Introduction
Factor V deficiency, also known as Owren disease or parahemophilia, is a rare bleeding disorder that may be inherited or acquired. Dr. Paul Owren first identified the condition in Norway in 1943.[1][2] The disease manifests similarly to other clotting factor deficiencies, with symptoms ranging from minor mucosal bleeding to serious and life-threatening hemorrhage.[3] The degree of bleeding generally correlates with factor Va levels. However, literature reports that factor Va levels below 1% may still present with only mild bleeding symptoms in some individuals.[4][5]
Factor V deficiency may be categorized as mild, moderate, or severe based on plasma factor V activity relative to normal. Mild factor V deficiency is identified by plasma activity greater than 10% of normal. Moderate deficiency shows activity from 1% to 10%, and severe deficiency presents with activity less than 1%. Initial laboratory evaluation shows prolonged prothrombin time and activated partial thromboplastin time (aPTT) with a normal thrombin time. A low plasma factor V level confirms the diagnosis.
Plasma mixing studies help distinguish between inherited and acquired factor V deficiency. These tests assess whether prolonged coagulation studies result from a factor deficiency or an inhibitor. Normal plasma is combined with the patient's plasma, which demonstrates prolonged prothrombin time and aPTT. Improvement of these parameters after mixing suggests an inherited form, with normal plasma replacing the missing factor. Persistent prothrombin time and aPTT prolongation after mixing indicates an acquired form, as an inhibitor in the patient's plasma prevents normalization. This test is not specific to factor V deficiency.[6]
Treatment varies depending on whether factor V deficiency is inherited or acquired. In inherited cases, treatment typically involves transfusion of fresh frozen plasma (FFP), which contains factor V. Mild cases may require antifibrinolytics to achieve hemostasis. In contrast, managing acquired factor V deficiency is more complex, requiring both bleeding symptom control and elimination of anti–factor V autoantibodies. Bleeding control may be achieved with transfusion of FFP, platelets, prothrombin complex concentrates, antifibrinolytics, or recombinant activated factor VII. Eradicating factor V inhibitor requires immunosuppression.[7]
Coagulation Physiology
The coagulation cascade orchestrates hemostasis through the intrinsic, extrinsic, and common pathways (see Image. Coagulation Cascade Diagram). In the intrinsic pathway, endothelial damage exposes collagen, activating factor XII. This factor catalyzes the subsequent activation of factors XI, IX, and X, culminating in thrombin generation and fibrin formation. The extrinsic pathway begins when tissue factor is released by endothelial cells, activating factor VII and merging with the intrinsic pathway to initiate factor X activation. Factor Xa subsequently catalyzes the conversion of prothrombin to thrombin, promoting fibrin polymerization and stabilizing the platelet plug.[8]
Factor V, also known as proaccelerin or labile factor, is a crucial nonenzymatic protein in the coagulation cascade. The liver produces approximately 80% of this glycoprotein, with the remaining 20% synthesized within the α-granules of platelets and megakaryocytes. Factor V circulates in plasma with a half-life of 12 to 36 hours.[9] Either thrombin or factor Xa converts factor V to the plasma cofactor of the prothrombinase complex. This complex, composed of calcium, phospholipids, factor Va, and factor Xa, is essential for converting prothrombin to thrombin. Thrombin then activates factor XIII and fibrin, leading to clot formation.
Factor Va is deactivated by protein C once hemostasis is achieved. Negative feedback mechanisms modulate the coagulation cascade to prevent excessive clot formation. Despite thrombin's role in clot formation, this factor also activates plasminogen to plasmin, aiding fibrinolysis. Thrombin induces antithrombin production, which inhibits thrombin and factor Xa activity. Factor V is also vital in the anticoagulation pathway, coordinating with protein C to deactivate factor VIII, thereby decreasing prothrombinase activity and, ultimately, thrombin and fibrin production. This mechanism reduces clot formation. Activated protein C (APC) shifts the balance toward coagulation inhibition.[10]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Factor V deficiency may either be inherited or acquired. These forms arise from different causes, explained below.
Inherited Factor V Deficiency
Inheritance follows an autosomal recessive pattern, with F5 gene (1q23) mutations transmitted either homozygously or heterozygously. Heterozygous carriers are typically asymptomatic. In contrast, homozygotes and compound heterozygotes, possessing germline variants of 2 different mutations at the same genetic locus, may exhibit a wide range of signs and symptoms, from mild to severe bleeding.
