Introduction
Aplasia cutis congenita (ACC) is a rare congenital skin defect involving focal or extensive absence of the epidermis, dermis, and, occasionally, subcutaneous tissue.[1] The exact etiology remains unclear, with proposed mechanisms including impaired prenatal skin development.[2][3] Initially reported by Cordon in 1767, ACC may present as solitary or multiple lesions on any body region, with 70% to 90% localized to the vertex of the scalp.[4][5] Classification includes 6 subtypes, some associated with congenital dermatologic syndromes. Most lesions heal spontaneously. However, certain locations and clinical features warrant thorough evaluation to detect underlying soft tissue anomalies that may be life-threatening.[6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
ACC lacks a single underlying cause and represents a visible manifestation of abnormal intrauterine skin development. The condition likely arises from diverse causes. Proposed etiologies include genetic factors, placental infarcts, trauma, teratogens such as methimazole, intrauterine infections, and neural tube defects (NTDs).[7] No specific genetic target had been identified until recently. However, new evidence has implicated the BMS1 gene as a possible contributor.[8]
Cutaneous defects noted at birth may initially be mistaken for obstetric trauma, including injuries from forceps or fetal scalp electrodes. One hypothesis suggests that tension-induced disruption of the overlying skin occurs at 10 to 15 weeks’ gestation, during the period of hair direction and pattern formation and rapid brain growth. The frequent proximity of scalp ACC to the hair whorl, believed to be the point of maximum tensile force, supports this theory and may explain the vertex predilection. Early amniotic membrane rupture has also been associated with several cases.
Bullous or membranous ACC demonstrates histologic features resembling encephaloceles and meningoceles, supporting the hypothesis that these forms may represent a forme fruste of neural tube closure defects. ACC can also occur as part of genetic syndromes, including Adams-Oliver, Bart, and Setleis syndromes. Adams-Oliver syndrome presents with scalp ACC, skull defects, cutis marmorata telangiectatica congenita, limb defects, and cardiac anomalies.[9] Bart syndrome features lower extremity ACC associated with epidermolysis bullosa.[10] Setleis syndrome manifests as bilateral temporal ACC with “leonine” facies.[11]
Epidemiology
ACC is a rare congenital condition with an incidence of approximately 1 to 3 per 10,000 births.[12][13] A population-based analysis using 28 registries from the European Surveillance of Congenital Anomalies (EUROCAT) network in 16 countries reported a prevalence of 5.1 per 100,000 from 1998 to 2017.[14] No significant gender or cultural predilection has been documented.[15] Defects are typically recognized at birth, although evaluation may be delayed for several months when lesions are asymptomatic. Sexual predisposition occurs only when ACC is associated with an X-linked malformation syndrome.
Pathophysiology
The most widely accepted pathophysiologic model describes tension that disrupts proper skin approximation during fetal development. Contributing exogenous factors may include ingested teratogens, fetal or placental ischemia, intrauterine infections, and NTDs. Recent investigations have identified dominant-negative mutations in the KCTD1 and KCTD15 genes as causes of ACC through functional impairment of KCTD1/KCTD15 complexes in cranial neural crest cells. This impairment disrupts the formation of normal midline cranial sutures and the overlying skin.[16]
Frieden proposed a classification system for ACC in 1986 that remains in use, defining 9 groups according to lesion number and location, as well as the presence or absence of associated anomalies. The categories are as follows:
- Group 1: Scalp ACC without multiple anomalies
- Group 2: Scalp ACC with limb abnormalities
- Group 3: Scalp ACC with epidermal and organoid nevi
- Group 4: ACC overlying congenital malformations
- Group 5: ACC with associated fetus papyraceus or placental infarct
- Group 6: ACC with epidermolysis bullosa
- Group 7: ACC localized to extremities without blistering
- Group 8: ACC due to specific teratogens
- Group 9: ACC associated with malformation syndromes
The interaction of exogenous insults and genetic mutations explains both the localization and variability of ACC defects. This mechanistic insight underlies the Frieden classification, which remains a valuable guide for evaluating associated anomalies and anticipating potential complications.
Histopathology
Although histopathologic evaluation may be useful in selected cases, the diagnosis of ACC is primarily clinical. Many clinicians avoid lesional biopsy due to the patient’s age and the frequent scalp location. When a biopsy is indicated, an appropriate preprocedure workup, including ultrasound or magnetic resonance imaging (MRI), is essential to exclude underlying malformations that could be injured during the procedure.
