Introduction
Platinum-based chemotherapeutic agents have revolutionized oncology practice since the accidental discovery of cisplatin's antineoplastic properties by Barnett Rosenberg in 1965 and its first use in clinical practice in the 1970s.[1] Agents such as cisplatin, carboplatin, and oxaliplatin have become vital first-line, second-line, and adjuvant therapies for several types of solid tumors, including ovarian, testicular, lung, head and neck, colorectal, and bladder cancers. To date, platinum compounds continue to play a central role in oncology practice, and approximately 40% to 80% of patients with cancer undergoing chemotherapy are exposed to platinum compounds.[2] However, the clinical utility of platinum compounds is significantly limited by 2 limitations—hypersensitivity reactions and dose-limiting organ toxicities.[3] These adverse events negatively impact a patient's quality of life and often necessitate a reduction in dose, prolongation of treatment duration, or switching therapies, which may compromise treatment efficacy.[3] Hypersensitivity reactions to platinum compounds vary in frequency, occurring in approximately 5% of patients treated with cisplatin, 12% to 27% with carboplatin, and 10% to 24% with oxaliplatin.[4] Several factors can influence the occurrence of hypersensitivity reactions, including the type of platinum compound used, the cumulative dose administered, any previous allergic reactions to medications, and possibly genetic predispositions.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The 3 main platinum compounds—cisplatin, carboplatin, and oxaliplatin—differ in their chemical structures, clinical applications, adverse effects, and indications for treatment. These differences include variations in hypersensitivity incidence (5%-10% for cisplatin, 9%-27% for carboplatin, and 10%-24% for oxaliplatin), primary dose-limiting toxicities, and onset patterns of adverse reactions, which are crucial for clinical decision-making and management approaches.[5] At the same time, platinum compounds produce a set of dose-limiting organ toxicities that affect nearly all major body systems. The dose-limiting toxicity of cisplatin is nephrotoxicity, which is observed in 20% to 30% of patients despite the use of adequate hydration regimens.[6] In contrast, carboplatin has reduced nephrotoxicity but is associated with higher rates of myelosuppression and thrombocytopenia, affecting approximately 40% of patients.[6] Additional adverse effects that impact patients' quality of life include neurotoxicity and ototoxicity.[7]
Organ-specific platinum toxicities follow epidemiological patterns that demonstrate complex characteristics. The incidence of cisplatin-induced nephrotoxicity ranges from 20% to 30% in patients, despite current hydration protocols, with higher rates observed in older patients and those with pre-existing renal impairment.[6] The occurrence of neurotoxicity differs significantly between agents and depends on the cumulative dose administered.[6] Cisplatin is associated with cumulative sensory neuropathy, affecting 30% to 40% of patients. In contrast, oxaliplatin induces acute cold-induced dysesthesias in approximately 90% of patients during infusion and causes cumulative sensory neurotoxicity in 50% of patients who receive more than 780 to 850 mg/m².[8]
Epidemiology
Hypersensitivity reactions vary significantly among platinum agents and patient populations. Cisplatin is associated with the lowest incidence, affecting 5% to 10% of patients.[9] In contrast, carboplatin carries higher rates, ranging from 9% to 27% and an overall incidence of 15% to 20% in patients receiving multiple treatment cycles.[10] Oxaliplatin has a two-step hypersensitivity reaction profile with immediate reactions occurring in approximately 10% to 12% of patients and delayed reactions occurring in an additional 10% of patients.[11]
Several factors have been identified that may increase the risk of hypersensitivity reactions to platinum compounds. Some of the most consistent associations with this risk include prior exposure to platinum, especially when there is a treatment-free interval of more than 12 months; a history of drug allergies; and higher cumulative platinum dose.[12] Additionally, disease-specific factors may also influence the risk of hypersensitivity reactions; for example, patients with ovarian cancer develop higher rates of carboplatin hypersensitivity compared to those with lung cancer.[13]
Table 1. Frequency and Key Risk Factors of Platinum Hypersensitivity Reactions Associated with Cisplatin, Carboplatin, and Oxaliplatin
| Platinum Agent | Incidence (%) | Median Cycle of Onset | Patient-Specific Risk Factors | Treatment-Related Risk Factors |
| Cisplatin | 5%-10% | 6-8 cycles | History of drug allergies and atopy | Cumulative dose >400 mg/m² |
| Carboplatin | 9%-27% | 8 cycles (range 2-25) |
Ovarian cancer diagnosis, female gender, and history of drug allergies |
Treatment-free interval >12 months, previous platinum exposure, and cumulative dose |
| Oxaliplatin | 10%-24% |
Bimodal: 1-3 cycles (immediate) or 7-9 cycles (delayed) |
History of drug allergies and atopy |
Multiple treatment courses and a weekly administration schedule |
Note: Incidence rates differ across studies and patient populations. Risk factors show different levels of association in the published literature.
