Introduction
Neck pain is a common condition that can cause significant discomfort and disability in patients of different ages. Workers who have experienced neck pain account for up to 40% of work absenteeism.[1][2][3] Cervical radiculopathy, on the other hand, is a condition in which the nerve root of a spinal nerve is compressed or impaired, causing pain and symptoms to spread beyond the neck and radiate to other areas of the body, such as the arms, neck, chest, upper back, and shoulders. Due to the nerve impingement, muscle weakness and impaired deep tendon reflexes are often observed.
Cervical radiculopathy is a common neck disorder that reduces function, productivity, and quality of life. The natural course is generally favorable. However, significant pain, progressive neurologic loss, or myelopathic signs warrant escalation of care.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Cervical radiculopathy develops when a spinal nerve root is compressed or chemically inflamed. In younger patients, typically in the third and fourth decades, disc trauma and herniation are the most frequent causes of impingement.[4] With increasing age, the causation is degenerative. Disc degeneration is the most common cause in the fifth and sixth decades of life. In the seventh decade, causation stems from foraminal narrowing due to arthritic change.
Beyond these age-related patterns, most cases arise from progressive spondylotic change at the uncovertebral and facet joints or protrusion of degenerated nucleus pulposus.[5][6] Osteophytes, loss of disc height, and a mixture of disc material with bony overgrowth gradually shrink the lateral recess and neuroforamen, trapping the exiting root and its venous plexus. Less frequent structural culprits include synovial or arachnoid cysts, primary or metastatic tumors, vascular malformations, traumatic root avulsion, and looping vertebral arteries that encroach on the foramen.
Mechanical stress represents just 1 aspect of the overall pathogenesis. Root compression blocks axoplasmic transport and venous return, setting off a local ischemic milieu. Fragments of degenerated disc and infiltrating inflammatory cells release proinflammatory substances, which amplify nociceptor activity and neural edema. The degree of mechanical load and biochemical irritation effects determines whether degeneration remains silent or becomes symptomatic.
Several host and environmental factors influence these effects. Family and twin studies suggest that genetic factors account for up to half of the variability in disc degeneration, likely through their influence on collagen composition and disc metabolism.[7] Tobacco smoking accelerates dehydrative change within the disc, diminishes end plate nutrition, and magnifies proinflammatory signaling, increasing the likelihood of clinically significant disease.[8] Occupational exposure to sustained neck flexion, repetitive work with the arms above shoulder height, and heavy upper limb loading raises the incidence of surgically treated spondylosis among construction workers and other manual laborers.[9] Mendelian randomization suggests that lower educational attainment predisposes to cervical spondylosis in part through higher body mass index and continued tobacco use, pointing toward modifiable societal levers.[10]
Epidemiology
The cervical radiculopathies are less frequent than lumbar radiculopathies. The C7 nerve root is most frequently impacted, with more than half of all cases affecting this level. Roughly a quarter of cases involve the C6 nerve root. Nerve roots C1 to C5 and C8 are less impacted. Risk factors for developing radicular disease include manual labor with heavy lifting, driving, or operating vibrating equipment. Chronic smoking history can increase the risk of radiculopathies.
A recent systematic review of 9 observational studies provides a comprehensive estimate of the burden of adult cervical radiculopathy. Pooled data from 2 high-quality cohorts and 1 lower-quality cohort indicated an annual incidence of approximately 0.8 to 1.8 new cases per 1,000 person-years. Point prevalence was more variable, ranging from 1.2 to 5.8 per 1,000 in 4 medium- to high-quality studies.[11]
Pathophysiology
Cervical radiculopathy develops when a spinal nerve root is subjected to combined mechanical, inflammatory, and vascular stressors. Mechanical deformation plays a dominant role in this process. Disc extrusion, disc-osteophyte complexes, or facet joint hypertrophy reduce foraminal volume, leading to direct root compression or dynamic tethering during neck movement.
This compression disrupts venous outflow and axoplasmic transport, resulting in intraradicular ischemia. Concurrently, chemical sensitization intensifies nociceptive signaling. Degenerating nucleus pulposus and damaged fibrocartilage release tumor necrosis factor-α, interleukins 1 and 6, substance P, and bradykinin. These mediators lower the activation threshold of dorsal root ganglion neurons, promoting pain hypersensitivity. Proinflammatory cytokines also contribute to neural edema and increase susceptibility to additional mechanical stress.
In many cases, symptoms improve as inflammatory substances subside and herniated disc material undergoes spontaneous resorption. However, the emergence or progression of neurologic deficits often reflects sustained nerve root compression and may necessitate surgical decompression to preserve function and prevent further damage.
