Introduction
Macrocytic anemia refers to macrocytosis, a mean corpuscular volume (MCV) >100 fL, in the setting of anemia. Generally, the following thresholds define anemia:
- For nonpregnant females: hemoglobin <12 g/dL or hematocrit (Hct) <36%
- For pregnant females: hemoglobin <11 g/dL
- For males: hemoglobin <13 g/dL or Hct <41%
Macrocytic anemia is divided into 2 forms: megaloblastic (hypersegmented neutrophils) and nonmegaloblastic. The megaloblastic form is due to impaired DNA synthesis from folate or vitamin B12 deficiencies, while the nonmegaloblastic moiety occurs from multiple mechanisms.[1][2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Megaloblastic Anemia
Megaloblastic anemia occurs from deficiencies in folic acid and vitamin B12. Folate deficiency is due to diminished intake (eg, alcohol abuse or malnutrition), increased consumption (eg, hemolysis or pregnancy), and malabsorption (eg, familial, gastric bypass, or medications like cholestyramine or metformin). Vitamin B12 deficiency appears in diminished intake (eg malnutrition), malabsorptive states (eg, atrophic gastritis, either autoimmune or nonautoimmune, from Helicobacter pylori or Zollinger-Ellison syndrome, Diphyllobothrium tapeworm infection, gastric bypass, or ileal resection), or the presence of antagonists (eg, nitrous oxide).[3] Drugs that impair DNA synthesis include folic acid analogs (eg, methotrexate and trimethoprim-sulfamethoxazole), nucleic acid analogs (eg, 5-fluorouracil), and others, including hydroxyurea, pentamidine, phenytoin, pyrimethamine, sulfasalazine, and triamterene. An increase in the MCV in patients with rheumatoid or psoriatic arthritis portends a good response to methotrexate (MTX).[4] However, an increase in the MCV also warrants concern over heme toxicity. Many patients with arthritis have concurrent anemia, and the toxicity of MTX compounds this.
Nonmegaloblastic Anemia
Nonmegaloblastic anemia, which lacks hypersegmented neutrophils, occurs in various settings. Benign conditions include alcohol consumption, red blood cell (RBC) toxicity, hereditary spherocytosis (ie, impaired volume regulation increases red cell size), hypothyroidism, liver disease due to lipid deposition in the cell membrane, and marked reticulocytoses from states of excess RBC consumption, such as hemolysis or turnover in pregnancy or primary bone marrow disease (ie, reticulocytes are larger than the average RBCs).
Other Macrocytosis Etiologies
Some cases of macrocytosis are normal variants associated with a genetic predisposition or found in infants, patients with Down syndrome, and pregnant women.[5] Specific mutations associated with macrocytosis include HGprt deficiency (Lesch-Nyhan disease), ALAS2, and KLF13.[6][5][7] Other spurious findings include hyperglycemia, concentrating the blood. When diluted, the RBCs swell with volume, leukocytosis, and paraproteinemia, increasing sample turbidity for overestimates of RBC size or operator error from occlusion of microscope aperture or sample left out at room temperature too long.[8][9]
Extreme macrocytosis (MCV >130 fL) can be seen with HIV medicines, particularly zidovudine, stavudine, and lamivudine). The resolution of the anemia from HIV is seen as a positive effect of HAART therapy.[10] However, the aforementioned agents are suspected of interfering with B12/DNA, which, in a patient who may be already malnourished, can compound the situation.
Epidemiology
Macrocytosis affects 2% to 4% of the population, 60% of whom have anemia. Alcohol use accounts for the majority, followed by deficiencies in folate and vitamin B12, and medications. Autoimmune causes are more common in middle-aged women. Hypothyroidism and primary bone marrow disease account for more cases of macrocytic anemia in older patients. The prevalence of vitamin B12 deficiency increases in patients older than 60.