Pathophysiology
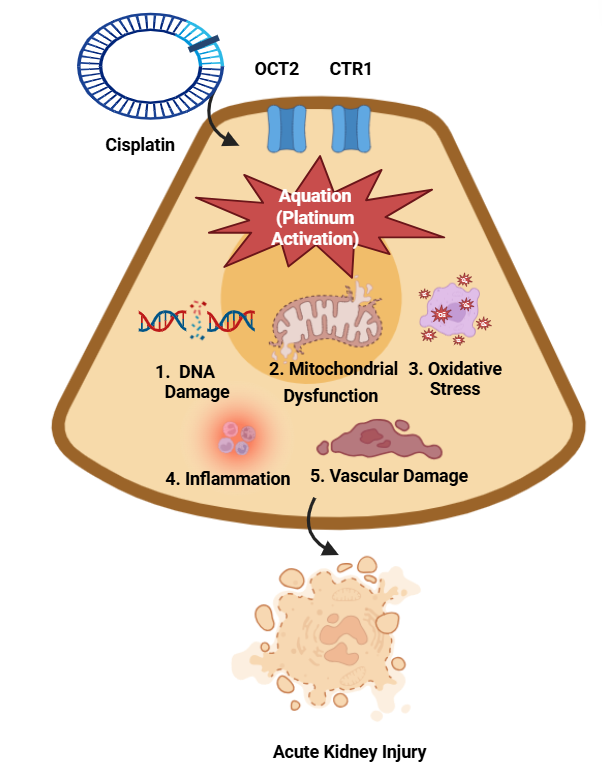
Platinum hypersensitivity reactions show diverse immunological origins, including Immunoglobulin E (IgE)-dependent (type I) and non–IgE-independent reactions. Platinum compounds bind to endogenous proteins, especially serum albumin, by forming stable haptens.[8] The processed platinum-protein complexes enable antigen-presenting cells to present antigens effectively to T cells and B cells, thereby activating them. The presence of platinum-specific IgE antibodies in 60% to 80% of patients who experience moderate-to-severe reactions supports that type I hypersensitivity plays a significant role in their reactions.[8] Several reactions show evidence of non–IgE-independent mechanisms, in addition to IgE-independent reactions, especially in cases of first-time exposure. The process involves the direct activation of mast cells and basophils, complement activation, and cytokine release. Sensitivity to platinum compounds exhibits frequent but imperfect cross-reactivity, as approximately 60% to 70% of patients develop reactions to multiple agents.[14] The pathogenesis of platinum-induced nephrotoxicity involves diverse cellular pathways (see Image. Pathophysiological Mechanisms of Platinum-Induced Nephrotoxicity). Platinum compounds enter renal tubular epithelial cells through organic cation transporters (OCT2) and copper transporters (CTR1). Once inside the cell, they undergo aquation, leading to DNA damage, mitochondrial dysfunction, oxidative stress, and damage to the vascular endothelium.[15] Tubular cell apoptosis and acute tubular necrosis result from multiple injury pathways that merge in the final stage to produce acute kidney injury. OCT2 and CTR1 facilitate the selective accumulation of cisplatin in proximal tubular epithelial cells, thereby achieving intracellular concentrations 5 times greater than those in plasma.[16]
The neurotoxic effects of platinum compounds exist through separate mechanisms. The neuronal damage caused by cisplatin and oxaliplatin occurs due to their ability to accumulate in the dorsal root ganglia, leading to nucleolar formation and the suppression of essential neuronal maintenance genes.[17] The rapid neurotoxic effects of oxaliplatin arise from its ability to bind extracellular calcium ions, which modifies sodium channel conductance properties and generates neuronal excitability.[18] The toxic effects of platinum compounds on the ears result from cochlear hair cell death due to their ability to accumulate. The production of reactive oxygen species, combined with glutathione depletion, induces lipid peroxidation and calcium dysregulation, ultimately leading to the apoptosis of hair cells.[19]
History and Physical
Hypersensitivity reactions to platinum-based chemotherapy agents—cisplatin, carboplatin, and oxaliplatin—present with distinct clinical manifestations. Acute hypersensitivity reactions to cisplatin occur immediately after infusion, presenting with symptoms such as facial swelling, flushing with urticaria, dyspnea, and hypotension.[8] Patients who develop hypersensitivity to carboplatin typically exhibit delayed reactions during their second treatment cycle. Clinical features may include cutaneous symptoms, respiratory compromise, gastrointestinal symptoms, and cardiovascular signs, such as hypotension.[20] The clinical presentation of oxaliplatin differs from that of other platinum agents because it causes both immediate and delayed hypersensitivity reactions. These reactions present as pruritus or rash and mild bronchospasm during the acute phase but can lead to generalized urticaria, hypotension, and anaphylaxis in delayed reactions.[21]
Evaluation