Factor V deficiency is categorized into types 1 and 2. Type 1 involves a quantitative reduction in factor V activity and antigen levels. Type 2 involves qualitative dysfunction, with decreased coagulant activity despite normal or low factor V antigen levels.[11][12] Over 190 mutations have been identified, predominantly missense and nonsense mutations, followed by small deletions, splicing mutations, and, less frequently, small and large insertions, large deletions, and complex rearrangements. Symptoms typically manifest before age 6. Rarely, factor V deficiency is coinherited with factor VIII deficiency.
Combined Congenital Factor V and Factor VIII Deficiency
Combined factor V and factor VIII deficiency (F5F8) is extremely rare, accounting for about 3% of all rare coagulopathies.[13] The condition occurs most often in regions where consanguineous marriages are common, such as the Middle East and South Asia.
F5F8 is inherited in an autosomal recessive pattern. The congenital deficiency results from mutations in either the LMAN1 or MCFD2 genes, which encode components of the transport apparatus responsible for moving factors V and VIII from the endoplasmic reticulum to the Golgi complex. The LMAN1 gene is located on chromosome 18 (18q21.32) and contains 13 exons, whereas the MCFD2 gene is located on chromosome 2 (2p21) and contains 4 exons. Combined F5F8 deficiency is associated with significant hemorrhagic complications, most notably obstetrical bleeding.
Factor V-related bleeding is managed with FFP and tranexamic acid. Bleeding from factor VIII deficiency requires the addition of factor VIII concentrate.
Acquired Factor V Deficiency
Acquired factor V deficiency is less common than the inherited form and results from the production of factor V inhibitors. Reported risk factors include surgery involving bovine thrombin, certain antibiotics (particularly β-lactams), malignancies, infections, liver disease, and autoimmune disorders.[14] COVID-19 vaccination has also been associated with acquired factor V deficiency, as well as deficiencies of factors VIII and XIII.[15]
Current clinical recommendations include performing mixing tests with prolonged incubation, as some factor V inhibitors may be “slow responders.”[16] Factor V deficiency has been reported in cases of chronic myelogenous leukemia, particularly during phases of extreme leukocytosis.[17] Predicting bleeding episodes in this condition is challenging, as symptoms may not consistently correlate with factor V inhibitor levels, the duration of inhibitor presence, aPTT and prothrombin time prolongation, or factor V activity.
Epidemiology
Factor V deficiency is a rare bleeding disorder with an estimated prevalence of 1 per 1 million live births. The condition is typically inherited in an autosomal recessive pattern, affecting both sexes equally, with nearly 200 confirmed mutations. No specific ethnic group has been identified as being at increased risk. However, the deficiency occurs more frequently in regions where consanguineous marriages are common.[18] Recent genetic work identified a homozygous mutation of exon 16 (Met1736Val) in a family in Taiwan.[19]
Pathophysiology
Congenital factor V deficiency, although rare, can cause uncontrolled bleeding, with the triggers for spontaneous episodes remaining unknown. Acquired factor V inhibitors, in contrast, are frequently associated with exposure to bovine thrombin. Such exposure may induce antibodies against bovine factor V that, through cross-reactivity, stimulate autoantibody production against human factor V.[20]
Family history is an important consideration in genetic bleeding disorders. However, the absence of similar complaints among the patient’s relatives suggests a possible noninheritable factor V deficiency, although further evidence is required. Factor V deficiency is distinct from the more common factor V Leiden mutation, which produces resistance to activated protein C and prevents inhibition of factor V’s procoagulant activity. Individuals with factor V Leiden mutations have an increased risk of venous thromboembolism.
History and Physical
History
Factor V deficiency can present with a wide array of bleeding symptoms. The inherited form can manifest in infants, while the acquired form can present at any age and may be more challenging to diagnose. When inherited, symptoms usually appear before age 6, though some mild congenital forms may not manifest until adulthood. Bleeding due to inherited factor V deficiency is indistinguishable from that in other coagulation disorders.
