Introduction
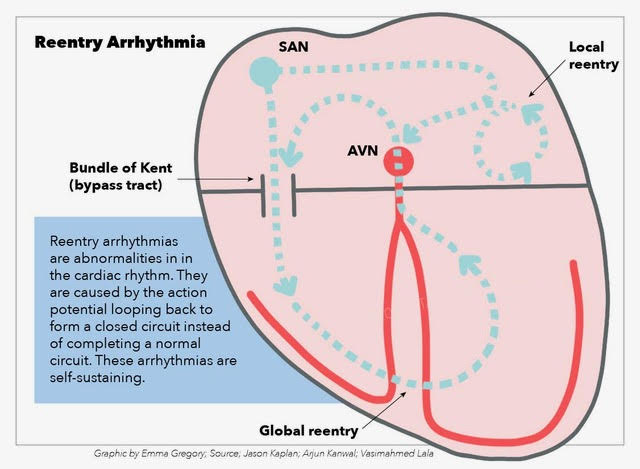
Reentry describes a self-sustaining cardiac rhythm abnormality. In reentry, the action potential propagates in a circus-like closed loop manner. It is a disorder of impulse conduction and thus describes one kind of arrhythmogenesis and is differentiated from disorders of impulse generation such as automaticity and triggered activity. First studies investigating reentry are over 100 years old and go back to Mines and Garrey.[1][2][3] Dysrhythmias based on the reentry mechanism include atrial tachyarrhythmias such as atrial flutter (AFlut), atrioventricular nodal reentry (AVNRT), atrioventricular reentry (AVRT) like Wolff-Parkinson-White (WPW) syndrome and ventricular reentry such as bundle branch reentry (BBR). The pathophysiologic importance of reentry and utility as a treatment target in atrial fibrillation (AFib) is part of ongoing research.
The main task of the heart is to produce cardiac output. Therefore myocardiocytes contract in a highly orchestrated and rhythmic manner. Proper electro-mechanic coupling (EMC) translates electric stimulation into contraction. The action potential runs from the sinus node (SN) via right atrial pathways through the atrioventricular node (AVN) and the His bundle down the Purkinje fibers (His-Purkinje system, HPS) to the ventricular myocardium. Cells are either in an excited or resting condition. From the resting potential, the cell is depolarized until the crossing of a critical threshold, which then causes an action potential (AP). Following the action potential, there is a period of refractoriness (refractory period, RP) when another trigger cannot activate the cell. Wavelength (WL) or cycle length (CL) is the time between two action potentials.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of reentry is diverse and includes:
- Electrolyte abnormalities and channelopathies form a substrate for reentry arrhythmias.
- A mechanical force such as the chest thump maneuver induces but also terminates reentry.[4]
- Chronic cardiac stretch leads to remodeling and fibrosis that changes electrical properties of the myocardiocytes.
- Scar tissue following infarction is the basis of anatomical reentry.
- Congenital heart disease and surgery performed to treat it can cause reentry.
- Genetic culprits are related reentry tachycardias. Roberts et al. identified genetic culprits SCN5A and LMNA as a cause of idiopathic bundle branch reentry ventricular tachycardia.[5]
The connection between congenital heart disease and reentry is of particular research interest and requires further attention. Congenital heart disease itself or scars following surgical repair poses a substrate for reentry circuits.[6] Patients with Ebstein anomaly are prone to supraventricular tachyarrhythmias because of multiple accessory pathways.
Muscular bridges crossing the fibrous atrioventricular skeleton can cause the Wolff-Parkinson-White syndrome in Ebstein patients.[7] Surgical procedures known to cause arrhythmias due to reentry include the Mustard Senning procedure for transposition of the great arteries or the Fontan Bjork operation for a univentricular heart.[8] Pericaval intra-atrial reentry tachycardia (IART) is specific to Fontan surgery with longer cycle lengths and zones of slow conduction compared to periannular IART. Both, pericaval und periannular IART are amenable to ablation.[9] Ventricular tachycardia following repair of congenital heart disease can be traced to anatomic isthmuses and thus be successfully ablated.[10]
Arrhythmogenic right ventricular dysplasia has characteristic fibrofatty atrophy of the right ventricular myocardium and accompanying ventricular arrhythmias. Ellison et al. described clustering of reentry circuits sites in areas like the right ventricular outflow tract and the tricuspid annulus that are affected by fibrotic changes.[11][12]
Epidemiology
As a pathophysiologic mechanism of arrhythmias, the incidence and prevalence depend on the arrhythmia ranging from highly prevalent diseases like atrial fibrillation to rare diseases like Wolff-Parkinson-White atrioventricular reentry tachycardia. Atrial fibrillation occurs in 10% of the over 60-years old. In patients undergoing surgery for mitral valve disease, it can be found in 60% of the cases. Atrial fibrillation is associated with increased mortality due to three problems: thromboembolism causing stroke, decreased cardiac output due to atrioventricular synchrony, and irregular heartbeat.[13] Ventricular tachyarrhythmias can cause sudden death in patients suffering from chronic heart failure.[14] 15% of adult patients with congenital heart disease have concomitant supraventricular tachycardias (SVT).[8]
Regarding the epidemiology of SVT, the AHA/ACC/HRS guideline from 2015 reports a prevalence of 2.29 per 1000 persons, an incidence of 36 per 100.000 persons per year and approximately 89.000 new cases per year and 570.000 people living with SVT. AVNRT is more common in middle-aged or older person, whereas AVRT is more prevalent in adolescents. ECG changes due to WPW syndrome can be found in 0.1 to 0.3% of the population.[15]
Pathophysiology
The study of arrhythmogenesis comprises disorders of impulse initiation and disorders of impulse conduction. Impulse initiation disturbances include automaticity and triggered activity with early and late depolarizations (EAD and DAD). Automaticity is the spontaneous depolarization of tissue which is physiologic in the sinus and atrioventricular node and the His bundle. This is in contrast to triggered activity, which is abnormal impulse generation in response to a previous stimulus.
Arrhythmias caused by early depolarizations include Torsade de Pointes and delayed depolarizations are exemplified by ventricular arrhythmia due to digitalis toxicity. Reentry is a mechanism of pathologic impulse conduction. Reentry is connected to structural heart disease whereas automaticity and triggered activity may be found in structurally normal hearts.[16]
Computer simulations play an essential role in electrophysiology research. Liu et al. used computer simulation to show that delayed after-depolarizations, which are spontaneous depolarization occurring late during an action potential, can trigger premature ventricular complexes (PVC) and in vulnerable tissue with properties of unidirectional block can cause reentry. This is called triggered activity with reentry.[17] As a general rule, it is possible to say that reentry is responsible for ventricular tachyarrhythmias following myocardial infarction, whereas automaticity and triggered activity lead to arrhythmia in chronic heart failure.[14]
Mines described three criteria to define reentry. For reentry to occur unidirectional block, return to point of origin and the possibility to interruption are required.[18] Heterogeneity of structural and electrophysiological properties increases the likelihood of unidirectional block and thus creates fertile ground for reentry.[19]
The wavelength of the reentry circuit is defined by the impulse conduction velocity times the refractory period.[20] Rensma et al. studied the characteristics of wavelength conduction velocity and the refractory period required to initiate atrial flutter and fibrillation. They showed that wavelength is the best predictor for inducibility of arrhythmias.[21]
Mechanic and electric properties of the cell are interdependent, which is called the mechanoelectrical feedback (MEF) or electrochemical coupling (ECC). Acute stretch induces cell currents causing depolarization (stretch-activated currents, SAC).[4] Regarding the physiologic mechanism, an acute stretch during systole shortens the action potential and the refractory period, and thus the wavelength which in general favors reentry. Chronic stretch leads to fibrosis and subsequently to different conduction across longitudinal and perpendicular cell axis, which is called anisotropy and leads to zig-zag conduction across cells.[22]
Gilmour et al. studied the electrophysiological mechanisms giving rise to reentry in a ring-like Purkinje muscle junction model. They imitated conditions that are present in the ischemic heart by chemically creating a unidirectional block, shortening the action potential duration, which finally initiated reentry. Furthermore, they described the relation between Purkinje fibers and muscle cells as source and sink, respectively. Asymmetrical impulse transmission, which can be found in the heart due to anisotropy, contributes to arrhythmia. Dispersion of refractoriness due to different membrane properties and cell coupling also plays a role in reentry mechanism.[23]
There are many concepts of functional reentry such as the leading circle reentry,[24] the spiral rotor wave, phase 2 reentry, and figure of eight reentry.