Nonhealed lesions typically demonstrate the absence of the epidermis, dermis, or both, with proliferation of blood vessels. Membranous-type ACC presents with a thin translucent covering.[17] Histologic features of membranous ACC include an atrophic, flattened epidermis, replacement of the dermis with loose connective tissue, and absence of adnexal structures. Other forms of ACC differ histologically, although healed lesions usually show scarring, loss of adnexa, and, in some cases, fragmented elastic tissue.[18]
History and Physical
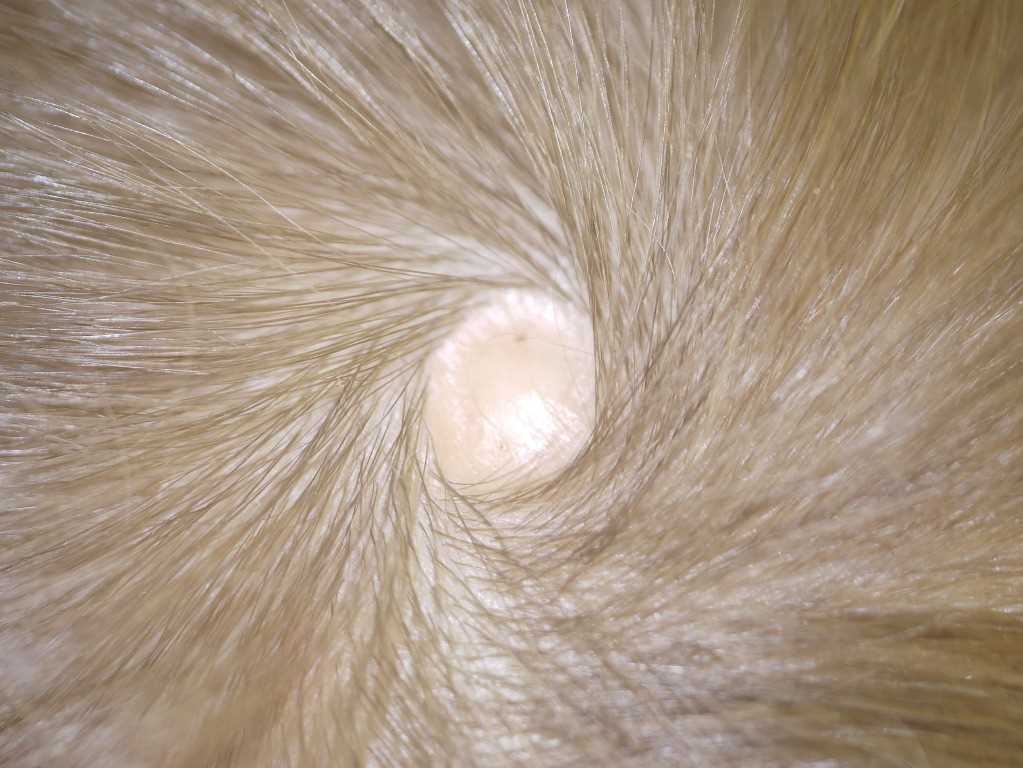
The scalp is the predominant site for ACC, accounting for over 85% of solitary lesions. Most scalp cases, whether membranous or irregular and scar-like, occur at or near the vertex, close to the parietal hair whorl (see Image. Scalp Defect in Aplasia Cutis Congenita). Approximately 25% of affected individuals have multiple lesions, sometimes arranged in a linear pattern.
Defects typically measure 1 to 2 cm in diameter but may range from 0.5 cm to over 10 cm. Clinical presentation at birth varies widely due to the condition’s heterogeneous causes. Lesions may appear as erosions, deep ulcerations, scars, or distinct ovoid defects covered by a membrane. The membranous variant is the most common.
Membranous lesions are usually located on the scalp and are often bordered by a hair collar, which may signify a forme fruste of a neural tube anomaly. These defects may also develop along embryonic fusion lines of the face. At birth, membranous ACC appears as a sharply demarcated (“punched-out”), circular or oval defect covered by a thin, translucent, shiny epithelial membrane. Some lesions contain serous fluid during the neonatal period, producing a bullous appearance. Over time, these bullae flatten and progress to atrophic scars.[19]
Solitary scalp lesions account for approximately 70% of all ACC cases, although multiple lesions involving the face, trunk, or extremities may also occur.[20] Sizes can range from a few millimeters to several centimeters. In cases without underlying defects, lesions gradually contract and ultimately heal with a scar.
A second main category of ACC comprises stellate or angulated lesions, believed to arise from vascular anomalies and intrauterine ischemic incidents. Examples encompass irregular midline scalp deformities in Adams–Oliver syndrome and large, symmetric lesions on the trunk and extremities linked to fetal papyraceus, placental infarction, or other types of vascular insufficiency. These abnormalities typically manifest as ulcers of varying depth, characterized by a raw, hemorrhagic, or granulating base.[21][22][23]
Additional patterns of ACC encompass an absence of skin covering embryological abnormalities, whether apparent or concealed, as well as extensive erosions with irregular margins on the extremities of infants affected with epidermolysis bullosa.[24] The former likely arises from the mechanical trauma of fetal movements in an environment of heightened skin fragility.
