Introduction
Human babesiosis is an emerging infectious disease caused by the tick-borne, eukaryotic, intraerythrocytic, parasitic protozoan Babesia.[1][2][3][4] The Babesia parasite can be transmitted to humans worldwide through the bites of infected ticks, most commonly the Ixodes scapularis tick, as well as by blood transfusions, organ transplantation, and transplacental transmission.[5] Out of the 100 species of Babesia that can infect wild and domestic animals, only a few infect humans, with Babesia microti being the predominant species worldwide.[4][6] Babesia primarily infects erythrocytes, causing a disease that can be either asymptomatic or symptomatic. Acute asymptomatic babesiosis is the most common presentation of the disease.[7] When it is symptomatic, it manifests as a viral-like, malaria-like syndrome, typically characterized by fever and laboratory abnormalities, including non-immune hemolytic anemia and frequently thrombocytopenia.[4][7] The clinical severity of babesiosis depends on the Babesia species present and the patient's immune competence.[4] Babesiosis can result in high morbidity and mortality, even progressing to multi-organ failure, especially in patients with weakened immune systems, eg, in older and very young patients and those who are immunocompromised. Asplenic patients are at a high risk for severe babesiosis, regardless of the Babesia species with which they are infected.[4]
Clinicians should have a high index of suspicion when evaluating patients with clinical symptoms and laboratory findings compatible with babesiosis, such as a febrile illness after travelling or residing in an endemic area within the last 2 months, or someone who has received a blood transfusion within the last 6 months.[4][8] Babesia may not be considered in the differential diagnosis, which can subsequently delay diagnosis and treatment. Additional barriers to the diagnosis of babesiosis include that when evaluating a peripheral blood smear for intraerythrocytic Babesia, the parasitic burden may be too low to be detected, making the diagnosis more elusive. A lack of diagnosis of babesiosis can not only lead to delays in appropriate treatment, causing an increase in morbidity and mortality, but also may, due to the prolonged, undiagnosed babesiosis, cause transmission to others through blood transfusions. It is therefore essential to enhance clinicians' knowledge of when to consider babesiosis as a diagnosis and to understand how to effectively evaluate and treat this condition promptly, leading to improved patient outcomes.[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Human babesiosis is an emerging infectious disease caused by the tick-borne, eukaryotic, intraerythrocytic, parasitic protozoan Babesia.[1][2][3][4] Babesia spp belong to the Order Piroplasmida, in the Phylum Apicomplexa. Plasmodia spp, eg, Plasmodium falciparum, also belong to the Phylum Apicomplexa, and Babesia spp and Plasmodia spp share microbiological and pathogenic characteristics.[7] More than 100 species of Babesia can infect wild and domestic animals; however, only a few species infect humans, and Babesia microti is the predominant species involved in human infections worldwide.[4] These include B microti, B divergens, B duncani, Babesia-like agent, B microti-like agent, and B venatorum.[7] Babesia microti is endemic in the Northeastern and upper Midwestern United States and has been occasionally reported from other areas of the world.[4][7] B divergens was the most prevalent species in Europe, but more recently, B microti and B venatorum have increased in numbers.[7]
Babesia microti is transmitted by the I scapularis tick, which carries the protozoa.[9] Babesia spp can also be acquired through blood transfusions, organ transplantation, and transplacentally.[10][11][12] The exact pathogenesis of infection following the transmission of Babesia spp has not been fully elucidated, as pathogenesis studies encompass numerous pathogenic mechanisms and host immunologic and pathophysiologic responses that don't allow for firm conclusions.[7] The host immune response is a crucial factor that can significantly influence the severity of babesiosis. Immune dysregulation and conditions such as asplenia can result in more severe infections.[7]
Epidemiology
More than 100 species of Babesia can infect wild and domestic animals; however, only a few species infect humans, and B microti is the predominant species involved in human infections worldwide.[4] These include B microti, B divergens, B duncani, Babesia-like agent, B microti-like agent, and B venatorum.[7] Babesia microti is endemic in the Northeastern and upper Midwestern United States, and has been occasionally reported from other areas of the world.[4][7] In Europe, B divergens, B microti, and B venatorum are the most prevalent species[13]. In Asia, the most prevalent species are B venatorum, B microti, and the Babesia crassa-like pathogen.[7][13] Babesia spp can also be acquired through blood transfusions, organ transplantation, and transplacentally. In the United States, B microti is one of the leading pathogens transmitted through blood transfusions, with an estimated mortality rate of around 20% in affected recipients. This level of fatality is similar to that observed in individuals with significant immunosuppression who contract the infection via tick bites. [9][10][11][14][15][16]