Comtois introduced the idea of spiral wave reentry. The spiral wave reentry is a continuously rotating electric potential around a point of phase singularity where wavetail and wavefront meet. It can be evaluated by dominant frequency analysis and complex fractionated electrogram (CFE).[25] Spiral wave reentry is the proposed mechanism for VF and polymorphic VT.[16][26][27]
Antzelevitch et al. observed differences in action potentials at different epicardial sites giving rise to reentry. The transmural voltage gradient is visible on electrocardiogram (ECG) as j-wave or st-segment elevation. Since these changes are related to the second phase of the action potential, this is called phase 2 reentry.[28] The concept of phase 2 reentry has been proposed by Lukas et al. and is connected to the syndromes of Brugada and early repolarization.[29][16]
The multiple wavelet theory was first proposed by Moe in 1959 researching the pathophysiologic basis of atrial fibrillation. This theory states that many wavefronts will not die out spontaneously. This idea found reinforcement with Allessie's finding that four to six simultaneous reentrant wavelets are needed to maintain atrial fibrillation.[20][30] Yagishita described localized reentry in atrial fibrillation and the difficulty to differentiate it from focal activation.[31]
Different mechanisms of arrhythmogenesis may be responsible for initiating and sustaining atrial fibrillation. Accepting the multiple wavelet theory as the sustaining force, Haissaguerre described ectopic foci at the point where the pulmonary veins insert into the left atrium for initiation of atrial fibrillation. This allowed for targeted radiofrequency ablation.[32] Pulmonary vein ectopic foci show high dominant frequency giving origin to fractionated rotors.[33] In some patients, both atrial fibrillation and atrial flutter can occur. This clinical interrelationship may reflect an underlying common pathophysiology such as atrial fibrillation can cause a functional line of block that creates a macro-reentrant circuit and atrial flutter. On the other side atrial flutter, due to short cycle length may result in atrial fibrillation.[34]
Histopathology
Reentry around an anatomical obstacle has been named anatomic reentry as compared to functional reentry. Cox et al. who developed the surgical treatment for atrial fibrillation observed that chronic atrial enlargement causes atrial fibrillation. The concept of the "two holes macro-reentry" including the left atrial appendage and the pulmonary vein orifice led to the idea of creating lesions to interrupt these anatomical reentry circuits.[13] Myocardial scars following infarction may serve as an anatomic obstacle around which a reentry circuit can form.[35] The joint ESC/NASPE expert group proposed according to Cosio the term macro-reentrant tachycardia (MRT) to describe many reentrant tachycardias around a large central obstacle. Typical atrial flutter is the most common MRT. Lower loop reentry around inferior vena cava is a variation of typical atrial flutter and upper loop reentry around superior vena cava.[36] Other examples of anatomic reentry include scar-related VT and BBRVT.
Identifying parts of the reentry circuit is essential for successful ablation maneuvers to terminate the arrhythmia.[37] The right atrium possesses the anatomical structures that form the atrial flutter circuit.[38][39] The cavotricuspid isthmus (CTI) is the narrow point between the inferior vena cava and the tricuspid annulus where typical atrial flutter can undergo ablation successfully.[40] Anatomical reentry in cases of atrial flutter can also form around barriers such as the crista terminalis, eustachian valve, inferior vena cava, coronary sinus, and tricuspid annulus.[41] A circuit inside the CTI may serve as reentry circuit known as intra-isthmus reentry.[42][43][44] The terminal crest has anisotropic property and forms a functional line of a block to conduction in the right atrium.[45][46]
Left atrial macro-reentry circuits have also been described. The Bachmann bundle is a large muscle bundle that conducts excitation under physiologic conditions from the right atrium to the left.[47][48] Macro-reentry circuits include the mitral isthmus and epicardial connections located in the ligament of Marshall known as the Marshall bundle causing peri-mitral atrial tachycardia (PMAT). Peri-mitral atrial tachycardias develop following pulmonary vein isolation (PVI) or mitral valve surgery.[49][50] There have also been reports of reentrant atrial tachycardia not involving the AV node. Adenosine-sensitive atrial tachycardia has variable locations and can shift to another site after ablation. In a case reported by Inagaki mapping identified the critical slow conduction zone close to the mitral annulus.[51]
The fibrous skeleton of the heart plays a role as electric insulation between atria and ventricles.[7] Usually, the AV node connects to the His bundle form the only electric connection between atria and ventricles. 60% of paroxysmal supraventricular tachycardias are AVNRT.[52] The dual pathway model of the AV node as proposed by Mendez et al. forms the pathohistological basis for AVNRT. According to this model, the upper part of the AV node comprises two pathways, an inferior-slow (shorter ERP but longer AV delay) and superior-fast (longer ERP but shorter AV delay) pathway, providing input to the His bundle. If transverse propagation fails, longitudinal propagation occurs. The transverse-versus-longitudinal electrical propagation inside the AV node results in dissociation in the distal node and dual input into the His bundle, a phenomenon described as His electrogram alternans. AVNRT uses these different pathways inside the AV node as reentry circuit. This also offers a target for successful ablation of AVNRT.
Electrophysiologists prefer ablation of the slow pathway since ablation of the fast pathway poses a higher risk for AV block.[53] Localizing the slow pathway in an electrophysiologic study can be done through an anatomic approach according to Haissaguerre or using an assessment of potentials as was proposed by Jackman. The slow pathway is located at the atrial septum along the tricuspid annulus midway between the His bundle recording site and the coronary sinus ostium, and the fast pathway is located anteriorly near the His bundle.[54] Spach observed reentry due to anisotropy as cells conduct currents different depending on longitudinal or transversal axis. He challenged Moe's idea of anatomic slow and fast pathways of the AV node and made anisotropy responsible for AVNRT.[55]
Atrioventricular reciprocating tachycardias form a reentry circuit via the atrioventricular node/his Purkinje system and one or more accessory pathways. Accessory pathways bridges span the atrioventricular plane in addition to the physiological pathway via the atrioventricular node and his bundle. Wolff, Parkinson, and White described the first preexcitation syndrome in 1930. Atrial fibrillation may conduct via the accessory pathway instead of the av node bypassing the frequency limiting effect. This leads to a fast, broad and irregular tachycardia and rarely to ventricular fibrillation.[56]
The bundle branch reentry ventricular tachycardia (BBRVT) runs via a macro-reentry circuit including the His bundle and the fascicular branches. It is common in dilatative cardiomyopathy with reduced left ventricular ejection fraction. The right bundle branch is more vulnerable to unidirectional block due to a long refractory period and slow retrograde conduction via the left bundle branch. The connection between the two bundle branches forms by bridges spanning the ventricular septum. The surface electrocardiogram (ECG) will show a left bundle branch block (LBBB) pattern. The contrariwise BBRVT showing right bundle branch block (RBBB) pattern on ECG is rare.
