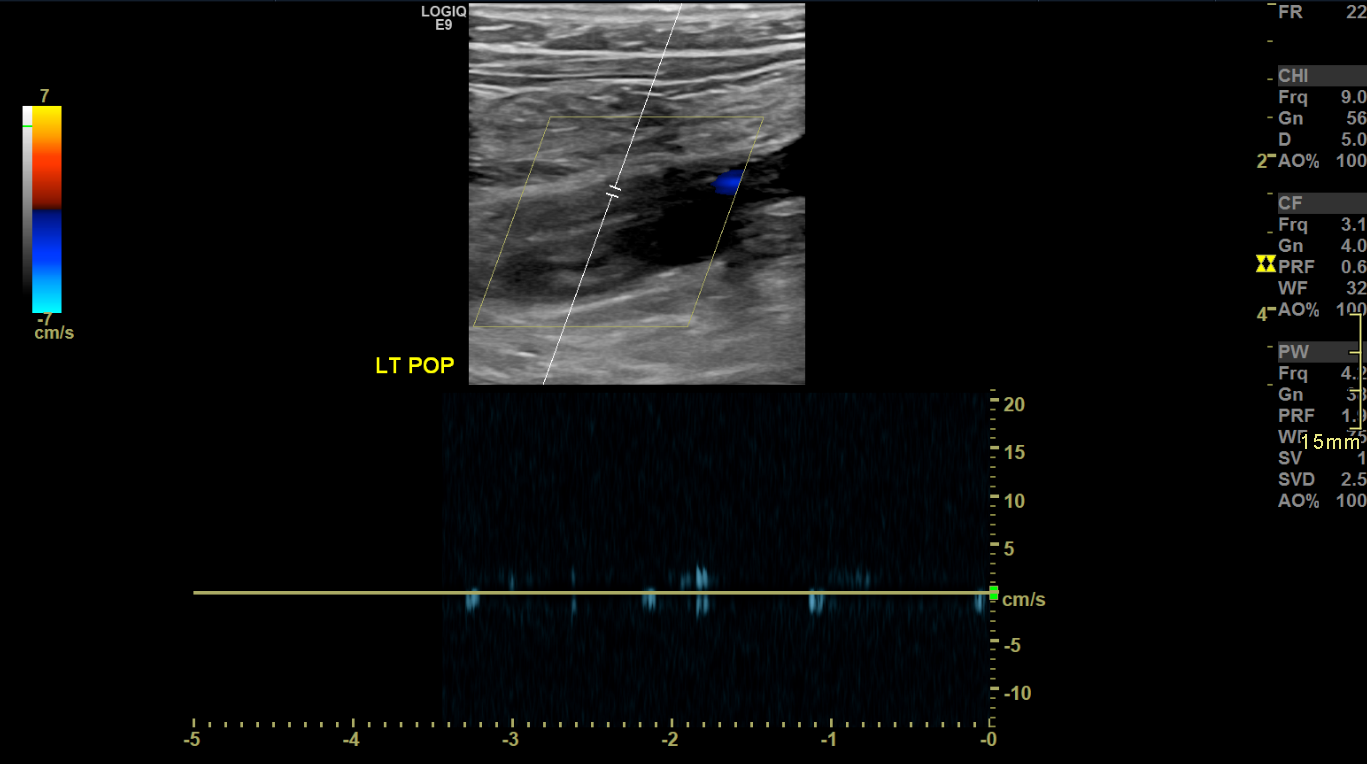
Chronic Deep Vein Thrombosis on Brightness Mode with Spectral Doppler Imaging, Long View. Longitudinal ultrasound image of the left popliteal vein demonstrates echogenic thrombus within a dilated, noncompressible vein segment. Minimal color flow is seen, consistent with chronic deep vein thrombosis. Spectral Doppler waveform (bottom panel) shows absent phasicity and low flow, supporting the diagnosis.