Mild macrocytic anemia (MCV 100 to 110 fL) is more likely to be caused by benign conditions compared to marked macrocytic anemia (MCV >110 fL), the latter of which is due to primary bone marrow disease or megaloblastic anemia from folate or vitamin B12 deficiencies.[11][12]
Pathophysiology
The equation for mean corpuscular volume [MCV (fL) = Hct (%) X 10/RBC (106/µg)] explains how macrocytic anemia represents sizable RBCs in comparison to the total amount. Folate and vitamin B12 are necessary for RBC nucleic acid synthesis. Without DNA or RNA, erythropoiesis is ineffective with nuclear/cytoplasmic asynchrony, resulting in larger erythrogenic precursors with abnormal nuclei (eg, hypersegmentation) but normal cytoplasms. Anemia occurring in the presence of macrocytosis and hypersegmented neutrophils is known as megaloblastic anemia. The absence of hypersegmented neutrophils characterizes nonmegaloblastic anemia due to abnormalities involving the RBC membrane, excess erythrocytic precursors, increased cell volume, or RBC toxicity.
The average individual's daily folate requirement is 100 to 200 µg; the body can absorb 400 µg/day. Healthy individuals typically have stored sufficient folate for 4 months. Folate is primarily absorbed in the small bowel. In comparison, the average individual's daily vitamin B12 requirement is 1 µg, and the body can absorb 2 to 3 µg/day. Most individuals have several years of B12 stores. Vitamin B12 is absorbed in the ileum when bound by an intrinsic factor, a protein the gastric parietal cells produce. Abnormalities in these cascades cause deficiencies in folate or vitamin B12, respectively.[13]
Histopathology
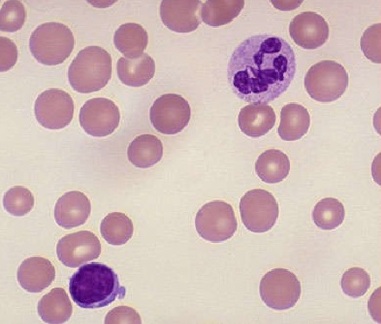
Megaloblastic macrocytic anemia contains hypersegmented neutrophils and macro-ovalocytes (macrocytes having an oval shape with a reduced or absent central pallor) on a peripheral blood smear (PBS) (see Image. Macrocytic Anemia). Anisocytosis and poikilocytosis are not uncommon due to ineffective erythropoiesis. Bone marrow evaluation will demonstrate hypercellularity with abnormal maturation and proliferation of myeloid cell lines, particularly the erythroid lineage.
Nonmegaloblastic macrocytic anemia will not have hypersegmented neutrophils; instead, its PBS will show round macrocytes or macro reticulocytes, in addition to other cells representative of the underlying etiology including acanthocytes from liver disease, myeloid dysplasia, or immaturity in primary bone marrow disease, polychromatophilic RBCs with reticulocytosis, schistocytes from hemolysis, or spherocytes in inherited hemolytic anemias, eg, hereditary spherocytosis. The term "polychromasia" describes the color variation in the peripheral blood smear (PBS) from reticulocytosis. Reticulocytes have a bluish-purple-like hue, contrasting with the erythrocyte red. This contrast, this variation, is suggestive of reticulocytosis, especially when considered in a field of younger, larger red blood cells.
History and Physical
The presentations of macrocytic anemia are dependent on the underlying cause.
Clinical History
Patients with vitamin B12 deficiency complain of mood disturbances and neurologic symptoms like loss of balance, memory loss, paresthesias, and peripheral neuropathy. Other symptoms are typically more specifically associated with an underlying cause for vitamin B12 deficiency, eg, constitutional symptoms from primary bone marrow disease, gastrointestinal upset secondary to enteral malabsorption, or hypoactive metabolism from hypothyroidism.
Folate deficiency has similar clinical features to vitamin B12 deficiency; however, Patients with folate deficiency typically do not have the neuropsychiatric complaints associated with vitamin B12 deficiency. Clinicians should also obtain a clinical history that may help to identify an underlying etiology. Prior surgeries associated with macrocytic anemia, including a gastric bypass for weight loss or ileal resection, are likely causations. Family or personal medical histories may reveal autoimmune diseases, hemolytic anemias, or an inherited cause.