Interfascicular tachycardia or left ventricular BBRVT includes antegrade conduction through the left-anterior and retrograde conduction via the left posterior fascicles, which manifests as right bundle branch block with left-posterior hemiblock on ECG. Criteria for the diagnosis of BBRVT through electrophysiologic examination include the following:
- LBBB pattern on ECG; slowed conduction of the His-Purkinje system
- Termination of BBRVT through blocking the His-Purkinje system proximal to distal activation of the His bundle and right bundle branch
- The His ventricular interval in BBRVT is equal or longer than the His ventricular interval in sinus rhythm
- Variations of the V-V interval that are preceded by variations of the H-H interval
BBRVT should be not inducible after ablation of the right bundle branch.[57][58][59][60]
In contrast to macro-reentry better resolution of mappings systems allowed to detect smaller reentry circuits, called localized reentry or micro-reentry. The resolution of mapping depends on the distance between electrodes, which is limited, per se. There is an ongoing debate about whether focal activity is micro-reentry. Some studies propose micro-reentry based on fractionated electrograms, areas of slow conduction or zig-zag pattern of activation. Ideker et al. estimated the electrode spacing needed to detect the smallest micro-reentry. Spach et al. described the smallest reentry circuit in superfused human atrial trabeculae spanning an area of 1.6 mm. Ideker argues that electrodes need not be as close together to register the reentry circuit just as a hurricane is noticeable from a great distance.[61]
El-Sherif illustrated the competing concept of disorders of impulse generation (rapid firing focus) against disorders of impulse conduction (reentry) using the clinical entity of SVT. He concludes that a rapid firing focus cannot be differentiated from a micro-reentry completely.[62] Garan and Ruskin induced myocardial infarction in animal experiments to study sustained monomorphic ventricular tachycardia with electrodes in proximity. They observed continuous electrical activity (CEA) in small areas of infarcted myocardium on the initiation, maintenance, and termination of VT. They interpreted these small locations of CEA as micro-reentry.[63] In a letter to the editor, Tai discusses a report of macro-reentrant interfascicular reentry with convincing data to exclude bundle branch reentry but not enough evidence to rule out micro-reentrant intra-fascicular reentry.[64]
History and Physical
Symptoms of SVT often start in young adulthood with a mean age of 32+-18 years for AVNRT compared to 23+-14 years for AVRT. Patients with AVNRT show a female predilection. Patients with SVT reported the following symptoms (% of cases): palpitations (22%), chest pain (5%), syncope (4%), and sudden cardiac death in (0.4%). Panic or anxiety may occur concomitantly. In AVNRT patients often complain about neck pounding, which may be attributed to cannon A waves. Higher right atrial pressure may lead to polyuria in AVNRT. Exertion or substances like coffee or alcohol can trigger AVNRT.[15]
Evaluation
To evaluate arrhythmias, different types of ECG (surface 12-lead, Holter, event recorder, intracardiac) and finally electrophysiologic studies using targeted stimuli are available. There are helpful ECG features to distinguish between AVNRT and AVRT. Features favoring AVRT include delta wave, lengthening of the tachycardia cycle length when bundle branch occurs ipsilateral to the accessory pathway, and the finding of QRS alternans. On the other hand features such as pseudo s in lead II or pseudo r' in lead V1 favor AVNRT.[41] The Brugada algorithm using the QRS morphology in the precordial leads and the Vereckei algorithm based on QRS changes in lead aVR assist in the differentiation between SVT and VT.[15]
The entrainment technique is required to diagnose reentry. It is useful to diagnose, ablate and map reentry. Mapping allows for identification of reentry circuit elements such as entry, exit, conduction barriers, bystanders, inner and outer loop, isthmus site, and zone of slow conduction (SCZ).[65][66] Stevenson et al. proposed a mapping site classification to guide ablation. Radiofrequency ablation success correlated with different locations in the reentry circuit. Successful ablation targets include zones of slow conduction (SCZ) and exit sites.[67][68][35]
Waldo formulated three criteria for entrainment. These are fixed fusion with constant pacing rate, progressive fusion at faster pacing, and resumption of tachycardia with captured not fused beat on termination of pacing.[69][16] Waldos observation lead to prophylactic implantation of electrodes to terminate postoperative atrial flutter by overdrive pacing. The excitable gap is the part of the reentry circuit between the tail of refractoriness and the next orthodromic excitation wave. An external stimulus can enter the reentry circuit at the excitable gap.[70] Through entrainment Waldo et al. differentiate between atrial flutter type I and II. Atrial flutter type I can be transiently entrained and interrupted by rapid atrial pacing since it is caused by reentry and has an excitable gap. Type I atrial flutter can show concealed entrainment with a zone of slow conduction. In contrast, type II atrial flutter has not been entrained, and the mechanism remains speculative.[69]
To entrain the tachyarrhythmia the paced cycle length needs to be 10 to 20ms shorter than the tachycardia cycle length (TCL). A fusion beat is the appearance of a QRS complex being formed by the addition of a paced or premature stimulus and the physiologic action potential. Constant fusion, progressive fusion, and variable fusion can be distinguished.[71] Entrainment with fusion proves that reentry is the cause of the investigated arrhythmia. The PPI-TCL duration gives information whether the place of stimulation is inside our outside the reentry circuit. PPI response pattern characterizes different electrophysiologic properties of the tissues (excitable gap, excited tissue, refractory tissue).[72] Similarly, the stimulus to QRS interval can be used to assess lead placement relative to the reentry circuit.[73]
Electrophysiologic studies are indicated for supraventricular or ventricular tachycardias. Several measurements help to investigate the pathophysiologic mechanism of arrhythmias and might reveal reentry as a cause. The A-H jump, which is a sudden increase of the atrial-hisian interval to more than 50ms with a prior stimulus, indicates dual AV node physiology and together with inducible tachycardia suggest AVNRT.[74]
The Wolff-Parkinson-White (WPW) syndrome and Lown-Ganong-Levine (LGL) syndrome belong to the group of atrioventricular reentry tachycardias (AVRT). Depending on the direction the accessory pathway is used, orthodromic (antegrade conduction via AV node-His bundle and retrograde conduction via AP) or antidromic (retrograde conduction via AV node-His bundle and antegrade conduction via an accessory pathway) AVRT are differentiated. Accessory pathways are muscle bridges connecting the atria and the ventricle along the tricuspid and mitral annulus spanning the fibrous cardiac skeleton thus offering additional conduction routes beside the AV node-His bundle. Atrioventricular accessory pathways are called Kent bundles, and atriofascicular accessory pathways are called Mahaim fibers.[75][76] Antegrade AP conduction manifests in the ECG as Delta wave, short PQ interval, and QRS prolongation. But ECG manifestations may be discrete or hidden such as with posterior AP or retrograde conduction. Ablation is the recommendation when the refractory period is shorter than 250 ms and in cases of inducible AVRT or symptomatic preexcitation syndrome.
