Introduction
Pythium insidiosum is a parasitic aquatic oomycete in the family Pythiaceae, commonly found in aquatic environments in tropical, subtropical, and temperate regions. The organism causes corneal damage that resembles fungal keratitis both clinically and morphologically, earning the label "fungus-like organism."[1] First reported as a systemic infection in 1884, P. insidiosum affects both animals and humans, causing systemic and ocular infections, with higher prevalence in male individuals, particularly field workers.[2][3][2] Clinically, Pythium keratitis presents with features such as dry stromal infiltrates, feathery margins, satellite lesions, hypopyon, and corneal perforation, similar to fungal keratitis.
While the organism’s appearance mimics fungi, P. insidiosum is an oomycete more closely related to algae. Zoospore formation on culture media helps with identification and diagnosis. While antifungals are commonly used, they are ineffective due to the organism's lack of ergosterol. Cyanoacrylate glue has also been used as part of the treatment approach. However, antibacterial agents like linezolid and azithromycin have demonstrated higher efficacy and are the preferred treatments.[4] In nonresolving cases, early therapeutic penetrating keratoplasty (TPK) is recommended. However, the rapid proliferation, high virulence, and diagnostic challenges, such as limited access to advanced diagnostic tools, pose ongoing management challenges.
Over the last decade, the incidence of Pythium keratitis has risen significantly, particularly in Southeast Asia and India, often following corneal trauma from exposure to contaminated water or vegetation. Despite advancements in microbiological diagnostics, early identification remains difficult, and the disease is frequently misdiagnosed as fungal keratitis, delaying appropriate treatment and leading to poor outcomes.[5]
Pythium keratitis often mimics fungal ulcers, presenting with feathery borders, satellite lesions, and hypopyon. However, distinctive features like tentacular projections, a dry stromal surface with reticular infiltrates, peripheral guttering, and resistance to conventional antifungals can help clinicians identify the disease early. Routine laboratory techniques, such as potassium hydroxide (KOH) mounts or gram staining, are unreliable in distinguishing Pythium from fungal pathogens, and cultures may be delayed or misinterpreted.
Histopathological techniques, including hematoxylin and eosin (H&E) or Gomori methenamine silver (GMS) stains, may reveal broad, sparsely septate hyphae-like filaments. However, definitive identification typically requires molecular methods like polymerase chain reaction (PCR) or zoospore induction, neither of which is widely accessible in all practice settings. Misdiagnosis and delayed treatment can result in severe corneal destruction, sometimes necessitating surgical intervention or enucleation in advanced cases.[6]
One of the primary challenges in managing Pythium keratitis is its poor response to traditional antifungal therapies. As an oomycete, Pythium lacks ergosterol in its cell membrane, rendering common antifungal agents such as natamycin, amphotericin B, and voriconazole ineffective. Encouragingly, antibacterial agents like linezolid and azithromycin have shown in vitro efficacy and clinical success when used early, sometimes combined with systemic therapy. However, in many cases, medical therapy alone is insufficient, and early TPK is often required for better outcomes. Success rates are higher when surgery is performed before the organism breaches the Descemet membrane or causes panophthalmitis.
The rising incidence of Pythium keratitis has prompted discussions on prevention, public health awareness, and updated diagnostic protocols. Clinicians in endemic areas must maintain a high index of suspicion, especially when patients present with rapidly progressive corneal ulcers unresponsive to antifungals. A thorough patient history, including potential exposure to contaminated freshwater or agricultural environments, is crucial in narrowing the differential diagnosis. Interprofessional collaboration with microbiologists and pathologists is vital for timely diagnosis and treatment. Advances in techniques such as real-time PCR, confocal microscopy, and zoospore culture methods have improved diagnostic yields in specialized centers, although access and turnaround time remain limitations in many low-resource settings.[7]
Pythium keratitis produces biflagellate zoospores that swim in aqueous environments and penetrate corneal tissue through minor abrasions. Once in the corneal stroma, P. insidiosum proliferates rapidly, triggering an intense inflammatory response. This response often leads to extensive tissue damage, contributing to high rates of corneal perforation and poor visual prognosis in untreated or late-treated cases. Understanding of the host immune response and resistance mechanisms remains limited, with ongoing research aiming to identify novel therapeutic targets.[7]
Epidemiological studies show a seasonal spike in cases, especially during the monsoon, when exposure to contaminated water is more likely. The disease predominantly affects young, economically active individuals in rural areas, highlighting the socioeconomic burden. In certain regions, Pythium accounts for a significant proportion of culture-positive microbial keratitis cases, often outnumbering bacterial and fungal infections. This trend calls for Pythium-specific diagnostic and treatment protocols to be integrated into national and international guidelines for keratitis.[8]
Managing Pythium keratitis challenges traditional classifications of corneal infections. Early and accurate diagnosis is critical for initiating nontraditional therapies and planning surgery. Ophthalmic centers have proposed scoring systems based on ulcer size, depth, location, hypopyon presence, and symptom duration to predict prognosis and guide treatment. Though research into adjunctive therapies like collagen cross-linking, immunomodulation, and topical antiseptics is ongoing, evidence remains limited and anecdotal.[9]
Beyond treatment, educating clinicians, trainees, and allied health professionals about this pathogen has become crucial. Including Pythium in academic curricula, cornea-focused continuous medical education activities, and residency training programs can enhance early recognition and improve patient outcomes. Equally important is patient education—informing at-risk populations about the dangers of untreated corneal trauma and the need for prompt medical attention. Public health campaigns in endemic areas can help reduce the burden of advanced cases requiring surgical intervention.[10]
Pythium keratitis presents a formidable challenge in corneal infectious diseases. The pathogen's unique microbiological profile, resistance to standard antifungal agents, and rapidly destructive clinical course distinguish it from more common pathogens, necessitating a tailored approach to diagnosis and treatment. Growing global awareness, collaborative research efforts, and evidence-based clinical strategies better position the ophthalmic community to combat this neglected but impactful disease. However, continued vigilance, innovation, and education remain essential in bridging the gap between current clinical practice and optimal patient care in the face of this evolving pathogen.[11]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Pythium belongs to the genus of parasitic oomycetes.[12] Once classified as fungi, the majority of Pythium species are plant parasites, but P. insidiosum has emerged as an important pathogen in humans and animals, causing both ocular and systemic pythiosis. Over 120 species of Pythium have been reported worldwide, with P. insidiosum being a notable cause of sight-threatening keratitis in humans. Various clinical forms, including ocular, cutaneous, subcutaneous, vascular, and disseminated pythiosis, have been identified. Systemic involvement manifests as ulcerative lesions and granulomas on the skin, face, limbs, and subcutaneous tissues. Vascular manifestations include aneurysms, thrombosis, and vasculitis.[13]
Systemic associations with P. insidiosum infection have been noted in conditions such as thalassemia or hemoglobinopathy syndrome, paroxysmal nocturnal hemoglobinuria, aplastic anemia, chronic arterial insufficiency syndrome, and cavernous sinus thrombophlebitis. Previous studies highlight risk factors related to environmental exposure, including injuries from mud, grass, soil, and sticks, particularly in occupations involving work in aquatic environments.
Pythium keratitis is caused by P. insidiosum, a filamentous, aquatic organism classified under the kingdom Straminipila and phylum Oomycota. Unlike true fungi, which contain ergosterol in their cell membranes and chitin-rich cell walls, Pythium species lack ergosterol and instead have cellulose and β-glucans in their cell walls. This structural difference not only has taxonomic significance but also impacts the organism’s resistance to antifungal agents, rendering standard antifungal therapies largely ineffective.[14]
P. insidiosum is a saprophytic pathogen found in stagnant water, swamps, flooded fields, and decaying vegetation, predominantly in tropical and subtropical regions. The microbe thrives in warm, humid environments and is endemic in Southeast Asia, India, Thailand, and northern Australia. The organism uniquely produces biflagellate zoospores—motile forms that swim actively in water. These zoospores are the infective units of P. insidiosum and can exhibit chemotaxis, allowing them to locate and adhere to damaged epithelial surfaces of the cornea.[15]
The typical route of corneal infection begins with trauma involving exposure to contaminated water or vegetation, particularly among agricultural workers, children, or individuals living in rural or flood-prone areas. Even minor abrasions to the corneal epithelium serve as portals of entry. Once the zoospores attach, they encyst and germinate, leading to hyphae-like filamentation into the corneal stroma. P. insidiosum then proliferates rapidly, triggering a significant inflammatory response and causing extensive tissue destruction.[16]
Interestingly, P. insidiosum does not require an immunocompromised host and primarily infects healthy individuals, distinguishing it from opportunistic corneal pathogens. Pythium keratitis pathogenesis is driven by both mechanical invasion and immunologically mediated damage. The organism’s hyphae extend deeply and diffusely through corneal tissue, often resulting in ring infiltrates, tentacle-like projections, and peripheral guttering, all hallmark features seen on slit-lamp examination.[17]
Thus, the etiology of Pythium keratitis revolves around a unique aquatic oomycete capable of causing aggressive corneal infection through direct traumatic inoculation. The microbe's motile, flagellated zoospores and ability to mimic fungal morphology while resisting antifungal therapy make it distinct and challenging to treat. Understanding the pathogen's environmental niche, structural biology, and infection mechanism is essential for early recognition and effective treatment.[18]
Epidemiology
The first case of systemic pythiosis was described in 1884 by British veterinarians in horses. However, sight-threatening ocular infection in the form of keratitis has been primarily reported from Thailand, the U.S., China, Israel, Australia, and India.[19]
The first case of systemic pythiosis in a human was documented in Thailand in 1985.[20] However, the first case of Pythium keratitis was recorded in 1988, and since then, large-scale studies from all over the globe have been conducted. In animals, the condition occurs in tropical, subtropical, and temperate climates, with cases reported from Australia, Argentina, Brazil, Costa Rica, India, and Indonesia. Studies quoting the incidence and prevalence of Pythium keratitis are scarce in the literature due to the rarity of the organism. P. insidiosum exists in all age groups, with a higher incidence in students and software professionals. In older adults, the majority of patients are men and outdoor workers.
Pythium keratitis is an emerging ocular infection with significant geographical and seasonal predilections. Once considered rare and primarily affecting animals, this infection has gained increasing clinical relevance as a cause of severe microbial keratitis in humans, especially in tropical and subtropical regions. Epidemiological evidence suggests that P. insidiosum is particularly prevalent in South and Southeast Asia, with the highest reported cases from India (especially in states like Tamil Nadu, West Bengal, and Maharashtra) and Thailand. Sporadic reports have also emerged from Australia, Taiwan, Sri Lanka, and the southern U.S., underscoring its expanding geographic footprint.[21]
Environmental conditions play a central role in the distribution of P. insidiosum infections. The organism thrives in stagnant water, paddy fields, flooded lands, and areas with high humidity and warmth, which promote the formation of its infective biflagellate zoospores. Pythium keratitis often peaks during the monsoon and post-monsoon seasons, when exposure to contaminated water is more likely due to increased agricultural activity and waterlogging. Such environmental factors make this infection particularly common in rural, agrarian communities.[22]
Demographically, Pythium keratitis most frequently affects young, healthy individuals, unlike other types of opportunistic keratitis that tend to occur in the elderly or immunocompromised. Most patients are male individuals in the 2nd to 4th decades of life, likely reflecting higher occupational exposure to soil and water through farming and outdoor work. A consistent history of minor corneal trauma, often with vegetative or waterborne material, is seen in the majority of cases. Unlike fungal keratitis, where diabetes or immunosuppression may play a role, P. insidiosum targets otherwise healthy corneas following mechanical epithelial disruption.[23]
While the exact global incidence of Pythium keratitis remains underreported, partly due to misdiagnosis as fungal keratitis, its proportion among culture-positive microbial keratitis cases is rising in endemic regions. Several tertiary eye care centers in India have reported that P. insidiosum now accounts for up to 5% to 10% of microbial keratitis cases in certain seasons, especially during monsoons. The apparent increase in incidence may be partially due to better awareness and improved diagnostic capabilities, including PCR-based testing and zoospore culture techniques.
P. insidiosum has not been recognized as a distinct pathogen for many years, contributing to underdiagnosis and mismanagement. Historically grouped with fungi due to its similar clinical and microscopic features, failing to distinguish P. insidiosum from fungal keratitis has led to poor outcomes, including higher rates of corneal perforation, TPK, and even enucleation or evisceration.
The epidemiology of Pythium keratitis highlights an environmentally driven disease, geographically concentrated in warm, humid, agrarian regions, and demographically distinct in its predilection for healthy young adults. The rising incidence of this ocular condition, particularly in endemic countries, demands continued surveillance, improved awareness among clinicians, and inclusion of Pythium-specific diagnostic algorithms in evaluating microbial keratitis.
Pathophysiology
Austwick and Copland stimulated the production of Pythium zoospores and proposed that these particles play a crucial role in pathogenesis.[24] Mendoza et al further analyzed that these zoospores are particularly inclined toward animal wounds, hairs, damaged skin, and mucosa of the intestine.[25]
Once the zoospores encounter wounded tissues, they encyst and secrete glycoproteins that facilitate adhesion to the surface. The host body temperature also acts as a stimulus for these zoospores. This process leads to the formation of hyphae, which extend into the infected tissue and penetrate blood vessels. The high incidence of Pythium keratitis in thalassemia patients may be linked to iron overload, with hemochromatosis being another significant predisposing factor. Iron overload inhibits the phagocytic actions of tumor necrosis factor α (TNF-α), interferon γ (IFN-γ), macrophages, and neutrophils.
Udnaen et al demonstrated that monocytes and macrophages in these patients produce lower levels of IFN-γ in response to zoospore exposure, indicating inhibition of the T helper 1 (Th1) cell response. This suppression may explain the increased susceptibility to infection in these individuals. P. insidiosum is also believed to induce T helper 2 (Th2) cells, and antigen immunotherapy can stimulate an immune shift from Th2 to Th1, which is thought to be curative for thalassemia patients. Another hypothesis suggests that P. insidiosum encodes a gene for ferrochelatase, a potential factor contributing to its high virulence in these patients.[26]
Pythium keratitis is a distinct corneal infection caused by an organism with unique biological and pathogenic characteristics. Unlike typical fungal or bacterial corneal pathogens, P. insidiosum is a filamentous aquatic organism from the class Oomycetes, phylum Oomycota—more closely related to algae than true fungi. This distinction plays a crucial role in its disease behavior, tissue invasion, and therapeutic challenges.[27]
The infective stage of P. insidiosum is the biflagellate zoospore, which is motile and responsive to chemotactic cues. These zoospores are found in stagnant water, marshes, paddy fields, and wet vegetation and can actively migrate toward damaged epithelial surfaces of the cornea. The zoospores germinate and produce hyphae-like structures that invade the corneal stroma upon contact with the corneal surface, often following minor trauma.[28]
Once within the corneal stroma, P. insidiosum exhibits aggressive tissue invasion. The pathogen's filamentous structures are broad, sparsely septate, and often aseptate, resembling fungal hyphae under microscopy. These hyphae penetrate deeply into the corneal lamellae, facilitated by the secretion of lytic enzymes and proteases that degrade collagen and the stromal extracellular matrix. Additionally, P. insidiosum may form biofilm-like aggregates within the corneal tissue, which likely contributes to its resistance against both host immune responses and conventional antimicrobial treatments.
A critical component of the pathophysiology is the intense neutrophilic and macrophage-mediated inflammatory response triggered by the invading oomycete. The host immune system mounts a strong but often ineffective response due to the organism's unique surface antigens and immune evasion strategies. The resulting inflammatory cascade leads to rapid tissue destruction, necrosis, and stromal melting, often out of proportion to the size of the initial ulcer. In advanced cases, the infection can reach the Descemet membrane and even involve the anterior chamber, leading to endothelial damage, corneal perforation, and, in some cases, panophthalmitis.[29]
P. insidiosum makes conventional antifungal agents like natamycin, voriconazole, and amphotericin B largely ineffective. The microbe's distinct cell wall biochemistry contributes to the difficulty in treating Pythium keratitis, highlighting the importance of early suspicion, accurate diagnosis, and timely surgical or alternative medical intervention.[30]
Histologically, infected corneal tissue shows infiltration by broad, ribbon-like hyphae with minimal septation, surrounded by an intense granulomatous and suppurative reaction. Vascular endothelial damage and necrosis can also occur, contributing to the clinical findings of ring infiltrates, tentacular extensions, and rapid peripheral corneal thinning or guttering. These features are often accompanied by a recalcitrant hypopyon and a dry-looking ulcer bed, which helps distinguish the condition from some forms of microbial keratitis.[31]
Overall, the pathophysiology involves aggressive stromal invasion by hyphal elements, enzymatic corneal degradation, and a robust yet insufficient host inflammatory response, all exacerbated by the pathogen's resistance to conventional antifungal therapy. This distinctive behavior underscores the importance of early recognition, differentiation from fungal keratitis, and targeted medical or surgical treatment initiation to prevent irreversible visual morbidity.[32]
Histopathology
A variety of histopathological diagnostic techniques are available for Pythium keratitis. On a 10% KOH wet mount and gram stain, the organism possesses slender, long, septate or aseptate hyaline hyphae with perpendicular lateral branches, mimicking fungal hyphae. However, numerous vesicles are often present in the hyphae of Pythium, distinguishing it from fungi.