The Imerslund-Grasbect syndrome is an autosomal recessive disorder manifesting a decreased B12 absorption, resulting in megaloblastic anemia.[14] This syndrome is found primarily in patients of Scandinavian descent. Furthermore, certain medical conditions may require medications that increase the risk of developing macrocytic anemia. Additionally, a review of dietary intake can reveal nutritional causes of vitamin B12 or folate deficiencies, eg, excessive alcohol consumption or limited food choices (“tea and toast”).
Physical Examination Findings
Physical exam may reveal nonspecific anemia findings (eg, conjunctival pallor) or neurologic deficits due to vitamin B12 deficiency (eg, impaired proprioception or vibration, positive Romberg sign). Stigmata of underlying diseases may also be noted, including glossitis from autoimmune atrophic gastritis, hepatosplenomegaly from familial hemolytic anemias, hypopigmentation from vitiligo, or jaundice and spider angiomata from alcohol use.
Evaluation
Initial evaluation of macrocytic anemia includes a thorough history and physical followed by focused laboratory studies, eg PBS, reticulocyte count, and serum B12. RBC indices alone will underestimate the presence of macrocytosis by 30% because MCV represents the mean distribution curve of all RBCs and fails to identify smaller macrocytes. If the PBS has normal findings, with no megaloblastic RBCs noted, additional assessments for liver and thyroid diseases (ie, aminotransferases and thyroid stimulating hormone) should be ordered, as these are the most common causes of nonmegaloblastic anemia.
The reticulocyte count should be evaluated if megaloblastic RBCs are demonstrated on PBS. A reticulocyte count <1% indicates underproduction, while a finding of >2% is associated with hyperproliferation due to hemolysis or hemorrhage, which should prompt a hemolytic anemia work-up. Additionally, if findings consistent with hyperproliferation are reported, a vitamin B12 level should be performed.
Vitamin B12 values <100 pg/mL indicate deficiency; an RBC folate level (not serum folate due to lack of sensitivity) should be ordered for a B12 >400 pg/mL. However, low levels may also indicate folate deficiency. Normal B12 levels may indicate the need for bone marrow evaluation. Homocysteine and methylmalonic acid (MMA) should be measured in patients with a B12 level of 100 pg/mL to 400 pg/mL, as these are biochemical compounds important in cell metabolism pathways and use folate and vitamin B12 as cofactors.[15] The measurement of serum B12 alone can miss upwards of 50% of cases.
When checked alone, the shortcomings of B12 elevations cast favor towards checking both the MMA and homocysteine measurements.[15] MMA is only elevated in patients with vitamin B12 deficiency; homocysteine is elevated in patients with folate deficiency. Normal values may warrant hematology consultation for bone marrow studies. Specialist consults are recommended in patients with abnormal myeloid morphology on PBS (disordered immaturity, hypogranulated or hyposegmented neutrophils, or additional cytopenias). Clinicians should remember that the patient may have macrocytic anemia with concurrent iron-deficient anemia. Under these circumstances, the MCV may be normal (ie, automated CBC measurements may "cancel out" the high and low indices, but the RDW would be increased).
Evaluations for specific causes of megaloblastic anemia should be based on presentation. Antibodies to intrinsic factors or parietal cells are indicative of pernicious anemia. The Schilling test, once used to measure vitamin B12 uptake before and after administration of intrinsic factors, has fallen out of favor.[8][16]
Treatment / Management
Macrocytic Anemia Management
Management of macrocytic anemia comprises deficiency correction. Therefore, macrocytic anemia should be treated by replacing folate or vitamin B12 to resolve the underlying cause. Folic acid, 1 mg to 5 mg orally daily, is recommended, and clinicians should encourage patients to consume folate-rich diets (eg, fortified cereals and leafy vegetables). Patients who are taking folate antimetabolites (eg, methotrexate) or pregnant women (especially those with a history of neural tube defects or taking antiepileptics) should take daily supplements to prevent deficiencies. Clinicians should not overlook vitamin B12 deficiency as a cause of macrocytic anemia.