The surface ECG algorithm helps to localize the accessory pathway. A positive delta wave in V1 and negative in I and aVL proposes a left-sided (posterior) AP otherwise a right-sided AP. A positive delta wave in III and aVF indicates superior location, contrariwise inferior location. Right atrial septal AP show R/S ratio larger than one in V3 or earlier. If it is not the case, free wall AP should be suspected. 60% of APs are located at the left atrial free wall, 25% at the septum, and 15% right free wall.[56][[41]
Veenhuyzen describes how to differentiate types of SVT such as AVNRT, AT and AVNRT using entrainment technique and observing manifest or concealed entrainment. Ventricular stimulation of SVT showing manifest entrainment suggests AVRT whereas ventricular stimulation of SVT showing concealed entrainment proposes AVNRT. The PPI-TCL is shorter in AVRT as compared to AVNRT. A post-pacing interval of A-A-V is specific for AT, whereas A-V indicates AVRT and AVNRT. The same applies to the atrial-hisian response. A-A-H response pattern indicates AT whereas A-H response pattern indicates AVRT or AVNRT. Concealed entrainment (AVNRT) is not the same as entrainment with concealed fusion (AVRT with pacing site close to AP). Entrainment pattern depends on the pacing site. Therefore, the response should be measured with different pacing locations (differential entrainment).[71]
Almendral examined resetting and entrainment phenomena in ventricular tachycardias. Resetting is the singular interaction between a stimulus and a given rhythm. Entrainment is the continuous resetting of the rhythm to repetitive stimuli. Almendral describes the concept of resetting a cardiac rhythm comparing the effect of a ventricular premature beat on continuous stimulation with V00 or VVI pacemaker. In V00 stimulation there will be no influence of the premature beat. Thus no resetting occurs. In contrast, in VVI the premature beat is sensed, a compensatory pause made, and thus the following stimuli are reset. Josephson described entrainment as the continuous resetting of a tachycardia.[77]
Treatment / Management
The AHA/ACC/HRS 2015 guideline makes recommendations on the treatment of SVT. Regular SVT should be treated with Valsalva maneuver and if unsuccessful with adenosine. If these fail or the patient is hemodynamically unstable cardioversion should be performed. Ongoing treatment includes beta blocker, calcium channel blockers, and sodium channel blockers, amiodarone or digoxin depending on the kind of SVT. The only curative treatment is ablation.[15](A1)
The Sicilian Gambit proposes a rational approach to antiarrhythmic drug selection. In contrast to empiric drug choice, this approach tries to identify the underlying mechanism of arrhythmogenesis to target drug therapy. The common form AVNRT uses slow pathway in antegrade conduction and retrograde fast pathway excitation. First-line therapy is the vagal maneuver. Drugs of choice include adenosine, beta blockers such as esmolol, and calcium channel blockers such as verapamil or diltiazem. All these drugs affect the slow conduction pathway. To target the fast pathway sodium channel blockers and amiodarone can be used. In orthodromic WPW syndrome, the excitation travels the AV node antegrade and the accessory pathway Kent bundle retrogradely. The length of the excitable gap can be evaluated in electrophysiologic studies. If the reentry is associated with a short excitable gap, potassium channel blocking agents such as amiodarone can be used to lengthen the refractory period of the accessory pathway. If the reentry is associated with a long excitable gap a sodium channel blocker may be chosen.[75]
Atrial flutter is based on a macro-reentry mechanism. Antitachycardic pacing is an option to treat atrial flutter. Stimulating the right atrium for 20 to 30 seconds with a rate of 10 beats per minute higher than flutter and up to 400 beats per minute can terminate the flutter circuit. Otherwise, ablation of a narrow part of the reentry pathway such as the cavotricuspid isthmus (CTI) can erase the reentry arrhythmia.[78][79]
Liu et al. reported a case of multiple (in this case bilateral) accessory pathways and three different appearances of AVRT. Multiple accessory pathways are present in 15% of WPW cases. Different combinations of conduction result in different morphologies of AVRT in the same patient.[80] In general, type A preexcitation uses antegrade conduction via AV node and retrograde via an accessory pathway resulting in a narrow QRS complex and type B preexcitation antegrade via an accessory pathway and retrograde via AV node resulting in a broad QRS complex. Radiofrequency ablation has success rates of 97 to 100% to eradicate accessory pathways in AVRT. Complications include inadvertent AV block and recurrence of arrhythmia.[54][81](B2)
The definition of complex fractionated atrial electrograms (CFAE) is electrograms having two fractionated deflections or a short cycle length (less than 120ms). Once ablation of CFAE is complete, proper conduction pattern can reorganize. Due to the distinct distribution of CFAE, they can are targetable by RFA.[82][83] Electrograms from healthy myocardium shows a simple morphology, but in dyssynchronous activation, they appear complex. Other authors have described CFAE proximity to areas of high dominant frequency, and CFAE also have correlations to reentry.[84] If PVI is unsuccessful electrophysiologist move to CFAE ablation.[85]
Narayan et al. reported that atrial fibrillation due to rotor activity underwent successful ablation by targeting the center of the spiral wave. This concept is called focal impulse and rotor modulation ablation (FIRM).[86] FIRM-guided RFA is used in addition to PVI ablation and atrial tachycardia/atrial flutter ablation. Reports of the long-term success of PVI are 50 to 60% in paroxysmal AF and less in persistent and permanent AF. Consistent with the findings of the CONFIRM trial Tomassoni et al. reported promising long-term success for FIRM-guided ablation for atrial fibrillation in addition to conventional ablation.[87]
The development of a surgical approach to atrial fibrillation and the pathophysiologic mechanisms behind it are closely related. In 1980 the left atrial isolation procedure was introduced. 1982 the Scheinmann catheter fulguration of the His bundle for electrical dissociation of atria and ventricles (functional total AV block) was invented: a procedure that dictates the placement of a pacemaker. Cox and other surgeons (among them Damiano, Kosakai, and Sueda) developed and improved the Maze procedure for atrial fibrillation. It has three goals: permanent ablation of AF, restoration of AV synchrony, preservation of atrial transport function. The different Cox Maze procedures try to eliminate multiple wave reentry by creating multiple lesions in both atria. Takahashi proposes to treat paroxysmal AF with PVI and persistent AF with Cox-Maze procedure although the decision should preferably be made considering individual patient factors.[88][89][13](B3)
Differential Diagnosis
Differentiating the mechanisms arrhythmogenesis may be difficult. Micro reentry circuits, left atrial reentry spreading via Bachmann bundle to the right atrium where single spread is detected, slow conduction zone, and cul-du-ac activity of the pulmonary veins may appear as a focal activity on electrophysiologic studies. When equal PPI-TCL values are obtained for widespread points, a large macro-reentrant circuit should be suspected. Then a reentry circuit spanning left and right atrium via Bachmann bundle and Marshall ligament is possible.[90]
An important differential diagnosis for atrial fibrillation is multifocal atrial tachycardia (MAT). The correct diagnosis was made in only 22% of cases in one retrospective study. MAT is characterized by at least three different non-sinus p waves in the same lead, an atrial rate of over 100 beats per minute, and isoelectricity between p waves. Triggered activity is the pathomechanism of MAT. Since electrical cardioversion is effective only in reentrant tachycardias, cardioversion would not be helpful in this case. Treatment consists of beta-blocker, calcium channel blockade, and magnesium. This emphasizes the importance of the underlying pathomechanism for effective treatment.[91][15]
Pertinent Studies and Ongoing Trials
Reentry offers unresolved questions needing further research. Despite the high prevalence of atrial fibrillation, only insufficient treatment options are available due to missing knowledge about the true mechanism and target for therapy.[92] There is speculation about highly organized reentrant activity despite the seemingly chaotic activity in atrial fibrillation. Maybe not a single but rather a combination of mechanisms cause atrial fibrillation.[20][93] A joint workforce proposed an open classification for atrial tachycardias including focal atrial tachycardia and macro-reentrant atrial tachycardia. But the workforce also admitted that other tachycardias (atypical atrial flutter, type II atrial flutter, inappropriate sinus tachycardia) could not be well classified properly due to an incomplete understanding of the corresponding mechanism.[94]
Consultations
Insufficient treatment response and undifferentiated arrhythmias should prompt electrophysiology consultation which is a subspecialty of cardiology.