Babesiosis in the United States is most common in the Northeastern states and the Upper Midwest, where B microti is the leading cause of infection. The disease is primarily transmitted by the I scapularis tick, and its natural reservoir is small animals, such as the white-footed mouse (Peromyscus leucopus).[9][10][11][14][15][16] The Ixodes scapularis tick progresses through three life stages: the larval, nymph, and adult stages. It requires a blood meal from a vertebrate host at each stage to develop to the next. [4][13] The life cycle of B microti relies on the interaction between these ticks and their small mammal reservoirs. Although larger mammals, such as deer, provide blood meals that support adult ticks, they do not act as reservoirs for the parasite; humans are incidental, dead-end hosts. Even though adult ticks can transmit B microti, most cases result from exposure to nymphal ticks, with the majority of cases occurring from late spring to fall, when people are more likely to encounter ticks and reservoir species.[4][13]
B microti completes distinct stages of development within both the tick vector and the mammalian reservoir host. During late summer, when larval ticks feed on infected mice, the parasite’s gametocytes accumulate in the tick’s gut and differentiate into gametes.[4] Fusion of gametes produces zygotes that traverse the gut epithelium into the hemolymph, where they mature into motile ookinetes. These ookinetes then migrate to the salivary glands and transform into dormant sporoblasts. Upon the tick’s progression to the nymph stage, feeding in the early summer of the following year triggers the activation of these sporoblasts, leading to the production of thousands of sporozoites, which are injected into the vertebrate host. Sporozoites bind to erythrocytes via interactions with glycosaminoglycans and sialoglycoproteins on the cell surface. Inside the erythrocytes, sporozoites mature into trophozoites that replicate by budding to produce four merozoites. The rupture of the host erythrocyte releases the merozoites, facilitating further infection of additional red blood cells.[4][13]
Symptoms typically develop 1 to 4 weeks after a tick bite from a B microti-infected tick, or 1 to 9 weeks after receiving contaminated blood products. However, in rare cases, onset may be delayed by up to 6 months.[4][17] The illness often begins gradually with a flu-like syndrome characterized by malaise and fatigue, followed by high fever. Common accompanying symptoms include chills, sweats, headache, myalgia, anorexia, nonproductive cough, arthralgia, and nausea.[4][17]
The incidence of infection in these areas has been increasing, and 2000 or more cases are being reported annually to the United States Centers for Disease Control and Prevention (CDC). These cases are likely an underestimation of the true incidence, as most are undiagnosed and underreported. Babesiosis has emerged as a public health threat, similar to Lyme disease, which is transmitted through the same tick.[4][13] The geographic spread of babesiosis parallels that of Lyme disease, as well as other Ixodes scapularis-transmitted diseases, such as Anaplasma phagocytophilum, the agent of human granulocytic anaplasmosis, and Ehrlichia chaffeensis, the agent of ehrlichiosis.[18][19] The expansion of babesiosis has occurred at a slower pace than Lyme disease; however, several factors contribute to the lower reported incidence of babesiosis compared to Lyme disease.[19] These include its more limited geographic distribution, a lower prevalence of Babesia infection in ticks, a higher rate of asymptomatic infections, limited clinician awareness, and the diagnostic challenges associated with identifying the disease.[20] The rise of babesiosis in these areas is largely due to the increasing populations of white-tailed deer and I scapularis, increasing human encroachment into wildlife habitats, increasing exposure of humans to ticks, and heightened awareness of the disease among both the public and healthcare providers.[4][13]
Pathophysiology
The nymphal stage of Ixodes ticks is the primary vector responsible for transmitting Babesia species to humans. Successful transmission generally requires the tick to remain attached for 36 to 72 hours to complete a full blood meal.[4] If the tick is infected with Babesia, sporozoites are most commonly transmitted during the latter part of this feeding period, typically on the second or third day of attachment. Once introduced into the human host, sporozoites invade erythrocytes, where they undergo maturation and asexual replication through binary fission, producing merozoites. These merozoites then rupture the host erythrocyte upon exit, subsequently invading additional erythrocytes and perpetuating the parasitic life cycle.[4][13][20]