Treatment of folate deficiency without addressing vitamin B12 deficiency, if present, will result in the resolution of the anemia but not the neurologic effects. Clinicians should prescribe 1000 µg of oral vitamin B12 daily for 1 month, followed by 125 to 250 µg daily, or administer 1000 µg of intramuscular B12 every week for 4 weeks, then monthly to replace vitamin B12 stores, the latter of which is preferred for patients with pernicious anemia or altered gastrointestinal anatomy. Clinicians may prescribe empiric folate supplementation (400 µg to 1 mg/d) in patients receiving vitamin B12 replacement. Reticulocytosis will improve within 1 to 2 weeks, and anemia should resolve after 4 to 8 weeks.
Treatment Monitoring
Monitoring RBC indices or rechecking folate or vitamin B12 levels and their metabolites during active treatment is unnecessary, though some clinicians check yearly complete blood counts in patients taking long-term vitamin B12 therapy. Neurologic symptoms from vitamin B12 deficiency take longer. Alcohol-related macrocytosis resolves with abstinence. Other nonmegaloblastic anemias improve when the underlying conditions are treated.
In other instances, macrocytosis indicates medical compliance (eg, methotrexate or zidovudine) and does not need additional management beyond supplements to prevent anemia.[17][18] Assessing MMA and serum B12 levels to judge the patient's compliance and efficacy of therapy is recommended.[19]
Differential Diagnosis
Several differential diagnoses should also be considered when evaluating macrocytic anemia.
Folate deficiency anemia
A subset of this entity is the "functional" or "facultative" folate deficiency.[20][21][22] This occurs in high-demand states such as pregnancy or severe hemolysis, where the body attempts to augment red cell production, increasing the demand for folate and exhausting the folate reserves. Inadequate folate reserves/intake during pregnancy is a leading cause of the development of neural tube defects (NTD) in newborns. For this reason, "fortification" of cereal products with folate has been mandated.
Folate administration can help compensate for the functional deficiency caused by increased erythrocyte production in cases of significant hemolysis, such as sickle cell disease. In infants, folate deficiency decreases the prevalence of NTD, anemia, and serum homocysteine, reducing the risk of developing cardiovascular disease.
Anemia due to liver disease
Anemia is a consistent factor in cirrhosis, with upwards of three-quarters of patients manifesting it.[23][24] Although factors exist to support macrocytosis, they are sometimes overshadowed by iron deficiency, the most common cause of anemia in these patients. For macrocytic anemia in cirrhosis, folate deficiency is prevalent in 40% of patients, while B12 deficiency is prevalent in 30% to 40%.
Alcohol use can lead to B12 and folate deficiency, as well as have a direct toxic effect on the erythroid precursors. B12 and folate coenzymes are required for Thymidylate and purine biosynthesis. A deficiency would cripple DNA synthesis leading to delayed nuclear maturation and resulting in macrocytosis.[24] In alcoholic liver disease, the resultant splenomegaly (from portal hypertension) can have a sequestration and hemolytic effect, thereby leading to macrocytosis.[23] By various pathways, alcohol can cause significant reticulocytosis, leading to an overall increase in the MCV (as reticulocytes are large cells).
With liver disease, an increased deposition of cholesterol is noted on the red cell membranes, leading to an increase in their surface area.[24] Macrocytic anemia is found to be associated with the severity of liver disease, a gauge, as it were, in following hepatic decompensation. However, cirrhosis remains the harbinger of variceal hemorrhage, spontaneous bacterial peritonitis, encephalopathy, and jaundice.[25] The 5-year survival for compensated cirrhosis is about 84%; it is <35% if decompensated.
Hypothyroidism
Hypothyroidism anemia is most commonly normocytic, with macrocytic and microcytic findings less frequent.[26] Its presentation may be somewhat variable if other comorbidities exist, such as bone marrow depression, decreased erythropoietin production, or deficiency of iron, B12, and folate. Hypothyroidism decreases the number and proliferative potential of erythroid cells in the bone marrow.