Deterrence and Patient Education
Reentry serves as a model for the pathophysiology of some self-sustaining cardiac arrhythmias and helps to diagnose and treat them.
Enhancing Healthcare Team Outcomes
Arrhythmogenesis research needs to combine computer, life sciences and clinical experts and translate new findings into evidence-based clinical care.[95] The demographic change requires broadening the knowledge about chronic and ischemic changes of the myocardium leading to arrhythmias and the importance of genetic research will increase.[96]
Media
(Click Image to Enlarge)
References
Paterson DJ. Arrhythmia: 100 years on from George Ralph Mines. The Journal of physiology. 2013 Sep 1:591(17):4065-6. doi: 10.1113/jphysiol.2013.262386. Epub [PubMed PMID: 24742770]
Wit AL, Cranefield PF. Reentrant excitation as a cause of cardiac arrhythmias. The American journal of physiology. 1978 Jul:235(1):H1-17 [PubMed PMID: 677321]
Level 3 (low-level) evidenceAguilar M, Nattel S. The pioneering work of George Mines on cardiac arrhythmias: groundbreaking ideas that remain influential in contemporary cardiac electrophysiology. The Journal of physiology. 2016 May 1:594(9):2377-86. doi: 10.1113/JP270506. Epub 2016 Jan 19 [PubMed PMID: 26607760]
Timmermann V, Dejgaard LA, Haugaa KH, Edwards AG, Sundnes J, McCulloch AD, Wall ST. An integrative appraisal of mechano-electric feedback mechanisms in the heart. Progress in biophysics and molecular biology. 2017 Nov:130(Pt B):404-417. doi: 10.1016/j.pbiomolbio.2017.08.008. Epub 2017 Aug 26 [PubMed PMID: 28851517]
Roberts JD, Gollob MH, Young C, Connors SP, Gray C, Wilton SB, Green MS, Zhu DW, Hodgkinson KA, Poon A, Li Q, Orr N, Tang AS, Klein GJ, Wojciak J, Campagna J, Olgin JE, Badhwar N, Vedantham V, Marcus GM, Kwok PY, Deo RC, Scheinman MM. Bundle Branch Re-Entrant Ventricular Tachycardia: Novel Genetic Mechanisms in a Life-Threatening Arrhythmia. JACC. Clinical electrophysiology. 2017 Mar:3(3):276-288. doi: 10.1016/j.jacep.2016.09.019. Epub 2016 Dec 21 [PubMed PMID: 29759522]
Delacretaz E, Ganz LI, Soejima K, Friedman PL, Walsh EP, Triedman JK, Sloss LJ, Landzberg MJ, Stevenson WG. Multi atrial maco-re-entry circuits in adults with repaired congenital heart disease: entrainment mapping combined with three-dimensional electroanatomic mapping. Journal of the American College of Cardiology. 2001 May:37(6):1665-76 [PubMed PMID: 11345382]
Saremi F, Sánchez-Quintana D, Mori S, Muresian H, Spicer DE, Hassani C, Anderson RH. Fibrous Skeleton of the Heart: Anatomic Overview and Evaluation of Pathologic Conditions with CT and MR Imaging. Radiographics : a review publication of the Radiological Society of North America, Inc. 2017 Sep-Oct:37(5):1330-1351. doi: 10.1148/rg.2017170004. Epub 2017 Aug 18 [PubMed PMID: 28820653]
Level 3 (low-level) evidenceCombes N, Derval N, Hascoët S, Zhao A, Amet D, Le Bloa M, Maltret A, Heitz F, Thambo JB, Marijon E. Ablation of supraventricular arrhythmias in adult congenital heart disease: A contemporary review. Archives of cardiovascular diseases. 2017 May:110(5):334-345. doi: 10.1016/j.acvd.2017.01.007. Epub 2017 Mar 27 [PubMed PMID: 28359691]
Mandapati R, Walsh EP, Triedman JK. Pericaval and periannular intra-atrial reentrant tachycardias in patients with congenital heart disease. Journal of cardiovascular electrophysiology. 2003 Feb:14(2):119-25 [PubMed PMID: 12693488]
Level 2 (mid-level) evidenceKapel GF, Reichlin T, Wijnmaalen AP, Piers SR, Holman ER, Tedrow UB, Schalij MJ, Stevenson WG, Zeppenfeld K. Re-entry using anatomically determined isthmuses: a curable ventricular tachycardia in repaired congenital heart disease. Circulation. Arrhythmia and electrophysiology. 2015 Feb:8(1):102-9. doi: 10.1161/CIRCEP.114.001929. Epub 2014 Nov 24 [PubMed PMID: 25422392]
Ellison KE, Friedman PL, Ganz LI, Stevenson WG. Entrainment mapping and radiofrequency catheter ablation of ventricular tachycardia in right ventricular dysplasia. Journal of the American College of Cardiology. 1998 Sep:32(3):724-8 [PubMed PMID: 9741518]
Stark SI, Arthur A, Lesh MD. Radiofrequency catheter ablation of ventricular tachycardia in right ventricular cardiomyopathy: use of concealed entrainment to identify the slow conduction isthmus bounded by an aneurysm and the tricuspid annulus. Journal of cardiovascular electrophysiology. 1996 Oct:7(10):967-71 [PubMed PMID: 8894939]
Level 3 (low-level) evidenceCox JL, Boineau JP, Schuessler RB, Kater KM, Ferguson TB Jr, Cain ME, Lindsay BD, Smith JM, Corr PB, Hogue CB. Electrophysiologic basis, surgical development, and clinical results of the maze procedure for atrial flutter and atrial fibrillation. Advances in cardiac surgery. 1995:6():1-67 [PubMed PMID: 7894763]
Level 3 (low-level) evidenceMilberg P, Pott C, Eckardt L, Breithardt G. [Heart hypertrophy and heart failure--experimental findings for arrhythmogenesis]. Deutsche medizinische Wochenschrift (1946). 2008 Dec:133 Suppl 8():S285-9. doi: 10.1055/s-0028-1100963. Epub 2008 Dec 15 [PubMed PMID: 19085808]
Level 3 (low-level) evidencePage RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, Estes NA 3rd, Field ME, Goldberger ZD, Hammill SC, Indik JH, Lindsay BD, Olshansky B, Russo AM, Shen WK, Tracy CM, Al-Khatib SM, Evidence Review Committee Chair‡. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016 Apr 5:133(14):e471-505. doi: 10.1161/CIR.0000000000000310. Epub 2015 Sep 23 [PubMed PMID: 26399662]
Level 1 (high-level) evidenceEnriquez A, Frankel DS, Baranchuk A. Pathophysiology of ventricular tachyarrhythmias : From automaticity to reentry. Herzschrittmachertherapie & Elektrophysiologie. 2017 Jun:28(2):149-156. doi: 10.1007/s00399-017-0512-4. Epub 2017 May 31 [PubMed PMID: 28567491]
Liu CF, Cheung JW, Ip JE, Thomas G, Yang H, Sharma S, Markowitz SM, Lerman BB. Unifying Algorithm for Mechanistic Diagnosis of Atrial Tachycardia. Circulation. Arrhythmia and electrophysiology. 2016 Aug:9(8):. pii: e004028. doi: 10.1161/CIRCEP.116.004028. Epub [PubMed PMID: 27516463]
Mines GR. On dynamic equilibrium in the heart. The Journal of physiology. 1913 Jul 18:46(4-5):349-83 [PubMed PMID: 16993210]
Abildskov JA, Lux RL. Spiral waves in a computer model of cardiac excitation. Pacing and clinical electrophysiology : PACE. 1994 May:17(5 Pt 1):944-52 [PubMed PMID: 7517529]