The host's splenic function and immune status play a crucial role in containing and managing infection with Babesia. As infected erythrocytes circulate, they are identified as abnormal by the spleen, and macrophages facilitate their clearance. Asplenic individuals, therefore, are at significantly increased risk of severe babesiosis, often presenting with high parasitemia. Other populations at heightened risk include those with HIV, patients receiving immunosuppressive therapies such as tumor necrosis factor (TNF) inhibitors or anti-CD20 monoclonal antibodies, neonates, and adults over 50 years of age.[4][13][20]
Upon examination of a peripheral blood smear in patients with babesiosis, erythrocytes containing the parasite may be characteristically oval or pear-shaped, and the intraerythrocytic Babesia ring forms can resemble those of Plasmodium falciparum. Despite this morphological similarity, babesiosis typically does not lead to the degree of hemolysis seen in falciparum malaria. Instead, the infection increases erythrocyte aggregation and membrane rigidity, leading to complications such as acute respiratory distress syndrome (ARDS) and noncardiogenic pulmonary edema.[4][13][20]
Additionally, erythrocyte fragmentation can lead to microvascular occlusion in various organs, with the potential for multi-organ failure. Throughout this process, the spleen often enlarges as it filters damaged erythrocytes. If patients are asplenic, they are more susceptible to a fulminant course with increased morbidity and mortality.[4][13][20]
Severe babesiosis necessitates hospitalization and may be associated with a range of serious complications. These can include profound anemia, acute respiratory distress syndrome (ARDS), disseminated intravascular coagulation (DIC), congestive heart failure, renal and hepatic dysfunction or failure, and septic shock. Additional complications may involve splenic infarction or rupture, the development of warm autoimmune hemolytic anemia, and, in some cases, a fatal outcome. Prompt recognition and management are essential to mitigate these risks and improve patient outcomes.[4][13][20]
History and Physical
A comprehensive history and physical examination should be conducted in all patients seeking medical care. Babesiosis should always be included in the list of differential diagnoses in areas where Babesia are endemic, especially when patients present with a malaria-like illness from spring to autumn, or when the weather is warmer. Babesiosis can present with a wide range of clinical signs and symptoms, and it can be entirely asymptomatic, symptomatic, or even lead to severe, fulminant illness that can be fatal. Asymptomatic babesiosis has been documented in approximately 50% of children and 20% of adults who are infected with the disease. Symptoms typically develop 1 to 4 weeks after a tick bite from a Babesia microti-infected tick, or 1 to 9 weeks after receiving contaminated blood products. However, in rare cases, onset may be delayed by up to 6 months.[4][17]
The illness often begins gradually with a flu-like syndrome characterized by malaise and fatigue, followed by high fever. Common accompanying symptoms include chills, sweats, headache, myalgia, anorexia, nonproductive cough, arthralgia, and nausea.[4][17] On physical examination, fever is the most consistent finding. Other possible signs include splenomegaly, hepatomegaly, pharyngeal erythema, hepatomegaly, jaundice, and retinopathy, characterized by splinter hemorrhages or retinal infarcts.[4][17] Rashes are not typically present unless there is a co-infection with Lyme disease. Severe disease typically occurs in high-risk populations, particularly in those with a history of asplenia. These patients may have multi-organ dysfunction, including respiratory distress, congestive heart failure, renal failure, splenic rupture, disseminated intravascular coagulation (DIC), hepatitis, or coma.[20]
Evaluation
In patients with an unexplained fever, clinicians should consider babesiosis as a potential diagnosis, particularly if there is a history of recent travel to or residence in an endemic region within the past two months, with or without a known tick bite, or if the patient has received a blood transfusion in the last six months.[20]
Laboratory studies in patients with babesiosis can typically reveal specific abnormalities, which include hemolytic anemia, leukopenia, and thrombocytopenia, as well as elevated lactate dehydrogenase levels, decreased haptoglobin, and features consistent with microangiopathic hemolytic anemia.[4] Laboratory findings may also include elevated liver enzymes, increased blood urea nitrogen and creatinine, and the presence of proteinuria. When the disease is severe, complications such as pulmonary edema, acute respiratory distress syndrome, congestive heart failure, and dysfunction of other organs can develop.[4] Even with appropriate antibiotic treatment, patients with severe babesiosis may experience a prolonged and relapsing course of illness, especially if complications arise.[21]