History and Physical
Accurate recognition of cervical radiculopathy relies on a detailed clinical history and a focused physical examination, as no single laboratory test or imaging modality definitively confirms the diagnosis. Clinicians must integrate symptom patterns, neurologic findings, and results of provocative maneuvers to establish a reliable working diagnosis.
Clinical History
Patients often report a sharp or electric pain that originates in the neck and radiates into the upper limb along the trajectory of the affected nerve root. This radiation frequently follows classic dermatomal patterns. Accompanying symptoms may include paresthesia, subjective numbness, and muscle weakness; the presence of these features together increases the likelihood of cervical radiculopathy.
Symptoms tend to present unilaterally and typically worsen with neck extension, rotation toward the symptomatic side, or overhead arm activity. Bilateral symptoms or involvement of multiple root levels—especially following trauma—warrant immediate imaging, as such findings may indicate a central disc extrusion with potential spinal cord compromise.
A comprehensive history should include the timing and nature of symptom onset, specific positions that provoke or alleviate discomfort, occupational activities that require sustained neck flexion or work above shoulder height, a history of prior neck injuries, and details of any treatments already attempted.
Distinct symptom patterns help differentiate between axial neck pain, cervical radiculopathy, and cervical myelopathy. Axial neck pain typically presents as load-dependent discomfort localized to the neck, often exacerbated by movement or posture. Cervical radiculopathy manifests with arm pain that follows the distribution of a specific nerve root and intensifies when the head tilts toward the affected side, reflecting nerve root compression or irritation.
Cervical myelopathy presents a different clinical picture. Patients often experience hand clumsiness, gait instability, or urinary urgency, even in the absence of significant neck pain. These signs suggest spinal cord involvement and warrant prompt evaluation due to the risk of progressive neurologic impairment.
Red-flag features
The following red-flag symptoms may indicate infection or malignancy and require prompt assessment:
- Fever
- Night sweats
- Unexplained weight loss
- Immunosuppression
- Persistent pain at rest
- Tenderness over the vertebrae
Physical Examination
Cervical range-of-motion testing provides an essential starting point in the physical examination. Guarded neck movements or a noticeable loss of neck extension often indicate underlying pathology that requires further evaluation.
A thorough neurologic survey follows. Motor strength testing in key myotomes—C5 (deltoid and biceps), C6 (wrist extensors), C7 (triceps), C8 (finger flexors), and T1 (interossei)—helps identify lower motor neuron weakness. Sensory assessment with light touch or pinprick targets dermatomal patterns and seeks deficits that correspond to the motor findings. Deep tendon reflexes, including the biceps (C5–6), brachioradialis (C6), and triceps (C7), are examined bilaterally; asymmetry or diminished responses on the symptomatic side can help pinpoint the affected nerve root.
Provocative maneuvers add valuable diagnostic information. The Spurling test—performed by applying axial compression while extending and rotating the neck toward the symptomatic side—serves as one of the most specific assessments for cervical radiculopathy. Although the test demonstrates high specificity, its sensitivity remains moderate, supporting its use for confirmation rather than screening.[12]
Evaluation for cervical myelopathy remains mandatory in all patients with suspected cervical spine pathology.[13] Degenerative cervical myelopathy may begin with subtle, often overlooked complaints. Approximately 40% of the total symptom burden stems from nontraditional signs, eg, dizziness, fatigue, or autonomic disturbances, and every patient typically reports at least one. A sensation of heavy legs emerges as the earliest and most predictive symptom for timely diagnosis.[14] More familiar features include hand clumsiness or numbness, diffuse paresthesia, limb weakness, and unsteady gait.
Although neck or shoulder pain affects just over half of patients, bladder dysfunction appears infrequently. On physical examination, the Tromner reflex and generalized hyperreflexia offer the highest sensitivity.[15] In contrast, signs such as Babinski, clonus, a positive Tromner sign, and the inverted supinator reflex demonstrate greater specificity for cervical myelopathy.[16]
Clinical Documentation
Key elements to document during the clinical assessment for cervical radiculopathy include:
-
Pain profile: Record the patient’s pain intensity on a 0 to 10 numeric rating scale and map its distribution, noting whether it follows the sensory dermatomes of the upper limb to localize the affected nerve roots.
- Symptom trajectory: Clarify whether pain, weakness, or sensory changes are stable, improving, or worsening over time.
-
Prior and current treatments: Detail any therapies already attempted or in progress. When reviewing prior management, ask specifically about non-steroidal anti-inflammatory drugs, oral corticosteroids, a structured physical-therapy program, cervical epidural steroid injections, and surgical interventions.