Waks JW, Josephson ME. Mechanisms of Atrial Fibrillation - Reentry, Rotors and Reality. Arrhythmia & electrophysiology review. 2014 Aug:3(2):90-100. doi: 10.15420/aer.2014.3.2.90. Epub 2014 Aug 30 [PubMed PMID: 26835073]
Rensma PL, Allessie MA, Lammers WJ, Bonke FI, Schalij MJ. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circulation research. 1988 Feb:62(2):395-410 [PubMed PMID: 3338122]
Level 3 (low-level) evidenceJanse MJ, Coronel R, Wilms-Schopman FJ, de Groot JR. Mechanical effects on arrhythmogenesis: from pipette to patient. Progress in biophysics and molecular biology. 2003 May-Jul:82(1-3):187-95 [PubMed PMID: 12732278]
Level 3 (low-level) evidenceGilmour RF Jr, Watanabe M. Dynamics of circus movement re-entry across canine Purkinje fibre-muscle junctions. The Journal of physiology. 1994 May 1:476(3):473-85 [PubMed PMID: 8057255]
Level 3 (low-level) evidenceAllessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The "leading circle" concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circulation research. 1977 Jul:41(1):9-18 [PubMed PMID: 862147]
Level 3 (low-level) evidenceComtois P, Kneller J, Nattel S. Of circles and spirals: bridging the gap between the leading circle and spiral wave concepts of cardiac reentry. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2005 Sep:7 Suppl 2():10-20 [PubMed PMID: 16102499]
Zykov V, Krekhov A, Bodenschatz E. Fast propagation regions cause self-sustained reentry in excitable media. Proceedings of the National Academy of Sciences of the United States of America. 2017 Feb 7:114(6):1281-1286. doi: 10.1073/pnas.1611475114. Epub 2017 Jan 25 [PubMed PMID: 28123066]
Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circulation research. 2013 Mar 1:112(5):849-62. doi: 10.1161/CIRCRESAHA.111.300158. Epub [PubMed PMID: 23449547]
Level 3 (low-level) evidenceAntzelevitch C. In vivo human demonstration of phase 2 reentry. Heart rhythm. 2005 Aug:2(8):804-6 [PubMed PMID: 16051113]
Lukas A, Antzelevitch C. Phase 2 reentry as a mechanism of initiation of circus movement reentry in canine epicardium exposed to simulated ischemia. Cardiovascular research. 1996 Sep:32(3):593-603 [PubMed PMID: 8881520]
Level 3 (low-level) evidenceChen YJ, Tai CT, Hsieh MH, Tsai CF, Lin WS, Chen SA. Dependence of electrogram duration in right posteroseptal atrium and atrium-pulmonary vein junction on pacing site: mechanism and implications regarding atrioventricular nodal reentrant tachycardia and paroxysmal atrial fibrillation. Journal of cardiovascular electrophysiology. 2000 May:11(5):506-15 [PubMed PMID: 10826929]
Yagishita A, Yamauchi Y, Miyamoto T, Hirao K. Electrophysiological evidence of localized reentry as a trigger and driver of atrial fibrillation at the junction of the superior vena cava and right atrium. HeartRhythm case reports. 2017 Mar:3(3):164-166. doi: 10.1016/j.hrcr.2016.10.005. Epub 2017 Jan 7 [PubMed PMID: 28491795]
Level 3 (low-level) evidenceHaïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England journal of medicine. 1998 Sep 3:339(10):659-66 [PubMed PMID: 9725923]
Suenari K, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Huang SY, Tai CT, Nakano Y, Kihara Y, Tsao HM, Wu TJ, Chen SA. Relationship between arrhythmogenic pulmonary veins and the surrounding atrial substrate in patients with paroxysmal atrial fibrillation. Journal of cardiovascular electrophysiology. 2011 Apr:22(4):405-10. doi: 10.1111/j.1540-8167.2010.01932.x. Epub 2010 Oct 19 [PubMed PMID: 20958838]
Waldo AL. Mechanisms of atrial flutter and atrial fibrillation: distinct entities or two sides of a coin? Cardiovascular research. 2002 May:54(2):217-29 [PubMed PMID: 12062328]
Level 3 (low-level) evidenceStevenson WG, Friedman PL, Sager PT, Saxon LA, Kocovic D, Harada T, Wiener I, Khan H. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. Journal of the American College of Cardiology. 1997 May:29(6):1180-9 [PubMed PMID: 9137211]
Cosío FG, Martín-Peñato A, Pastor A, Nuñez A, Goicolea A. Atypical flutter: a review. Pacing and clinical electrophysiology : PACE. 2003 Nov:26(11):2157-69 [PubMed PMID: 14622320]
Cabrera JA, Sanchez-Quintana D, Ho SY, Medina A, Anderson RH. The architecture of the atrial musculature between the orifice of the inferior caval vein and the tricuspid valve: the anatomy of the isthmus. Journal of cardiovascular electrophysiology. 1998 Nov:9(11):1186-95 [PubMed PMID: 9835263]
ROSENBLUETH A, GARCIA RAMOS J. Studies on flutter and fibrillation; the influence of artificial obstacles on experimental auricular flutter. American heart journal. 1947 May:33(5):677-84 [PubMed PMID: 20238579]
Puech P. The P wave: correlation of surface and intra-atrial electrograms. Cardiovascular clinics. 1974:6(1):43-68 [PubMed PMID: 4140029]
Tai CT, Chen SA. Cavotricuspid isthmus: anatomy, electrophysiology, and long-term outcome of radiofrequency ablation. Pacing and clinical electrophysiology : PACE. 2009 Dec:32(12):1591-5. doi: 10.1111/j.1540-8159.2009.02555.x. Epub 2009 Oct 19 [PubMed PMID: 19843312]
Obel OA, Camm AJ. Supraventricular tachycardia. ECG diagnosis and anatomy. European heart journal. 1997 May:18 Suppl C():C2-11 [PubMed PMID: 9152669]
Yang Y, Varma N, Badhwar N, Tanel RE, Sundara S, Lee RJ, Lee BK, Tseng ZH, Marcus GM, Kim AM, Olgin JE, Scheinman MM. Prospective observations in the clinical and electrophysiological characteristics of intra-isthmus reentry. Journal of cardiovascular electrophysiology. 2010 Oct:21(10):1099-106. doi: 10.1111/j.1540-8167.2010.01778.x. Epub [PubMed PMID: 20455984]
Latcu DG, Bun SS, Saoudi N. Intra-isthmus reentry: diagnosis at-a-glance. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2014 Feb:16(2):251. doi: 10.1093/europace/eut288. Epub 2013 Oct 24 [PubMed PMID: 24158259]
Level 3 (low-level) evidenceSaoudi N, Latcu DG. Intra-isthmus reentry: another form of typical atrial flutter? Journal of cardiovascular electrophysiology. 2010 Oct:21(10):1107-8. doi: 10.1111/j.1540-8167.2010.01819.x. Epub [PubMed PMID: 20550609]
Sánchez-Quintana D, Anderson RH, Cabrera JA, Climent V, Martin R, Farré J, Ho SY. The terminal crest: morphological features relevant to electrophysiology. Heart (British Cardiac Society). 2002 Oct:88(4):406-11 [PubMed PMID: 12231604]