Flat, feathery colonies form on blood agar and Sabouraud dextrose agar (SDA), along with zoospore formation. Zoospore presence is confirmed by the leaf incarnation method. Staining with iodine potassium iodide (IKI), combined with sulfuric acid (IKI-H2SO4), is another method for identifying hyphae. Pythium hyphae turn blue or bluish-black in IKI, indicating a positive result, while yellow or yellowish-brown staining is negative. Trypan blue can also help identify both aseptate and septate hyphae, particularly in rural settings where other diagnostic methods may not be available.[33]
Periodic acid-Schiff (PAS) staining is used by treating slides with 0.5% and 1% periodic acid for varying times and applying Schiff reagent for 10 minutes. Positive staining appears pink, while negative results show no color change. On H&E staining, Pythium filaments appear pale pinkish to ghost-like. GMS staining turns Pythium filaments brown, with the stroma appearing greenish. The filaments on GMS may be septate or aseptate, broad or narrow, and may exhibit obtuse to perpendicular branching, swollen hyphae, or twisted, folded, collapsed, or hollow tube morphologies.[34]
Histopathological examination is pivotal in definitively diagnosing Pythium keratitis, especially in cases where microbiological methods yield inconclusive or delayed results. Given P. insidiosum's clinical resemblance to fungal keratitis, careful tissue analysis is essential for accurate differentiation, as treatment strategies differ significantly.
The hallmark histopathological finding in Pythium keratitis is the presence of broad, ribbon-like, sparsely septate or aseptate hyphal elements infiltrating the corneal stroma. These filaments are often irregular in width, with a characteristic right-angle branching pattern and occasional bulbous terminal ends. Although these features may mimic those of fungi such as Zygomycetes, closer evaluation, in conjunction with clinical history and staining characteristics, aids in correct identification.
On H&E staining, the corneal tissue shows dense inflammatory infiltration surrounding the hyphal elements, primarily composed of neutrophils and macrophages. Necrosis of stromal collagen and edema are common. The organism itself often appears as refractile, pale-staining structures with indistinct walls on H&E, making further staining crucial for identification.
Special stains such as PAS and GMS are widely used to highlight the organism more clearly. On GMS staining, P. insidiosum filaments appear as black, thick-walled hyphae that are aseptate or sparsely septate and show branching at right angles. PAS may stain the filaments weakly, a useful clue for differentiation from true fungal organisms, which typically stain more intensely due to chitin-rich walls. Additionally, Calcofluor white stain (CFW), which binds to cellulose and chitin, may be used in fluorescent microscopy to demonstrate the presence of P. insidiosum filaments, which have cellulose in the cell walls.
An important distinguishing feature from fungal keratitis is the absence of angioinvasion and lack of fruiting bodies or spores, which are often observed in fungal infections. Instead, the organism elicits a strong suppurative and granulomatous host response, which, although intense, is often ineffective in controlling the spread of infection. Occasionally, eosinophilic material surrounding the filaments (Splendore-Hoeppli phenomenon) may be seen, but it is not pathognomonic.[35]
When deeper stromal or full-thickness biopsies are examined, with samples often obtained during TPK, the organism can be seen extending up to the Descemet membrane and, in some cases, infiltrating into the anterior chamber or the iris, especially in advanced disease. The extent of stromal invasion is often disproportionate to the superficial lesion, reflecting the organism’s aggressive tissue-destructive potential.
Staining Differences Between Pythium and Fungi
The filaments of P. insidiosum stain differently from fungi, with PAS staining the filaments weakly or inconsistently, ranging from pink to pale magenta. This variation is due to the cellulose in the P. insidiosum cell wall, which reacts less strongly with pectin and chitin compared to fungal cell walls. The staining intensity also depends on the duration and concentration of periodic acid.
On H&E staining, P. insidiosum filaments appear as pale, poorly staining, ribbon-like structures. The filaments are broad, sparsely or completely aseptate, with indistinct borders, making them difficult to visualize without special stains. The inflammatory background often consists of neutrophils, and stromal necrosis is pronounced. In contrast, fungal filaments, such as those of Fusarium or Aspergillus, stain more intensely, showing regular septation, thinner and more uniform walls, and dichotomous branching at 45° angles, making them easier to detect without the need for special stains.
GMS staining is particularly useful for identifying P. insidiosum, as the hyphae stain black, are broad (5–10 μm), and exhibit right-angle branching, often being aseptate or sparsely septate. The GMS stain highlights these filaments against a green background, which helps distinguish them from host tissues. Fungal hyphae also stain black with GMS but are typically more slender, exhibit frequent septation, and show regular dichotomous branching, as seen with Aspergillus at 45° angles or Fusarium, which may have more irregular branching patterns.[36]
In PAS staining, P. insidiosum generally shows weak or inconsistent results due to its lack of chitin and ergosterol in its cell wall, making it less reactive to PAS compared to fungi, whose chitin-rich walls strongly bind to the stain, appearing magenta or reddish-purple. This difference in PAS staining can provide a useful diagnostic clue.[37]
CFW binds to cellulose and β-glucans, components of the P. insidiosum cell wall, resulting in fluorescence, although the intensity may be lower than in fungi. Fungal cell walls, which contain chitin, bind strongly to CFW, leading to bright fluorescence under UV light, making it an effective tool for detecting fungal infections, though it is not specific and may also highlight P. insidiosum.[38]
Morphological Clues Beyond Staining
P. insidiosum filaments are typically aseptate or sparsely septate, with a broad hyphal width of 5 to 10 μm, and they display irregular, right-angle branching. The cell wall is composed of cellulose and β-glucans, which results in weak PAS staining. GMS staining, however, strongly highlights the hyphae. In contrast, fungal hyphae are septate, with a narrower width of 2 to 5 μm and acute-angle (45°), dichotomous branching. The pathogen's cell wall, composed primarily of chitin and glucans, shows strong PAS staining and also stains strongly with GMS. Fungi, such as Aspergillus, may also form spores or fruiting bodies, unlike P. insidiosum, which lacks these structures.
Misidentifying P. insidiosum as a fungus based on morphology alone can result in inappropriate treatment. Antifungal agents like voriconazole or amphotericin B are ineffective since P. insidiosum lacks ergosterol. Accurate differentiation through histopathology and staining is crucial to ensure early treatment with appropriate interventions, such as linezolid, azithromycin, or TPK.[39]
Toxicokinetics
In Pythium keratitis, the traditional concept of toxicokinetics, focusing on absorption, distribution, metabolism, and excretion of toxic substances, does not directly apply to the disease process. P. insidiosum is an infectious organism, not a toxic entity. However, toxicokinetic principles are relevant when managing the infection, especially in the off-label use of antibacterial agents like linezolid and azithromycin, which have demonstrated in vitro inhibitory effects against P. insidiosum.[40]
Understanding the ocular pharmacokinetics and tissue penetration of linezolid and azithromycin is crucial to ensure effective therapeutic concentrations in the cornea and aqueous humor while minimizing systemic toxicity. Linezolid, an oxazolidinone antibiotic, shows good corneal penetration when used topically, achieving therapeutic levels in ocular tissues. Although systemic linezolid may be used adjunctively in severe cases, it carries risks of bone marrow suppression, optic neuropathy, and serotonin syndrome with prolonged use.
Azithromycin, a macrolide antibiotic, binds well to tissues and has a long half-life, achieving sustained concentrations in ocular tissues when applied topically. This agent's systemic absorption is minimal, reducing concerns about systemic toxicokinetics.
Toxicokinetics is not central to the pathophysiology of Pythium keratitis. Nevertheless, ocular bioavailability and potential systemic toxicity of repurposed drugs are important to consider when treating this infection.
History and Physical
Patients with Pythium keratitis present with a history of pain, redness, photophobia, blurred vision, irritation, watering, and occasional discharge. An antecedent history of trauma, such as stick injury, mud injury, dust fall, clay injury, or contact lens use, may be noted. However, no history of trauma is elicited in some cases.[41]
Anterior segment findings in Pythium keratitis are similar to those seen in fungal corneal ulcers. These findings include lid edema, blepharospasm, conjunctival congestion, discharge, epithelial defects, stromal infiltrates, feathery margins, satellite lesions, and stromal edema. Other features that may mimic fungal keratitis include corneal melt, descemetocele, endothelial plaque, hypopyon, anterior chamber exudates, ring infiltrates, Wessely’s immune ring, and corneal perforation (see Image. Pythium Keratitis with Central Perforation and Limbal Spread).[42] The clinical signs that specifically distinguish P. insidiosum from other infective keratitis include stromal infiltrates with hyphal edges, multifocal lesions, tentacular projections, peripheral furrowing with thinning, guttering, and a tendency for early spread toward the limbus.[43]
Rare clinical features resembling Acanthamoeba keratitis include radial keratoneuritis with stromal infiltrates, often seen in mixed infections with Acanthamoeba and P. insidiosum, particularly in patients with a history of contact lens use. Some features may also mimic atypical Mycobacteria, such as epithelial breaches, grayish-white stromal infiltrates, dry, cracked windshield-like appearance of the infiltrates, stromal edema, and Descemet membrane folds.[44]
Patients with Pythium keratitis often have a history suggestive of this rare oomycete infection, typically following minor corneal trauma. Most cases occur in tropical or subtropical regions, with many patients reporting a preceding corneal injury, which could be as trivial as a dust particle or insect strike. Exposure to contaminated water is a notable risk factor, such as splashes of pond or river water into an eye with an epithelial defect, introducing Pythium zoospores. Other reported triggers include vegetative matter injury, soil or dust exposure, and trauma from agricultural debris or cement.
Demographically, Pythium keratitis commonly affects young, otherwise healthy adults, particularly rural farmers and urban dwellers. Male individuals may be more frequently affected due to greater outdoor exposure and agricultural work. Unlike fungal infections, which often occur in immunocompromised patients, Pythium keratitis typically affects immunocompetent hosts. However, the condition has also been reported in patients with systemic vulnerabilities, such as those with thalassemia or on immunosuppressive therapy for conditions like Crohn disease.
An uncommon but notable risk is contact lens use combined with water exposure, with reports of Pythium keratitis in contact lens wearers who swam in freshwater or had mud contamination on their lenses. Thus, a history of minor eye trauma in a wet, rural, or agricultural setting, such as muddy water or plant matter contacting the eye, should raise suspicion for Pythium keratitis. This history, along with a compatible exam, is crucial for early diagnosis in endemic areas.
Symptoms
Patients with Pythium keratitis present with acute eye pain and redness, typical of severe corneal infections. These individuals often report noticing a "white spot" or visible whitish opacity in the cornea. Common symptoms include intense ocular pain, foreign-body sensation, tearing, and photophobia. Blurred vision in the affected eye is also typical. Mucopurulent or watery discharge may occur, though it is less prominent compared to bacterial infections.
The time from corneal injury or symptom onset to presentation varies, with patients seeking care anywhere from a couple of days to several weeks after the initial event, with documented cases ranging from about 2 days to 2 months. The symptom profile of Pythium keratitis closely resembles other forms of microbial keratitis, making clinical history and exam findings crucial for accurate diagnosis.[45]
Slit-Lamp Examination Findings
On examination, Pythium keratitis closely resembles a severe fungal corneal ulcer, earning it the nickname "parafungal" infection. Slit-lamp findings often overlap with those of filamentous fungal keratitis, including a large corneal infiltrate with a feathery appearance, surrounding inflammation, and sometimes a hypopyon. However, several characteristic clues on the ocular exam can help suggest Pythium as the cause.[46]
Corneal Ulcer Morphology
The corneal ulcer typically appears as a dense grayish-white infiltrate in the corneal stroma, which may be central or paracentral, though peripheral presentation is also possible. The infiltrate is often large (several millimeters in diameter) and can be full-thickness. The surface of the ulcer is often raised with necrotic slough or flocculent white material attached, giving it a "cotton wool" or fluffy appearance. The edges of the infiltrate are usually ill-defined and serrated, with feathery margins commonly observed in Pythium keratitis, contributing to its fungus-like appearance. An epithelial defect (corneal ulceration) is often present over the infiltrated area, typically with undermined edges of epithelium at the lesion's margins.[47]
A distinguishing feature of the ulcer’s morphology is the presence of multiple foci. Patients may have multifocal infiltrates or satellite lesions around the main abscess, resembling fungal ulcers. These satellite lesions are small, white, secondary stromal opacities separate from the primary lesion. Additionally, a ring-shaped infiltrate (annular lesion) may develop in some cases, another feature shared with advanced fungal keratitis.
The cornea around the ulcer is typically edematous, appearing hazy, due to inflammation. Rapid stromal melting can occur as the infection progresses, and the ulcer may deepen to the Descemet membrane, leading to a descemetocele, ultimately risking perforation if not managed. This aggressive tissue destruction is characteristic of Pythium's fulminant course, often progressing despite standard antimicrobial therapy, with corneal perforation being a known outcome in advanced cases, similar to severe fungal keratitis.[48]
Characteristic Stromal Features
While many aspects of the ulcer resemble fungal keratitis, Pythium keratitis has several distinctive stromal features that experienced observers recognize. These features include unique patterns of infiltration in the stroma and corneal periphery. One of the most notable signs is the presence of "tentacle-like" extensions that radiate from the main infiltrate into the surrounding cornea. These tentacular projections appear as slender linear opacities or hyphae-like strands that extend radially from the edges of the ulcer into the clearer cornea. These projections can be single or multiple and often reach into midstromal depth. The structures represent strands of organisms and inflammatory material tracking through the stroma and are highly suggestive of Pythium. In contrast, fungal ulcers typically show feathery, indistinct margins rather than discrete linear "tentacles."[49]
Another characteristic finding is the pattern of small, pinpoint infiltrates in the anterior stroma, often surrounding the main lesion. These patchy, reticular, dot-like infiltrates create a net-like pattern under the epithelium, resembling numerous tiny white opacities in the subepithelial or superficial stromal layer. Over time, these infiltrates may coalesce or expand. This pattern of expanding dot infiltrates is a strong diagnostic clue, as it is not commonly seen in typical bacterial or fungal keratitis. The presence of both "tentacle lesions" and pinhead infiltrates strongly supports the diagnosis of Pythium keratitis.[50]
Additionally, Pythium keratitis often causes distinctive peripheral stromal thinning around the edge of the corneal infiltrate. This thinning, known as peripheral guttering or furrowing, appears as a circular or crescent-shaped excavation at the periphery of the ulcer. This annular trough of stromal melt is most visible with optic section illumination, showing intense enzymatic destruction of the stroma at the lesion’s margin. Peripheral furrowing is considered a hallmark feature of Pythium and is less commonly seen in other infectious keratitis, though a similar thinning may occasionally occur in severe immune-mediated ulcers. Notably, this guttering becomes more pronounced as the infection heals and the central infiltrate contracts.[51]
A striking feature in many P. insidiosum cases is the rapid spread of infection to the limbus and beyond. In these cases, the infiltrates often extend toward the corneal periphery, with limbal-scleral involvement occurring earlier than in fungal ulcers. On slit-lamp examination, the corneal infiltrate may reach the limbal border or even cause adjacent scleral whitening and inflammation. In aggressive cases, 360° limbal involvement has been observed, and some patients develop adjacent scleritis. This rapid peripheral spread is a key differentiator from fungal keratitis, which typically begins centrally and gradually extends outward.[52]
Alongside peripheral guttering, corneal thinning (melt) can progress rapidly in Pythium infections. Within days, an ulcer can advance from superficial to near-perforation depth, reflecting the severe nature of the infection. One proposed severity grading system categorizes "early melt" as a hallmark of severe Pythium keratitis, while fungal ulcers usually take longer to cause such extensive melt. Clinically, early corneal thinning can present as a markedly thinned ulcer bed with a high risk of perforation, sometimes requiring urgent TPK.