Various direct and indirect tests can be used to diagnose babesiosis, including identifying Babesia on blood smears, detecting Babesia DNA using the polymerase chain reaction (PCR), or documenting a four-fold rise in anti-Babesia antibody titers in acute and convalescent sera.[20][22][23]
The recent Infectious Diseases Society of America (IDSA) guidelines recommend diagnosing babesiosis by visualizing the protozoa on a peripheral blood smear or by detecting Babesia DNA using polymerase chain reaction (PCR), rather than relying solely on antibody testing.[22]
Serology
Serology is performed via indirect immunofluorescent antibody testing and can help confirm the diagnosis. A single positive serology cannot distinguish between acute and previous infection, but a four-fold rise in acute and convalescent titers confirms a recent infection.[20] Serology should not replace a blood smear or a DNA PCR because antibodies may be absent early in the infection.[20]
Peripheral smears
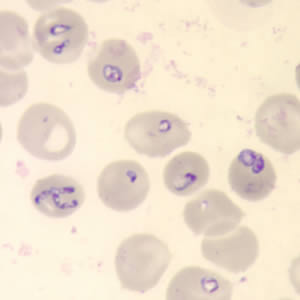
More specifically, diagnosis of babesiosis is typically made by identifying the organism on microscopic examination of Giemsa-stained or Wright-stained thin smears of peripheral blood.[22] Thick smears are not recommended because the organism is too small (<3 μm) to detect and may be missed.[20][22][23] Reviewing multiple fields may furthermore be necessary to visualize the parasite if the parasitemia is low, and detecting the parasite will depend not only on the level of parasitemia but also on the reviewer's competence and experience. In early infection, it is recommended that multiple thin smears be examined as parasite burden may initially be low.[22]
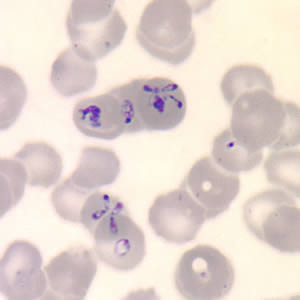
Peripheral blood smears stained with Wright or Giemsa typically reveal intraerythrocytic ring forms characterized by central pallor in cases of Babesia infection.[22] A distinctive feature of babesiosis is the presence of merozoites arranged in a tetrad formation, commonly referred to as the "Maltese cross," which is considered pathognomonic for the disease.[20] However, in individuals with low-level parasitemia or asymptomatic infection, blood smears may yield negative results due to insufficient parasite burden.[22]
Real-time polymerase chain
Several real-time PCR assays have proven effective for detecting low-level Babesia microti parasitemia in peripheral blood specimens, offering greater sensitivity than traditional blood smear microscopy.[20][22][23] These molecular tests exhibit high diagnostic accuracy and are designed to avoid amplification of DNA from Plasmodium species, thus avoiding an incorrect diagnosis of malaria.[23] PCR is particularly valuable during the early stages of infection, when parasitemia may be too low to detect microscopically. However, its utility in monitoring treatment response is limited, as B. microti DNA may persist and remain detectable by PCR for several weeks or even months after parasites are no longer visible on peripheral blood smears.[23]
Treatment / Management
Recent guidelines for babesiosis have been published, including recommendations for therapy.[22] Treatment is indicated in symptomatic cases or in asymptomatic patients who have a positive blood smear or PCR for more than three months.[19][22] Treatment is recommended even for patients with mild babesiosis. Without appropriate therapy, the infection may persist, potentially leading to progression to severe disease or inadvertent transmission through blood donation. In asymptomatic individuals, consideration should be given to initiating treatment if parasitemia persists beyond three months.[4](A1)
The recommended first-line treatment is oral atovaquone plus oral azithromycin for 7 to 10 days. An alternative treatment option is oral quinine plus oral clindamycin for 7-10 days.[4][22][24] Generally, the two regimens used for treating mild to moderate disease are equally effective; however, the atovaquone/azithromycin regimen has a more favorable adverse event profile than the quinine/clindamycin regimen.[22][24](A1)
Severe disease, requiring hospitalization and which typically occurs in high-risk populations or in those infected with the B divergens species will require treatment with high-dose oral IV azithromycin plus oral clindamycin until symptoms pass and then a step-down to all oral regimens.[4] An alternate recommended regimen is IV clindamycin plus PO quinine until all symptoms resolve, and then transition to an all-oral regimen.[22] Intravenous quinidine may also be used, but patients require monitoring for QT prolongation, and oftentimes it is discontinued for that reason.[22] (A1)