Cosío FG. The right atrium as an anatomic set-up for re-entry: electrophysiology goes back to anatomy. Heart (British Cardiac Society). 2002 Oct:88(4):325-7 [PubMed PMID: 12231579]
Kawamura I, Fukamizu S, Miyazawa S, Hojo R. Biatrial tachycardia utilizing Bachmann bundle. Heart rhythm. 2018 Aug:15(8):1277. doi: 10.1016/j.hrthm.2018.04.001. Epub 2018 Apr 6 [PubMed PMID: 29627434]
Miyazawa S, Fukamizu S, Kawamura I, Hojo R. Macroreentrant atrial tachycardia detouring the epicardium at the anterior wall of the left atrium. Journal of cardiovascular electrophysiology. 2019 Feb:30(2):263-264. doi: 10.1111/jce.13766. Epub 2018 Nov 2 [PubMed PMID: 30288841]
Wilber DJ, Kopp DE, Glascock DN, Kinder CA, Kall JG. Catheter ablation of the mitral isthmus for ventricular tachycardia associated with inferior infarction. Circulation. 1995 Dec 15:92(12):3481-9 [PubMed PMID: 8521570]
Hayashi T, Fukamizu S, Mitsuhashi T, Kitamura T, Aoyama Y, Hojo R, Sugawara Y, Sakurada H, Hiraoka M, Fujita H, Momomura SI. Peri-Mitral Atrial Tachycardia Using the Marshall Bundle Epicardial Connections. JACC. Clinical electrophysiology. 2016 Feb:2(1):27-35. doi: 10.1016/j.jacep.2015.08.011. Epub 2015 Nov 10 [PubMed PMID: 29766850]
Inagaki D, Hojo R, Fukamizu S, Sakurada H, Hiraoka M. Adenosine-sensitive atrial tachycardia originating from the anterior mitral annulus. HeartRhythm case reports. 2018 Nov:4(11):542-544. doi: 10.1016/j.hrcr.2018.08.003. Epub 2018 Aug 14 [PubMed PMID: 30479956]
Level 3 (low-level) evidenceHeidbüchel H. How to ablate typical 'slow/fast' AV nodal reentry tachycardia. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2000 Jan:2(1):15-9 [PubMed PMID: 11225592]
Zhang Y. His electrogram alternans (Zhang's phenomenon) and a new model of dual pathway atrioventricular node conduction. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing. 2016 Jan:45(1):19-28. doi: 10.1007/s10840-015-0079-0. Epub 2015 Nov 27 [PubMed PMID: 26614299]
Yu JC, Lauer MR, Young C, Liem LB, Hou C, Sung RJ. Localization of the origin of the atrioventricular junctional rhythm induced during selective ablation of slow-pathway conduction in patients with atrioventricular node reentrant tachycardia. American heart journal. 1996 May:131(5):937-46 [PubMed PMID: 8615313]
Spach MS, Josephson ME. Initiating reentry: the role of nonuniform anisotropy in small circuits. Journal of cardiovascular electrophysiology. 1994 Feb:5(2):182-209 [PubMed PMID: 8186887]
Level 3 (low-level) evidenceVoss F, Eckardt L, Busch S, Estner HL, Steven D, Sommer P, von Bary C, Neuberger HR. [AV-reentrant tachycardia and Wolff-Parkinson-White syndrome : Diagnosis and treatment]. Herzschrittmachertherapie & Elektrophysiologie. 2016 Dec:27(4):381-389 [PubMed PMID: 27878364]
Reithmann C. [Bundle branch reentry VT : Diagnosis, mapping, and ablation]. Herzschrittmachertherapie & Elektrophysiologie. 2017 Jun:28(2):193-198. doi: 10.1007/s00399-017-0502-6. Epub 2017 May 8 [PubMed PMID: 28484841]
Blanck Z, Jazayeri M, Dhala A, Deshpande S, Sra J, Akhtar M. Bundle branch reentry: a mechanism of ventricular tachycardia in the absence of myocardial or valvular dysfunction. Journal of the American College of Cardiology. 1993 Nov 15:22(6):1718-22 [PubMed PMID: 8227845]
Level 3 (low-level) evidenceBlanck Z, Dhala A, Deshpande S, Sra J, Jazayeri M, Akhtar M. Bundle branch reentrant ventricular tachycardia: cumulative experience in 48 patients. Journal of cardiovascular electrophysiology. 1993 Jun:4(3):253-62 [PubMed PMID: 8269297]
Nageh MF, Schwartz J, Mokabberi R, Dabiesingh D, Kalamkarian N. Bundle branch reentry with His dissociation-The His bundle: Bystander or participant? HeartRhythm case reports. 2018 Aug:4(8):378-381. doi: 10.1016/j.hrcr.2018.05.006. Epub 2018 May 23 [PubMed PMID: 30116713]
Level 3 (low-level) evidenceIdeker RE, Rogers JM, Fast V, Li L, Kay GN, Pogwizd SM. Can mapping differentiate microreentry from a focus in the ventricle? Heart rhythm. 2009 Nov:6(11):1666-9. doi: 10.1016/j.hrthm.2009.07.012. Epub 2009 Jul 15 [PubMed PMID: 19793684]
el-Sherif N. The mechanism of supraventricular tachycardia. Ectopic (parasystolic) focus versus re-entry. Journal of electrocardiology. 1974:7(2):169-78 [PubMed PMID: 4132557]
Garan H, Ruskin JN. Localized reentry. Mechanism of induced sustained ventricular tachycardia in canine model of recent myocardial infarction. The Journal of clinical investigation. 1984 Aug:74(2):377-92 [PubMed PMID: 6746899]
Level 3 (low-level) evidenceTai YT, Lee KL. Interfascicular macroreentry versus intrafascicular microreentry. Journal of cardiovascular electrophysiology. 1996 Mar:7(3):275 [PubMed PMID: 8867302]
Level 3 (low-level) evidenceKocovic DZ, Harada T, Friedman PL, Stevenson WG. Characteristics of electrograms recorded at reentry circuit sites and bystanders during ventricular tachycardia after myocardial infarction. Journal of the American College of Cardiology. 1999 Aug:34(2):381-8 [PubMed PMID: 10440149]
Level 2 (mid-level) evidenceStevenson WG, Khan H, Sager P, Saxon LA, Middlekauff HR, Natterson PD, Wiener I. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993 Oct:88(4 Pt 1):1647-70 [PubMed PMID: 8403311]
Stevenson WG, Sager PT, Friedman PL. Entrainment techniques for mapping atrial and ventricular tachycardias. Journal of cardiovascular electrophysiology. 1995 Mar:6(3):201-16 [PubMed PMID: 7620645]
Waldo AL, Henthorn RW. Use of transient entrainment during ventricular tachycardia to localize a critical area in the reentry circuit for ablation. Pacing and clinical electrophysiology : PACE. 1989 Jan:12(1 Pt 2):231-44 [PubMed PMID: 2466258]
Waldo AL. Atrial flutter: entrainment characteristics. Journal of cardiovascular electrophysiology. 1997 Mar:8(3):337-52 [PubMed PMID: 9083885]
Level 3 (low-level) evidenceBrugada P, Wellens HJ. Entrainment as an electrophysiologic phenomenon. Journal of the American College of Cardiology. 1984 Feb:3(2 Pt 1):451-4 [PubMed PMID: 6693633]
Veenhuyzen GD, Quinn FR. Principles of entrainment: diagnostic utility for supraventricular tachycardia. Indian pacing and electrophysiology journal. 2008 Feb 1:8(1):51-65 [PubMed PMID: 18270602]
Almendral J. Resetting and entrainment of reentrant arrhythmias: part II: informative content and practical use of these responses. Pacing and clinical electrophysiology : PACE. 2013 May:36(5):641-61. doi: 10.1111/pace.12075. Epub 2013 Jan 18 [PubMed PMID: 23330693]