Not all hallmark features are present in every case of Pythium keratitis. Some cases may initially lack tentacle-like projections or reticular dots, resembling a routine fungal ulcer. However, the presence of characteristic stromal signs—such as tentacle-like radial infiltrates, pinhead-sized dots, peripheral furrowing, or early limbal spread—strongly suggests Pythium in the differential diagnosis.[53]
Anterior Chamber Reaction
The reaction within the anterior segment in Pythium keratitis can range from mild to severe, depending on the disease stage. Early in the infection, the reaction may be minimal, with a few cells and flare, or even a quiet segment. A hypopyon (layered pus) is often absent, which is atypical for such a severe corneal ulcer. In bacterial or fungal infections, a hypopyon is more common with large infiltrates. In moderate cases, a small hypopyon may develop, but as the infection progresses, a more pronounced hypopyon is frequently observed. In severe cases of Pythium keratitis, hypopyon is present in most instances, often accompanied by fibrinous debris in the chamber. The hypopyon may be less responsive to steroid or antifungal treatment, as antifungals are ineffective against the pathogen, and may persist or enlarge until proper therapy is started.
Endothelial exudates or plaques on the posterior cornea are also commonly seen, appearing as white fibrinous deposits beneath the ulcer, signaling severe inflammation. These plaques may also be seen in fungal ulcers, making them a common feature rather than a distinguishing one. While posterior synechiae or fibrin in the pupil is rare at presentation, it can occur if the inflammation is severe or treatment is delayed, resulting in iridocyclitis spillover.[54]
Conjunctival and Other Ocular Findings
The external examination typically reveals a red eye with marked conjunctival injection and ciliary flush. Lid edema is frequently present due to intense anterior segment inflammation. Mild scleritis adjacent to the ulcer may also be noted, particularly if the infection has spread to the limbus. Despite the severe corneal involvement, frank endophthalmitis (infection inside the eye) is rare at the time of presentation, with the pathogen largely confined to the corneal tissues unless the infection is very advanced or follows surgical intervention. However, panophthalmitis may eventually develop if the ulcer remains uncontrolled.[55]
Key Differentiators from Fungal Keratitis
Distinguishing Pythium keratitis from the more common filamentous fungal keratitis is challenging but essential, as their management differs. Both can appear similar on slit-lamp examination, but certain features favor Pythium infection. One such characteristic is the presence of tentacle-like stromal projections. Pythium often shows linear tentacular infiltrates radiating from the main lesion, a feature rarely seen in fungal ulcers, which typically have feathery, branching margins instead of distinct tentacles.
Another key indicator is the presence of reticular "dot" infiltrates. The appearance of numerous pinpoint subepithelial infiltrates in a reticular pattern is characteristic of Pythium. Fungal keratitis typically lacks this peppering of tiny lesions. While fungi may produce satellite lesions or a ring infiltrate, they do not form the fine reticular dots characteristic of Pythium.[56]
Peripheral furrowing and thinning around the ulcer are also commonly seen in Pythium keratitis. Although fungal ulcers can cause thinning, a pronounced furrow at the margin, reminiscent of peripheral ulcerative keratitis, is much more suggestive of Pythium. Early corneal melt and thinning in the course of infection are also more typical for Pythium than for most fungal ulcers.
Rapid extension to the limbus or beyond serves as a warning sign for Pythium. Fungal keratitis often remains more localized initially, taking longer to reach the limbus unless it is very advanced. Therefore, Pythium or other aggressive etiologies should be considered if limbal or scleral involvement is observed early in an ulcer’s course.[57]
A hypopyon can occur in both Pythium and fungal keratitides, but this finding is less consistently present in early Pythium cases. Most fungal ulcers of comparable size will present with a hypopyon, whereas a Pythium ulcer might not have one or may only have a small one until the infection becomes severe. Thus, a large corneal infiltrate without a hypopyon (or with an unusually fibrinous, thin hypopyon) could suggest Pythium. Conversely, the presence of a large hypopyon does not exclude Pythium, but it is not a distinguishing feature.
Host factors and the response to treatment also provide important clues. Fungal keratitis, particularly when caused by filamentous fungi like Fusarium or Aspergillus, often follows plant trauma in otherwise healthy individuals, a scenario that overlaps with Pythium keratitis. However, a lack of improvement despite appropriate antifungal therapy is a key clinical clue. Pythium should be suspected if a presumed fungal ulcer does not respond to potent antifungal drops. This pathogen's cell wall lacks ergosterol, making typical antifungal medications ineffective. In contrast, true fungal keratitis usually shows some response to antifungals like natamycin or voriconazole over time. Therefore, a refractory keratitis that appears fungal but does not improve with antifungal therapy warrants reconsideration for Pythium infection.[58]
Pythium keratitis should be included in the differential diagnosis of any aggressive corneal ulcer in a patient with aquatic or agricultural exposure, particularly when the slit-lamp examination reveals tentacular stromal infiltrates or a pattern of fine dot infiltrates with peripheral guttering. Although Pythium keratitis can closely resemble fungal keratitis, with features like a white, fluffy infiltrate, satellite lesions, and even hypopyon, the combination of distinguishing signs and the patient’s history can help clinicians suspect Pythium early.
A high index of suspicion is critical because confirmation requires specific diagnostic tests, and the management, often involving surgery and antibacterial agents, differs from standard fungal keratitis treatments. Recognizing the classic clinical presentation of Pythium keratitis can prompt timely therapy and improve patient outcomes.[59]
Evaluation
Evaluation of Pythium keratitis requires a thorough clinical and laboratory assessment to differentiate it from other forms of keratitis. Timely diagnosis is critical, as the pathogen is resistant to typical antifungal treatments, and early intervention can significantly impact outcomes.
The clinical history is vital, with patients often reporting exposure to contaminated water, agricultural environments, or organic matter. Symptoms typically develop insidiously, progressing over days to weeks, and may not respond to conventional antifungal therapy. Pain is often disproportionate to the clinical signs, particularly in the early stages, and may become intense in later stages due to corneal melt. A careful history of exposure to stagnant or muddy water is often key to the diagnosis, as is the fact that patients are typically immunocompetent, unlike individuals with fungal keratitis, where systemic immunosuppression is more common.
Slit-lamp biomicroscopy reveals a characteristic infiltrate pattern in Pythium keratitis, with tentacle-like stromal projections radiating from the central lesion best seen with diffuse illumination. The leading edge of the infiltrate is typically hyphated or finger-like, with active edges that fail to respond to antifungal treatment. The peripheral guttering, a hallmark of the infection, represents necrotic stromal digestion by the pathogen. The hypopyon in Pythium cases may be sterile and nonshifting, unlike in fungal keratitis. Corneal melt often occurs early, without microbial response, which is a red flag for Pythium infection, and descemetoceles are often observed at the preperforation stage. In contrast to fungal keratitis, satellite lesions are absent in Pythium infections.[60]
Microbiological evaluation of P. insidiosum begins with corneal scrapings examined using 10% KOH, which reveals sparse, broad, ribbon-like filaments with perpendicular branching. CFW stain enhances the visibility of these filaments under fluorescence microscopy, while gram staining is typically negative or shows ghost hyphae-like structures. Lactophenol Cotton Blue (LPCB) stain shows poor uptake. Cultures on blood agar typically produce flat, colorless, nonpigmented, nonsporulating colonies within 3 to 5 days, whereas SDA offers less reliable growth. Cornmeal agar helps rule out Candida by showing no chlamydospore formation. Pythium-specific media, such as hemp seed agar, enhances isolation.
Special techniques, such as the leaf incubation method, use plant leaves (eg, autoclaved grass) to foster sporangia formation. The zoospore induction assay confirms Pythium species.
Laboratory evaluation of Pythium keratitis begins with corneal scrapings collected under 5% proparacaine topical anesthesia using a Kimura spatula, a number 15 Bard-Parker blade, a 26-gauge needle, a hypodermic needle, or a platinum spatula. The scrapings undergo wet mount direct microscopic examination using 10% KOH, CFW, and gram stain.
The presence of septate or aseptate hyphae with perpendicular or obtuse lateral branching is observed. The material is also cultured on blood agar, chocolate agar, SDA, or any nutritional agar, where Pythium appears as white, creamy colonies with zoospore formation. Zoospore growth, including numerous vesicles, is confirmed using the leaf incubation method. Histopathological analysis is assisted with iodine IKI and IKI-H2SO4, H&E, PAS, or GMS stain.
Molecular diagnostics, including PCR targeting the internal transcribed spacer (ITS) region of the ribosomal DNA, provide species-level identification. Real-time PCR enables faster results, while mass spectrometry-based methods like matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and point-of-care loop-mediated isothermal amplification (LAMP) assays offer alternative diagnostic approaches. Metagenomic next-generation sequencing (mNGS) is useful in atypical or smear-negative cases, helping to detect rare pathogens, including Pythium.
Serological analysis of P. insidiosum relies on detecting serum antibodies using immunodiffusion, hemagglutination, immunochromatography, enzyme-linked immunosorbent assay (ELISA), and Western blot. The sensitivity of immunodiffusion is approximately 61%.[61][62][63] In contrast, ELISA demonstrates a reported sensitivity and specificity of 100%.[64]
Molecular diagnosis primarily involves PCR targeting the ITS region, ribosomal intergenic spacer region, and cytochrome oxidase gene, allowing rapid detection from both isolates and direct clinical specimens.[65] In vivo confocal microscopy (IVCM) offers a noninvasive method to visualize Pythium hyphae as beaded, branching, hyperreflective structures or as slender, thin, hyperreflective lines measuring 90 to 400 μm in length and 1.5 to 7.5 μm in diameter, with a branching angle of approximately 78.6°. Ultrasound B-scan aids in identifying complications such as retinal detachment, choroidal detachment, vitritis, and endophthalmitis following TPK.
IVCM reveals hyperreflective, thick, aseptate filaments without dichotomous branching, often arranged in parallel or tangential patterns. This diagnostic tool helps differentiate Pythium from septate fungal filaments, which characteristically show branching and septations.[66]
Anterior segment optical coherence tomography (AS-OCT) quantifies the depth of stromal involvement and clearly delineates areas of descemetocele or corneal thinning. This technique is particularly useful for serial monitoring of disease progression or therapeutic response, often showing hyperreflective stromal lesions and zones of localized thinning.[67]
Histopathological analysis following keratoplasty or tissue excision demonstrates poor PAS stain uptake in Pythium, helping to differentiate it from fungal elements. GMS staining faintly highlights Pythium filaments, while H&E staining reveals necrotic stroma containing broad, embedded filaments with inflammatory cell infiltrates. Eosinophilic inflammatory infiltration, relatively more common in Pythium infections, is another characteristic finding.
Immunohistochemistry and in situ hybridization are emerging diagnostic techniques, currently limited to research settings. These methods assist in species differentiation and confirmatory diagnosis, with monoclonal antibody–based assays under development for future clinical use.
Ancillary investigations include monitoring visual acuity and refraction to assess disease response, measuring intraocular pressure, since an elevation of this parameter can indicate anterior segment inflammation or angle closure, and performing color vision testing when optic nerve involvement is suspected. Fundus examination should be performed through clear media or after healing. If media opacity exists, B-scan ultrasonography is recommended for posterior segment evaluation.[68]
Pythium keratitis poses significant diagnostic challenges due to its clinical resemblance to fungal keratitis and its poor response to conventional antifungal therapy. Early suspicion, supported by targeted microbiological or molecular testing such as culture or PCR, is essential to avoid delays in initiating appropriate treatment.[69]
Treatment / Management
Pythium keratitis is a vision-threatening corneal infection caused by an aquatic oomycete, often misdiagnosed as fungal keratitis due to its similar clinical appearance. The condition tends to be rapidly progressive, leading to severe corneal destruction if not promptly treated. Over the past decade, cases of P insidiosum infection have surged, highlighting the challenges in diagnosis and the poor response to conventional antifungal therapy. Recent research has improved diagnostic techniques and led to the development of evolving management protocols. Below is a comprehensive overview of current treatment and management strategies, suited for general understanding and academic reference.
Pharmacologic Management
Management of Pythium keratitis often begins with medical therapy, but the organism's unique microbiology means that standard antifungals are frequently ineffective. Clinicians have increasingly turned to off-label use of certain antibacterial agents that have shown efficacy against P insidiosum in laboratory and animal studies. A tailored approach combines antifungal coverage—initially, if diagnosis is uncertain—with specific anti-Pythium drugs.
Antifungals
Since P insidiosum was historically misidentified as a fungus, topical antifungals such as 5% natamycin, 1% voriconazole, and 1% itraconazole were once considered 1st-line treatments. However, Pythium’s cell wall lacks ergosterol, the target of many antifungals, rendering these drugs largely ineffective in most cases. Numerous reports document poor outcomes with antifungal monotherapy, even with broad-spectrum azoles, polyenes such as amphotericin B, and echinocandins.
For example, the echinocandin caspofungin demonstrated slightly better activity than some antifungals. However, overall results remained inconsistent. Antifungal therapy still has a role before a definitive diagnosis is made, since fungal keratitis is more common, and patients often start on antifungals until Pythium is confirmed. In practice, if a corneal ulcer exhibits classic features of Pythium keratitis, such as reticular-dot infiltrates or tentacle-like extensions, clinicians may empirically combine an antifungal with anti-Pythium drugs while awaiting culture and PCR results. This approach ensures fungal pathogens are covered early on, though antifungals rarely cure Pythium keratitis.
Antibacterials
Recent evidence has established certain antibacterial agents as 1st-line therapy for Pythium keratitis, despite this strategy being unconventional for a corneal infection. In vitro susceptibility studies and animal models have identified several antibiotics with potent anti-Pythium activity, notably linezolid, an oxazolidinone, and azithromycin, a macrolide. These drugs, used in topical form, have become the cornerstone of medical treatment for Pythium keratitis.
Topical linezolid 0.2% is prepared by compounding an intravenous formulation into eye drops, which are initially applied hourly. Linezolid inhibits protein synthesis initiation in Pythium, and its relatively low molecular weight aids corneal penetration. Studies report that linezolid is highly effective, often outperforming other agents, such as tigecycline or azithromycin, when used alone.
Topical azithromycin 1% is initially used hourly as eye drops, sometimes as an ointment at night. Azithromycin, a protein synthesis inhibitor, also has anti-Pythium effects and is typically combined with linezolid for synergy. A topical azithromycin 1% ophthalmic ointment is often applied twice daily, especially at bedtime, in conjunction with the drops. Oral azithromycin 500 mg daily is frequently added for systemic support during the first 1 to 2 weeks.
Combination therapy with linezolid and azithromycin has become a preferred regimen, particularly for sight-threatening or deep stromal infections. Typically, both agents are administered hourly for at least 48 hours, then tapered based on the response. This dual therapy has significantly improved outcomes, with nearly 50% to 60% of Pythium keratitis cases now resolving with medical therapy alone, and a median treatment duration of approximately 3 months. This trend represents a remarkable shift from past reliance on surgery. Clinical improvement is marked by a reduction in the density and number of “tentacles” in the infiltrate, as well as the appearance of peripheral scarring or vascularization, indicating that the infection is under control.
Other off-label antibiotics have also been used in Pythium keratitis management. For example, a combination of minocycline, chloramphenicol, and linezolid successfully cured a case in one report. Macrolides such as clarithromycin, tetracyclines like tigecycline, lincosamides such as clindamycin, streptogramins like quinupristin-dalfopristin, phenicols like chloramphenicol, and aminoglycosides have all shown in vitro or animal efficacy against Pythium.
In practice, the combination of linezolid and azithromycin remains the therapy of choice due to its demonstrated clinical success, with alternatives like tigecycline reserved for special cases. Tigecycline can cause ocular surface irritation and yellow discoloration. Notably, all these treatments are off-label, requiring special compounding and close monitoring. Patients are closely followed, with evaluations every few days initially, to ensure the infection is responding. Surgical intervention must be performed without delay if no improvement is observed or if progression occurs despite aggressive medical therapy.
While topical antibiotics have traditionally formed the mainstay of therapy in Pythium keratitis, the role of oral antibiotics, particularly in severe or longstanding infections with possible intraocular extension, deserves greater emphasis. Oral linezolid, with its excellent ocular penetration, achieves therapeutic levels in aqueous and vitreous compartments irrespective of inflammation, making it a rational adjunct in fulminant or recalcitrant cases. Moreover, oral tetracyclines such as doxycycline and minocycline may demonstrate enhanced stromal penetration in corneas with chronic vascularization—a feature frequently encountered in prolonged Pythium keratitis.