In patients with a known family history, cord blood sampling at birth can enable early diagnosis and intervention. Neonatal signs and symptoms reported in the literature include umbilical stump bleeding, nipple bleeding, epistaxis, gum bleeding, and subcutaneous hematomas. Life-threatening intracranial hemorrhage may also occur and often initially presents as hydrocephalus or seizures in infants, warranting an immediate workup to prevent significant morbidity and mortality.[21][22]
Manifestations of factor V deficiency in older children and adults often include mucocutaneous and soft tissue bleeding such as ecchymosis, easy bruising, petechiae, epistaxis, hemoptysis, hematemesis, melena, hemarthrosis, hematuria, menorrhagia in women, and prolonged bleeding after surgery or trauma. Serious internal hemorrhage may also occur, and if not recognized early, mortality can reach 15% to 20%.
For the congenital type, obtaining an accurate family history of bleeding diathesis is key to early diagnosis. Identifying consanguinity within the family may also help. For the acquired type, critical information includes recent surgical procedures with bovine thrombin exposure, malignancies, infections, liver disease, antibiotic use (particularly the β-lactam group), or autoimmune disease.
Physical Examination
Bleeding in patients with factor V deficiency may be external or internal. A thorough physical examination is critical to diagnosis and management. Vital signs must be checked to assess hemodynamic status. Tachycardia and hypotension may indicate hemodynamic compromise in a bleeding patient. The oral and nasal cavities must be inspected for mucosal bleeding. Chest auscultation is essential for patients presenting with hemoptysis. Trauma-prone areas such as the extremities must be inspected for ecchymoses, petechiae, or subcutaneous hematomas. The genitals should also be examined, particularly in individuals with hematuria or menorrhagia.
Patients presenting with hematemesis or melena require a detailed abdominal examination, including a digital rectal examination. Abdominal tenderness or distension may be noted. Patients with neurological manifestations such as seizures, altered mental status, or focal neurological deficits should undergo a detailed neurological assessment. A bulging anterior fontanelle suggests increased intracranial pressure in infants. Unresponsiveness, apnea, and pulselessness are signs of cardiorespiratory arrest. Resuscitation measures must be initiated immediately in such cases, regardless of the cause.
Evaluation
Laboratory evaluation should be initiated when clinical findings suggest a bleeding disorder. Studies that may yield the appropriate diagnosis include coagulation tests, factor assays, inhibitor screening, and molecular genetic testing, as explained below.
Coagulation Tests
Factor V deficiency prolongs both prothrombin time and aPTT but leaves thrombin time unaffected. These prolonged clotting times are not specific to factor V deficiency but suggest a defect within the common pathway. Additional testing for other common pathway factors, such as fibrinogen, factor II, and factor X, is necessary to specify the defect. Liver disease can also prolong prothrombin time and aPTT due to decreased clotting factor synthesis. After confirming factor V deficiency, mixing studies help distinguish between a lack of factor V and the presence of an anti–factor V inhibitor. Correction of clotting times after mixing indicates factor V deficiency, whereas failure to correct suggests the presence of an inhibitor.
Factor Assays
Factor V assays reveal reduced activity levels, with the deficiency classified as mild (>10%), moderate (1% to 10%), or severe (<1%). All factor activity levels should be measured to determine whether a coexisting deficiency is present, as in combined factors V and VIII deficiency.
Inhibitor Screening
Nonspecific inhibitor screening may involve lupus anticoagulant testing and enzyme-linked immunosorbent assay (ELISA) for β-2 glycoprotein and cardiolipin antibodies, which may be elevated in acquired factor V deficiency. The Bethesda assay, which measures inhibitors against a specific coagulation factor in the blood, confirms the diagnosis.
Molecular Genetic Analysis
The F5 gene, located on chromosome 1q24.2 and spanning 25 exons, consists of 6 domains: A1, A2, A3, B, C1, and C2. Sequencing DNA from peripheral blood leukocytes can identify mutations within this gene to diagnose inherited factor V deficiency. Missense mutations are most often located (61.5%) in domains A2 and C2, with an additional 20% in the B domain. A single heterozygous mutation typically results in factor V activity levels around 50%, whereas homozygous or compound heterozygous mutations often lead to levels below 10%. Mutations in the MCFD2 and LMAN1 genes cause combined factor V and VIII deficiency, as these genes are involved in the transport of these coagulation factors.[23]
Treatment / Management
Treatment depends on the condition's etiology and severity. Mild inherited factor V deficiency may be managed with antifibrinolytics, while more severe phenotypes often require FFP transfusions to replenish factor V levels. Factor V deficiency is uncommon. Thus, a specific factor V concentrate is not commercially available. Other blood products (eg, platelets, prothrombin complex, or cryoprecipitate) contain minimal factor V, making FFP the primary therapy for moderate-to-severe cases. FFP sustains factor V levels above 25% to 30% during bleeding episodes or invasive procedures, with higher levels required to ensure hemostasis in severe or life-threatening hemorrhages.