Contributed by Nathaniel Shapiro, MD
(Click Image to Enlarge)
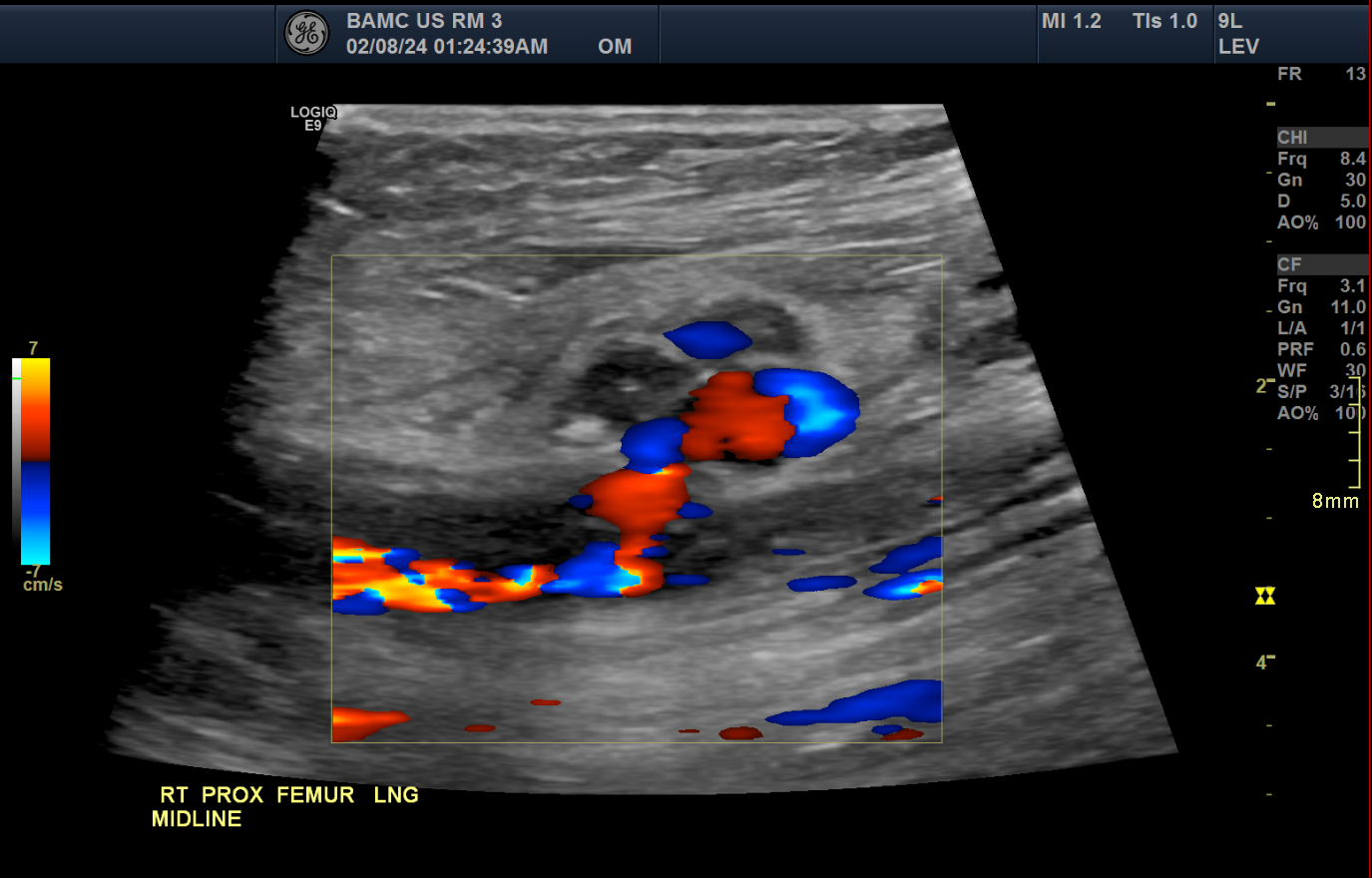
Common Femoral Artery Pseudoaneurysm on Doppler Ultrasound, Long View. Longitudinal color Doppler ultrasound of the right proximal femur demonstrates a pseudoaneurysm arising from the common femoral artery. The image shows characteristic swirling bidirectional flow, often described as the “yin-yang” sign, within the pseudoaneurysm sac. This finding is consistent with turbulent flow due to arterial leakage into a contained hematoma.