Beyond their antimicrobial action, tetracyclines possess matrix metalloproteinase-inhibitory properties, which can reduce the risk of corneal melt and perforation—a dreaded complication of Pythium keratitis. Incorporating oral agents may also facilitate the earlier tapering of topical antibiotics, thereby accelerating epithelial healing and minimizing complications associated with toxicity. Hence, a multimodal strategy including systemic antibiotics may offer better control of the infection, while preserving corneal integrity, especially in cases with deep stromal or intraocular involvement. Future prospective studies may better delineate the optimal duration and combination protocols involving oral agents for Pythium management.
Surgical Interventions
Despite advances in medical treatment, a large subset of Pythium keratitis cases still requires surgical management. In aggressive infections, surgery removes the infected tissue and prevents further spread of the organism. The primary surgical modality is TPK, although further measures, such as enucleation, may be necessary in extreme cases. Surgical timing and technique differ somewhat from those in fungal keratitis due to Pythium’s behavior.
Therapeutic penetrating keratoplasty
Early TPK or full-thickness corneal transplant is often considered the definitive treatment for Pythium keratitis that does not respond rapidly to medical therapy. Most experts advocate for prompt TPK within 10 to 14 days of presentation for ulcers that are large, rapidly progressive, or associated with any of the following: more than 90% stromal thinning or perforation, limbal or scleral involvement, or endotheliitis/endophthalmitis. The surgical goal is to excise all infected tissue with a wide margin of clear tissue. Unlike fungal ulcers, where a 0.5 mm clear margin is typical, Pythium requires at least a 1- to 1.5-mm larger trephine than the infiltrate to ensure no residual oomycete filaments are left at the edges. This aggressive margin is needed because of Pythium’s tendency for radial extension (the “tentacles”) and frequent recurrence if any is left behind.
In practice, if the infection has extended to the limbus or beyond, surgeons may perform an extended keratoplasty with scleral rim excision and conjunctival resection to remove all affected areas, often combined with adjunctive measures to sterilize the margins. TPK can be very effective in saving the eye. However, recurrence of infection in the graft or at the interface is a known problem. Studies from large cohorts report recurrence rates of 50% to 60% after TPK without adjunct therapy.
Repeated keratoplasty may be attempted if a recurrence occurs. However, outcomes tend to decline with each subsequent graft. Fortunately, the use of intraoperative adjuncts like cryotherapy and alcohol application has drastically lowered post-TPK recurrence to approximately 7% in some series. Thus, the surgical strategy for Pythium often pairs TPK with these adjunct techniques to improve success.
After a successful TPK, where the infection is eradicated and the graft remains clear, patients typically receive intensive topical antibiotics such as linezolid and azithromycin for weeks to months and are closely monitored for any signs of recurring infection at the graft edge. Topical steroids are withheld until the infection is completely quiescent, given the risk of immunosuppression on residual Pythium.
Once the cornea has fully scarred and remained stable, usually a minimum of 6 months post-TPK, the patient may undergo a secondary optical penetrating keratoplasty (OPK) for visual rehabilitation. This elective graft, done under the cover of topical steroids, can significantly improve vision if the initial therapeutic graft is opaque. In some cases, if the only issue is endothelial failure of an otherwise clear graft, a Descemet stripping endothelial keratoplasty (DSEK) may be performed to restore clarity.
Advanced or globe-salvaging procedures
If Pythium keratitis is not controlled early, the infection can spread to involve intraocular structures, leading to endophthalmitis or panophthalmitis. Unfortunately, globe salvage is rarely possible once the infection breaches the cornea and enters the posterior segment. In such cases, or when corneal perforation is so large that keratoplasty is not feasible, evisceration or enucleation may be necessary to remove the eye and prevent life-threatening spread of the infection. These drastic procedures are a last resort but have been reported in a notable percentage of cases with delayed presentation. A study from Thailand noted that over half of their patients ultimately required evisceration, highlighting the severe impact of Pythium when not diagnosed and treated early. Early diagnosis and intervention are thus critical to preventing such outcomes.
Adjunctive Therapies
Several adjunctive treatment strategies have been explored in Pythium keratitis to augment standard medical and surgical management. These adjuncts range from immunologic therapies to physical or chemical measures aimed at eliminating residual organisms. While some have shown promise, others remain experimental or of limited efficacy. Key adjunctive therapies are discussed in this section.
One such approach is P. insidiosum antigen immunotherapy (PIAI), which is modeled after treatments for systemic pythiosis used in veterinary cases. This approach involves a series of subcutaneous injections of Pythium antigen to stimulate the patient’s immune system to fight the infection. Thanathanee et al reported using PIAI in humans, administering 3 injections of 100 to 200 mL of antigen at biweekly intervals. However, the role of immunotherapy remains controversial, as it has not reliably prevented the need for surgical intervention or improved globe salvage rates in clinical practice. As a result, PIAI is not a routine therapy and is typically considered on a case-by-case basis or in research settings.[70]
Intraoperative cryotherapy and topical ethanol have also been adopted as adjuncts to reduce recurrence after TPK. Cryotherapy, which involves freezing the corneal rim and adjacent sclera, induces local cell death that can kill any remaining Pythium filaments. Ethanol is then applied to devitalize residual organisms on the host tissue.
Agarwal et al demonstrated that adding cryotherapy—a single freeze-thaw at the limbus—and ethanol application at the graft margins resulted in only a 7.1% recurrence rate, compared to 51.8% with TPK alone and 100% if only medical therapy were used in advanced cases. This approach is especially useful when the ulcer extends to the limbus or beyond. After trephining the host cornea, surgeons apply a nitrous oxide cryoprobe to the limbal sclera and may swab the peripheral cornea or sclera with 95% alcohol before placing the graft. These additional steps increase the complexity and duration of the surgery but have become an important adjunct in eradicating occult Pythium and preventing regrowth at the graft edges.
Cyanoacrylate tissue adhesive, traditionally used to seal small corneal perforations or areas of thinning, has also found application in Pythium keratitis management to stabilize the cornea and prevent perforation. This agent acts as a chemical barrier with some antimicrobial properties. Applying cyanoacrylate glue over an area of thinning can buy time for medical therapy to work, potentially avoiding or delaying the need for urgent TPK.
Gurnani et al reported the successful use of this treatment in a pediatric Pythium keratitis case, where it allowed the infection to be controlled without immediate grafting. The glue’s exothermic reaction and its chemical content are thought to damage the organism’s cellulose-rich cell wall, lending it inherent anti-Pythium activity. The glue is often combined with a bandage contact lens (BCL) to enhance comfort and act synergistically with topical linezolid or azithromycin by maintaining the medication in contact with the cornea for a longer period. While not a definitive treatment, tissue adhesive is a useful adjunct for corneal integrity, especially in rapidly melting corneas or in children, where urgent keratoplasty poses higher risks.
Photodynamic antimicrobial therapy has also been investigated for refractory Pythium infections, where photosensitizing agents like riboflavin or rose bengal are applied to the cornea and activated with light to generate reactive oxygen species that kill the organism. Though early laboratory experiments suggest that this approach may have an anti-Pythium effect, no large-scale clinical studies have proven its efficacy in Pythium keratitis. The treatment remains a potential adjunctive treatment for cases that do not respond to conventional approaches.
Additional adjuncts have been tested in vitro or animal models, expanding the range of potential therapies for Pythium keratitis. Nitrofurantoin, a urinary antiseptic, inhibited Pythium mycelial growth in laboratory tests, while mupirocin, a topical antibiotic, demonstrated activity with a minimum inhibitory concentration of or below 4 µg/mL.
Disulfiram, a drug used to treat alcoholism, also exhibited anti-Pythium effects at certain concentrations. Moreover, natural compounds such as xanthyletin, a plant-derived coumarin, have been shown to alter the pathogen’s cell wall. Silver nanoparticles or metabolites from bacteria like Pseudomonas stutzeri can damage Pythium hyphae. These findings expand the list of potential adjunctive therapies, although their clinical use in keratitis remains experimental.
Recent Advances and Future Directions
Management of Pythium keratitis is an evolving field, with ongoing research focused on improving both treatment modalities and diagnostic tools. Some of the most notable recent advances have significantly expanded our understanding and approach to this challenging infection.
One area of focus is the development of novel drugs and repurposed therapies. Due to the limitations of current antifungals and antibiotics, researchers are exploring alternative agents to combat Pythium. Miltefosine, an antileishmanial drug, has shown potential antifungal activity against Pythium. Copper compounds, such as copper acetate, and various plant extracts have also demonstrated inhibitory effects in experimental settings.
In addition to exploring new compounds, repurposing existing medications is a key strategy. Tetracyclines, macrolides, and other classes of drugs have already proven effective against Pythium, while additional agents like procyclidine, an antiprotozoal drug, have been identified for their anti-Pythium properties. These findings, mostly from in vitro studies and treatments for systemic pythiosis, offer promise but will require clinical trials to confirm their efficacy in ocular infections. Nonetheless, the growing pipeline of potential anti-Pythium drugs raises hope for more effective medical treatments in the future.
In parallel with drug development, advancements in diagnostic techniques are enhancing the ability to detect Pythium infections early. Rapid and accurate identification is critical, as early intervention can significantly improve outcomes and preserve vision. While traditional culture remains the gold standard for diagnosis, revealing characteristic zoospore-forming colonies on blood agar, newer molecular diagnostics, such as PCR, provide faster and more specific confirmation of P insidiosum.
IVCM is another valuable tool, enabling visualization of the corneal infiltrate at the cellular level. This technique can sometimes reveal the distinctive “tentacle” hyphal pattern of Pythium, which differs from that of fungal filaments. Serological tests, including ELISA and immunofluorescence assays, have also been explored to detect Pythium antigens or antibodies. These advanced diagnostic tools are becoming increasingly available, allowing clinicians to distinguish Pythium keratitis from fungal keratitis early on, leading to more appropriate treatment choices and better outcomes. Timely diagnosis using these methods has played a significant role in reducing delays associated with ineffective antifungal therapies.
Another exciting development is the integration of artificial intelligence (AI) in the diagnosis and prognosis of Pythium keratitis. Researchers are exploring AI and deep learning models to assist in diagnosing Pythium infections. By analyzing large datasets of corneal images and clinical parameters, AI algorithms could potentially identify Pythium infections from the very first day, differentiating them from fungal or bacterial ulcers based on subtle features.
Early studies in infectious keratitis have demonstrated that machine learning models can accurately analyze corneal photographs or confocal scans. In the near future, ophthalmologists may use a smartphone or slit-lamp images, paired with an AI app, to quickly assess the likelihood of Pythium versus fungal keratitis and initiate prompt therapy. AI could also play a key role in predicting prognosis, identifying which cases are likely to fail medical treatment and require early intervention, such as keratoplasty. Although still in the experimental phase, the use of AI in corneal infection management represents an exciting frontier that could revolutionize Pythium keratitis care by facilitating ultra-early targeted treatment.[71]
Medical management of Pythium keratitis has increasingly focused on specific agents like linezolid and azithromycin, which have shown promising results. Surgical intervention, particularly TPK, remains essential for advanced cases. Adjunctive treatments, such as cryotherapy, glue, and experimental immunotherapies, further enhance the chances of eradicating this resilient pathogen. Ongoing innovations, including new drug trials and AI-assisted diagnostics, offer hope for improving clinical outcomes and reducing blindness. Given the lack of standardized guidelines, clinicians must stay informed and adapt treatment strategies to individual cases, ensuring the best possible outcome for patients.
Differential Diagnosis
The differential diagnosis of Pythium keratitis includes various infectious and noninfectious keratitides, each with distinct clinical features. Fungal keratitis caused by Fusarium spp presents with feathery margins, satellite lesions, and a response to antifungal treatment. Aspergillus spp typically causes dry ulcers with pigmented margins, while Curvularia spp infection results in dark pigmented ulcers commonly associated with agricultural activities. Alternaria spp infections are characterized by dry infiltrates, often seen following vegetative trauma, and Scedosporium spp is known for being multidrug resistant and slow-growing.[72] Pythium mimics fungal ulcers but lacks pigmentation. Filaments are aseptate and stain poorly on KOH and CFW staining.
Bacterial keratitis, including Pseudomonas aeruginosa, presents with a greenish hue, mucopurulent discharge, and rapid corneal melt. Staphylococcus aureus forms focal dense infiltrates with surrounding edema. Streptococcus pneumoniae is typically associated with central ulcers and hypopyon. Nocardia spp shows a wreath-like stromal pattern with slow response to treatment. Mycobacterium spp typically has an indolent course and is often misdiagnosed. Pythium shows reticular stromal infiltration and hyphated edges. Bacterial ulcers respond well to antibiotics.
Protozoal infections, such as Acanthamoeba keratitis, present with ring infiltrates, severe pain, and perineural infiltrates. Pythium keratitis lacks radial perineural patterns, and pain may be less intense. Parasitic keratitis, such as onchocerciasis, is marked by granulomatous inflammation, systemic features, and endemic distribution. Pythium keratitis, on the other hand, is confined to the cornea and does not cause systemic symptoms.
Viral infections like herpes simplex virus produce dendritic or geographic ulcers and a decrease in corneal sensation. Herpes zoster ophthalmicus, on the other hand, presents with a rash, neurotrophic changes, and sectoral iris atrophy.[73] Pythium keratitis lacks a dendritic pattern, and corneal sensation is usually preserved.
Rare pathogens such as Microsporidia cause superficial punctate and granular lesions, mainly involving the epithelium, but may also cause stromal edema. Pythium keratitis is more stromal and infiltrative. Candida spp typically produces round yeast cells on stains with central lesions. Systemic involvement is seen in Histoplasma and Blastomyces, which are often associated with endemic exposure.[74]
Autoimmune and inflammatory conditions, such as peripheral ulcerative keratitis, cause crescent-shaped ulcers typically associated with systemic diseases like rheumatoid arthritis and systemic lupus erythematosus. Mooren ulcers present with peripheral ulceration, overhanging edges, and adjacent inflammation. Sclerokeratitis involves corneoscleral inflammation with systemic associations. Marginal keratitis is related to staphylococcal hypersensitivity, often presenting as marginal infiltrates.
Neurotrophic and exposure keratopathies also need to be considered. Neurotrophic keratopathy involves absent corneal sensation and persistent epithelial defects (PEDs), while exposure keratopathy is caused by lagophthalmos and is associated with an exposure zone of the cornea.[75]
Contact lens-related or toxic keratitis can present as multiple sterile infiltrates in lens users or chronic inflammation due to preservatives in preserved drops. Corneal melt can also occur after using topical nonsteroidal anti-inflammatory drugs (NSAIDs), leading to toxic reactions.[76]
Metabolic and nutritional causes, such as vitamin A deficiency, lead to bilateral dryness, Bitot spots, and xerosis, while dry eye-associated keratopathy may cause filamentary keratitis with punctate epithelial erosions. Degenerative or mechanical conditions like Terrien marginal degeneration result in peripheral thinning without inflammation. Keratoconus with hydrops is marked by sudden hydrops with pain and edema. Salzmann nodular degeneration presents with nodules and blue-white opacities. Hereditary and congenital disorders, including Reis-Bücklers dystrophy with subepithelial haze and recurrent erosions, Meesmann dystrophy with intraepithelial cysts, and Lattice dystrophy with refractile lattice lines and amyloid deposits, are also differential diagnoses.[77]
Pertinent Studies and Ongoing Trials
Recent years have seen a surge in investigations targeting the unique challenges posed by Pythium keratitis. Key studies have reshaped the understanding of diagnosis, therapeutics, and host response. For instance, a landmark multicenter, randomized controlled trial conducted from 2021 to 2023 compared the combined linezolid-azithromycin therapy with standard antifungal regimens, finding significantly better outcomes with the antibacterial combination and challenging the conventional antifungal approach.[78] Studies from India, notably from Aravind Eye Hospital, have demonstrated the ineffectiveness of voriconazole and natamycin in culture-proven Pythium cases, reinforcing the importance of early, aggressive surgical intervention.