The form and distribution of skin defects, along with the presence or absence of accompanying abnormalities, provide significant clues as to the origin of ACC. Uncommon forms vary from an isolated defect on the ventral penis to a severe type characterized by the lack of skin and subcutaneous tissue throughout more than 90% of the body surface area, accompanied by extracutaneous anomalies.
A capillary malformation may occasionally be observed in the skin encircling membranous ACC lesions, and dilated scalp veins may also be apparent, particularly in those with Adams–Oliver syndrome.[25] ACC manifesting in a blaschkoid distribution has also been reported.[26]
An uncommon bullous type of ACC has been documented, presenting as a tense yellow vesicle or cyst on the scalp. Typically, this form is observed after the bullae have been absorbed, resulting in a flat scar. Consequently, several authors associate bullous ACC with membranous ACC.
Evaluation
ACC may be associated with underlying morphologic abnormalities in approximately 37% of cases, according to Mesrati et al.[27] These abnormalities may include underlying bony defects, vascular anomalies, or neurologic malformations. Thus, clinicians must assess disease involvement using imaging techniques.
A scalp lesion at the midline vertex, a hair collar sign, and vascular stains have all been shown to be strong indicators of cranial or central nervous system (CNS) involvement.[28] Small scalp lesions are less likely to be associated with underlying defects and typically heal on their own within a couple of months. Therefore, monitoring these lesions without further imaging is acceptable.
For larger, ulcerative lesions, ultrasound offers a relatively inexpensive evaluation and avoids significant discomfort for the child. Further workup with MRI is warranted if ultrasound findings suggest underlying defects. MRI is more sensitive and specific for detecting associated lesions, according to a 2017 retrospective multicenter study. However, this technology is more costly than ultrasound and typically requires sedating the child, making it a poor choice for initial screening.
A laboratory workup, including a complete blood count, blood cultures, and wound cultures, is advised if the lesion is purulent or surrounded by erythema.[29] Dermoscopy generally reveals an absence of follicular openings, the presence of telangiectatic vessels, and hair follicles arranged radially and horizontally at the periphery, resembling a “starburst” pattern (see Image. Dermoscopy Findings in Membranous Aplasia Cutis Congenita).[30]
Treatment / Management
The approach to managing ACC depends on lesion size and the presence or absence of associated defects. For small lesions (<4 cm) without additional abnormalities, treatment typically involves daily cleansing and the application of topical antibacterial ointment until complete healing occurs.[31] Management primarily aims to restore the skin’s mechanical and immunological integrity, as well as reduce the risk of fluid loss or organ exposure. Small lesions generally heal within weeks to a few months, leaving an atrophic, hairless scar.(B2)
Larger lesions (>4 cm) are more often linked to associated defects and carry a higher risk of complications such as hemorrhage, venous thrombosis, and infection. Early surgical repair is advised to reduce these risks. Before surgery, patients should be evaluated for signs of epidermolysis bullosa. Skin grafts or flap techniques are frequently employed, especially for defects spanning several centimeters.
The use of advanced materials, including cultured epithelial autografts and biodegradable matrices, has expanded treatment possibilities for extensive skin loss.[32] Basic fibroblast growth factor has been applied in conservative therapy to accelerate healing and is proposed as a means to strengthen the epithelium for later reconstruction in large ACC lesions.[33] The successful use of a synthetic dermal substitute to treat full-thickness scalp ACC without grafting has been documented. Amniotic membrane dressings have also been reported to improve cosmetic results, minimize hypertrophic scarring, and promote reepithelialization.[34] Innovative techniques, such as tissue expansion, have been successfully implemented to close residual defects and enhance aesthetic outcomes in patients with ACC.[35]
Differential Diagnosis
ACC is often easily identified with a thorough clinical examination. However, conditions with similar presentations at birth must also be considered and excluded.
NTDs, such as meningoceles, are important differentials. A meningocele involves protrusion of the neural sac from the CNS and often appears as a midline skin- or membrane-covered defect, commonly on the scalp. Imaging is essential to confirm the CNS connection required for diagnosis.[36]
Membranous-type ACC lesions can resemble congenital dermoid cysts, although ACC typically has a more translucent surface. Birth trauma from vacuum extraction, forceps delivery, or fetal scalp monitor electrodes may also produce erosions mimicking ACC. Some lesions heal in utero, leaving atrophic hairless scars that may be mistaken for epidermal nevi.[37]
Erosions from epidermolysis bullosa should be differentiated from ACC, particularly because ACC may be detected before additional epidermolysis bullosa lesions develop. Other infectious causes include neonatal herpes, which may present as scalp erosions but rarely occurs at delivery, and fetal varicella infection, which has been linked to similar lesions.