Additionally, the bone marrow ground substance undergoes a gelatinous transformation, as shown by mucopolysaccharide accumulation. For patients with an increased RDW, in the absence of an iron deficiency, thyroid function tests are advocated, along with the testing of B12 and folate. B12 deficiency can be found with hypothyroidism in the setting of autoimmune disease.[27][26] Thyroid dysfunction should also be considered if treatment-resistant or refractory anemia should occur.
Myelodysplastic syndrome
Macrocytosis (MCV >100 fL) is considered a hallmark of megaloblastic anemia, but other findings and incongruities can obscure the diagnosis and even point towards incorrect ones, like myelodysplasia (MDS).[28][22] Characteristics of MDS that would help avoid a misdiagnosis include the lack of clonality and cytogenetic anomalies, bilineage cytopenias, granulocytic hyposegmentation or hypogranulation, megakaryocytic hypo lobulation, hypogranular platelets, and the presence of increased blasts.
Although the presence of bilineage cytopenias does support a diagnosis of MDS, leukopenia, and thrombocytopenia occur in megaloblastic anemias. The bone marrow in this entity does show megaloblastic precursors with nuclear-cytoplasmic dysynchrony. Indirectly, evidence of hemolysis may be present, eg, an increased LDH or a decreased haptoglobin. Hypercellularity may be evident. Giant metamyelocytes and bands, hyperpigmented neutrophils (felt most specific for megaloblastic anemia), and hyperlobulated megakaryocytes may be present. The proper diagnosis can be cloaked in dysmorphisms,
Alcohol use disorder
The etiology of macrocytosis in alcoholism can be associated with folate deficiency alone but is often multifactorial.[29] The liver stores 50% of the body's folate in deposits, but additional deposits exist in the connective tissue, erythrocytes, kidneys, and the gastrointestinal tract. About 1 in 4 patients with megaloblastic anemia show low serum folate levels and 1 in 10 show low deposits. Megaloblastic anemia with ringed sideroblasts is a well-recognized entity in alcoholism.[30] Macrocytosis in alcoholism is reversible.
Alcohol causes anemia via 2 mechanisms. The first is the development of megaloblastic hematopoiesis by inducing folate deficiency. The second is nonmegaloblastic, macrocytic anemia due to the direct toxic effect of alcohol on the erythroid precursors in the marrow, independent of folate depletion. These bone marrow anomalies are reversible. Folate deficiency in alcoholism is often attributed to dietary deficiency. However, other situations can cause it, eg, cancer (with its increased folate requirement), renal dialysis, increased renal excretion, malabsorption, drugs (eg, antiseizure medications and metformin), and impaired hepatic uptake leading to scanty deposits.[29] Abstinence from alcohol can bring some reversibility to this damage, allowing some macrocytic anemia to be treated without medication.[30]
Prognosis
The prognosis for macrocytic anemia is excellent, provided the underlying cause is identified and treated early. Specialist referral is rarely needed unless the anemia is resistant to therapy or there is evidence of underlying myelodysplasia or leukemia.
Complications
Patients with chronic megaloblastic anemia from vitamin B12 deficiency can develop permanent neurologic damage, referred to as subacute combined neurodegeneration. Patients will have gait ataxia, memory loss, peripheral neuropathy, and psychiatric disturbances. Patients with macrocytic anemia from underlying conditions will experience complications of their respective diseases.
Deterrence and Patient Education
Macrocytic anemia is a special blood disorder in which the body is not able to form enough blood cells because it lacks the necessary nutrients. Some patients do not eat enough foods with these nutrients (folate or vitamin B12), while others cannot absorb it or have an underlying condition that makes it difficult for the body to keep up with its needs. Patients may feel tired, have memory or mood disturbances, or notice tingling in the arms and legs. Physicians can diagnose the anemia during an office visit and from laboratory work. Treatment involves addressing the underlying cause and nutrient supplementation.
Pearls and Other Issues
The prognosis for macrocytic anemia is excellent, with early identification and treatment of the underlying cause. This anemia is the most underestimated of all forms, and physicians should not hesitate to treat it due to its unique adverse effects.