The diagnosis of platinum drug hypersensitivity requires a comprehensive clinical evaluation and specific diagnostic tests. This process involves obtaining a detailed medical history, assessing reaction patterns, and evaluating the severity of reactions along with the patient's history of drug exposure. Skin tests, including skin prick and intradermal tests, help identify IgE-mediated sensitivities to platinum drugs, such as carboplatin and oxaliplatin. These tests help clinicians safely administer subsequent chemotherapy doses.[8][21] Although skin tests provide reliable predictions of sensitivities, their negative results do not eliminate the possibility of sensitivity. Patients who require platinum-based chemotherapy after experiencing hypersensitivity reactions need drug re-challenge or rapid desensitization protocols as their current treatment approach.[21]
Treatment / Management
The management of hypersensitivity reactions to platinum-based chemotherapeutic agents requires personalized approaches that minimize adverse outcomes, enabling ongoing effective treatment. The first approach for treating patients involves premedication with antihistamines and corticosteroids to reduce the severity and occurrence of reactions.[22] Preconditioning with medication shows unpredictable benefits among platinum compounds since it works better with taxanes than with platinum drugs.[22] The extension of infusion duration helps minimize symptoms in patients with mild hypersensitivity reactions, allowing them to complete the planned treatment uninterrupted.[20](A1)
Rapid drug desensitization protocols have proven effective for patients experiencing moderate-to-severe hypersensitivity reactions. The doses of platinum agents are administered gradually through a carefully designed protocol that spans multiple hours under close supervision to minimize the risk of severe reactions and complete planned treatments.[23] Patients who develop hypersensitivity reactions during platinum-based chemotherapy can switch to a different platinum agent, such as oxaliplatin, which provides an effective treatment option with preserved efficacy and good tolerability.[24](B2)
Table 2. Clinical Management of Platinum-Induced Hypersensitivity Reactions
| Severity | Clinical Features | Immediate Actions | Recommended Management Strategy |
| Mild | Flushing, rash, and pruritus | Stop infusion and administer antihistamines | Restart with a slower infusion rate and premedication [22] |
| Moderate | Urticaria, nausea, and mild dyspnea | Add corticosteroids and monitor closely | Consider skin testing and future desensitization [10][21] |
| Severe | Hypotension, bronchospasm, and anaphylaxis | Emergency management with epinephrine and intravenous fluids | Refer to allergists or immunologists for evaluation and initiate a desensitization protocol or agent substitution [12][23] |
Emerging Therapeutics and Future Directions
The development of new therapeutic methods for managing hypersensitivity reactions to platinum-based chemotherapy focuses on biological treatment approaches and improved clinical procedures. Research has investigated the preventive use of monoclonal antibodies, including omalizumab, which binds IgE to prevent mast cell activation and histamine release, thus minimizing platinum drug allergic reactions.[25] Initial studies suggest that omalizumab as a premedication substantially decreases the occurrence of reactions, thereby enhancing the safety profile of platinum-based chemotherapy for patients who have shown hypersensitivity.[25] Research focuses on improving chemotherapy desensitization procedures by developing outpatient treatment strategies that ensure safe care, improve patient convenience, and promote more efficient healthcare delivery. Traditional inpatient desensitization procedures require extensive medical supervision and a prolonged hospital stay. Outpatient protocols now use shorter dosing increments to establish drug tolerance rapidly under medical supervision, allowing patients to continue their treatments outside of hospital settings.[26] Initial data indicate that these simplified outpatient desensitization protocols are safe and effective while demonstrating strong clinical and economic benefits.[26]