Transient bullous dermolysis of the newborn is a rare, benign blistering disorder present at or shortly after birth. The condition resolves within the first months of life without leaving dystrophic scars.
Prognosis
Although isolated ACC without an associated defect can have a relatively benign course, complications significantly increase the risk of mortality. The average recovery duration with conservative treatment is 27.9 days.[38] The estimated mortality rate ranges from 20% to 55% due to serious complications.[39] Full-thickness defects of the scalp, skull, and dura are associated with a mortality rate exceeding 50%.[40]
Complications
Scalp ACC involves the underlying skull in 10% to 30% of cases, with a higher risk associated with midline vertex location, hair collar sign, and accompanying capillary stain. Extensive or irregular lesions are more likely to infiltrate deeper structures, potentially affecting the dura mater and leptomeninges.[41]
Deep scalp ACC can result in life-threatening sagittal sinus hemorrhage or thrombosis. Since prolonged healing increases the likelihood of complications, early surgical repair is recommended for large stellate scalp lesions or those with a dural defect or exposed sagittal sinus.
Patients face an increased risk of cutaneous infection due to loss or impairment of the skin’s barrier against environmental microbes. Severe infection can progress to meningitis if untreated.[42] Prompt surgical management of large scalp lesions can help prevent these complications.
Deterrence and Patient Education
Preventive care includes early dermatologic evaluation, infection surveillance, and timely surgical intervention when indicated. Parents should be offered genetic counseling if a strong family history of ACC is elicited or their child presents with ACC and a malformation syndrome.
Pearls and Other Issues
ACC is a heterogeneous group of disorders characterized by the congenital absence of skin in localized or extensive areas at birth. Most minor lesions resolve within the first few months of life, leaving an atrophic scar or, less commonly, a hypertrophic scar. In such cases, daily cleansing and application of petrolatum or a topical antibiotic ointment until complete healing is generally sufficient.
Underlying skull defects often close spontaneously during infancy. Preoperative imaging is necessary to map vascular structures before any surgical procedure. Without surgery, residual scarred or hairless patches often become less conspicuous as the child grows. For cosmetic improvement, excision or hair transplantation may be considered later in life.