Asymptomatic patients with acquired factor V deficiency do not require treatment because the inhibitors present in the blood are transient. A multifactorial approach is often necessary for patients undergoing surgery or those experiencing significant bleeding. Measures to control bleeding include transfusion of FFP, platelets, prothrombin complex concentrate, and recombinant activated factor VII. Although platelet α-granules contain minimal factor V, platelet transfusions alone have achieved a 71% success rate in treating acquired factor V deficiency by protecting factor V from inhibitors and promoting hemostasis.
Recombinant activated factor VII, commonly used for inhibitors in hemophilia A or B, can also manage bleeding by directly activating factor X, bypassing the requirement for factor V or other coagulation factors.[24] Immunosuppression remains the gold standard for eliminating factor V inhibitors. Corticosteroids, cyclophosphamide, and rituximab, alone or in combination, suppress anti–factor V autoantibody production with a 63% success rate. High-dose intravenous immunoglobulin (60% success rate) and plasmapheresis (53% success rate) also reduce circulating factor V inhibitors.[25]
Differential Diagnosis
Disorders affecting the coagulation cascade and platelet function cannot be differentiated based solely on clinical presentation. Additional diagnostic testing is required for accurate identification of the condition.
Coagulation Cascade Abnormalities
Coagulation studies can determine specific protein and factor deficiencies. Hemophilia A and B, the most common inherited bleeding disorders, are routinely included in the differential diagnosis. Hemophilia A (factor VIII deficiency), B (factor IX deficiency), and C (factor XI deficiency) typically present with isolated aPTT prolongation. These disorders are distinguished from factor V deficiency by measuring factors VIII, IX, or XI, which are decreased, with a normal prothrombin time.
Disorders producing prolonged prothrombin time and aPTT with a normal thrombin time include deficiencies of common pathway factors (II or X), combined factor V and VIII co-deficiency, vitamin K-dependent factor deficiencies (II, VII, IX, X), and liver disease. Afibrinogenemia, hypofibrinogenemia, and dysfibrinogenemia also cause prolonged prothrombin time and aPTT, but thrombin time is likewise prolonged in these conditions. Thrombotic or hemorrhagic events can occur, although many patients remain asymptomatic.
Platelet Dysfunction
Von Willebrand disease, the most common inherited bleeding disorder, occurs with a variable clinical spectrum ranging from mild mucosal bleeding in type 1 to severe hemorrhage resembling hemophilia in type 3. Laboratory findings include prolonged or normal aPTT, normal prothrombin time, normal-to-low platelet count, and normal-to-low factor VIII level. Less common inherited platelet function disorders include Glanzmann thrombasthenia (αIIbβ3 integrin deficiency or defect) and Bernard–Soulier syndrome (GPIb-IX-V complex deficiency or defect). These disorders show normal prothrombin time and aPTT, reflecting impaired platelet adhesion or aggregation independent of the coagulation cascade.[26]
Prognosis
Congenital and acquired factor V deficiency are both exceedingly rare. Data are insufficient to determine the long-term prognosis of affected individuals. However, mortality rates as high as 21% have been documented in patients who present with severe bleeding manifestations.
Complications
Complications of factor V deficiency may occur as early as the neonatal period. Although uncommon, intracranial hemorrhage poses diagnostic challenges in newborns and carries a high mortality rate. Thrombotic events, while less frequent, can arise from factor V's prothrombotic effect, with reported cases including deep vein thrombosis, cerebral infarction, and limb gangrene. Prompt recognition of factor V deficiency is essential to initiate treatment and prevent potentially life-threatening outcomes.
Consultations
A hematologist should be consulted at the earliest suspicion of a bleeding disorder. Pregnant patients with a family history of bleeding diatheses should be referred to a pediatric hematologist to enable early diagnosis. Cord blood sampling at birth facilitates the diagnosis of coagulation disorders in neonates and reduces the need for large blood volumes. Early hematology involvement is essential, particularly in patients with active bleeding who require urgent blood product support, as accurate testing before transfusion prevents diagnostic and treatment delays caused by posttransfusion interference with coagulation studies.