Future management of platinum-induced hypersensitivity reactions focuses on individualized treatment strategies guided by genetic and biomarker analysis to predict the risk of severe reactions in patients. Research has identified genetic polymorphisms associated with increased sensitivity to platinum agents, enabling healthcare providers to develop personalized prevention methods and alternative treatments through genetic testing.[27] Research continues to explore alternative chemotherapeutic agents that exhibit reduced immunogenicity, aiming to develop safer treatments with equivalent effectiveness. These innovative strategies will improve patient outcomes by reducing treatment disruptions and enhancing safety measures of cancer chemotherapy.[27]
Complications
Platinum-Induced Hypersensitivity Reactions
Hypersensitivity reactions to platinum-based chemotherapeutic agents, such as cisplatin, carboplatin, and oxaliplatin, are a growing concern as these agents are widely used in the treatment of various malignancies.[1][3][4] The pathogenesis of platinum-induced hypersensitivity reactions is not fully understood but is believed to be cell-mediated and possibly IgE-independent, and is related to the dose and number of exposures.[4][9][11] Although there has been an improvement in understanding the biological processes underlying these reactions, they are still challenging to predict. Notably, oxaliplatin tends to cause a bimodal distribution of hypersensitivity reactions, often manifesting early or after multiple treatment cycles, whereas carboplatin reactions typically arise after the sixth exposure.[4][11][13] The diagnosis is made on clinical grounds, and although skin testing is useful in detecting platinum-specific sensitization, the negative predictive value is not very high.[10][8] Several strategies have been developed to minimize the occurrence of platinum-related hypersensitivity reactions, including premedication regimens, prolonged infusion schedules, and, when feasible, substitution with an alternative platinum agent.[22] Among these approaches, rapid drug desensitization has become a particularly useful tool, thus allowing continued treatment in patients who otherwise have to discontinue therapy.[12][23][25] These protocols, typically involving the administration of the drug in graded doses under observation, have been successful and safe in both inpatient and outpatient settings.[23][25] Additionally, oxaliplatin has been used as an alternative to carboplatin in certain cases of hypersensitivity, which can aid in decision-making in clinical practice.[24]
However, current evidence is mainly based on observational studies and single-center experiences, and there is a lack of high-quality randomized controlled trials to make definitive recommendations.[4] Lack of uniformity in hypersensitivity reaction grading, diagnostic cut-off, and reporting makes it difficult to develop universal guidelines. Furthermore, immunocompromised patients, older patients, and pediatric patients are underrepresented in the literature and may be at a higher risk for adverse outcomes.[8][17][18] Future research should prioritize the standardization of diagnostic criteria, a prospective evaluation of desensitization efficacy, and the identification of predictive biomarkers for individualized risk assessment. Ensuring safe and uninterrupted cancer care for patients with platinum hypersensitivity remains critical.