The evaluation of ACC begins with a detailed personal, obstetric, and family history, followed by a comprehensive physical examination. Special attention should be given to identifying associated anomalies or malformation syndromes, particularly limb and digit deformities. In the neonatal period, additional tests may include placental examination and viral testing for herpes simplex virus and varicella-zoster virus.
Imaging is indicated for extensive, deep, irregular, or membranous scalp lesions, especially when located at the midline vertex or surrounded by a hair collar, to assess for osseous defects, vascular anomalies, or cerebral malformations. Elevated α-fetoprotein in midtrimester maternal serum and amniotic fluid, along with increased acetylcholinesterase in amniotic fluid, may suggest ACC prenatally. However, these findings lack both sensitivity and specificity.[43]
Enhancing Healthcare Team Outcomes
The treatment of ACC is generally straightforward unless underlying abnormalities are present. Complications can occur, requiring an interprofessional team to diagnose and treat affected newborns. Complex ACC cases have increased morbidity and mortality, highlighting the need for excellent communication between providers. Determining whether imaging is warranted can be challenging when a newborn presents with a lesion suggestive of ACC. Although no formal imaging guidelines exist, studies indicate that the following findings are more often associated with underlying abnormalities that merit appropriate imaging:
- Lesion size greater than 4 cm
- Presence of hair tufting
- Neurologic deficits
- Hemorrhagic lesion
- Membranous-type ACC
If ultrasound or MRI reveals an underlying abnormality, surgical intervention is usually required to prevent complications, such as superior sagittal sinus hemorrhage, meningitis, and thrombosis. In isolated cases without underlying defects, the prognosis is favorable with simple wound care and close monitoring by pediatricians and dermatologists. Although complications are uncommon, a thorough understanding of ACC is essential to ensure prompt and appropriate evaluation and consultation.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
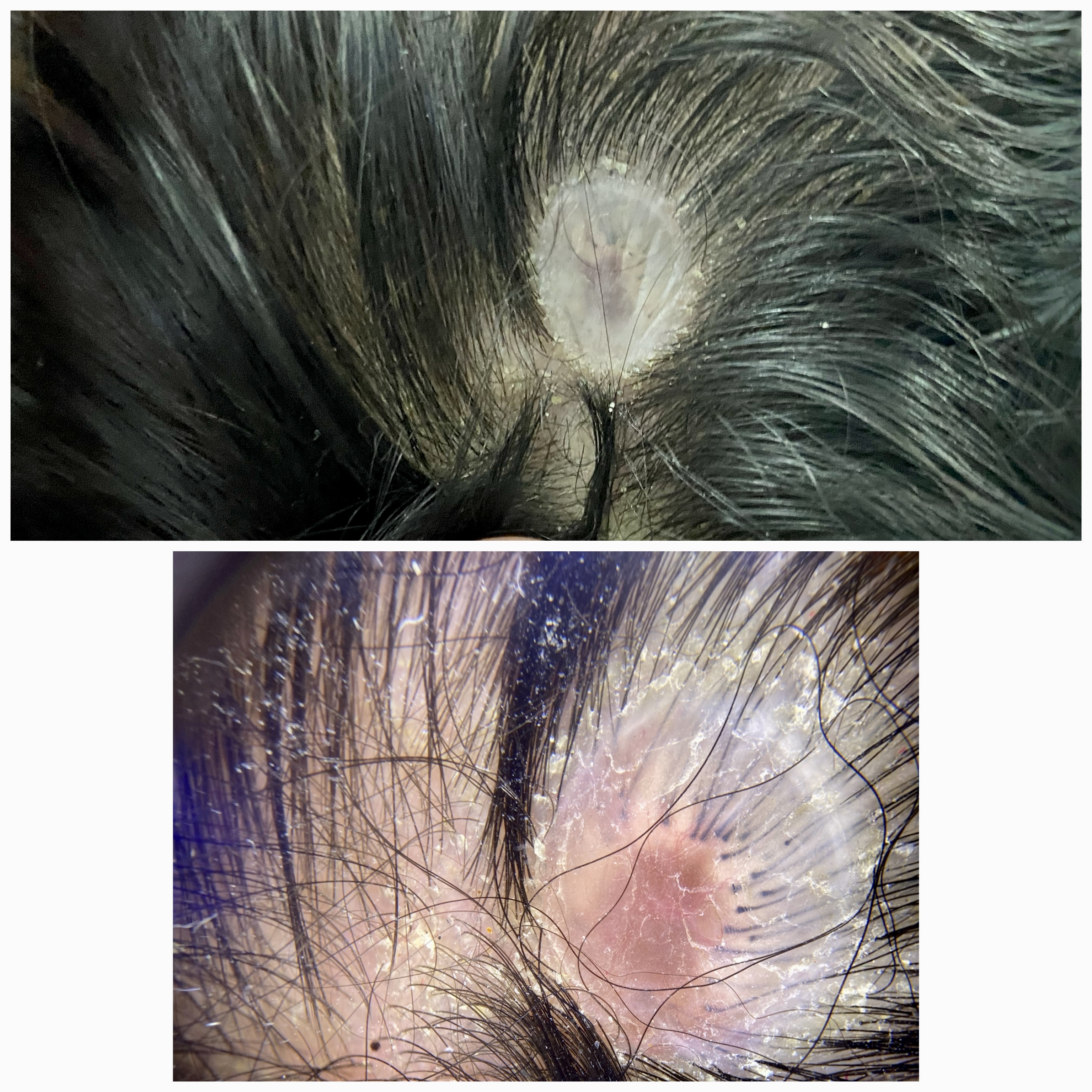
Dermoscopy Findings in Membranous Aplasia Cutis Congenita. The top image shows a well-demarcated, translucent scalp lesion covered by a thin, shiny epithelial membrane—classic for the membranous type of this condition. The bottom image demonstrates the absence of skin appendages and marked thinning of the epidermis within the lesion, consistent with the diagnosis.