Although the duration of treatment is typically recommended to last 7 to 10 days, it is essential to monitor both the clinical and laboratory responses while the patient is undergoing therapy.[4] Patients who are immunocompromised may develop persistent or relapsing disease. In such cases, a course of at least six weeks is recommended, with treatment continued for at least two weeks after parasites are no longer detected on blood smears. In these patients, high-dose azithromycin combined with atovaquone has been used.[23] Other suggested regimens for immunocompromised patients with persistent or relapsing disease include two-drug combinations, such as azithromycin with atovaquone or clindamycin with atovaquone, or three-drug regimens, such as atovaquone with azithromycin and clindamycin, or atovaquone with clindamycin and artemisinin.[23] Additionally, atovaquone/proguanil has been used as a component of various therapeutic protocols, reflecting ongoing efforts to optimize treatment, particularly in severe or refractory cases.[25]
For patients who fail to respond to standard therapy, alternative regimens have been used, including atovaquone plus azithromycin plus clindamycin, atovaquone plus azithromycin plus doxycycline, and atovaquone plus clindamycin plus doxycycline. No particular anti-parasitic combination therapeutic drug regimen has demonstrated superiority. When possible, it is recommended to reduce underlying immunosuppression. [22][26](A1)
Partial or complete red blood cell (RBC) exchange transfusion is indicated in patients presenting with a parasitemia of 10% or more and severe hemolytic anemia.[22] Consideration for exchange transfusion should be strongly given in those with infection due to B divergens, pulmonary, renal, or hepatic dysfunction, regardless of parasitemia level.[22][27][28](A1)
Differential Diagnosis
-
The differential diagnosis of babesiosis encompasses several infectious and non-infectious conditions that can present with similar clinical and laboratory features, particularly in patients presenting with a febrile illness, hemolytic anemia, and/or a history of tick exposure. Some conditions to consider include:
- Human Anaplasmosis (Anaplasma phagocytophilum)
- Lyme disease (Borrelia burgdorferi)
- Tick-Borne Relapsing Fever (Borrelia spp)
- Colorado Tick Fever
- Rocky Mountain spotted fever (Rickettsia rickettsii)
- Other Rickettsial infections
- Malaria
- Human monocytic ehrlichiosis ( Ehrlichia chaffeensis)
- Q fever
- Epstein-Barr Virus (EBV) and Cytomegalovirus (CMV)
- Typhoid fever
- Mycoplasma pneumoniae
- Thrombotic Thrombocytopenic Purpura (TTP)
- Hemolytic Uremic Syndrome (HUS)
- Disseminated Intravascular Coagulation (DIC)
Prognosis
The prognosis of babesiosis is generally favorable in immunocompetent individuals, and no treatment is necessary if they have no risk factors for severe disease, eg, underlying diseases, age over 50, or asplenia. Thus, most immunocompetent and healthy patients will remain asymptomatic or experience a mild, self-limiting febrile illness.[4] In these patients, symptoms typically resolve spontaneously within 6 to 8 weeks, although parasitemia can persist subclinically for months or even years, and monitoring may be warranted to ensure clearance of parasitemia.[23]
In contrast, patients with severe babesiosis require hospitalization, and the prognosis is more guarded in high-risk populations, eg, asplenic individuals, patients over 50 years of age, patients with immunosuppression, and those with significant comorbidities such as hemoglobinopathies, cancer, or cardiac disease.[4] These patients are at greater risk for severe or relapsing disease, which can be complicated by multiorgan dysfunction, ARDS, congestive heart failure, renal and hepatic failure, or death.[4] One multivariate analysis demonstrated that poor outcomes are also associated with male sex, elevated alkaline phosphatase levels (> 125 U/L), and a white blood cell count greater than 5 x 109/L.[29]
Persistent parasitemia after treatment is a significant prognostic concern in babesiosis. Current recommendations advise that any patient who remains positive on peripheral blood smear or PCR testing for Babesia DNA three months after completing therapy should be re-treated, even in the absence of symptoms, to reduce the risk of relapse or disease progression.[23] Furthermore, co-infection with other tick-borne pathogens, particularly B burgdorferi and A phagocytophilum, occurs in approximately 20% of cases.[23] These co-infections can complicate the clinical picture and may lead to a more prolonged or severe illness, generally requiring treatment for these other conditions.[23] Co-infection with other tick-borne illnesses can obscure the diagnosis of any of these illnesses and may delay diagnosis and appropriate therapy.
Ultimately, early diagnosis and initiation of appropriate, targeted therapy are key to improving patient outcomes. However, careful and continuous follow-up is necessary, especially in those with underlying risk factors that predispose them to persistent infection or severe complications.