Deterrence and Patient Education
Patients diagnosed with factor V deficiency should receive counseling given the condition's rarity. Women of childbearing age require education about the condition's heritable nature and potential complications during pregnancy and delivery. Screening the proband's relatives for hereditary factors is important for genetic counseling. Family members should also be informed regarding preventive measures and possible perioperative complications. Wearing an alert bracelet enables rapid recognition and intervention during life-threatening bleeding events.
In acquired factor V deficiency, bleeding prevention involves treating the underlying cause, such as discontinuing inciting medications or managing conditions that trigger antibody formation. Coagulation parameters should be closely monitored, and appropriate blood products, including FFP, administered as needed to maintain hemostasis. Although often transient, the condition warrants patient education on trauma avoidance, particularly avoiding high-risk activities such as contact sports. Regardless of etiology, patients should have regular follow-up with a hematologist and maintain prompt communication with healthcare providers in the event of bleeding.
Pearls and Other Issues
Factor V deficiency disrupts the coagulation cascade due to dysfunction or absence of the glycoprotein. The condition may be congenital or acquired. Bleeding severity ranges from mild episodes to life-threatening hemorrhage. Inherited factor V deficiency usually presents in infancy, warranting a detailed family history and cord blood sampling for early diagnosis. Acquired factor V deficiency can develop at any age, commonly from factor V antibodies triggered by bovine thrombin exposure, medications, or underlying conditions such as liver disease and malignancy.
Diagnosis requires coagulation studies, factor assays, inhibitor screening, and molecular genetic analysis. Management depends on etiology. Inherited cases are generally treated with FFP transfusions, while mild cases may respond to antifibrinolytics. Acquired cases are managed by controlling bleeding and eliminating anti–factor V autoantibodies, often through immunosuppression and transfusion of blood products such as FFP, platelets, or prothrombin complex concentrates. Prevention strategies include patient education, wearing alert bracelets for prompt recognition, and avoiding trauma to reduce bleeding risk.