Contributed by Nathaniel Shapiro, MD
References
Dempsey PJ. The history of breast ultrasound. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2004 Jul:23(7):887-94 [PubMed PMID: 15292555]
Smith-Bindman R, Miglioretti DL, Larson EB. Rising use of diagnostic medical imaging in a large integrated health system. Health affairs (Project Hope). 2008 Nov-Dec:27(6):1491-502. doi: 10.1377/hlthaff.27.6.1491. Epub [PubMed PMID: 18997204]
Meissner MH, Moneta G, Burnand K, Gloviczki P, Lohr JM, Lurie F, Mattos MA, McLafferty RB, Mozes G, Rutherford RB, Padberg F, Sumner DS. The hemodynamics and diagnosis of venous disease. Journal of vascular surgery. 2007 Dec:46 Suppl S():4S-24S. doi: 10.1016/j.jvs.2007.09.043. Epub [PubMed PMID: 18068561]
Taylor KJ, Holland S. Doppler US. Part I. Basic principles, instrumentation, and pitfalls. Radiology. 1990 Feb:174(2):297-307 [PubMed PMID: 2404309]
Boote EJ. AAPM/RSNA physics tutorial for residents: topics in US: Doppler US techniques: concepts of blood flow detection and flow dynamics. Radiographics : a review publication of the Radiological Society of North America, Inc. 2003 Sep-Oct:23(5):1315-27 [PubMed PMID: 12975518]
Foshager MC, Finlay DE, Longley DG, Letourneau JG. Duplex and color Doppler sonography of complications after percutaneous interventional vascular procedures. Radiographics : a review publication of the Radiological Society of North America, Inc. 1994 Mar:14(2):239-53 [PubMed PMID: 8190950]
Revzin MV, Imanzadeh A, Menias C, Pourjabbar S, Mustafa A, Nezami N, Spektor M, Pellerito JS. Optimizing Image Quality When Evaluating Blood Flow at Doppler US: A Tutorial. Radiographics : a review publication of the Radiological Society of North America, Inc. 2019 Sep-Oct:39(5):1501-1523. doi: 10.1148/rg.2019180055. Epub 2019 Aug 9 [PubMed PMID: 31398088]
Level 2 (mid-level) evidenceAnaya-Ayala JE, Younes HK, Kaiser CL, Syed O, Ismail N, Naoum JJ, Davies MG, Peden EK. Prevalence of variant brachial-basilic vein anatomy and implications for vascular access planning. Journal of vascular surgery. 2011 Mar:53(3):720-4. doi: 10.1016/j.jvs.2010.09.072. Epub 2010 Dec 8 [PubMed PMID: 21144691]
Level 2 (mid-level) evidenceBehravesh S, Yakes W, Gupta N, Naidu S, Chong BW, Khademhosseini A, Oklu R. Venous malformations: clinical diagnosis and treatment. Cardiovascular diagnosis and therapy. 2016 Dec:6(6):557-569. doi: 10.21037/cdt.2016.11.10. Epub [PubMed PMID: 28123976]
Lewis BD, James EM, Charboneau JW, Reading CC, Welch TJ. Current applications of color Doppler imaging in the abdomen and extremities. Radiographics : a review publication of the Radiological Society of North America, Inc. 1989 Jul:9(4):599-631 [PubMed PMID: 2667050]
Needleman L, Cronan JJ, Lilly MP, Merli GJ, Adhikari S, Hertzberg BS, DeJong MR, Streiff MB, Meissner MH. Ultrasound for Lower Extremity Deep Venous Thrombosis: Multidisciplinary Recommendations From the Society of Radiologists in Ultrasound Consensus Conference. Circulation. 2018 Apr 3:137(14):1505-1515. doi: 10.1161/CIRCULATIONAHA.117.030687. Epub [PubMed PMID: 29610129]
Level 3 (low-level) evidenceGuideline developed in collaboration with the American College of Radiology, Society of Pediatric Radiology, Society of Radiologists in Ultrasound. AIUM Practice Guideline for the Performance of Peripheral Venous Ultrasound Examinations. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2015 Aug:34(8):1-9. doi: 10.7863/ultra.34.8.15.13.0002. Epub [PubMed PMID: 26206814]
Level 1 (high-level) evidence. AIUM Practice Parameter for the Performance of a Peripheral Venous Ultrasound Examination. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2020 May:39(5):E49-E56. doi: 10.1002/jum.15263. Epub 2020 Mar 12 [PubMed PMID: 32162338]
Hangiandreou NJ. AAPM/RSNA physics tutorial for residents. Topics in US: B-mode US: basic concepts and new technology. Radiographics : a review publication of the Radiological Society of North America, Inc. 2003 Jul-Aug:23(4):1019-33 [PubMed PMID: 12853678]
Kesteven P, Robinson B. Superficial thrombophlebitis followed by pulmonary embolism. Journal of the Royal Society of Medicine. 2001 Apr:94(4):186-7 [PubMed PMID: 11317624]
Level 3 (low-level) evidenceOrtel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, Hutten BA, Jaff MR, Manja V, Schulman S, Thurston C, Vedantham S, Verhamme P, Witt DM, D Florez I, Izcovich A, Nieuwlaat R, Ross S, J Schünemann H, Wiercioch W, Zhang Y, Zhang Y. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood advances. 2020 Oct 13:4(19):4693-4738. doi: 10.1182/bloodadvances.2020001830. Epub [PubMed PMID: 33007077]
Level 3 (low-level) evidence