Enhancing Healthcare Team Outcomes
Effective management of macrocytic anemia requires a collaborative, interprofessional approach to enhance patient-centered care, safety, and outcomes. Physicians and advanced practitioners play a critical role in diagnosing and determining the underlying etiology, whether due to vitamin deficiencies, medication side effects, or systemic conditions. They must integrate laboratory findings with clinical presentations to develop individualized treatment plans. Nurses contribute by monitoring patient symptoms, educating patients on dietary modifications, and ensuring adherence to prescribed therapies. Pharmacists provide essential medication counseling, particularly for patients on drugs that may contribute to macrocytic anemia, such as reverse transcriptase inhibitors. They also play a vital role in ensuring proper supplementation, preventing drug interactions, and encouraging regular blood tests to monitor treatment efficacy and safety.
Interprofessional communication and care coordination are essential in optimizing outcomes for patients with macrocytic anemia. Physicians and advanced practitioners must collaborate with dietitians to educate patients on folate- and cobalamin-rich diets, particularly for those at risk due to poor nutrition or alcohol abuse. Social workers can assist in addressing barriers to dietary compliance, particularly for older adults or those with limited access to nutritious foods. Regular follow-ups and clear communication among healthcare professionals ensure early detection of complications, such as anemia-related cardiac stress or irreversible neurological damage. By fostering teamwork and patient education, the healthcare team can improve adherence to treatment plans, enhance patient safety, and ultimately lead to better long-term outcomes.
Media
(Click Image to Enlarge)
References
Lanier JB, Park JJ, Callahan RC. Anemia in Older Adults. American family physician. 2018 Oct 1:98(7):437-442 [PubMed PMID: 30252420]
Válka J, Čermák J. [Differential diagnosis of anemia]. Vnitrni lekarstvi. 2018 Summer:64(5):468-475 [PubMed PMID: 30193515]
Wolffenbuttel BHR, McCaddon A, Ahmadi KR, Green R. A Brief Overview of the Diagnosis and Treatment of Cobalamin (B12) Deficiency. Food and nutrition bulletin. 2024 Jun:45(1_suppl):S40-S49. doi: 10.1177/03795721241229500. Epub [PubMed PMID: 38987879]
Level 3 (low-level) evidenceBaek IW, Park KS, Kim KJ. An erythrocyte macrocytosis by methotrexate is associated with early initiation of biologic or targeted synthetic agents in patients with rheumatoid arthritis. Journal of rheumatic diseases. 2025 Jan 1:32(1):30-37. doi: 10.4078/jrd.2024.0073. Epub 2024 Sep 2 [PubMed PMID: 39712246]
Cakmakli HF, Torres RJ, Menendez A, Yalcin-Cakmakli G, Porter CC, Puig JG, Jinnah HA. Macrocytic anemia in Lesch-Nyhan disease and its variants. Genetics in medicine : official journal of the American College of Medical Genetics. 2019 Feb:21(2):353-360. doi: 10.1038/s41436-018-0053-1. Epub 2018 Jun 6 [PubMed PMID: 29875418]
Sankaran VG, Ulirsch JC, Tchaikovskii V, Ludwig LS, Wakabayashi A, Kadirvel S, Lindsley RC, Bejar R, Shi J, Lovitch SB, Bishop DF, Steensma DP. X-linked macrocytic dyserythropoietic anemia in females with an ALAS2 mutation. The Journal of clinical investigation. 2015 Apr:125(4):1665-9. doi: 10.1172/JCI78619. Epub 2015 Feb 23 [PubMed PMID: 25705881]