Complications
Most complications are related to the intravascular hemolysis and include:
- Jaundice
- Shock
- Hemoglobinuria
- Splenic rupture
- Death
- ARDS
- Renal dysfunction
- Noncardiogenic heart failure
Deterrence and Patient Education
Individuals with asplenia and those who are immunocompromised are at significantly increased risk for severe and potentially life-threatening babesiosis, particularly when residing in or traveling to Babesia spp endemic regions. To mitigate exposure to the vector I scapularis, these high-risk populations are advised to avoid environments characterized by dense vegetation, tall grass, and wooded areas where tick populations are prevalent. The application of protective measures, including the use of clothing treated with N, N-diethyl-meta-toluamide (DEET), dimethyl phthalate, or permethrin, is recommended when exposure to tick habitats is unavoidable.[4][20]
When exposed to environments that could harbor ticks, children and adults should perform full-body systematic tick checks with special focus on warm areas, such as the axillae, groin, and hairy regions, because ticks can burrow in these areas.[4][20] Early detection and removal of these ticks can reduce the risk of pathogen transmission, a time-dependent process. Embedded ticks should be extracted promptly using fine-tipped forceps, grasping the tick as close to the skin surface as possible, and applying steady, direct traction without compressing the tick’s abdomen to avoid regurgitation of infective material.[4][20]
Regarding transfusion safety, individuals with a confirmed history of babesiosis are subject to permanent deferral from blood donation due to the risk of transfusion-transmitted infection. While the U.S. Food and Drug Administration (FDA) has not yet approved a universal screening assay for B. microti, certain states in endemic areas have implemented donor screening protocols that utilize a combination of serologic testing and PCR to identify asymptomatic carriers and reduce the risk of transfusion-related transmission.[4][20]
Pearls and Other Issues
Babesiosis became a reportable disease in the United States in January 2011, and its incidence has been increasing, partly due to the geographic expansion of the vector. Because Ixodes scapularis is also the vector for B burgdorferi and A phagocytophilum, coinfections do occur and should especially be considered in patients failing to improve on therapy.
Enhancing Healthcare Team Outcomes
Babesiosis is an emerging tick-borne disease that is endemic in several regions of the United States and globally. As tick populations carrying Babesia spp continue to grow, the geographic range of the infection is expanding, increasing the risk of human transmission. Individuals may be infected with Babesia alone or may experience coinfection with other tick-borne pathogens, such as B burgdorferi, the causative agent of Lyme disease and A phagocytophilum the agent of human granulocytic anaplasmosis. The infection can impact multiple organ systems and requires coordinated care from an interprofessional healthcare team.
Diagnosing babesiosis can be challenging, as it may resemble malaria or other tick-borne illnesses. Asymptomatic individuals generally do not require immediate treatment but should be monitored, particularly if parasitemia persists beyond three months, at which point therapy is warranted. Importantly, individuals infected with the virus should refrain from donating blood until the infection is fully resolved.
Upon diagnosis, the case should be reported to public health authorities, and consultation with an infectious disease specialist is advised to guide treatment. Pharmacists play a crucial role in ensuring that patients understand and adhere to their prescribed antimicrobial regimens. Nurses can provide education on tick-bite prevention strategies, such as wearing protective clothing when visiting areas where ticks are endemic. Delayed diagnosis and treatment can result in significant morbidity and mortality, especially in asplenic patients. Effective management of babesiosis relies on timely recognition and a collaborative, multidisciplinary approach.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Patel KM, Johnson JE, Reece R, Mermel LA. Babesiosis-associated Splenic Rupture: Case Series From a Hyperendemic Region. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019 Sep 13:69(7):1212-1217. doi: 10.1093/cid/ciy1060. Epub [PubMed PMID: 30541016]