Diagnostic advances have further refined the approach to this infection. A 2022 study introduced loop-mediated isothermal amplification and ITS-based PCR, demonstrating 95% to 98% specificity in identifying Pythium DNA, enhancing rapid bedside diagnosis. Additionally, a pilot study conducted by the All India Institute of Medical Sciences and the Indian Institute of Technology in Delhi explored the use of AI-based image recognition software for early-stage slit-lamp image classification using deep learning models, which showed promising accuracy and is currently undergoing validation.[79]
Surgical outcomes have emphasized the role of early intervention. A retrospective study involving 84 eyes compared primary TPK with medical therapy and found a significantly lower recurrence rate with early surgical management. The adjunctive use of freeze-thaw cryotherapy around the graft margin during TPK has also been associated with reduced recurrence risk, and a prospective trial is underway to quantify this benefit.[80]
Understanding host-pathogen interactions has provided insight into the aggressive clinical course of the disease. Immunohistochemistry-based studies have demonstrated heightened neutrophilic infiltration and poor granulomatous containment in infected corneas. Genomic investigations are examining polymorphisms in innate immunity genes among Indian patients with recurrent disease.[81]
Ongoing trials continue to explore novel therapeutic strategies. A multicenter trial sponsored by the Indian Council of Medical Research is evaluating a combination topical therapy consisting of linezolid, azithromycin, and cyclosporine A for early epithelial Pythium keratitis. A Phase I clinical trial is underway for a recombinant anti-Pythium vaccine in animal models, with the goal of future human application. Trials investigating adjunctive nanocarrier-based delivery systems for antibacterial agents are also being conducted at tertiary centers.[82]
Treatment Planning
Early recognition and targeted management of Pythium keratitis are critical. Diagnosis should be suspected in cases refractory to antifungal agents, especially when reticular stromal infiltrates, tentacle-like extensions, and rapid progression are noted. Antifungal therapy should be discontinued once the infection is confirmed, given the inherent resistance of Pythium. The disease should be categorized based on stage (epithelial, stromal, or perforated) and the size and location of infiltrates (small peripheral or large central). Confirmation relies on microbiological smear, culture, or PCR-based methods such as ITS or LAMP assays.
First-line medical therapy includes topical linezolid 0.2% administered hourly during waking hours and topical azithromycin 1% ointment applied twice daily. Systemic azithromycin (500 mg/day) may be added when deeper stromal involvement is present. Intrastromal injections of linezolid or azithromycin may be considered in cases with severe stromal infiltration. Cycloplegic agents, such as homatropine, are useful for pain relief and anterior chamber inflammation. Early surgical intervention is indicated in cases where infiltrates fail to resolve within 48 to 72 hours of therapy, or evidence of corneal thinning, impending perforation, or panstromal involvement is elicited. TPK with at least 1 mm clear margins remains the preferred approach, and adjunctive measures such as intraoperative cryotherapy or ethanol irrigation may reduce the risk of recurrence.
Postoperative management involves the continued use of topical linezolid and azithromycin for 4 to 6 weeks while avoiding corticosteroids in the early postoperative phase. Regrafting or the use of a scleral patch may be necessary in the event of recurrence or graft melt. Post-TPK, microbiological clearance should be confirmed with repeat cultures or smears from the graft rim or bed. Close monitoring is essential, with daily follow-up during the 1st week and gradual tapering based on clinical response. Key parameters include graft clarity, signs of recurrence such as satellite lesions or stromal melt, and intraocular pressure. Long-term visual rehabilitation may require rigid contact lenses or secondary optical keratoplasty. Optical keratoplasty may be planned once active inflammation has subsided to optimize visual outcomes.[83]
Supportive care plays an important role and includes lubrication with preservative-free artificial tears and management of secondary glaucoma if necessary. Given the significant visual morbidity, particularly in young patients, psychological support and low-vision aids should also be provided to help with adjustment to visual impairment.[84]
Toxicity and Adverse Effect Management
Linezolid may cause bone marrow suppression (anemia, thrombocytopenia), optic neuropathy, or lactic acidosis. Weekly monitoring of the complete blood count is essential, and visual acuity and color vision should be assessed if the drug is used for more than 2 weeks. The drug should be discontinued if hematologic toxicity occurs and switched to an alternative agent if vision changes arise.
Azithromycin may lead to gastrointestinal upset, QT prolongation, and hepatotoxicity. Patients with cardiac conditions should undergo electrocardiogram monitoring, and liver function tests should be conducted during prolonged or systemic use. Caution is necessary if QT-prolonging drugs are concurrently prescribed. This drug should be discontinued if hepatic symptoms emerge.
Intrastromal injections can result in local inflammation and, in rare cases, corneal melting. Regular slit-lamp exams are advised to monitor stromal changes and thinning. If these manifestations are observed, the injection frequency should be reduced or topical therapy switched, and lubricants or a BCL should be considered for comfort.
Systemic therapy may result in drug-drug interactions, gastrointestinal intolerance, or, in rare cases, neuropathy, particularly when linezolid is given. Regular clinical evaluations and medication reconciliations are important. The regimen must be adjusted as needed based on patient tolerance and side effect profiles.
General strategies include avoiding corticosteroids during active infections, as they may worsen prognosis. For prolonged cases, supportive therapies like artificial tears, BCLs, and vitamin supplements can be beneficial. An interprofessional team should be involved if systemic toxicity is suspected.
Staging
The severity-based classification for Pythium keratitis outlines the clinical progression of the disease from early to advanced stages. This classification helps guide management decisions based on the severity and ocular involvement.
In Stage I (early/localized), symptoms are mild, with a solitary, superficial, dry infiltrate accompanied by mild pain and redness. Topical therapy with linezolid and azithromycin is recommended, along with frequent monitoring. Stage II (progressive/central involvement) presents with central extension and worsening pain or vision, as well as multiple satellite lesions, tentacular projections, and early hypopyon. Intensive medical therapy with dual antibiotics is required, and early surgical planning, including possible TPK, should be considered.
Stage III (deep stromal involvement) features severe keratitis with corneal melting and dense infiltrates. TPK and systemic antibiotics, including azithromycin and linezolid, are necessary to manage the infection. In Stage IV (extrascleral extension), signs of scleritis, limbal ischemia, and panophthalmitis emerge, with globe-threatening signs such as corneal perforation and scleral necrosis. Globe salvage is unlikely, and evisceration or enucleation with systemic Pythium-targeted therapy is required. Stage V (systemic dissemination, rare) is marked by sepsis and cutaneous or gastrointestinal symptoms, with ocular signs often coexisting or preceding the systemic symptoms. Interprofessional care, including infectious disease specialists, surgical intervention, and intensive care, is essential.
The Gurnani and Kaur classification is used to categorize the severity of the infection based on ulcer size, location, depth, and the presence of complications. This classification guides treatment decisions and helps assess the prognosis of the disease.
In Grade I (mild), the ulcer is less than 4 mm, peripheral, and superficial with no hypopyon or scleral involvement. The cornea presents with a dry ulcer and no tentacles. Treatment involves topical linezolid and azithromycin, with close monitoring for 48 to 72 hours. Grade II (moderate) is characterized by a 4- to 6-mm ulcer, located paracentral or central, and midstromal in depth. A small hypopyon (< 1 mm) may be present, but no scleral involvement is seen. Early tentacles and satellite lesions appear. Treatment includes adding systemic linezolid (600 mg twice daily) and initiating surgical planning.
In Grade III (severe), the ulcer exceeds 6 mm, is centrally located, and involves deep stromal or full-thickness layers. Hypopyon of 1 mm or more and scleral involvement may be noted. The cornea displays dense plaque, tentacle margins, and rapid progression. Urgent TPK and systemic therapy are required.
Grade IV (very severe) presents with an ulcer greater than 8 mm or total, diffuse, or limbal involvement, full-thickness with descemetocele or perforation. A large hypopyon (> 2 mm) and extensive spread are seen, often accompanied by scleritis, corneal melt, and panophthalmitis features. The treatment approach includes evisceration or enucleation, systemic antibiotics, and supportive or palliative management.
Key points to remember include tentacle-like margins, dry surface, and antifungal resistance, which should prompt suspicion of Pythium. Rapid progression within 24 to 48 hours highlights the urgency for early surgical referral, improving outcomes.[85]
Prognosis
The prognosis of Pythium keratitis depends on factors such as the size, depth, and extent of the infiltrate, associated complications, the timing of treatment, and follow-up. Patients with early superficial stromal infiltrates that do not involve the visual axis generally have good visual outcomes if treated promptly and monitored closely. However, those with full-thickness infiltrates, corneal perforation, and anterior chamber exudates require early keratoplasty to prevent posterior segment involvement and irreversible complications like phthisis bulbi.
Endophthalmitis, panophthalmitis, scleritis, and choroidal or retinal detachment are associated with a guarded prognosis. Timely TPK can restore anatomical integrity and prevent corneal opacification, which may later be managed with OPK for visual rehabilitation.
Key prognostic factors include ulcer size and location, depth of infiltration, presence of hypopyon, scleral extension, delayed diagnosis, and response to medical therapy. Larger ulcers (>6 mm) with central or limbal involvement, deep stromal or full-thickness ulcers, and significant hypopyon (≥1 mm) correlate with worse visual and anatomical outcomes. Scleral extension suggests advanced infection and a poor chance of globe salvage. Misdiagnosis, particularly as fungal keratitis, can delay proper treatment and increase the risk of complications, such as corneal melt and vision loss. Unresponsiveness to medical therapy within 48 to 72 hours warrants surgical intervention to avoid worsening complications.
Outcomes vary by disease stage. Early-stage keratitis (Grades I–II) may allow for globe salvage with preserved vision if managed aggressively with antibiotics and closely monitored. Advanced cases (Grades III–IV) often necessitate TPK, and many progress to evisceration or enucleation, particularly with limbal or scleral involvement.[86]
Visual prognosis is favorable in patients diagnosed early with limited disease but poor in late-stage cases with deep stromal involvement, limbal extension, or scleral involvement. Globe salvage rates range from 30% to 60%, depending on the timing and effectiveness of the intervention. Post-TPK recurrence rates remain high (~30%), especially if surgical margins are not adequately cleared or if systemic antibiotics are not continued after surgery.
Overall, the prognosis for Pythium keratitis is closely tied to early diagnosis, timely Pythium-specific antibiotic therapy, and prompt surgical intervention. Collaborative care, involving a corneal surgeon, microbiologist, and infectious disease specialist, is key to improving patient outcomes.[87]
Complications
Complications often arise as the infection progresses or when initial treatment fails to control the disease effectively. These conditions may develop due to delayed diagnosis, inadequate therapeutic response, or the aggressive nature of P insidiosum, leading to severe ocular and systemic consequences.
Corneal melt is the rapid and progressive thinning of the corneal stroma, leading to structural compromise and a high risk of perforation due to severe inflammation or enzymatic degradation. Descemetocele is a severe corneal ulceration where only the Descemet membrane remains intact, creating a translucent bulge that signals imminent perforation. Corneal perforation often results from rapid stromal melt and progressive necrosis. Scleral extension, which involves infiltration of the adjacent sclera, indicates severe disease and increases the risk of globe loss.
Refractory infection can occur due to Pythium's resistance to antifungals and antibiotics. Recurrent graft infection is seen in 30% to 40% of patients post-TPK. Endophthalmitis, which extends into the posterior segment, may lead to panophthalmitis in advanced cases. Phthisis bulbi represents an end-stage outcome of chronic inflammation and uncontrolled infection. Anterior chamber reaction with hypopyon can persist even after treatment. Chronic inflammation may cause iris atrophy and synechiae, while secondary glaucoma may result from trabecular meshwork blockage or steroid response. Cataract formation often arises due to steroid use or chronic inflammation.
Limbal stem cell deficiency (LSCD) is especially common after repeated TPKs or when limbal involvement occurs. Corneal neovascularization can develop with chronic inflammation or multiple grafts. PEDs are due to LSCD or limbal ischemia. Panophthalmitis, a severe extension of infection involving all eye coats, is rare. Toxic anterior segment syndrome (TASS) can cause a sterile inflammatory reaction following intervention. Orbital cellulitis or abscess may arise from scleral involvement, although these conditions are rare. Uveal prolapse can occur following perforation or during graft failure, requiring repeated surgical interventions, such as TPKs, iridectomy, or evisceration (see Image. Failed Graft Posttherapeutic Keratoplasty in Pythium Keratitis).
Amblyopia, particularly in pediatric cases, can develop from poor visual rehabilitation or delayed patching. Cosmetic disfigurement may occur due to phthisis, evisceration, or multiple failed grafts. Systemic spread, though rare, may occur in immunocompromised patients and lead to systemic pythiosis. Orbital deformity postevisceration complicates socket reconstruction and prosthetic fitting, and facial asymmetry and psychological impacts may result from enucleation, visual loss, or disfigurement. Visual impairment or blindness is the most feared long-term outcome, resulting from corneal opacification, LSCD, or globe loss.
Post-TPK, poor graft clarity may be caused by recurrent inflammation, vascularization, or interface infection. Choroidal detachment, typically due to hypotony or intraocular surgery, threatens visual prognosis when fluid or blood accumulates between the choroid and sclera. Although rare, retinal detachment can occur following chronic inflammation or postsurgical complications, leading to the separation of the neurosensory retina from the underlying retinal pigment epithelium and significant vision loss.
Complications from Pythium keratitis can have significant long-term consequences, including visual impairment, cosmetic disfigurement, and systemic spread. Early intervention and careful management are essential to preventing these outcomes and improving prognosis.
Postoperative and Rehabilitation Care
After TPK, the patient should begin with hourly topical 0.2% linezolid and 1% azithromycin, 6 to 8 times a day, with the dose gradually tapered based on the clinical response. Additional postoperative medications include 1% homatropine twice daily to reduce pain from ciliary spasm, 0.5% timolol eye drops twice daily to prevent secondary glaucoma, and low-dose topical antibiotics like 0.5% moxifloxacin or 0.5% gatifloxacin 4 times daily to prevent secondary bacterial infection for at least 2 weeks. If presurgical cultures yield P insidiosum and the post-TPK button culture also returns positive, steroids can be introduced after 3 weeks, ensuring no residual hyphae are present.[88]
However, if the presurgical culture results grow P insidiosum but the button culture is negative, steroids can be started after 2 weeks of anti-Pythium treatment, with 0.1% prednisolone or 0.1% dexamethasone administered 4 times daily for 2 months, then gradually tapered over the following 3 months. Postoperative rehabilitation involves thorough counseling and regular follow-up to discuss the potential need for OPK once the infection is resolved.
Postoperative monitoring is essential, particularly within the first 72 hours, to detect signs of reinfection, graft edema, hypopyon, or early graft melt. Follow-up visits should occur daily to biweekly in the 1st month, depending on the severity and patient response. AS-OCT and ultrasound B-scan may be used to assess deeper structures.[89]
Topical medications should continue for 6 to 8 weeks post-TPK, including linezolid, azithromycin, and polymyxin B. Topical corticosteroids are cautiously introduced only if the epithelium heals and the infection is under control. Lubricants and cycloplegics help promote healing and reduce discomfort, while antiglaucoma agents may be necessary if steroid-induced or inflammatory glaucoma develops.[90]
Frequent slit-lamp evaluations are necessary to monitor graft clarity, edge integrity, and suture health, with early suture removal recommended if inflammation, neovascularization, or graft vascularization is observed. BCLs, punctal plugs, or amniotic membrane transplantation (AMT) may be utilized for PEDs. Tarsorrhaphy should be considered in nonhealing PEDs or cases of lagophthalmos.[91]
In the event of graft rejection, prompt treatment with topical or systemic steroids is necessary after infection clearance. Monitoring for LSCD, cataracts, or secondary glaucoma is important, with management strategies prepared accordingly.[92] If graft melt or reinfection occurs, repeat TPK or tectonic grafts may be needed.
Visual rehabilitation should include spectacle correction for aphakia or residual refractive error, with rigid gas permeable lenses or scleral lenses offering better outcomes. Low-vision aids and occupational therapy should be provided early to patients with nonsalvageable vision.
Psychological and social support is essential, especially for patients who are young or have monocular vision. For individuals with globe loss, planning for ocular prosthesis should begin within 6 to 8 weeks after evisceration.[93] Long-term follow-up is necessary every 1 to 3 months during the first year, and then twice a year, to monitor for recurrence, visual function, and progress in rehabilitation. Adjunctive therapies, such as ocular surface reconstruction for severe LSCD (eg, stem cell limbal transplantation or cultured limbal epithelial transplantation), corneal neurotization for neurotrophic keratopathy, or prosthetic cornea implantation in end-stage disease, may be considered as part of comprehensive care.
Consultations
A high index of suspicion and specialized expertise are essential for accurate diagnosis. Referral to a cornea and external disease specialist is recommended to ensure targeted and meticulous treatment. An infectious disease specialist can assist in guiding systemic therapy, particularly when cultures are ambiguous or mixed with bacterial mimickers or rare pathogens. A microbiologist or ocular pathologist is crucial for confirming the diagnosis through zoospore staining, differentiation from Lagenidium, and molecular testing, which improves treatment outcomes. Early involvement of a cornea specialist helps in planning advanced diagnostics, such as confocal microscopy, and timely surgical interventions like TPK.