Pearls and Other Issues
Limitations of Current Evidence and Clinical Applications
The management of hypersensitivity reactions to platinum-based chemotherapy agents has several challenges in current evidence, mainly due to a lack of prospective studies. Most of the current data is based on retrospective analyses and observational studies with small cohorts, which limits the external validity of findings and their generalizability to other patient populations.[28] Differences in the definition of hypersensitivity, variability in the assessment of the severity of reactions, and heterogeneity of the studies make it difficult to develop evidence-based clinical guidelines.[28] Therefore, the absence of well-conducted randomized controlled trials hampers the evaluation and standardization of the treatment protocols, including premedication and desensitization strategies, and clinicians have to rely on the consensus and guidelines, including individual patient management.[28] Due to the scarcity of large-scale prospective trials that compare different therapeutic interventions for platinum-induced hypersensitivity reactions, many of the current recommendations for management are based on expert opinion or observational evidence. This limitation emphasizes the issues with the optimal time of intervention, the criteria for selecting patients for desensitization, and long-term safety.[28] Hence, clinicians must be quite cautious and make many clinical decisions, especially in patients with complex backgrounds, including immunocompromised patients or the elderly, for whom evidence is weakest. These limitations have clinical implications, and therefore, individualized patient management and close monitoring of patients receiving platinum-based chemotherapy are crucial. Improving clinical awareness and reporting standards for hypersensitivity reactions is essential for improving knowledge in this area. Future research should focus on multicentre randomized controlled trials, the development of standardized reaction classification systems, and well-defined patient cohorts to generate more credible evidence.
Enhancing Healthcare Team Outcomes
Platinum-based chemotherapeutic agents such as cisplatin, carboplatin, and oxaliplatin have revolutionized oncological practice. Although numerous targeted therapies have been developed, platinum compounds are still crucial in current oncological practice, with patients with cancer often receiving platinum-based chemotherapy at some stage in their treatment course. However, their clinical utility is substantially limited by hypersensitivity reactions and dose-limiting organ toxicities, which negatively affect quality of life. Several factors can potentially increase the risk of hypersensitivity, including previous exposure to platinum drugs, especially if there is a long period between treatments; a history of drug allergies; and the total dose of platinum agents used. The toxicities of each platinum compound are different. The primary dose-limiting toxicity of cisplatin is nephrotoxicity, which can be prevented by hydration protocols. Although carboplatin is less nephrotoxic than the other 2 drugs, it causes more severe myelosuppression. Neurotoxicity mechanisms differ among platinum compounds, with oxaliplatin exhibiting unique acute neurotoxicity and cumulative sensory neurotoxicity with ongoing treatment. Hypersensitivity reactions manifest clinically from mild cutaneous symptoms to anaphylaxis. Preventive measures include premedication with antihistamines and corticosteroids, prolonged infusion protocols, and drug substitution. Rapid desensitization protocols have demonstrated notable success, involving the careful and controlled administration of incrementally increasing doses under close supervision. New approaches include the prophylactic administration of monoclonal antibodies, such as omalizumab, which binds to IgE and prevents mast cell degranulation, and the improvement of outpatient desensitization protocols. Future research aims to implement personalized medicine strategies based on genetic and biomarker profiles to identify at-risk patients and investigate new chemotherapeutic agents with reduced immunogenic potential. There is a lack of high-quality randomized controlled trials needed to validate standardized protocols.