Level 2 (mid-level) evidenceTonnetti L, Townsend RL, Deisting BM, Haynes JM, Dodd RY, Stramer SL. The impact of Babesia microti blood donation screening. Transfusion. 2019 Feb:59(2):593-600. doi: 10.1111/trf.15043. Epub 2018 Nov 30 [PubMed PMID: 30499595]
Beard CB, Occi J, Bonilla DL, Egizi AM, Fonseca DM, Mertins JW, Backenson BP, Bajwa WI, Barbarin AM, Bertone MA, Brown J, Connally NP, Connell ND, Eisen RJ, Falco RC, James AM, Krell RK, Lahmers K, Lewis N, Little SE, Neault M, Pérez de León AA, Randall AR, Ruder MG, Saleh MN, Schappach BL, Schroeder BA, Seraphin LL, Wehtje M, Wormser GP, Yabsley MJ, Halperin W. Multistate Infestation with the Exotic Disease-Vector Tick Haemaphysalis longicornis - United States, August 2017-September 2018. MMWR. Morbidity and mortality weekly report. 2018 Nov 30:67(47):1310-1313. doi: 10.15585/mmwr.mm6747a3. Epub 2018 Nov 30 [PubMed PMID: 30496158]
Vannier E, Krause PJ. Human babesiosis. The New England journal of medicine. 2012 Jun 21:366(25):2397-407. doi: 10.1056/NEJMra1202018. Epub [PubMed PMID: 22716978]
Bloch EM, Krause PJ, Tonnetti L. Preventing Transfusion-Transmitted Babesiosis. Pathogens (Basel, Switzerland). 2021 Sep 13:10(9):. doi: 10.3390/pathogens10091176. Epub 2021 Sep 13 [PubMed PMID: 34578209]
Martinot M, Zadeh MM, De Briel D. Human babesiosis. The New England journal of medicine. 2012 Sep 13:367(11):1070; author reply 1071-2. doi: 10.1056/NEJMc1208515. Epub [PubMed PMID: 22970965]
Puri A, Bajpai S, Meredith S, Aravind L, Krause PJ, Kumar S. Babesia microti: Pathogen Genomics, Genetic Variability, Immunodominant Antigens, and Pathogenesis. Frontiers in microbiology. 2021:12():697669. doi: 10.3389/fmicb.2021.697669. Epub 2021 Sep 3 [PubMed PMID: 34539601]
Leiby DA. Transfusion-transmitted Babesia spp.: bull's-eye on Babesia microti. Clinical microbiology reviews. 2011 Jan:24(1):14-28. doi: 10.1128/CMR.00022-10. Epub [PubMed PMID: 21233506]
Spielman A. Human babesiosis on Nantucket Island: transmission by nymphal Ixodes ticks. The American journal of tropical medicine and hygiene. 1976 Nov:25(6):784-7 [PubMed PMID: 1008124]
Abraham A, Brasov I, Thekkiniath J, Kilian N, Lawres L, Gao R, DeBus K, He L, Yu X, Zhu G, Graham MM, Liu X, Molestina R, Ben Mamoun C. Establishment of a continuous in vitro culture of Babesia duncani in human erythrocytes reveals unusually high tolerance to recommended therapies. The Journal of biological chemistry. 2018 Dec 28:293(52):19974-19981. doi: 10.1074/jbc.AC118.005771. Epub 2018 Nov 21 [PubMed PMID: 30463941]
Villatoro T, Karp JK. Transfusion-Transmitted Babesiosis. Archives of pathology & laboratory medicine. 2019 Jan:143(1):130-134. doi: 10.5858/arpa.2017-0250-RS. Epub 2018 Oct 30 [PubMed PMID: 30376376]
Drews SJ, Kjemtrup AM, Krause PJ, Lambert G, Leiby DA, Lewin A, O'Brien SF, Renaud C, Tonnetti L, Bloch EM. Transfusion-transmitted Babesia spp.: a changing landscape of epidemiology, regulation, and risk mitigation. Journal of clinical microbiology. 2023 Oct 24:61(10):e0126822. doi: 10.1128/jcm.01268-22. Epub 2023 Sep 26 [PubMed PMID: 37750699]
Krause PJ, Auwaerter PG, Bannuru RR, Branda JA, Falck-Ytter YT, Lantos PM, Lavergne V, Meissner HC, Osani MC, Rips JG, Sood SK, Vannier E, Vaysbrot EE, Wormser GP. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA): 2020 Guideline on Diagnosis and Management of Babesiosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 Jan 27:72(2):e49-e64. doi: 10.1093/cid/ciaa1216. Epub [PubMed PMID: 33252652]
Level 1 (high-level) evidenceLeiby DA, O'Brien SF, Wendel S, Nguyen ML, Delage G, Devare SG, Hardiman A, Nakhasi HL, Sauleda S, Bloch EM, WPTTID Subgroup on Parasites. International survey on the impact of parasitic infections: frequency of transmission and current mitigation strategies. Vox sanguinis. 2019 Jan:114(1):17-27. doi: 10.1111/vox.12727. Epub 2018 Dec 6 [PubMed PMID: 30523642]