Stevenson WG, Sager PT, Natterson PD, Saxon LA, Middlekauff HR, Wiener I. Relation of pace mapping QRS configuration and conduction delay to ventricular tachycardia reentry circuits in human infarct scars. Journal of the American College of Cardiology. 1995 Aug:26(2):481-8 [PubMed PMID: 7608454]
Level 2 (mid-level) evidenceAttin M. Electrophysiology study: a comprehensive review. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 2001 Jul:10(4):260-73; quiz 274-5 [PubMed PMID: 11432214]
Lévy S, Ricard P. Using the right drug: a treatment algorithm for regular supraventricular tachycardias. European heart journal. 1997 May:18 Suppl C():C27-32 [PubMed PMID: 9152672]
Touboul P, Vexler RM, Chatelain MT. Re-entry via Mahaim fibres as a possible basis for tachycardia. British heart journal. 1978 Jul:40(7):806-11 [PubMed PMID: 687479]
Level 3 (low-level) evidenceAlmendral J, Caulier-Cisterna R, Rojo-Álvarez JL. Resetting and entrainment of reentrant arrhythmias: part I: concepts, recognition, and protocol for evaluation: surface ECG versus intracardiac recordings. Pacing and clinical electrophysiology : PACE. 2013 Apr:36(4):508-32. doi: 10.1111/pace.12064. Epub 2013 Jan 10 [PubMed PMID: 23305213]
Cosío FG. Atrial Flutter, Typical and Atypical: A Review. Arrhythmia & electrophysiology review. 2017 Jun:6(2):55-62. doi: 10.15420/aer.2017.5.2. Epub [PubMed PMID: 28835836]
Moreira W, Timmermans C, Wellens HJ, Mizusawa Y, Philippens S, Perez D, Rodriguez LM. Can common-type atrial flutter be a sign of an arrhythmogenic substrate in paroxysmal atrial fibrillation? Clinical and ablative consequences in patients with coexistent paroxysmal atrial fibrillation/atrial flutter. Circulation. 2007 Dec 11:116(24):2786-92 [PubMed PMID: 18040030]
Liu PH, Wang WB, Wang DJ, Shieh SM, Sung PK. Three types of atrioventricular reciprocating tachycardia using bilateral accessory pathways in a patient with Wolff-Parkinson-White syndrome. Journal of electrocardiology. 1989 Apr:22(2):173-80 [PubMed PMID: 2708934]
Level 3 (low-level) evidenceChen TH, Tsai ML, Chang PC, Wo HT, Chou CC, Wen MS, Wang CC, Yeh SJ, Wu D. Risk factors of recurrence and complication in radiofrequency catheter ablation of atrioventricular reentrant tachycardia in children and adolescents. Cardiology in the young. 2013 Oct:23(5):682-91. doi: 10.1017/S1047951112001655. Epub 2013 Jan 18 [PubMed PMID: 23328409]
Level 2 (mid-level) evidenceNademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. Journal of the American College of Cardiology. 2004 Jun 2:43(11):2044-53 [PubMed PMID: 15172410]
Caldwell J, Redfearn D. Ablation of complex fractionated atrial electrograms in catheter ablation for AF; where have we been and where are we going? Current cardiology reviews. 2012 Nov:8(4):347-53 [PubMed PMID: 22920481]
Lau DH, Zeemering S, Maesen B, Kuklik P, Verheule S, Schotten U. Catheter Ablation Targeting Complex Fractionated Atrial Electrogram in Atrial Fibrillation. Journal of atrial fibrillation. 2013 Oct-Nov:6(3):907. doi: 10.4022/jafib.907. Epub 2013 Oct 31 [PubMed PMID: 28496893]
Berenfeld O, Jalife J. Complex fractionated atrial electrograms: is this the beast to tame in atrial fibrillation? Circulation. Arrhythmia and electrophysiology. 2011 Aug:4(4):426-8. doi: 10.1161/CIRCEP.111.964841. Epub [PubMed PMID: 21846887]
Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. Journal of the American College of Cardiology. 2012 Aug 14:60(7):628-36. doi: 10.1016/j.jacc.2012.05.022. Epub 2012 Jul 18 [PubMed PMID: 22818076]
Krummen DE, Swarup V, Narayan SM. The role of rotors in atrial fibrillation. Journal of thoracic disease. 2015 Feb:7(2):142-51. doi: 10.3978/j.issn.2072-1439.2014.11.15. Epub [PubMed PMID: 25713729]
Takahashi S, Sueda T. Development of the Maze procedure and the contribution of Japanese surgeons. General thoracic and cardiovascular surgery. 2017 Mar:65(3):144-152. doi: 10.1007/s11748-016-0728-y. Epub 2016 Nov 16 [PubMed PMID: 27854045]
Sueda T. History and development of surgical procedures for atrial fibrillation. Surgery today. 2015 Dec:45(12):1475-80. doi: 10.1007/s00595-015-1140-4. Epub 2015 Mar 4 [PubMed PMID: 25735737]
Asirvatham SJ, Stevenson WG. Mapping Reentry: In, Out, Into, or In Two? Circulation. Arrhythmia and electrophysiology. 2016 May:9(5):e003609. doi: 10.1161/CIRCEP.116.003609. Epub [PubMed PMID: 27153878]
Schwartz M, Rodman D, Lowenstein SR. Recognition and treatment of multifocal atrial tachycardia: a critical review. The Journal of emergency medicine. 1994 May-Jun:12(3):353-60 [PubMed PMID: 8040593]
Haissaguerre M, Shah AJ, Cochet H, Hocini M, Dubois R, Efimov I, Vigmond E, Bernus O, Trayanova N. Intermittent drivers anchoring to structural heterogeneities as a major pathophysiological mechanism of human persistent atrial fibrillation. The Journal of physiology. 2016 May 1:594(9):2387-98. doi: 10.1113/JP270617. Epub [PubMed PMID: 26890861]
Kapa S, Asirvatham SJ. Atrial fibrillation: focal or reentrant or both?: a new autonomic lens to examine an old riddle. Circulation. Arrhythmia and electrophysiology. 2009 Aug:2(4):345-8. doi: 10.1161/CIRCEP.109.888081. Epub [PubMed PMID: 19808489]
Saoudi N, Cosio F, Waldo A, Chen SA, Iesaka Y, Lesh M, Saksena S, Salerno J, Schoels W. Classification of atrial flutter and regular atrial tachycardia according to electrophysiologic mechanism and anatomic bases: a statement from a joint expert group from the Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Journal of cardiovascular electrophysiology. 2001 Jul:12(7):852-66 [PubMed PMID: 11469446]
Level 3 (low-level) evidenceLau DH, Volders PG, Kohl P, Prinzen FW, Zaza A, Kääb S, Oto A, Schotten U, European Heart Rhythm Association. Opportunities and challenges of current electrophysiology research: a plea to establish 'translational electrophysiology' curricula. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2015 May:17(5):825-33. doi: 10.1093/europace/euu301. Epub 2015 Feb 17 [PubMed PMID: 25691491]
Level 1 (high-level) evidenceAlbert CM, Stevenson WG. The Future of Arrhythmias and Electrophysiology. Circulation. 2016 Jun 21:133(25):2687-96. doi: 10.1161/CIRCULATIONAHA.116.023519. Epub [PubMed PMID: 27324363]