Contributed by Haitham M. Saleh, MSc
References
Blionas A, Giakoumettis D, Antoniades E, Drosos E, Mitsios A, Plakas S, Sfakianos G, Themistocleous MS. Aplasia cutis congenita: Two case reports and discussion of the literature. Surgical neurology international. 2017:8():273. doi: 10.4103/sni.sni_188_17. Epub 2017 Nov 9 [PubMed PMID: 29204308]
Level 3 (low-level) evidenceBelkhou A, François C, Bennis Y, Duquennoy-Martinot V, Guerreschi P. [Aplasia cutis congenita: Update and management]. Annales de chirurgie plastique et esthetique. 2016 Oct:61(5):450-461. doi: 10.1016/j.anplas.2016.07.003. Epub 2016 Aug 5 [PubMed PMID: 27503278]
Magliah T, Alghamdi F. Aplasia Cutis Congenita: A Case Report. Case reports in dermatology. 2018 May-Aug:10(2):182-186. doi: 10.1159/000490786. Epub 2018 Jul 5 [PubMed PMID: 30057534]
Level 3 (low-level) evidenceFrieden IJ. Aplasia cutis congenita: a clinical review and proposal for classification. Journal of the American Academy of Dermatology. 1986 Apr:14(4):646-60 [PubMed PMID: 3514708]
Lonie S, Phua Y, Burge J. Technique for Management of Aplasia Cutis Congenita of the Scalp With a Skin Allograft. The Journal of craniofacial surgery. 2016 Jun:27(4):1049-50. doi: 10.1097/SCS.0000000000002610. Epub [PubMed PMID: 27171959]
Humphrey SR, Hu X, Adamson K, Schaus A, Jensen JN, Drolet B. A practical approach to the evaluation and treatment of an infant with aplasia cutis congenita. Journal of perinatology : official journal of the California Perinatal Association. 2018 Feb:38(2):110-117. doi: 10.1038/jp.2017.142. Epub 2017 Oct 19 [PubMed PMID: 29048413]
Sachs C, Tebacher-Alt M, Mark M, Cribier B, Lipsker D. [Aplasia cutis congenita and antithyroid drugs during pregnancy: Case series and literature review]. Annales de dermatologie et de venereologie. 2016 Jun-Jul:143(6-7):423-35. doi: 10.1016/j.annder.2016.02.018. Epub 2016 Mar 28 [PubMed PMID: 27033749]
Level 2 (mid-level) evidenceMarneros AG. BMS1 is mutated in aplasia cutis congenita. PLoS genetics. 2013 Jun:9(6):e1003573. doi: 10.1371/journal.pgen.1003573. Epub 2013 Jun 13 [PubMed PMID: 23785305]
Saeidi M, Ehsanipoor F. A Case of Adams-Oliver Syndrome. Advanced biomedical research. 2017:6():167. doi: 10.4103/2277-9175.221861. Epub 2017 Dec 28 [PubMed PMID: 29387678]
Level 3 (low-level) evidenceAlfayez Y, Alsharif S, Santli A. A Case of Aplasia Cutis Congenita Type VI: Bart Syndrome. Case reports in dermatology. 2017 May-Aug:9(2):112-118. doi: 10.1159/000478889. Epub 2017 Aug 3 [PubMed PMID: 29033814]
Level 3 (low-level) evidenceGraul-Neumann LM, Stieler KM, Blume-Peytavi U, Tzschach A. Autosomal dominant inheritance in a large family with focal facial dermal dysplasia (Brauer-Setleis syndrome). American journal of medical genetics. Part A. 2009 Feb 15:149A(4):746-50. doi: 10.1002/ajmg.a.32728. Epub [PubMed PMID: 19291768]
Level 3 (low-level) evidenceE Rogvi R, Sommerlund M, Vestergaard ET. [Aplasia cutis congenita is a rare and possibly overlooked congenital anomaly]. Ugeskrift for laeger. 2014 Nov 24:176(48):. pii: V05140276. Epub [PubMed PMID: 25430571]
O'Neill JK, Carter M, Warr RP. Aplasia cutis congenita. A case of scalp defect repair using two opposing bipedicled local flaps. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2010 Mar:63(3):e242-4. doi: 10.1016/j.bjps.2009.06.005. Epub 2009 Jul 4 [PubMed PMID: 19577972]
Level 3 (low-level) evidenceCoi A, Barisic I, Garne E, Pierini A, Addor MC, Aizpurua Atxega A, Ballardini E, Braz P, Broughan JM, Cavero-Carbonell C, de Walle HEK, Draper ES, Gatt M, Häusler M, Kinsner-Ovaskainen A, Kurinczuk JJ, Lelong N, Luyt K, Mezzasalma L, Mullaney C, Nelen V, Odak L, O'Mahony MT, Perthus I, Randrianaivo H, Rankin J, Rissmann A, Rouget F, Schaub B, Tucker D, Wellesley D, Wiśniewska K, Yevtushok L, Santoro M. Epidemiology of aplasia cutis congenita: A population-based study in Europe. Journal of the European Academy of Dermatology and Venereology : JEADV. 2023 Mar:37(3):581-589. doi: 10.1111/jdv.18690. Epub 2022 Nov 11 [PubMed PMID: 36300660]