Level 3 (low-level) evidenceRautenbach Y, Schoeman J, Goddard A. Prevalence of canine Babesia and Ehrlichia co-infection and the predictive value of haematology. The Onderstepoort journal of veterinary research. 2018 Oct 9:85(1):e1-e5. doi: 10.4102/ojvr.v85i1.1626. Epub 2018 Oct 9 [PubMed PMID: 30326715]
Gray JS, Estrada-Peña A, Zintl A. Vectors of Babesiosis. Annual review of entomology. 2019 Jan 7:64():149-165. doi: 10.1146/annurev-ento-011118-111932. Epub 2018 Oct 1 [PubMed PMID: 30272993]
Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infectious disease clinics of North America. 2008 Sep:22(3):469-88, viii-ix. doi: 10.1016/j.idc.2008.03.010. Epub [PubMed PMID: 18755385]
Level 3 (low-level) evidenceSsentongo P, Venugopal N, Zhang Y, Chinchilli VM, Ba DM. Beyond Human Babesiosis: Prevalence and Association of Babesia Coinfection with Mortality in the United States, 2015-2022: A Retrospective Cohort Study. Open forum infectious diseases. 2024 Oct:11(10):ofae504. doi: 10.1093/ofid/ofae504. Epub 2024 Oct 8 [PubMed PMID: 39381028]
Level 2 (mid-level) evidenceWormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006 Nov 1:43(9):1089-134 [PubMed PMID: 17029130]
Level 3 (low-level) evidenceVannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infectious disease clinics of North America. 2015 Jun:29(2):357-70. doi: 10.1016/j.idc.2015.02.008. Epub [PubMed PMID: 25999229]
Hoversten K, Bartlett MA. Diagnosis of a tick-borne coinfection in a patient with persistent symptoms following treatment for Lyme disease. BMJ case reports. 2018 Sep 27:2018():. pii: bcr-2018-225342. doi: 10.1136/bcr-2018-225342. Epub 2018 Sep 27 [PubMed PMID: 30262525]
Level 3 (low-level) evidenceKrause PJ, Auwaerter PG, Bannuru RR, Branda JA, Falck-Ytter YT, Lantos PM, Lavergne V, Meissner HC, Osani MC, Rips JG, Sood SK, Vannier E, Vaysbrot EE, Wormser GP. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA): 2020 Guideline on Diagnosis and Management of Babesiosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 Jan 27:72(2):185-189. doi: 10.1093/cid/ciab050. Epub [PubMed PMID: 33501959]
Level 1 (high-level) evidenceSanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: A Review. JAMA. 2016 Apr 26:315(16):1767-77. doi: 10.1001/jama.2016.2884. Epub [PubMed PMID: 27115378]
Krause PJ, Lepore T, Sikand VK, Gadbaw J Jr, Burke G, Telford SR 3rd, Brassard P, Pearl D, Azlanzadeh J, Christianson D, McGrath D, Spielman A. Atovaquone and azithromycin for the treatment of babesiosis. The New England journal of medicine. 2000 Nov 16:343(20):1454-8 [PubMed PMID: 11078770]
Level 3 (low-level) evidenceKrause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, Furman RR, Neuhaus E, Skowron G, Gupta S, McCalla C, Pesanti EL, Young M, Heiman D, Hsue G, Gelfand JA, Wormser GP, Dickason J, Bia FJ, Hartman B, Telford SR 3rd, Christianson D, Dardick K, Coleman M, Girotto JE, Spielman A. Persistent and relapsing babesiosis in immunocompromised patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008 Feb 1:46(3):370-6. doi: 10.1086/525852. Epub [PubMed PMID: 18181735]
Paparone P, Paparone PW. Variable clinical presentations of babesiosis. The Nurse practitioner. 2018 Oct:43(10):48-54. doi: 10.1097/01.NPR.0000545000.07640.11. Epub [PubMed PMID: 30234826]
Lempereur L, Beck R, Fonseca I, Marques C, Duarte A, Santos M, Zúquete S, Gomes J, Walder G, Domingos A, Antunes S, Baneth G, Silaghi C, Holman P, Zintl A. Guidelines for the Detection of Babesia and Theileria Parasites. Vector borne and zoonotic diseases (Larchmont, N.Y.). 2017 Jan:17(1):51-65. doi: 10.1089/vbz.2016.1955. Epub [PubMed PMID: 28055573]
Choi E, Pyzocha NJ, Maurer DM. Tick-Borne Illnesses. Current sports medicine reports. 2016 Mar-Apr:15(2):98-104. doi: 10.1249/JSR.0000000000000238. Epub [PubMed PMID: 26963018]
White DJ, Talarico J, Chang HG, Birkhead GS, Heimberger T, Morse DL. Human babesiosis in New York State: Review of 139 hospitalized cases and analysis of prognostic factors. Archives of internal medicine. 1998 Oct 26:158(19):2149-54 [PubMed PMID: 9801183]
Level 3 (low-level) evidence