Platinum hypersensitivity and toxicity pose significant challenges to cancer therapy practice today because they affect numerous patients and require treatment adjustments that reduce treatment effectiveness. Although current management techniques have improved patient safety, significant knowledge gaps remain regarding how treatments work and how they can be optimized—gaps that researchers must address to further enhance patient care. Research should focus on creating validated predictive models that identify toxicity risk levels through clinical and molecular assessments. The evaluation of targeted interventions for toxicity should prioritize agents that provide both nephroprotective and neuroprotective effects while retaining antitumor efficacy. The addition of platinum to combination therapies, including immunotherapy and molecularly targeted therapies, requires a careful evaluation of toxicity to identify potential combined adverse effects. The foundation for this research requires standardized assessment and reporting frameworks that enable researchers to compare results across different studies and clinical environments. Scientific progress in platinum toxicity research and prevention methods should continue while the clinical practice relies on established methods to reduce risks through proper patient selection and hydration protocols, including dose adjustments and close patient monitoring. By integrating mechanistic insights with new preventive methods and optimized management techniques, the therapeutic ratio of these fundamental agents can be enhanced, thereby improving both treatment outcomes and patient well-being. Platinum-based chemotherapeutic agents produce clinically relevant hypersensitivity reactions that hinder the treatment of solid tumors and hematologic malignancies. Although research has advanced understanding of reaction mechanisms, patients still experience unexpected severe reactions during treatment, and skin testing provides only limited predictive value.[4][8] Several management approaches, including premedication, drug substitution, prolonged infusion protocols, and desensitization, facilitate continued treatment, although the available evidence is based on retrospective research and expert consensus.[12][23][25]
Managing hypersensitivity reactions to platinum-based chemotherapy agents requires a coordinated, interprofessional approach to ensure patient safety and maintain effective cancer treatment. Oncologists play a central role in identifying at-risk patients prone to hypersensitivity reactions, based on their clinical history, the number of prior cycles, and biomarkers. They collaborate closely with allergists and pharmacists to select safe, alternative treatments or initiate desensitization protocols. Allergists and immunologists assess patients with a history of hypersensitivity reactions. They may perform skin testing or drug provocation tests to determine the risk of recurrence and design and supervise desensitization procedures. Pharmacists play a crucial role in preparing desensitization solutions, verifying drug compatibility, and ensuring accurate dosing schedules, particularly during complex, multi-step desensitization protocols. Nurses play a frontline role in monitoring patients during infusion, recognizing early signs of hypersensitivity, and ensuring adherence to safety protocols. They are also key communicators between patients and the care team during desensitization. Emergency physicians or critical care teams are often consulted in facilities where high-risk desensitization takes place, ensuring rapid response capabilities in the event of anaphylaxis. Case reviews and morbidity and mortality rounds help refine approaches, identify system gaps, and improve safety. Psychosocial support is often provided by social workers or patient navigators, particularly for patients with anxiety related to previous hypersensitivity reactions or those undergoing repeated desensitizations.
Future research demands prospective multicenter investigations to standardize diagnostic criteria and validate predictive tools while evaluating the long-term safety and effectiveness of desensitization protocols. The integration of genetic and immunologic biomarkers into clinical practice should be a future priority to improve personalized risk assessment and treatment protocols. Clinicians need to follow a practical, patient-focused approach that weighs the need for treatment against potential side effects until new data become available for safe cancer treatment delivery.