In pediatric patients, referral to a pediatric ophthalmologist ensures specialized postoperative care, prevention of amblyopia, and optimal visual rehabilitation, especially in cases with bilateral infection or a risk of LSCD. An oculoplastics or orbit specialist becomes important in managing orbital extension, severe scleral thinning, or postevisceration socket reconstruction to optimize cosmetic and functional outcomes.
In cases of recurrent or chronic Pythium keratitis, consultation with a rheumatologist or immunologist is valuable to evaluate underlying immune dysregulation. When systemic azoles or antibiotics are prescribed long-term, collaboration with a renal physician or hepatologist is essential to monitor for renal or hepatic toxicity, particularly with agents like polymyxins or azithromycin.
If systemic spread is suspected, an otolaryngologist or pulmonologist can assess for nasopharyngeal or pulmonary involvement, which is rare but possible in immunocompromised patients. Public health officials or epidemiologists should be consulted for outbreak analysis, water source evaluations, and environmental assessments in endemic regions or clustered cases. A psychologist or rehabilitation counselor plays a key role in helping patients adapt to vision loss, cope with anxiety, and improve quality of life after evisceration or prosthesis fitting, especially among children and those with monocular status.
Deterrence and Patient Education
Patients must be educated on proper hygienic practices when applying and using contact lenses. Agricultural work-related trauma remains the leading cause of Pythium keratitis, making the use of eyeshields and protective goggles essential for individuals working in fields. Education on the consistent application of medications, the importance of follow-up care, and the potential need for early keratoplasty in nonresolving cases must also be emphasized.
Preventing Pythium keratitis begins with widespread community education and environmental caution, particularly in endemic regions such as tropical and subtropical areas of India and Southeast Asia. Patients should be counseled to avoid exposure to stagnant or contaminated water, especially during agricultural activities, gardening, or the monsoon season. Protective eyewear is strongly advised for individuals working in paddy fields, sewage treatment, or other high-risk occupations.
Early recognition of symptoms—including pain disproportionate to clinical signs, tentacular infiltrates, and rapid stromal melt—is crucial. Patients must be urged to seek prompt ophthalmic evaluation rather than resorting to self-medication or delaying treatment. After initial management, strict follow-up is critical due to the aggressive recurrence risk and potential for graft failure.
Education on hygiene, wound care following keratoplasty, adherence to topical polyene or macrolide therapy, and early identification of recurrence signs must be reinforced. Training community health workers and primary care physicians to recognize early manifestations of Pythium keratitis ensures timely referral and intervention. Long-term discussions on visual rehabilitation, psychosocial support, and realistic expectations help foster patient trust and adherence to care plans. Patient-friendly leaflets in regional languages and audiovisual campaigns can further enhance awareness and prevention in vulnerable populations.
Pearls and Other Issues
Pythium keratitis is a vision-threatening ocular condition that demands a high index of suspicion for diagnosis and prompt, meticulous management to salvage vision. Classical clinical features include tentacular projections, peripheral furrowing, and early limbal spread. Since Pythium hyphae are difficult to distinguish from fungal hyphae on smear alone, culture remains mandatory for zoospore identification. Antibacterials have shown better outcomes than antifungals and should be used as 1st-line therapy, with early keratoplasty often required for optimal anatomical and functional outcomes.
Although Pythium keratitis mimics fungal ulcers, distinctive signs such as reticular dot infiltrates, tentacular extensions, and peripheral guttering should raise suspicion, even when cultures are negative for fungus.[94] Standard microbiological methods may misidentify Pythium as a fungus or yield negative results due to poor growth on common media. Therefore, diagnostic tools like Lagenidium/Pythium-specific PCR, ITS sequencing, and wet-mount zoospore identification are critical. Early differentiation is vital because Pythium does not respond to conventional antifungals, and delayed identification can lead to rapid corneal melting and poor visual outcomes.
Nonantifungal therapies, particularly linezolid and azithromycin, form the cornerstone of medical management, as antifungals are largely ineffective. Early surgical intervention, such as TPK, is often necessary even before a definitive diagnosis due to the organism’s aggressive stromal invasion. Postoperative recurrence remains a significant concern, making wide surgical margins and adjunctive preoperative and postoperative medical therapy essential to reduce recurrence rates.
Histopathological examination can offer important clues, with broad, sparsely septate hyphae that are PAS-negative and GMS-positive, helping to differentiate Pythium from fungal infections, which are typically PAS-positive. Found in stagnant water and soil, Pythium affects both healthy individuals, particularly those involved in agriculture, and immunocompromised hosts, setting it apart from many common fungal pathogens.[95]
Emerging diagnostic tools such as MALDI-TOF mass spectrometry, AS-OCT, confocal microscopy, and AI-driven platforms are improving early detection and clinical decision-making. Research into antigen- and serology-based rapid detection kits also shows promise, particularly for early bedside diagnosis in rural and resource-limited settings. Although Pythium infections are more prevalent in tropical regions like India and Thailand, increasing reports from temperate zones highlight the growing global threat they pose.[96]
Artificial intelligence and predictive modeling are under investigation, with algorithms being trained to distinguish fungal from Pythium ulcers based on clinical photographs.[97] Ongoing research focuses on combination drug regimens, nanoparticle drug delivery systems, and immunomodulatory therapies to enhance treatment outcomes.[98] Veterinary parallels, particularly pythiosis in horses and dogs, have provided valuable insights, including the potential use of immunotherapy.[99]
The psychosocial impact of delayed diagnosis, repeated procedures, and vision loss is profound, significantly affecting patients’ quality of life. Structured rehabilitation and psychological support must be integrated into the management plan to address the long-term consequences of Pythium keratitis.
Enhancing Healthcare Team Outcomes
Any patient with Pythium keratitis presenting to the emergency department with pain, redness, and photophobia must be urgently referred to an ophthalmologist, preferably a corneal disease specialist. Prompt diagnosis and tailored therapy are critical to preventing blindness.
Smear and culture are mandatory for diagnosing Pythium keratitis, with the in-house microbiologist playing a crucial role in facilitating rapid identification. Treatment must begin with topical 0.2% linezolid and 1% azithromycin as 1st-line agents, continued for at least 2 to 3 months. The ophthalmologist is also responsible for patient education and counseling regarding hygiene practices, meticulous contact lens use, and proper medication adherence after infection. Close follow-up is essential, as the organism is highly virulent and can cause permanent blindness. The nursing team and pharmacist likewise play key roles in reinforcing patient education and medication compliance.
Managing Pythium keratitis requires an integrated, interprofessional approach that extends beyond the ophthalmologist’s purview. Optimal outcomes are achieved through proactive collaboration among specialists in cornea care, microbiology, pathology, infectious diseases, and pharmacology, from diagnosis through rehabilitation.
Early clinical recognition by ophthalmologists, particularly in endemic regions, is critical to preventing vision loss. Timely sample collection and close communication with microbiology teams expedite the accurate identification of Pythium through wet mount examination, culture, or molecular diagnostics. Histopathological confirmation by ocular pathologists further aids differentiation from fungal keratitis, especially in surgically excised specimens.
Pharmacologists and infectious disease specialists contribute by guiding the evidence-based use of novel agents such as linezolid and azithromycin and by monitoring for systemic toxicity. The role of these providers in antibiotic stewardship ensures that empirical treatment remains effective and rational. Additionally, input from researchers and geneticists developing rapid diagnostics, molecular tools, and AI-aided triage systems continues to enhance the accuracy and speed of diagnosis.
In cases requiring surgical intervention, coordination among the surgical team, anesthesiologists, and perioperative nursing staff is essential to minimize intraoperative risks and reduce postoperative recurrences. Postsurgical rehabilitation teams, including low vision specialists and psychological counselors, provide critical support to patients coping with visual disability.
Regular team briefings, shared care protocols, and the use of electronic health records help minimize decision-making delays and improve continuity of care. Patient education, conducted jointly by nurses, counselors, and ophthalmologists, reinforces adherence to treatment and infection prevention measures.[100]
Fostering clear communication, streamlining referral pathways, and conducting regular interprofessional training on emerging pathogens such as Pythium significantly enhance clinical outcomes and patient satisfaction, particularly in high-risk areas facing a growing disease burden.[101]
Media
(Click Image to Enlarge)
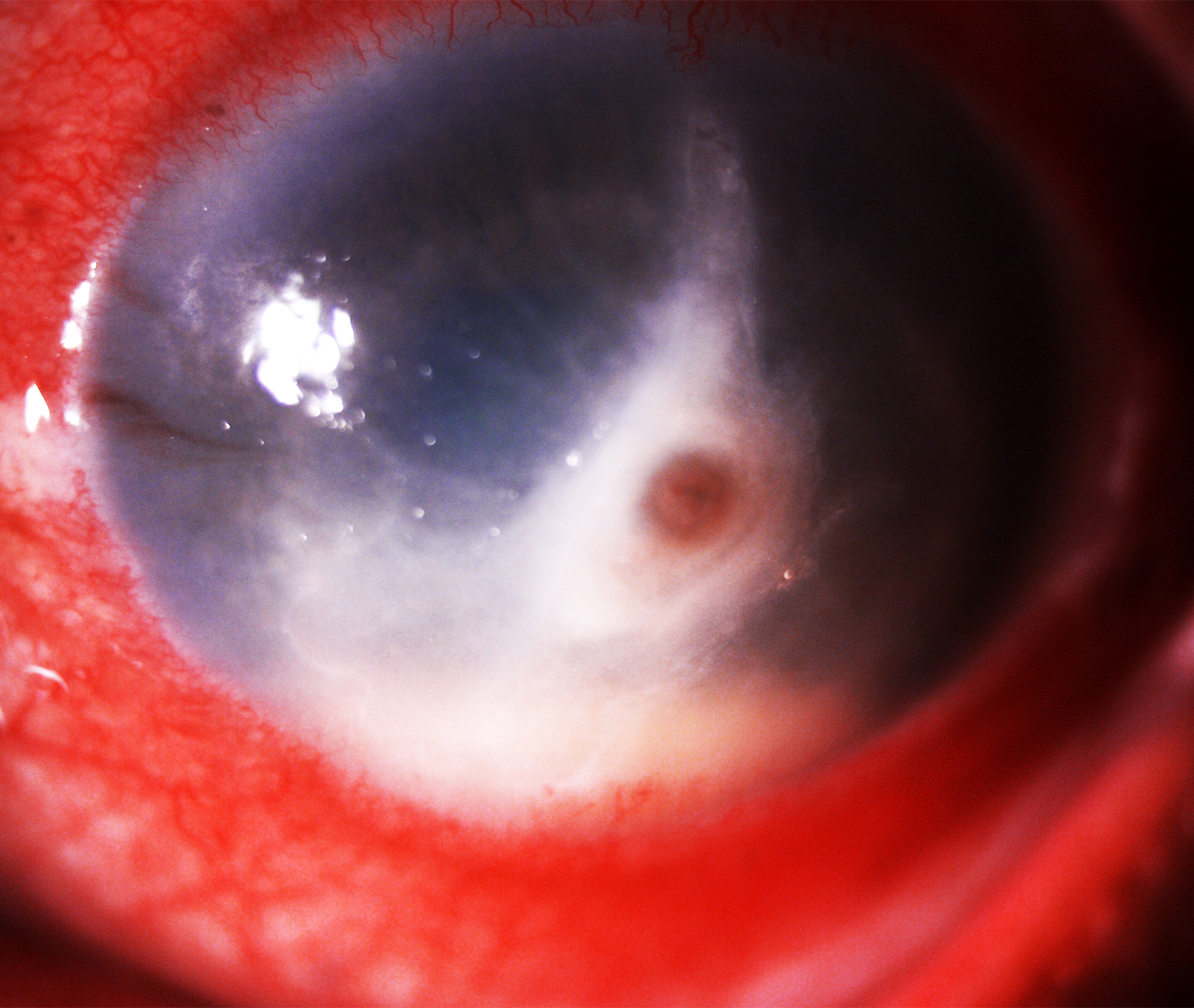
Pythium Keratitis with Central Perforation and Limbal Spread. Slit-lamp photograph showing conjunctival congestion, a 4 mm × 3 mm central full-thickness corneal infiltrate with perforation, tentacular projections, inferior limbal extension, and anterior chamber hypopyon—features suggestive of Pythium keratitis.
Contributed by Bharat Gurnani, MD
(Click Image to Enlarge)
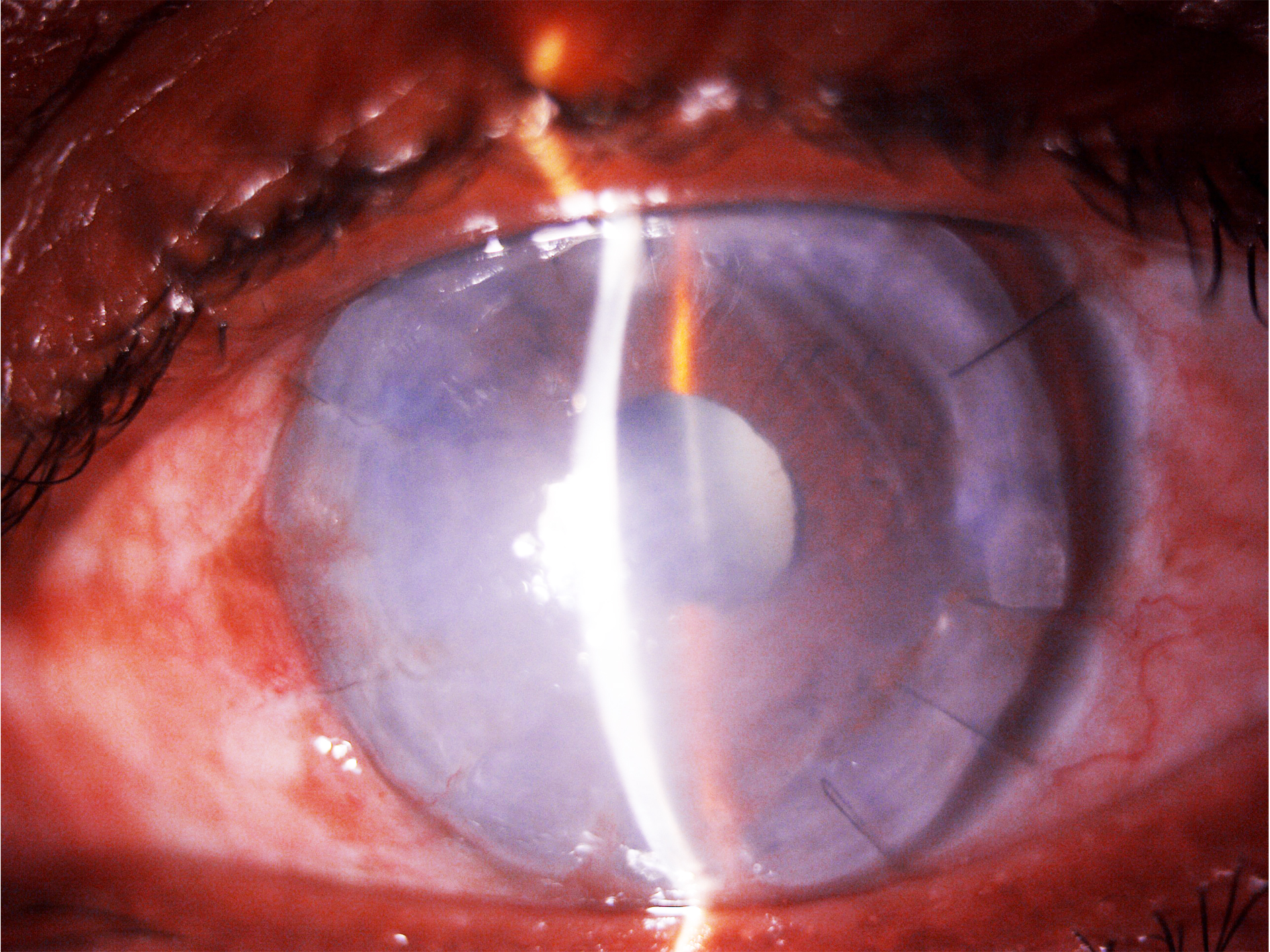
Failed Graft Posttherapeutic Keratoplasty in Pythium Keratitis. Slit-lamp image of a patient with Pythium keratitis following therapeutic keratoplasty, showing mild conjunctival congestion, a large 10-mm failed graft with well-apposed graft–host junction and edge lift from 1 to 2 o’clock, a well-formed anterior chamber, and an immature cataract.