Spielmann M, Reichelt G, Hertzberg C, Trimborn M, Mundlos S, Horn D, Klopocki E. Homozygous deletion of chromosome 15q13.3 including CHRNA7 causes severe mental retardation, seizures, muscular hypotonia, and the loss of KLF13 and TRPM1 potentially cause macrocytosis and congenital retinal dysfunction in siblings. European journal of medical genetics. 2011 Jul-Aug:54(4):e441-5. doi: 10.1016/j.ejmg.2011.04.004. Epub 2011 Apr 29 [PubMed PMID: 21596161]
Phillips J, Henderson AC. Hemolytic Anemia: Evaluation and Differential Diagnosis. American family physician. 2018 Sep 15:98(6):354-361 [PubMed PMID: 30215915]
Turner J, Parsi M, Badireddy M. Anemia. StatPearls. 2025 Jan:(): [PubMed PMID: 29763170]
Ezeamama AE, Sikorskii A, Bajwa RK, Tuke R, Kyeyune RB, Fenton JI, Guwatudde D, Fawzi WW. Evolution of Anemia Types During Antiretroviral Therapy-Implications for Treatment Outcomes and Quality of Life Among HIV-Infected Adults. Nutrients. 2019 Mar 31:11(4):. doi: 10.3390/nu11040755. Epub 2019 Mar 31 [PubMed PMID: 30935133]
Level 2 (mid-level) evidenceArshad M, Jaberian S, Pazouki A, Riazi S, Rangraz MA, Mokhber S. Iron deficiency anemia and megaloblastic anemia in obese patients. Romanian journal of internal medicine = Revue roumaine de medecine interne. 2017 Mar 1:55(1):3-7. doi: 10.1515/rjim-2016-0046. Epub [PubMed PMID: 27648630]
Stouten K, Riedl JA, Droogendijk J, Castel R, van Rosmalen J, van Houten RJ, Berendes P, Sonneveld P, Levin MD. Prevalence of potential underlying aetiology of macrocytic anaemia in Dutch general practice. BMC family practice. 2016 Aug 19:17(1):113. doi: 10.1186/s12875-016-0514-z. Epub 2016 Aug 19 [PubMed PMID: 27542607]
Oo TH, Rojas-Hernandez CM. Challenging clinical presentations of pernicious anemia. Discovery medicine. 2017 Sep:24(131):107-115 [PubMed PMID: 28972879]
Gräsbeck R. Imerslund-Gräsbeck syndrome (selective vitamin B(12) malabsorption with proteinuria). Orphanet journal of rare diseases. 2006 May 19:1():17 [PubMed PMID: 16722557]
Level 3 (low-level) evidenceIltar U, Göçer M, Kurtoğlu E. False elevations of vitamin B12 levels due to assay errors in a patient with pernicious anemia. Blood research. 2019 Jun:54(2):149-151. doi: 10.5045/br.2019.54.2.149. Epub 2019 Jun 25 [PubMed PMID: 31309095]
Long B, Koyfman A. Emergency Medicine Evaluation and Management of Anemia. Emergency medicine clinics of North America. 2018 Aug:36(3):609-630. doi: 10.1016/j.emc.2018.04.009. Epub 2018 Jun 12 [PubMed PMID: 30037447]
Nagao T, Hirokawa M. Diagnosis and treatment of macrocytic anemias in adults. Journal of general and family medicine. 2017 Oct:18(5):200-204. doi: 10.1002/jgf2.31. Epub 2017 Apr 13 [PubMed PMID: 29264027]
Green R, Datta Mitra A. Megaloblastic Anemias: Nutritional and Other Causes. The Medical clinics of North America. 2017 Mar:101(2):297-317. doi: 10.1016/j.mcna.2016.09.013. Epub 2016 Dec 14 [PubMed PMID: 28189172]
Khera S, Dhingra S. Co-existing Iron Deficiency and Compliance Issues in Nutritional Macrocytic Anemia in Children. Indian pediatrics. 2023 Feb 15:60(1):152 [PubMed PMID: 36786187]