Enhancing Healthcare Team Outcomes
Coordination among specialists is critical in managing rare disorders such as factor V deficiency. Patients require routine follow-up with a hematologist. For elective surgeries, prophylaxis and bleeding control should be planned collaboratively between the hematologist and the surgical team. In hemorrhage-related emergencies, hematologists and transfusion medicine physicians must be involved immediately to ensure timely product availability, with rapid pharmacy notification for urgent requests.
Close communication among healthcare teams is essential for patient safety and optimal outcomes. Pregnant patients should be monitored in a high-risk obstetrics clinic because of potential complications. Counseling families on the hereditary nature of the condition and considering genetic consultation may aid in early detection and prevention. Neonatologists and pediatricians should remain alert to family history and clinical presentation in undiagnosed pediatric cases. Prompt diagnosis of intracranial hemorrhage is vital, as untreated cases can be fatal, particularly when the etiology is unknown.
Media
(Click Image to Enlarge)
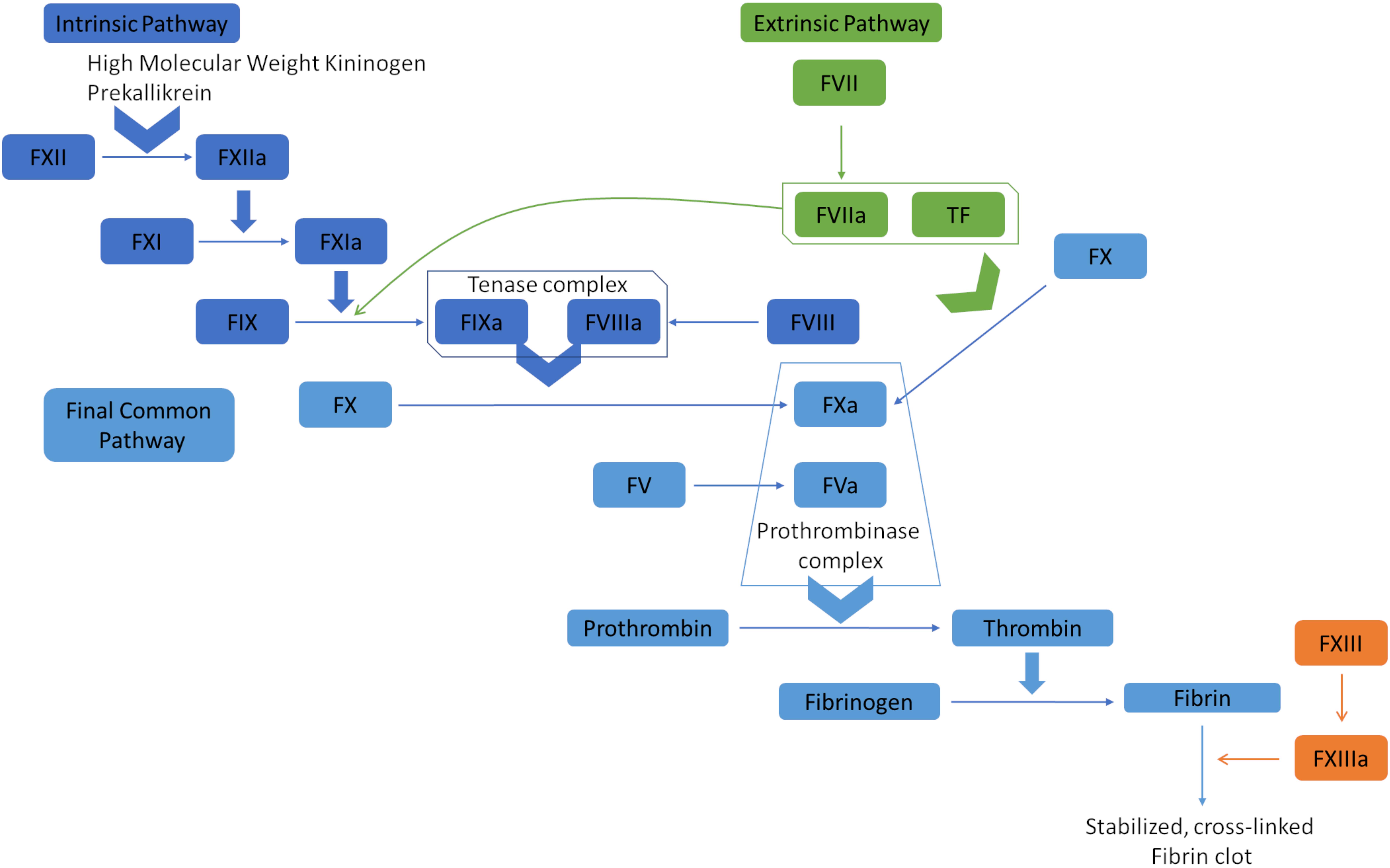
Coagulation Cascade Diagram. The extrinsic pathway, or tissue factor pathway, begins with the release of tissue factor, which combines with factor VIIa. This complex can convert factor X to factor Xa and factor IX to factor IXa. Factors IXa and Xa then activate factors VIII and V, respectively. The prothrombinase complex, containing factors Va and Xa, converts prothrombin (factor II) into thrombin, which transforms fibrinogen into fibrin. Fibrin polymerization leads to the formation of stable and cross-linked fibrin clots. The intrinsic pathway, or contact pathway, is initiated by Factor XII, prekallikrein, and high molecular weight kininogen. Factor XIIa activates factor XIa, which subsequently activates factor IX. These pathways converge at the common pathway, resulting in clot formation.
Top O, Geisen U, Decker EL, Reski R. Critical evaluation of strategies for the production of blood coagulation factors in plant-based systems. Front Plant Sci. 2019;10:261.
doi: 10.3389/fpls.2019.00261.