Martinez-Regueira S, Vazquez-Lopez ME, Somoza-Rubio C, Morales-Redondo R, Gonzalez-Gay MA. Aplasia cutis congenita in a defined population from northwest Spain. Pediatric dermatology. 2006 Nov-Dec:23(6):528-32 [PubMed PMID: 17155992]
Level 3 (low-level) evidenceMarneros AG. Aplasia Cutis Congenita Pathomechanisms Reveal Key Regulators of Skin and Skin Appendage Morphogenesis. The Journal of investigative dermatology. 2024 Nov:144(11):2399-2405. doi: 10.1016/j.jid.2024.05.014. Epub 2024 Jul 17 [PubMed PMID: 39023472]
Bessis D, Bigorre M, Malissen N, Captier G, Chiaverini C, Abasq C, Barbarot S, Boccara O, Bourrat E, El Fertit H, Eschard C, Hubiche T, Lacour JP, Leboucq N, Mahé E, Mallet S, Marque M, Martin L, Mazereeuw-Hautier J, Milla N, Phan A, Plantin P, Picot MC, Puzenat E, Rigau V, Vabres P, Fraitag S, Boralevi F, Groupe de Recherche Clinique en Dermatologie Pédiatrique. The scalp hair collar and tuft signs: A retrospective multicenter study of 78 patients with a systematic review of the literature. Journal of the American Academy of Dermatology. 2017 Mar:76(3):478-487. doi: 10.1016/j.jaad.2016.08.046. Epub 2016 Oct 11 [PubMed PMID: 27742172]
Level 2 (mid-level) evidenceBassi A, Greco A, de Martino M. Aplasia cutis with 'hair collar sign'. Archives of disease in childhood. 2014 Nov:99(11):1003. doi: 10.1136/archdischild-2014-306663. Epub 2014 Sep 8 [PubMed PMID: 25202133]
Level 3 (low-level) evidenceDrolet BA, Baselga E, Gosain AK, Levy ML, Esterly NB. Preauricular skin defects. A consequence of a persistent ectodermal groove. Archives of dermatology. 1997 Dec:133(12):1551-4 [PubMed PMID: 9420540]
Brzezinski P, Pinteala T, Chiriac AE, Foia L, Chiriac A. Aplasia cutis congenita of the scalp--what are the steps to be followed? Case report and review of the literature. Anais brasileiros de dermatologia. 2015 Jan-Feb:90(1):100-3. doi: 10.1590/abd1806-4841.20153078. Epub [PubMed PMID: 25672305]
Level 3 (low-level) evidenceMempel M, Abeck D, Lange I, Strom K, Caliebe A, Beham A, Kautza M, Worret WI, Neubauer BA, Ring J, Schröder H, Fölster-Holst R. The wide spectrum of clinical expression in Adams-Oliver syndrome: a report of two cases. The British journal of dermatology. 1999 Jun:140(6):1157-60 [PubMed PMID: 10354089]
Level 3 (low-level) evidenceShaheen R, Faqeih E, Sunker A, Morsy H, Al-Sheddi T, Shamseldin HE, Adly N, Hashem M, Alkuraya FS. Recessive mutations in DOCK6, encoding the guanidine nucleotide exchange factor DOCK6, lead to abnormal actin cytoskeleton organization and Adams-Oliver syndrome. American journal of human genetics. 2011 Aug 12:89(2):328-33. doi: 10.1016/j.ajhg.2011.07.009. Epub 2011 Aug 4 [PubMed PMID: 21820096]
Lane W, Zanol K. Duodenal atresia, biliary atresia, and intestinal infarct in truncal aplasia cutis congenita. Pediatric dermatology. 2000 Jul-Aug:17(4):290-2 [PubMed PMID: 10990578]
Mariath LM, Santin JT, Frantz JA, Doriqui MJR, Schuler-Faccini L, Kiszewski AE. Genotype-phenotype correlations on epidermolysis bullosa with congenital absence of skin: A comprehensive review. Clinical genetics. 2021 Jan:99(1):29-41. doi: 10.1111/cge.13792. Epub 2020 Jun 29 [PubMed PMID: 32506467]
Baselga E, Torrelo A, Drolet BA, Zambrano A, Alomar A, Esterly NB. Familial nonmembranous aplasia cutis of the scalp. Pediatric dermatology. 2005 May-Jun:22(3):213-7 [PubMed PMID: 15916567]
Vinay K, Yadav S, Parsad D, Abhijit C. Aplasia cutis congenita in a Blaschkoid distribution: a lesser known variant. International journal of dermatology. 2016 Apr:55(4):e217-9. doi: 10.1111/ijd.12877. Epub 2016 Jan 11 [PubMed PMID: 26755449]
Mesrati H, Amouri M, Chaaben H, Masmoudi A, Boudaya S, Turki H. Aplasia cutis congenita: report of 22 cases. International journal of dermatology. 2015 Dec:54(12):1370-5. doi: 10.1111/ijd.12707. Epub 2015 May 27 [PubMed PMID: 26016611]
Level 3 (low-level) evidencePatel DP, Castelo-Soccio L, Yan AC. Aplasia cutis congenita: Evaluation of signs suggesting extracutaneous involvement. Pediatric dermatology. 2018 Jan:35(1):e59-e61. doi: 10.1111/pde.13340. Epub 2017 Nov 27 [PubMed PMID: 29178194]