Contributed by Bharat Gurnani, MD
References
Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian journal of ophthalmology. 2021 May:69(5):1095-1101. doi: 10.4103/ijo.IJO_1808_20. Epub [PubMed PMID: 33913840]
Level 2 (mid-level) evidenceHasika R, Lalitha P, Radhakrishnan N, Rameshkumar G, Prajna NV, Srinivasan M. Pythium keratitis in South India: Incidence, clinical profile, management, and treatment recommendation. Indian journal of ophthalmology. 2019 Jan:67(1):42-47. doi: 10.4103/ijo.IJO_445_18. Epub [PubMed PMID: 30574890]
Bagga B, Sharma S, Madhuri Guda SJ, Nagpal R, Joseph J, Manjulatha K, Mohamed A, Garg P. Leap forward in the treatment of Pythium insidiosum keratitis. The British journal of ophthalmology. 2018 Dec:102(12):1629-1633. doi: 10.1136/bjophthalmol-2017-311360. Epub 2018 Mar 15 [PubMed PMID: 29545414]
Gurnani B, Narayana S, Christy J, Rajkumar P, Kaur K, Gubert J. Successful management of pediatric pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. European journal of ophthalmology. 2022 Sep:32(5):NP87-NP91. doi: 10.1177/11206721211006564. Epub 2021 Mar 28 [PubMed PMID: 33779337]
Level 3 (low-level) evidenceGurnani B, Kaur K. Anti-infective therapies for Pythium insidiosum keratitis. Expert review of anti-infective therapy. 2024 Oct:22(10):805-817. doi: 10.1080/14787210.2024.2403146. Epub 2024 Sep 13 [PubMed PMID: 39268901]
Gurnani B, Kaur K. Letter to the editor: Case series: Mixed infectious keratitis by Pythium insidiosum and fungal species. Optometry and vision science : official publication of the American Academy of Optometry. 2024 May 1:101(5):236. doi: 10.1097/OPX.0000000000002147. Epub [PubMed PMID: 38857033]
Level 2 (mid-level) evidenceGurnani B, Natarajan R, Mohan M, Kaur K. Breaking-Down Barriers: Proposal of Using Cellulose Biosynthesis Inhibitors and Cellulase Enzyme as a Novel Treatment Modality for Vision Threatening Pythium Insidiosum Keratitis. Clinical ophthalmology (Auckland, N.Z.). 2024:18():765-776. doi: 10.2147/OPTH.S450665. Epub 2024 Mar 11 [PubMed PMID: 38495678]
Byrd LB, Gurnani B, Martin N. Corneal Ulcer. StatPearls. 2025 Jan:(): [PubMed PMID: 30969511]
Gurnani B, Kaur K. Understanding barriers, recommended solutions, and future prospects for the diagnosis and management of Pythium insidiosum keratitis. Indian journal of ophthalmology. 2023 Dec 1:71(12):3584-3586. doi: 10.4103/IJO.IJO_1041_23. Epub 2023 Nov 20 [PubMed PMID: 37991287]
Level 3 (low-level) evidenceGurnani B, Kaur K. Leap forward in clinical and photographic diagnosis of Pythium insidiosum keratitis. Indian journal of ophthalmology. 2023 Sep:71(9):3263-3264. doi: 10.4103/IJO.IJO_355_23. Epub [PubMed PMID: 37602622]
Gurnani B, Kaur K, Tandon A. Letter Regarding: Randomized Double-Masked Placebo-Controlled Trial for the Management of Pythium Keratitis: Combination of Antibiotics Versus Monotherapy. Cornea. 2023 Dec 1:42(12):e22-e23. doi: 10.1097/ICO.0000000000003350. Epub 2023 Jul 18 [PubMed PMID: 37487172]
Level 1 (high-level) evidenceVishwakarma P, Mohanty A, Kaur A, Das S, Priyadarshini SR, Mitra S, Mittal R, Sahu SK. Pythium keratitis: Clinical profile, laboratory diagnosis, treatment, and histopathology features post-treatment at a tertiary eye care center in Eastern India. Indian journal of ophthalmology. 2021 Jun:69(6):1544-1552. doi: 10.4103/ijo.IJO_2356_20. Epub [PubMed PMID: 34011738]
Mendoza L, Newton JC. Immunology and immunotherapy of the infections caused by Pythium insidiosum. Medical mycology. 2005 Sep:43(6):477-86 [PubMed PMID: 16320491]
Level 3 (low-level) evidenceGurnani B, Kaur K. Pythium Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 34424645]
Cao B, Gonugunta VT, Radhakrishnan N, Lalitha P, Gurnani B, Kaur K, Iyer G, Agarwal S, Srinivasan B, Keenan JD, Prajna NV. Outcomes of Pythium keratitis: a meta-analysis of individual patient data. Current ophthalmology reports. 2022 Dec:10(4):198-208. doi: 10.1007/s40135-022-00302-7. Epub 2022 Nov 10 [PubMed PMID: 37250102]
Level 1 (high-level) evidenceGurnani B, Kaur K, Kumar T. Commentary: Current concepts, recent updates, and future treatment options for Pythium insidiosum keratitis. Indian journal of ophthalmology. 2023 May:71(5):1874-1876. doi: 10.4103/IJO.IJO_80_23. Epub [PubMed PMID: 37203047]
Level 3 (low-level) evidenceGurnani B, Kaur K. Predicting Prognosis Based on Regional Prevalence, Ulcer Morphology and Treatment Strategy in Vision-Threatening Pythium insidiosum Keratitis. Clinical ophthalmology (Auckland, N.Z.). 2023:17():1307-1314. doi: 10.2147/OPTH.S412274. Epub 2023 May 5 [PubMed PMID: 37181081]
Gurnani B, Christy J, Kaur K, Moutappa F, Gubert J. Successful Management of Pythium insidiosum Keratitis Masquerading as Dematiaceous Fungal Keratitis in an Immunosuppressed Asian Male. Ocular immunology and inflammation. 2024 Jul:32(5):583-586. doi: 10.1080/09273948.2023.2179495. Epub 2023 Feb 22 [PubMed PMID: 36812410]
Tanhehco TY, Stacy RC, Mendoza L, Durand ML, Jakobiec FA, Colby KA. Pythium insidiosum keratitis in Israel. Eye & contact lens. 2011 Mar:37(2):96-8. doi: 10.1097/ICL.0b013e3182043114. Epub [PubMed PMID: 21252687]
Level 3 (low-level) evidenceBadenoch PR, Mills RA, Chang JH, Sadlon TA, Klebe S, Coster DJ. Pythium insidiosum keratitis in an Australian child. Clinical & experimental ophthalmology. 2009 Nov:37(8):806-9. doi: 10.1111/j.1442-9071.2009.02135.x. Epub [PubMed PMID: 19878227]
Level 3 (low-level) evidenceGurnani B, Kaur K, Agarwal S, Lalgudi VG, Shekhawat NS, Venugopal A, Tripathy K, Srinivasan B, Iyer G, Gubert J. Pythium insidiosum Keratitis: Past, Present, and Future. Ophthalmology and therapy. 2022 Oct:11(5):1629-1653. doi: 10.1007/s40123-022-00542-7. Epub 2022 Jul 5 [PubMed PMID: 35788551]
Gurnani B, Kaur K. Comment on: Sensitivity and specificity of potassium hydroxide and calcofluor white stain to differentiate between fungal and Pythium filaments in corneal scrapings from patients of Pythium keratitis. Indian journal of ophthalmology. 2022 Jun:70(6):2204. doi: 10.4103/ijo.IJO_345_22. Epub [PubMed PMID: 35648022]
Level 3 (low-level) evidenceGurnani B, Kaur K, Venugopal A, Srinivasan B, Bagga B, Iyer G, Christy J, Prajna L, Vanathi M, Garg P, Narayana S, Agarwal S, Sahu S. Pythium insidiosum keratitis - A review. Indian journal of ophthalmology. 2022 Apr:70(4):1107-1120. doi: 10.4103/ijo.IJO_1534_21. Epub [PubMed PMID: 35325996]
Austwick PK, Copland JW. Swamp cancer. Nature. 1974 Jul 5:250(461):84 [PubMed PMID: 4276247]
Level 3 (low-level) evidenceMiller RI. Investigations into the biology of three 'phycomycotic' agents pathogenic for horses in Australia. Mycopathologia. 1983 Jan 17:81(1):23-8 [PubMed PMID: 6682179]
Level 3 (low-level) evidencePermpalung N, Worasilchai N, Chindamporn A. Human Pythiosis: Emergence of Fungal-Like Organism. Mycopathologia. 2020 Oct:185(5):801-812. doi: 10.1007/s11046-019-00412-0. Epub 2019 Dec 16 [PubMed PMID: 31845178]
Hu L, Huang X, Yee NH, Meng H, Jiang L, Liang L, Chen X. Pythium insidiosum: an emerging pathogen that is easily misdiagnosed and given treatment as a fungus. Frontiers in cellular and infection microbiology. 2024:14():1430032. doi: 10.3389/fcimb.2024.1430032. Epub 2024 Aug 29 [PubMed PMID: 39268488]
Lu SH, Qiao DN, Dong PF. A nursing report on a corneal contact lens wearer receiving keratoplasty due to corneal ulcer and perforation caused by Pythium insidiosum infection: A case report. Medicine. 2024 Apr 5:103(14):e37663. doi: 10.1097/MD.0000000000037663. Epub [PubMed PMID: 38579080]
Level 2 (mid-level) evidenceKulkarni U. Commentary: Pythium keratitis: The masquerading menace! Indian journal of ophthalmology. 2023 Feb:71(2):516-517. doi: 10.4103/ijo.IJO_2252_22. Epub [PubMed PMID: 36727352]
Level 3 (low-level) evidenceSridhar U, Tripathy K. Commentary: Clinico-microbiological differentiation between Pythium and fungal keratitis. Indian journal of ophthalmology. 2023 Feb:71(2):515-516. doi: 10.4103/ijo.IJO_2251_22. Epub [PubMed PMID: 36727351]
Level 3 (low-level) evidenceHou H, Wang Y, Tian L, Wang F, Sun Z, Chen Z. Pythium insidiosum keratitis reported in China, raising the alertness to this fungus-like infection: a case series. Journal of medical case reports. 2021 Dec 17:15(1):619. doi: 10.1186/s13256-021-03189-3. Epub 2021 Dec 17 [PubMed PMID: 34915928]
Level 2 (mid-level) evidenceOng HS, Sharma N, Phee LM, Mehta JS. Atypical microbial keratitis. The ocular surface. 2023 Apr:28():424-439. doi: 10.1016/j.jtos.2021.11.001. Epub 2021 Nov 10 [PubMed PMID: 34768003]
Zhang XY, Qi XL, Lu XH, Gao H. [Clinical features and treatment prognoses of Pythium keratitis]. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2021 Aug 11:57(8):589-594. doi: 10.3760/cma.j.cn112142-20201023-00703. Epub [PubMed PMID: 34344119]
Level 2 (mid-level) evidenceKalra P, Bagga B, Garg P. Pythium Insidiosum Keratitis: Histopathology and Rapid Novel Diagnostic Staining Technique. Cornea. 2018 Mar:37(3):e14. doi: 10.1097/ICO.0000000000001463. Epub [PubMed PMID: 29176454]
Behera HS, Barik MR, Das S, Sharma S. Simple polymerase chain reaction assay to differentiate between fungal and Pythium insidiosum keratitis. Clinical & experimental ophthalmology. 2021 Aug:49(6):630-632. doi: 10.1111/ceo.13939. Epub 2021 May 18 [PubMed PMID: 33931931]
Maeno S, Oie Y, Sunada A, Tanibuchi H, Hagiwara S, Makimura K, Nishida K. Successful medical management of Pythium insidiosum keratitis using a combination of minocycline, linezolid, and chloramphenicol. American journal of ophthalmology case reports. 2019 Sep:15():100498. doi: 10.1016/j.ajoc.2019.100498. Epub 2019 Jun 18 [PubMed PMID: 31289761]
Level 3 (low-level) evidenceBernheim D, Dupont D, Aptel F, Dard C, Chiquet C, Normand AC, Piarroux R, Cornet M, Maubon D. Pythiosis: Case report leading to new features in clinical and diagnostic management of this fungal-like infection. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2019 Sep:86():40-43. doi: 10.1016/j.ijid.2019.06.011. Epub 2019 Jun 15 [PubMed PMID: 31212104]
Level 3 (low-level) evidenceAppavu SP, Prajna L, Rajapandian SGK. Genotyping and phylogenetic analysis of Pythium insidiosum causing human corneal ulcer. Medical mycology. 2020 Feb 1:58(2):211-218. doi: 10.1093/mmy/myz044. Epub [PubMed PMID: 31073609]
Raghavan A, Bellamkonda P, Mendoza L, Rammohan R. Pythium insidiosum and Acanthamoeba keratitis in a contact lens user. BMJ case reports. 2018 Dec 3:11(1):. doi: 10.1136/bcr-2018-226386. Epub 2018 Dec 3 [PubMed PMID: 30567163]
Level 3 (low-level) evidenceNeufeld A, Seamone C, Maleki B, Heathcote JG. Pythium insidiosum keratitis: a pictorial essay of natural history. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2018 Apr:53(2):e48-e50. doi: 10.1016/j.jcjo.2017.07.002. Epub 2017 Sep 22 [PubMed PMID: 29631838]
Nonpassopon M, Jongkhajornpong P, Aroonroch R, Koovisitsopit A, Lekhanont K. Predisposing Factors, Clinical Presentations, and Outcomes of Contact Lens-Related Pythium Keratitis. Cornea. 2021 Nov 1:40(11):1413-1419. doi: 10.1097/ICO.0000000000002651. Epub [PubMed PMID: 33470674]
Agarwal S, Iyer G, Srinivasan B, Benurwar S, Agarwal M, Narayanan N, Lakshmipathy M, Radhika N, Rajagopal R, Krishnakumar S, K LT. Clinical profile, risk factors and outcome of medical, surgical and adjunct interventions in patients with Pythiuminsidiosum keratitis. The British journal of ophthalmology. 2019 Mar:103(3):296-300. doi: 10.1136/bjophthalmol-2017-311804. Epub 2018 Sep 11 [PubMed PMID: 30206158]
Puangsricharern V, Chotikkakamthorn P, Tulvatana W, Kittipibul T, Chantaren P, Reinprayoon U, Kasetsuwan N, Satitpitakul V, Worasilchai N, Chindamporn A. Clinical Characteristics, Histopathology, and Treatment Outcomes of Pythium Keratitis: A Retrospective Cohort Study. Clinical ophthalmology (Auckland, N.Z.). 2021:15():1691-1701. doi: 10.2147/OPTH.S303721. Epub 2021 Apr 23 [PubMed PMID: 33935486]
Level 2 (mid-level) evidenceRamappa M, Nagpal R, Sharma S, Chaurasia S. Successful Medical Management of Presumptive Pythium insidiosum Keratitis. Cornea. 2017 Apr:36(4):511-514. doi: 10.1097/ICO.0000000000001162. Epub [PubMed PMID: 28207431]
Rathi A, Chakrabarti A, Agarwal T, Pushker N, Patil M, Kamble H, Titiyal JS, Mohan R, Kashyap S, Sharma S, Sen S, Satpathy G, Sharma N. Pythium Keratitis Leading to Fatal Cavernous Sinus Thrombophlebitis. Cornea. 2018 Apr:37(4):519-522. doi: 10.1097/ICO.0000000000001504. Epub [PubMed PMID: 29319595]
Anutarapongpan O, Thanathanee O, Worrawitchawong J, Suwan-Apichon O. Role of Confocal Microscopy in the Diagnosis of Pythium insidiosum Keratitis. Cornea. 2018 Feb:37(2):156-161. doi: 10.1097/ICO.0000000000001466. Epub [PubMed PMID: 29176453]
Chatterjee S, Agrawal D. Azithromycin in the Management of Pythium insidiosum Keratitis. Cornea. 2018 Feb:37(2):e8-e9. doi: 10.1097/ICO.0000000000001419. Epub [PubMed PMID: 29095755]
Agarwal S, Iyer G, Srinivasan B, Agarwal M, Panchalam Sampath Kumar S, Therese LK. Clinical profile of pythium keratitis: perioperative measures to reduce risk of recurrence. The British journal of ophthalmology. 2018 Feb:102(2):153-157. doi: 10.1136/bjophthalmol-2017-310604. Epub 2017 Sep 13 [PubMed PMID: 28903964]
He H, Liu H, Chen X, Wu J, He M, Zhong X. Diagnosis and Treatment of Pythium Insidiosum Corneal Ulcer in a Chinese Child: A Case Report and Literature Review. The American journal of case reports. 2016 Dec 27:17():982-988 [PubMed PMID: 28025573]