References
Ehtisham M, Shafiq MA, Shafique M, Mumtaz H, Shahzad MN. Owren's Disease: A Rare Deficiency. Cureus. 2021 Aug:13(8):e17047. doi: 10.7759/cureus.17047. Epub 2021 Aug 10 [PubMed PMID: 34522525]
Bernal S, Pelaez I, Alias L, Baena M, De Pablo-Moreno JA, Serrano LJ, Camero MD, Tizzano EF, Berrueco R, Liras A. High Mutational Heterogeneity, and New Mutations in the Human Coagulation Factor V Gene. Future Perspectives for Factor V Deficiency Using Recombinant and Advanced Therapies. International journal of molecular sciences. 2021 Sep 8:22(18):. doi: 10.3390/ijms22189705. Epub 2021 Sep 8 [PubMed PMID: 34575869]
Level 3 (low-level) evidencePark CH, Park MS, Lee KO, Kim SH, Park YS, Kim HJ. Congenital factor V deficiency from compound heterozygous mutations with a novel variant c.2426del (p.Pro809Hisfs*2) in the F5 gene: A case report. Medicine. 2020 Jan:99(5):e18947. doi: 10.1097/MD.0000000000018947. Epub [PubMed PMID: 32000417]
Level 3 (low-level) evidenceChoi S, Song M. Successful coronary stenting in a patient with factor V deficiency in the absence of fresh frozen plasma transfusion: Case report. Medicine. 2017 Dec:96(50):e9274. doi: 10.1097/MD.0000000000009274. Epub [PubMed PMID: 29390379]
Level 3 (low-level) evidenceDuckers C, Simioni P, Spiezia L, Radu C, Gavasso S, Rosing J, Castoldi E. Low plasma levels of tissue factor pathway inhibitor in patients with congenital factor V deficiency. Blood. 2008 Nov 1:112(9):3615-23. doi: 10.1182/blood-2008-06-162453. Epub 2008 Aug 11 [PubMed PMID: 18695002]
Level 2 (mid-level) evidenceSubramanian H, Kar R, Charles D, Babu H, Ambika P, Dutta TK. A Confounding Case of Inherited Factor V Deficiency Complicated by Inhibitors at First Presentation. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2017 Apr:44(2):114-117. doi: 10.1159/000450793. Epub 2016 Dec 13 [PubMed PMID: 28503128]
Level 3 (low-level) evidenceWang X, Qin X, Yu Y, Wang R, Liu X, Ji M, Zhou M, Chen C. Acquired factor V deficiency in a patient with a urinary tract infection presenting with haematuria followed by multiple haemorrhages with an extremely low level of factor V inhibitor: a case report and review of the literature. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2017 Jun:28(4):334-341. doi: 10.1097/MBC.0000000000000581. Epub [PubMed PMID: 27428013]
Level 2 (mid-level) evidenceChaudhry R, Killeen RB, Babiker HM. Physiology, Coagulation Pathways. StatPearls. 2025 Jan:(): [PubMed PMID: 29489185]
Yang J, Mao H, Sun L. Congenital coagulation factor V deficiency with intracranial hemorrhage. Journal of clinical laboratory analysis. 2022 Nov:36(11):e24705. doi: 10.1002/jcla.24705. Epub 2022 Sep 20 [PubMed PMID: 36125894]
Franchini M, Marano G, Pupella S, Vaglio S, Masiello F, Veropalumbo E, Piccinini V, Pati I, Catalano L, Liumbruno GM. Rare congenital bleeding disorders. Annals of translational medicine. 2018 Sep:6(17):331. doi: 10.21037/atm.2018.08.34. Epub [PubMed PMID: 30306070]
Paraboschi EM, Menegatti M, Peyvandi F, Duga S, Asselta R. Understanding the Impact of Aberrant Splicing in Coagulation Factor V Deficiency. International journal of molecular sciences. 2019 Feb 20:20(4):. doi: 10.3390/ijms20040910. Epub 2019 Feb 20 [PubMed PMID: 30791524]
Level 3 (low-level) evidenceAbou Mourad Y, Shamseddine A, Hamdan A, Koussa S, Taher A. Report of a rare co-incidence of congenital factor V deficiency and thalassemia intermedia in a family. Annals of Saudi medicine. 2004 Jul-Aug:24(4):301-2 [PubMed PMID: 15387502]
Level 3 (low-level) evidenceYakovleva E, Zhang B. Clinical, Laboratory, Molecular, and Reproductive Aspects of Combined Deficiency of Factors V and VIII. Seminars in thrombosis and hemostasis. 2025 Mar:51(2):116-127. doi: 10.1055/s-0044-1789019. Epub 2024 Aug 29 [PubMed PMID: 39209292]