Ismail S, Eljazzar S, Ganji V. Intended and Unintended Benefits of Folic Acid Fortification-A Narrative Review. Foods (Basel, Switzerland). 2023 Apr 11:12(8):. doi: 10.3390/foods12081612. Epub 2023 Apr 11 [PubMed PMID: 37107407]
Level 3 (low-level) evidenceWilliams BA, McCartney H, Adams E, Devlin AM, Singer J, Vercauteren S, Wu JK, Karakochuk CD. Folic acid supplementation in children with sickle cell disease: study protocol for a double-blind randomized cross-over trial. Trials. 2020 Jun 29:21(1):593. doi: 10.1186/s13063-020-04540-7. Epub 2020 Jun 29 [PubMed PMID: 32600389]
Level 1 (high-level) evidenceSocha DS, DeSouza SI, Flagg A, Sekeres M, Rogers HJ. Severe megaloblastic anemia: Vitamin deficiency and other causes. Cleveland Clinic journal of medicine. 2020 Mar:87(3):153-164. doi: 10.3949/ccjm.87a.19072. Epub [PubMed PMID: 32127439]
Manrai M, Dawra S, Kapoor R, Srivastava S, Singh A. Anemia in cirrhosis: An underestimated entity. World journal of clinical cases. 2022 Jan 21:10(3):777-789. doi: 10.12998/wjcc.v10.i3.777. Epub [PubMed PMID: 35127894]
Level 3 (low-level) evidenceYang J, Yan B, Yang L, Li H, Fan Y, Zhu F, Zheng J, Ma X. Macrocytic anemia is associated with the severity of liver impairment in patients with hepatitis B virus-related decompensated cirrhosis: a retrospective cross-sectional study. BMC gastroenterology. 2018 Nov 1:18(1):161. doi: 10.1186/s12876-018-0893-9. Epub 2018 Nov 1 [PubMed PMID: 30384828]
Level 2 (mid-level) evidenceZhao TY, Cong QW, Liu F, Yao LY, Zhu Y. Nonlinear Relationship Between Macrocytic Anemia and Decompensated Hepatitis B Virus Associated Cirrhosis: A Population-Based Cross-Sectional Study. Frontiers in pharmacology. 2021:12():755625. doi: 10.3389/fphar.2021.755625. Epub 2021 Sep 20 [PubMed PMID: 34616304]
Level 2 (mid-level) evidenceSzczepanek-Parulska E, Hernik A, Ruchała M. Anemia in thyroid diseases. Polish archives of internal medicine. 2017 May 31:127(5):352-360. doi: 10.20452/pamw.3985. Epub 2017 Mar 28 [PubMed PMID: 28400547]
Aktaş HŞ. Vitamin B12 and Vitamin D Levels in Patients with Autoimmune Hypothyroidism and Their Correlation with Anti-Thyroid Peroxidase Antibodies. Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2020:29(4):364-370. doi: 10.1159/000505094. Epub 2019 Nov 29 [PubMed PMID: 31779003]
Diamantopoulos PT, Solomou E, Symeonidis A, Pappa V, Kotsianidis I, Galanopoulos A, Pontikoglou C, Anagnostopoulos A, Vassilopoulos G, Zikos P, Hatzimichael E, Papaioannou M, Megalakaki A, Vassilakopoulos T, Dimou M, Tsokanas D, Papoutselis MK, Papageorgiou S, Kourakli A, Papadaki H, Panayiotidis P, Viniou NA. The prognostic significance of macrocytosis in patients with myelodysplastic neoplasms. American journal of hematology. 2023 May:98(5):E119-E122. doi: 10.1002/ajh.26886. Epub 2023 Mar 1 [PubMed PMID: 36808739]
Sanvisens A, Zuluaga P, Pineda M, Fuster D, Bolao F, Juncà J, Tor J, Muga R. Folate deficiency in patients seeking treatment of alcohol use disorder. Drug and alcohol dependence. 2017 Nov 1:180():417-422. doi: 10.1016/j.drugalcdep.2017.08.039. Epub 2017 Sep 27 [PubMed PMID: 28988003]
Imashuku S, Kudo N, Kaneda S. Spontaneous resolution of macrocytic anemia: old disease revisited. Journal of blood medicine. 2012:3():45-7. doi: 10.2147/JBM.S34304. Epub 2012 Aug 2 [PubMed PMID: 22915983]
Level 3 (low-level) evidence