Harvey G, Solanki NS, Anderson PJ, Carney B, Snell BJ. Management of aplasia cutis congenita of the scalp. The Journal of craniofacial surgery. 2012 Nov:23(6):1662-4. doi: 10.1097/SCS.0b013e31826542de. Epub [PubMed PMID: 23147310]
Level 2 (mid-level) evidenceVerzì AE, Lacarrubba F, Micali G. Starburst hair follicles: A dermoscopic clue for aplasia cutis congenita. Journal of the American Academy of Dermatology. 2016 Oct:75(4):e141-e142. doi: 10.1016/j.jaad.2016.02.1216. Epub [PubMed PMID: 27646756]
Betancourth-Alvarenga JE, Vázquez-Rueda F, Vargas-Cruz V, Paredes-Esteban RM, Ayala-Montoro J. [Surgical management of aplasia cutis congenita]. Anales de pediatria (Barcelona, Spain : 2003). 2015 Nov:83(5):341-5. doi: 10.1016/j.anpedi.2015.02.005. Epub 2015 Mar 21 [PubMed PMID: 25804551]
Level 2 (mid-level) evidencePollock M, Leung R, Low NCK. A Novel Approach to Aplasia Cutis Congenita With PolyNovo BTM. The Journal of craniofacial surgery. 2025 May 1:36(3):e296-e297. doi: 10.1097/SCS.0000000000010918. Epub 2024 Dec 4 [PubMed PMID: 39630956]
Orgun D, Horiguchi M, Hayashi A, Shimoji K, Arai H, Mizuno H. Conservative Treatment of Large Aplasia Cutis Congenita of the Scalp With Bone Defect With Basic Fibroblast Growth Factor Application. The Journal of craniofacial surgery. 2017 Mar:28(2):e154-e158. doi: 10.1097/SCS.0000000000003347. Epub [PubMed PMID: 28045831]
Kadivar M, Sangsari R, Rostamli S, Sotoudeh S, Mirnia K. Amniotic membrane dressings for treatment of aplasia cutis in newborns. Pediatric dermatology. 2024 May-Jun:41(3):445-450. doi: 10.1111/pde.15540. Epub 2024 Feb 26 [PubMed PMID: 38409959]
Komuro Y, Yanai A, Seno H, Ichida M, Inoue M, Miyajima M, Arai H, Sato K. Surgical treatment of aplasia cutis congenita of the scalp associated with bilateral coronal synostosis. The Journal of craniofacial surgery. 2002 Jul:13(4):513-9 [PubMed PMID: 12140414]
Gao Z, Massimi L, Rogerio S, Raybaud C, Di Rocco C. Vertex cephaloceles: a review. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2014 Jan:30(1):65-72. doi: 10.1007/s00381-013-2249-7. Epub 2013 Aug 29 [PubMed PMID: 23989428]
Level 3 (low-level) evidenceAlShehri W, AlFadil S, AlOthri A, Alabdulkarim AO, Wani SA, Rabah SM. Aplasia Cutis Congenita of the Scalp with a Familial Pattern: A Case Report. World journal of plastic surgery. 2016 Sep:5(3):298-302 [PubMed PMID: 27853695]
Level 3 (low-level) evidenceTempark T, Shwayder TA. Aplasia cutis congenita with fetus papyraceus: report and review of the literature. International journal of dermatology. 2012 Dec:51(12):1419-26. doi: 10.1111/j.1365-4632.2012.05545.x. Epub [PubMed PMID: 23171007]
Ribuffo D, Costantini M, Gullo P, Houseman ND, Taylor GI. Aplasia cutis congenita of the scalp, the skull, and the dura. Scandinavian journal of plastic and reconstructive surgery and hand surgery. 2003:37(3):176-80 [PubMed PMID: 12841620]
Level 3 (low-level) evidenceJohnson R, Offiah A, Cohen MC. Fatal superior sagittal sinus hemorrhage as a complication of aplasia cutis congenita: a case report and literature review. Forensic science, medicine, and pathology. 2015 Jun:11(2):243-8. doi: 10.1007/s12024-014-9645-5. Epub 2015 Jan 23 [PubMed PMID: 25614301]
Level 3 (low-level) evidenceKuemmet TJ, Miller JJ, Michalik D, Lew SM, Maheshwari M, Humphrey SR. Low risk of clinically important central nervous system dysraphism in a cohort study of 69 patients with isolated aplasia cutis congenita of the head. Pediatric dermatology. 2020 May:37(3):455-460. doi: 10.1111/pde.14117. Epub 2020 Feb 13 [PubMed PMID: 32053222]
Suara RO, Dermody TS. Enterococcal meningitis in an infant complicating congenital cutis aplasia. The Pediatric infectious disease journal. 2000 Jul:19(7):668-9 [PubMed PMID: 10917233]
Level 3 (low-level) evidenceEvers ME, Steijlen PM, Hamel BC. Aplasia cutis congenita and associated disorders: an update. Clinical genetics. 1995 Jun:47(6):295-301 [PubMed PMID: 7554362]