Ishimori N, Wakabayashi M, Sakurai K, Suda A, Souri M, Osaki T, Ichinose A. [Autoimmune coagulation factor V/5 deficiency during chronic disseminated intravascular coagulation]. [Rinsho ketsueki] The Japanese journal of clinical hematology. 2023:64(2):113-118. doi: 10.11406/rinketsu.64.113. Epub [PubMed PMID: 36990730]
Tasaki A, Fukuda A, Kudo A, Nishikawa E, Koumatsu N, Wada M, Okita J, Maruo M, Uchida H, Nakata T, Itani K, Shibata H. A First Case Report of Autoimmune Acquired Factor V Deficiency After Severe Acute Respiratory Syndrome Coronavirus 2 mRNA Vaccination at the Time of Initiating Haemodialysis. Nephrology (Carlton, Vic.). 2025 Feb:30(2):e70003. doi: 10.1111/nep.70003. Epub [PubMed PMID: 39887486]
Level 3 (low-level) evidenceMa XL, Wang WC, Du C, Zhang T, Li TF, Guo Y, Zhu JH. A case of unusual acquired factor V deficiency. World journal of emergency medicine. 2023:14(1):78-80. doi: 10.5847/wjem.j.1920-8642.2023.003. Epub [PubMed PMID: 36713339]
Level 3 (low-level) evidenceTo AQ, Ciurea SO, Kongtim P. Acquired Factor V Deficiency: A New Cause of Bleeding in Patients with Chronic Myeloid Leukemia and Extreme Leukocytosis. Acta haematologica. 2023:146(6):546-549. doi: 10.1159/000524630. Epub 2022 May 2 [PubMed PMID: 35500558]
Ozkaya H, Akcan AB, Aydemir G, Akcan M, Kul M. Factor v deficiency associated with congenital cardiac disorder and intracranial hemorrage. Indian journal of hematology & blood transfusion : an official journal of Indian Society of Hematology and Blood Transfusion. 2013 Jun:29(2):99-101. doi: 10.1007/s12288-012-0149-8. Epub 2012 Mar 21 [PubMed PMID: 24426348]
Level 3 (low-level) evidenceChang YS, Lan YC, Chen YJ, Huang JS, Yang CN, Huang CF, Yeh KY. A Novel Phenotype of the Factor 5 Gene Mutation (Homozygote Met1736Val and Heterozygote Asp68His) Is Associated With Moderate Factor V Deficiency. Frontiers in medicine. 2022:9():870269. doi: 10.3389/fmed.2022.870269. Epub 2022 Jun 9 [PubMed PMID: 35755047]
Wu AH, Manje Gowda A, Peng S, Kaur S, Maroules M. Successful Management of Life-threatening Pelvic Hemorrhage From Acquired Factor V Deficiency With immunosuppressive Therapy. Cureus. 2020 Aug 23:12(8):e9972. doi: 10.7759/cureus.9972. Epub 2020 Aug 23 [PubMed PMID: 32983675]
Chingale A, Eisenhut M, Gadiraju A, Liesner R. A neonatal presentation of factor V deficiency: a case report. BMC pediatrics. 2007 Feb 21:7():8 [PubMed PMID: 17313676]
Level 3 (low-level) evidenceLee WS, Chong LA, Begum S, Abdullah WA, Koh MT, Lim EJ. Factor V inhibitor in neonatal intracranial hemorrhage secondary to severe congenital factor V deficiency. Journal of pediatric hematology/oncology. 2001 May:23(4):244-6 [PubMed PMID: 11846304]
Level 3 (low-level) evidenceCastaman G, Linari S. Diagnosis and Treatment of von Willebrand Disease and Rare Bleeding Disorders. Journal of clinical medicine. 2017 Apr 10:6(4):. doi: 10.3390/jcm6040045. Epub 2017 Apr 10 [PubMed PMID: 28394285]
Castaman G. The role of recombinant activated factor VII in the haematological management of elective orthopaedic surgery in haemophilia A patients with inhibitors. Blood transfusion = Trasfusione del sangue. 2017 Sep:15(5):478-486. doi: 10.2450/2017.0369-16. Epub 2017 May 16 [PubMed PMID: 28686157]
Tanaka KA, VanDyck K, Shettar SS, Gomes M. Perioperative Management of Hereditary Factor V Deficiency: Timing of Plasma Administration is Critical in Maximizing Hemostatic Potency of Transfused Factor V. Journal of cardiothoracic and vascular anesthesia. 2022 Jun:36(6):1811-1812. doi: 10.1053/j.jvca.2022.01.020. Epub 2022 Jan 19 [PubMed PMID: 35181237]
Mathews N, Rivard GE, Bonnefoy A. Glanzmann Thrombasthenia: Perspectives from Clinical Practice on Accurate Diagnosis and Optimal Treatment Strategies. Journal of blood medicine. 2021:12():449-463. doi: 10.2147/JBM.S271744. Epub 2021 Jun 11 [PubMed PMID: 34149292]
Level 3 (low-level) evidence