Level 3 (low-level) evidenceKosrirukvongs P, Chaiprasert A, Canyuk C, Wanachiwanawin W. Comparison of Nested PCR and Culture Identification of Pythium insidiosum in Patients with Pythium Keratitis. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2016 Sep:99(9):1033-8 [PubMed PMID: 29927209]
Acharya M, Singh A, Nidhi V, Tiwari A, Gandhi A, Chaudhari I. Outcomes of keratoplasty in a cohort of Pythium insidiosum keratitis cases at a tertiary eye care center in India. Indian journal of ophthalmology. 2024 Aug 1:72(8):1124-1129. doi: 10.4103/IJO.IJO_3108_23. Epub 2024 Jul 29 [PubMed PMID: 39078955]
Level 3 (low-level) evidenceMendoza L, Taylor JW, Walker ED, Vilela R. Description of three novel Lagenidium (Oomycota) species causing infection in mammals. Revista iberoamericana de micologia. 2016 Apr-Jun:33(2):83-91. doi: 10.1016/j.riam.2015.07.005. Epub 2016 Feb 28 [PubMed PMID: 26924580]
Sharma S, Balne PK, Motukupally SR, Das S, Garg P, Sahu SK, Arunasri K, Manjulatha K, Mishra DK, Shivaji S. Pythium insidiosum keratitis: clinical profile and role of DNA sequencing and zoospore formation in diagnosis. Cornea. 2015 Apr:34(4):438-42. doi: 10.1097/ICO.0000000000000349. Epub [PubMed PMID: 25738236]
Level 2 (mid-level) evidencePermpalung N, Worasilchai N, Plongla R, Upala S, Sanguankeo A, Paitoonpong L, Mendoza L, Chindamporn A. Treatment outcomes of surgery, antifungal therapy and immunotherapy in ocular and vascular human pythiosis: a retrospective study of 18 patients. The Journal of antimicrobial chemotherapy. 2015:70(6):1885-92. doi: 10.1093/jac/dkv008. Epub 2015 Jan 27 [PubMed PMID: 25630647]
Level 2 (mid-level) evidenceThanathanee O, Enkvetchakul O, Rangsin R, Waraasawapati S, Samerpitak K, Suwan-apichon O. Outbreak of Pythium keratitis during rainy season: a case series. Cornea. 2013 Feb:32(2):199-204. doi: 10.1097/ICO.0b013e3182535841. Epub [PubMed PMID: 22902492]
Level 2 (mid-level) evidenceBarequet IS, Lavinsky F, Rosner M. Long-term follow-up after successful treatment of Pythium insidiosum keratitis in Israel. Seminars in ophthalmology. 2013 Jul:28(4):247-50. doi: 10.3109/08820538.2013.788676. Epub 2013 Apr 29 [PubMed PMID: 23627654]
Hung C, Leddin D. Keratitis caused by Pythium insidiosum in an immunosuppressed patient with Crohn's disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014 Oct:12(10):A21-2. doi: 10.1016/j.cgh.2014.04.023. Epub 2014 Apr 30 [PubMed PMID: 24793025]
Kosrirukvongs P, Chaiprasert A, Uiprasertkul M, Chongcharoen M, Banyong R, Krajaejun T, Wanachiwanawin W. Evaluation of nested pcr technique for detection of Pythium insidiosum in pathological specimens from patients with suspected fungal keratitis. The Southeast Asian journal of tropical medicine and public health. 2014 Jan:45(1):167-73 [PubMed PMID: 24964666]
Lelievre L, Borderie V, Garcia-Hermoso D, Brignier AC, Sterkers M, Chaumeil C, Lortholary O, Lanternier F. Imported pythium insidiosum keratitis after a swim in Thailand by a contact lens-wearing traveler. The American journal of tropical medicine and hygiene. 2015 Feb:92(2):270-3. doi: 10.4269/ajtmh.14-0380. Epub 2014 Dec 22 [PubMed PMID: 25535313]
Gurnani B, Feroze KB, Patel BC. Neurotrophic Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 28613758]
Pracharktam R, Changtrakool P, Sathapatayavongs B, Jayanetra P, Ajello L. Immunodiffusion test for diagnosis and monitoring of human pythiosis insidiosi. Journal of clinical microbiology. 1991 Nov:29(11):2661-2 [PubMed PMID: 1774283]
Jindayok T, Piromsontikorn S, Srimuang S, Khupulsup K, Krajaejun T. Hemagglutination test for rapid serodiagnosis of human pythiosis. Clinical and vaccine immunology : CVI. 2009 Jul:16(7):1047-51. doi: 10.1128/CVI.00113-09. Epub 2009 Jun 3 [PubMed PMID: 19494087]
Level 3 (low-level) evidenceChareonsirisuthigul T, Khositnithikul R, Intaramat A, Inkomlue R, Sriwanichrak K, Piromsontikorn S, Kitiwanwanich S, Lowhnoo T, Yingyong W, Chaiprasert A, Banyong R, Ratanabanangkoon K, Brandhorst TT, Krajaejun T. Performance comparison of immunodiffusion, enzyme-linked immunosorbent assay, immunochromatography and hemagglutination for serodiagnosis of human pythiosis. Diagnostic microbiology and infectious disease. 2013 May:76(1):42-5. doi: 10.1016/j.diagmicrobio.2013.02.025. Epub 2013 Mar 26 [PubMed PMID: 23537786]
Worasilchai N, Leelahavanichkul A, Permpalung N, Kuityo C, Phaisanchatchawan T, Palaga T, Reantragoon R, Chindamporn A. Antigen host response differences between the animal-type strain and human-clinical Pythium insidiosum isolates used for serological diagnosis in Thailand. Medical mycology. 2019 Jun 1:57(4):519-522. doi: 10.1093/mmy/myy072. Epub [PubMed PMID: 30165659]
Level 3 (low-level) evidenceKulandai LT, Lakshmipathy D, Sargunam J. Novel Duplex Polymerase Chain Reaction for the Rapid Detection of Pythium insidiosum Directly From Corneal Specimens of Patients With Ocular Pythiosis. Cornea. 2020 Jun:39(6):775-778. doi: 10.1097/ICO.0000000000002284. Epub [PubMed PMID: 32118672]
Gurnani B, Kaur K, Lalgudi VG, Kundu G, Mimouni M, Liu H, Jhanji V, Prakash G, Roy AS, Shetty R, Gurav JS. Role of artificial intelligence, machine learning and deep learning models in corneal disorders - A narrative review. Journal francais d'ophtalmologie. 2024 Sep:47(7):104242. doi: 10.1016/j.jfo.2024.104242. Epub 2024 Jul 15 [PubMed PMID: 39013268]
Level 3 (low-level) evidenceMaghsoudlou P, Sood G, Gurnani B, Akhondi H. Cornea Transplantation. StatPearls. 2025 Jan:(): [PubMed PMID: 30969512]
Gurnani B, Kim J, Tripathy K, Mahabadi N, Edens MA. Iritis. StatPearls. 2025 Jan:(): [PubMed PMID: 28613659]
Gurnani B, Kaur K. Therapeutic Keratoplasty. StatPearls. 2025 Jan:(): [PubMed PMID: 37276297]
Thanathanee O, Bhoomibunchoo C, Anutarapongpan O, Suwan-Apichon O, Charoensuk K, Chindamporn A. Role of Immunotherapy in Pythium insidiosum Keratitis. The American journal of tropical medicine and hygiene. 2022 Jul 13:107(1):110-112. doi: 10.4269/ajtmh.22-0015. Epub 2022 Jun 6 [PubMed PMID: 35895358]
Murugan SRB, Sanjay S, Somanath A, Mahendradas P, Patil A, Kaur K, Gurnani B. Artificial Intelligence in Uveitis: Innovations in Diagnosis and Therapeutic Strategies. Clinical ophthalmology (Auckland, N.Z.). 2024:18():3753-3766. doi: 10.2147/OPTH.S495307. Epub 2024 Dec 14 [PubMed PMID: 39703602]
Lopez Montes T, Gurnani B, Stokkermans TJ. Assessment of the Watery Eye. StatPearls. 2025 Jan:(): [PubMed PMID: 36508543]
Ahmad B, Gurnani B, Patel BC. Herpes Simplex Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 31424862]
Gurnani B, Kaur K. Comment on: Clinical and mycological profile of fungal keratitis from North and North-East India. Indian journal of ophthalmology. 2023 Jun:71(6):2607-2608. doi: 10.4103/ijo.IJO_1655_22. Epub [PubMed PMID: 37322697]
Level 3 (low-level) evidenceGurnani B, Kaur K, Chaudhary S, Balakrishnan H. Ophthalmic manifestations of monkeypox infection. Indian journal of ophthalmology. 2023 May:71(5):1687-1697. doi: 10.4103/ijo.IJO_2032_22. Epub [PubMed PMID: 37203020]
Gurnani B, Kaur K, Gurav J. Natasol as a future management option to combat fungal keratitis. Indian journal of ophthalmology. 2023 May:71(5):2302-2303. doi: 10.4103/IJO.IJO_190_23. Epub [PubMed PMID: 37202986]
Gurnani B, Christy J, Narayana S, Kaur K, Moutappa F. Corneal Perforation Secondary to Rosacea Keratitis Managed with Excellent Visual Outcome. Nepalese journal of ophthalmology : a biannual peer-reviewed academic journal of the Nepal Ophthalmic Society : NEPJOPH. 2022 Jan:14(27):162-167. doi: 10.3126/nepjoph.v14i1.36454. Epub [PubMed PMID: 35996914]
Tanna V, Bagga B, Sharma S, Ahirwar LK, Kate A, Mohamed A, Joseph J. Randomized Double-Masked Placebo-Controlled Trial for the Management of Pythium Keratitis: Combination of Antibiotics Versus Monotherapy. Cornea. 2023 Dec 1:42(12):1544-1550. doi: 10.1097/ICO.0000000000003251. Epub 2023 Feb 14 [PubMed PMID: 36796011]
Level 1 (high-level) evidenceHtun ZM, Rotchanapreeda T, Rujirawat T, Lohnoo T, Yingyong W, Kumsang Y, Sae-Chew P, Payattikul P, Yurayart C, Limsivilai O, Sonthayanon P, Mangmee S, Chongtrakool P, Krajaejun T. Loop-mediated Isothermal Amplification (LAMP) for Identification of Pythium insidiosum. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2020 Dec:101():149-159. doi: 10.1016/j.ijid.2020.09.1430. Epub 2020 Sep 26 [PubMed PMID: 32987181]
Roozbahani M, Hammersmith KM, Nagra PK, Ma JF, Rapuano CJ. "Therapeutic Penetrating Keratoplasty: A Retrospective Review". Eye & contact lens. 2018 Nov:44 Suppl 2():S433-S441. doi: 10.1097/ICL.0000000000000522. Epub [PubMed PMID: 29944500]
Level 2 (mid-level) evidenceBouley DM, Ghori N, Mercer KL, Falkow S, Ramakrishnan L. Dynamic nature of host-pathogen interactions in Mycobacterium marinum granulomas. Infection and immunity. 2001 Dec:69(12):7820-31 [PubMed PMID: 11705964]
Sane SS, Madduri B, Mohan N, Mittal R, Raghava JV, Fernandes M. Improved Outcome of Pythium Keratitis With a Combined Triple Drug Regimen of Linezolid and Azithromycin. Cornea. 2021 Jul 1:40(7):888-893. doi: 10.1097/ICO.0000000000002503. Epub [PubMed PMID: 32947406]
Kaur K, Gurnani B. Targeting the blind years, not the blind eyes - Need to update current pediatric ophthalmology practices. Indian journal of ophthalmology. 2023 Nov:71(11):3573-3574. doi: 10.4103/IJO.IJO_838_23. Epub [PubMed PMID: 37870029]
Gurnani B, Kaur K. Re: Dot et al.: Incidence of retinal detachment, macular edema, and ocular hypertension after neodymium:yttrium-aluminum-garnet capsulotomy: a population-based nationwide study-The French YAG 2 Study (Ophthalmology. 2022;130:478-487). Ophthalmology. 2023 Dec:130(12):e43. doi: 10.1016/j.ophtha.2023.08.012. Epub 2023 Sep 20 [PubMed PMID: 37737811]
Kaur K, Srividya KS, Kabra N, Saranath R, Gurnani B, Venkatesh R. Patterns of ophthalmic emergencies presenting to a tertiary eye care hospital in India. Indian journal of ophthalmology. 2024 Feb 1:72(2):296-297. doi: 10.4103/IJO.IJO_1578_23. Epub 2024 Jan 25 [PubMed PMID: 38273691]
Gurnani B, Kaur K. Bacterial Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 34662023]
Gurnani B, Kaur K. Contact Lens–Related Complications. StatPearls. 2025 Jan:(): [PubMed PMID: 36512659]
Shukla UV, Gurnani B, Kaufman EJ. Intraocular Hemorrhage. StatPearls. 2025 Jan:(): [PubMed PMID: 33620856]
Venugopal A, Gurnani B, Ravindran M, Uduman MS. Management of symblepharon with Gore-tex as a novel treatment option for ocular chemical burns. European journal of ophthalmology. 2024 Mar 5:():11206721241238302. doi: 10.1177/11206721241238302. Epub 2024 Mar 5 [PubMed PMID: 38444229]
Nizami AA, Gurnani B, Gulani AC. Cataract. StatPearls. 2025 Jan:(): [PubMed PMID: 30969521]
Lee SY, Gurnani B, Mesfin FB. Blindness. StatPearls. 2025 Jan:(): [PubMed PMID: 28846303]
Gurnani B, Kaur K. Molecular and epigenetic mechanisms governing ocular surface squamous neoplasia: opportunities for diagnostics. Expert review of molecular diagnostics. 2023 Dec 22:():1-15. doi: 10.1080/14737159.2023.2298681. Epub 2023 Dec 22 [PubMed PMID: 38131180]
Alabdulrazaq ES, Gurnani B. Anophthalmic Socket. StatPearls. 2024 Jan:(): [PubMed PMID: 39163446]
Gurnani B, Kaur K. Navigating the challenges of infective keratitis: A critical analysis of treatment and diagnostic approaches. Indian journal of ophthalmology. 2024 Aug 1:72(8):1227-1228. doi: 10.4103/IJO.IJO_706_24. Epub 2024 Jul 29 [PubMed PMID: 39078975]
Parmar GS, Agrawal A, Meena A, Mutha P, Gurnani B. Corset suture: A novel technique of overlay appositional continuous sutures with air tamponade for management of large acute corneal hydrops. Indian journal of ophthalmology. 2024 Apr 1:72(4):592-595. doi: 10.4103/IJO.IJO_1006_23. Epub 2024 Mar 8 [PubMed PMID: 38546470]
Gurnani B, Kaur K, Chaudhary S, Kaur RP, Nayak S, Mishra D, Balakrishnan H, Parkash RO, Morya AK, Porwal A. Pediatric corneal transplantation: techniques, challenges, and outcomes. Therapeutic advances in ophthalmology. 2024 Jan-Dec:16():25158414241237906. doi: 10.1177/25158414241237906. Epub 2024 Mar 25 [PubMed PMID: 38533487]
Level 3 (low-level) evidenceGurnani B, Kaur K. Leveraging ChatGPT for ophthalmic education: A critical appraisal. European journal of ophthalmology. 2024 Mar:34(2):323-327. doi: 10.1177/11206721231215862. Epub 2023 Nov 16 [PubMed PMID: 37974429]
Schnider C, Yuen L, Rampat R, Zhu D, Dhallu S, Trinh T, Gurnani B, Abdelmaksoud A, Bhogal-Bhamra G, Wolffsohn JS, Naroo SA. BCLA CLEAR presbyopia: Management with intraocular lenses. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2024 Aug:47(4):102253. doi: 10.1016/j.clae.2024.102253. Epub 2024 Jul 2 [PubMed PMID: 39068141]
Pathak M, Sahu V, Kumar A, Kaur K, Gurnani B. Current Concepts and Recent Updates of Optical Biometry- A Comprehensive Review. Clinical ophthalmology (Auckland, N.Z.). 2024:18():1191-1206. doi: 10.2147/OPTH.S464538. Epub 2024 May 2 [PubMed PMID: 38711575]
Gurnani B, Kaur K. Comment on "Impact of the COVID-19 pandemic on research publications in emergency medicine". World journal of emergency medicine. 2025:16(2):172-173. doi: 10.5847/wjem.j.1920-8642.2025.026. Epub [PubMed PMID: 40135213]
Level 3 (low-level) evidenceBurrow MK, Gurnani B, Patel BC. Keratoconjunctivitis. StatPearls. 2025 Jan:(): [PubMed PMID: 31194419]