Media
(Click Image to Enlarge)
References
Harper BW, Krause-Heuer AM, Grant MP, Manohar M, Garbutcheon-Singh KB, Aldrich-Wright JR. Advances in platinum chemotherapeutics. Chemistry (Weinheim an der Bergstrasse, Germany). 2010 Jun 25:16(24):7064-77. doi: 10.1002/chem.201000148. Epub [PubMed PMID: 20533453]
Level 3 (low-level) evidenceSirohi B, Ashley S, Norton A, Popat S, Hughes S, Papadopoulos P, Priest K, O'Brien M. Early response to platinum-based first-line chemotherapy in non-small cell lung cancer may predict survival. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2007 Aug:2(8):735-40 [PubMed PMID: 17762340]
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. European journal of pharmacology. 2014 Oct 5:740():364-78. doi: 10.1016/j.ejphar.2014.07.025. Epub 2014 Jul 21 [PubMed PMID: 25058905]
Picard M, Castells MC. Re-visiting Hypersensitivity Reactions to Taxanes: A Comprehensive Review. Clinical reviews in allergy & immunology. 2015 Oct:49(2):177-91. doi: 10.1007/s12016-014-8416-0. Epub [PubMed PMID: 24740483]
Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nature reviews. Drug discovery. 2005 Apr:4(4):307-20 [PubMed PMID: 15789122]
Perazella MA. Onco-nephrology: renal toxicities of chemotherapeutic agents. Clinical journal of the American Society of Nephrology : CJASN. 2012 Oct:7(10):1713-21. doi: 10.2215/CJN.02780312. Epub 2012 Aug 9 [PubMed PMID: 22879440]
Calvert AH, Egorin MJ. Carboplatin dosing formulae: gender bias and the use of creatinine-based methodologies. European journal of cancer (Oxford, England : 1990). 2002 Jan:38(1):11-6 [PubMed PMID: 11750834]
Caiado J, Castells M. Presentation and Diagnosis of Hypersensitivity to Platinum Drugs. Current allergy and asthma reports. 2015 Apr:15(4):15. doi: 10.1007/s11882-015-0515-3. Epub [PubMed PMID: 26130472]
Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, Belinson J. Clinical features of hypersensitivity reactions to carboplatin. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999 Apr:17(4):1141 [PubMed PMID: 10561172]
Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to Carboplatin and Paclitaxel in an outpatient oncology infusion center: a 5-year review. The journal of allergy and clinical immunology. In practice. 2014 Jul-Aug:2(4):428-33. doi: 10.1016/j.jaip.2014.04.010. Epub 2014 May 23 [PubMed PMID: 25017531]
Maindrault-Goebel F, André T, Tournigand C, Louvet C, Perez-Staub N, Zeghib N, De Gramont A. Allergic-type reactions to oxaliplatin: retrospective analysis of 42 patients. European journal of cancer (Oxford, England : 1990). 2005 Oct:41(15):2262-7 [PubMed PMID: 16154353]
Level 2 (mid-level) evidenceCastells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI, Laidlaw TM, Legere HJ, Nallamshetty SN, Palis RI, Rao JJ, Berlin ST, Campos SM, Matulonis UA. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. The Journal of allergy and clinical immunology. 2008 Sep:122(3):574-80. doi: 10.1016/j.jaci.2008.02.044. Epub 2008 May 27 [PubMed PMID: 18502492]
Level 3 (low-level) evidenceGadducci A, Tana R, Teti G, Zanca G, Fanucchi A, Genazzani AR. Analysis of the pattern of hypersensitivity reactions in patients receiving carboplatin retreatment for recurrent ovarian cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2008 Jul-Aug:18(4):615-20 [PubMed PMID: 18754135]
Level 2 (mid-level) evidencePagani M, Bonadonna P, Senna GE, Antico A. Standardization of skin tests for diagnosis and prevention of hypersensitivity reactions to oxaliplatin. International archives of allergy and immunology. 2008:145(1):54-7 [PubMed PMID: 17703101]
Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney international. 2008 May:73(9):994-1007. doi: 10.1038/sj.ki.5002786. Epub 2008 Feb 13 [PubMed PMID: 18272962]
Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstädt H, Lanvers-Kaminsky C, am Zehnhoff-Dinnesen A, Schinkel AH, Koepsell H, Jürgens H, Schlatter E. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. The American journal of pathology. 2010 Mar:176(3):1169-80. doi: 10.2353/ajpath.2010.090610. Epub 2010 Jan 28 [PubMed PMID: 20110413]
Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: A current review. Annals of neurology. 2017 Jun:81(6):772-781. doi: 10.1002/ana.24951. Epub 2017 Jun 5 [PubMed PMID: 28486769]
Webster RG, Brain KL, Wilson RH, Grem JL, Vincent A. Oxaliplatin induces hyperexcitability at motor and autonomic neuromuscular junctions through effects on voltage-gated sodium channels. British journal of pharmacology. 2005 Dec:146(7):1027-39 [PubMed PMID: 16231011]
Waissbluth S, Daniel SJ. Cisplatin-induced ototoxicity: transporters playing a role in cisplatin toxicity. Hearing research. 2013 May:299():37-45. doi: 10.1016/j.heares.2013.02.002. Epub 2013 Mar 1 [PubMed PMID: 23467171]
Makrilia N, Syrigou E, Kaklamanos I, Manolopoulos L, Saif MW. Hypersensitivity reactions associated with platinum antineoplastic agents: a systematic review. Metal-based drugs. 2010:2010():. pii: 207084. doi: 10.1155/2010/207084. Epub 2010 Sep 20 [PubMed PMID: 20886011]
Level 1 (high-level) evidenceLeguy-Seguin V, Jolimoy G, Coudert B, Pernot C, Dalac S, Vabres P, Collet E. Diagnostic and predictive value of skin testing in platinum salt hypersensitivity. The Journal of allergy and clinical immunology. 2007 Mar:119(3):726-30 [PubMed PMID: 17258305]
Lee C, Gianos M, Klaustermeyer WB. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2009 Mar:102(3):179-87; quiz 187-9, 222. doi: 10.1016/S1081-1206(10)60078-6. Epub [PubMed PMID: 19354063]
Madrigal-Burgaleta R, Berges-Gimeno MP, Angel-Pereira D, Ferreiro-Monteagudo R, Guillen-Ponce C, Pueyo C, Gomez de Salazar E, Alvarez-Cuesta E. Hypersensitivity and desensitization to antineoplastic agents: outcomes of 189 procedures with a new short protocol and novel diagnostic tools assessment. Allergy. 2013 Jul:68(7):853-61. doi: 10.1111/all.12105. Epub 2013 May 6 [PubMed PMID: 23647576]
Kolomeyevskaya NV, Lele SB, Miller A, Riebandt GC, Blum BL, Odunsi KO, Frederick PJ. Oxaliplatin is a safe alternative option for patients with recurrent gynecologic cancers after hypersensitivity reaction to Carboplatin. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2015 Jan:25(1):42-8. doi: 10.1097/IGC.0000000000000307. Epub [PubMed PMID: 25356535]
Level 2 (mid-level) evidenceCastells M, Sancho-Serra Mdel C, Simarro M. Hypersensitivity to antineoplastic agents: mechanisms and treatment with rapid desensitization. Cancer immunology, immunotherapy : CII. 2012 Sep:61(9):1575-84. doi: 10.1007/s00262-012-1273-x. Epub 2012 May 11 [PubMed PMID: 22576054]
Sloane D, Govindarajulu U, Harrow-Mortelliti J, Barry W, Hsu FI, Hong D, Laidlaw T, Palis R, Legere H, Bunyavanich S, Breslow R, Wesemann D, Barrett N, Brennan P, Chong HJ, Liu A, Fernandez J, Fanning L, Kyin T, Cahill K, Bankova L, Lynch A, Berlin S, Campos S, Fuchs C, Mayer R, Matulonis U, Castells M. Safety, Costs, and Efficacy of Rapid Drug Desensitizations to Chemotherapy and Monoclonal Antibodies. The journal of allergy and clinical immunology. In practice. 2016 May-Jun:4(3):497-504. doi: 10.1016/j.jaip.2015.12.019. Epub 2016 Feb 16 [PubMed PMID: 26895621]
Pagani M, Bavbek S, Alvarez-Cuesta E, Berna Dursun A, Bonadonna P, Castells M, Cernadas J, Chiriac A, Sahar H, Madrigal-Burgaleta R, Sanchez Sanchez S. Hypersensitivity reactions to chemotherapy: an EAACI Position Paper. Allergy. 2022 Feb:77(2):388-403. doi: 10.1111/all.15113. Epub 2021 Oct 26 [PubMed PMID: 34587281]
Roselló S, Blasco I, García Fabregat L, Cervantes A, Jordan K, ESMO Guidelines Committee. Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Annals of oncology : official journal of the European Society for Medical Oncology. 2017 Jul 1:28(suppl_4):iv100-iv118. doi: 10.1093/annonc/mdx216. Epub [PubMed PMID: 28881914]
Level 1 (high-level) evidence