-
Motor deficits: Identify and grade any muscle weakness.
-
Functional impact: Describe how the radiculopathy limits daily activities or occupation. For example, even mild symptoms may significantly hinder a surgeon who depends on fine upper-extremity control.
Evaluation
Imaging Studies
Plain radiographs of the cervical spine are frequently obtained to assess neck and upper extremity pain. Lateral views often demonstrate disc space narrowing, along with additional indicators of degenerative disc disease. Oblique views may reveal foraminal narrowing corresponding to the location of radicular symptoms. Open-mouth views serve a specific role when the disruption of the atlantoaxial joint is suspected.[17][18][19][20] Flexion and extension views provide valuable information when spinal instability is a concern.
Computed tomography (CT) proves especially useful in the acute evaluation of traumatic injuries associated with radicular symptoms. While CT offers excellent resolution of bony structures and can accurately define osseous foraminal stenosis, limited soft tissue contrast reduces its utility in nontraumatic settings (see Image: Osseous Foraminal Stenosis).
Magnetic resonance imaging (MRI) remains the preferred imaging modality for evaluating cervical radiculopathy. MRI excels in identifying soft tissue abnormalities, including disc herniations and nerve root compressions. Although a strong correlation exists between imaging findings such as herniated discs or foraminal narrowing and clinical symptoms, these abnormalities do not always account for the patient’s complaints, and false positives remain a known limitation. MRI can also detect signs of degenerative disc disease, including Modic changes, when present.[21]
Additional Diagnostic Studies
Electromyography serves to confirm dysfunction in the affected nerve root, particularly when clinical and imaging findings lack clarity.
Selective nerve root blocks can simultaneously provide short-term symptom relief and help verify the specific nerve root responsible for radiating pain.
Treatment / Management
Cervical radiculopathy management is stepwise. Goals include rapid analgesia, restoration of neurologic function, and lasting prevention of recurrence. Over 85% of acute cervical radiculopathy resolves without any specific treatments within 8 to 12 weeks.[22][23][24] (A1)
Conservative Management
Most acute cases improve with conservative care. Education, continued daily activity, and structured home exercise should begin immediately. However, to facilitate reduced nerve root inflammation and improve radiculopathy, implementing nonsurgical treatments, including oral anti-inflammatory drugs, physical therapy, and translaminar epidural steroid injections, is important.
An aggressive, well-designed physical therapy program can provide significant relief. In the setting of surgical intervention, physical therapy can speed recovery. Using a cervical pillow at nighttime can help alleviate symptoms and make sleeping easier during recovery. Short-term use of a soft cervical collar can provide some relief.
Since the main cause of pain in cervical radiculopathy is inflammation, the use of non-steroidal anti-inflammatory drugs (NSAIDs) for 1 to 2 weeks can provide symptom relief and treat the proximate cause. The use of oral steroids should be limited to the short term due to controversies surrounding their use. Opioid pain medications are not recommended for routine use, but they can be useful in managing radicular pain. It should be noted that the use of opioid medications is a risk factor for slow recovery and delayed return to work for patients where surgical intervention is clinically necessary.[25][26](B2)
Additionally, a short oral corticosteroid taper can help alleviate pain in select acute presentations. Neuropathic pain agents, eg, gabapentin and pregabalin, can be considered. Studies have shown that epidural steroids can provide significant pain relief and accelerate the return to normal function for many patients. Relief from a single treatment can be significant and long-lasting. Half the treated patients reported at least 50% relief for weeks following the injection. The interlaminar approach for the injection is preferred because catastrophic neurologic events are rarer than with transforaminal access. Fluoroscopic or digital subtraction imaging is recommended, and nonparticulate dexamethasone is the steroid of choice. Repeated injections are reserved for relapsing symptoms and must respect cumulative dose limits.
Using acupuncture as an adjunctive therapy has also been shown to provide significant symptomatic relief. Chiropractic or direct osteopathic manipulation can worsen radicular symptoms. Conversely, indirect osteopathic techniques can help alleviate symptoms. Tricyclic antidepressants and drugs, eg, gabapentin, are useful adjuncts in the treatment of chronic cervical radiculopathy.
Surgical Management
Surgical intervention becomes appropriate when imaging confirms degenerative cervical radiculopathy due to disc herniation or bony stenosis and when symptoms persist despite 6 to 12 weeks of structured, conservative treatment. Cases involving disabling pain or progressive motor weakness that interfere with function warrant earlier surgical consideration.
Two main surgical approaches address cervical radiculopathy: anterior and posterior. The anterior approach involves complete discectomy followed by either spinal fusion or disc replacement. Anterior foraminotomy or discectomy without fusion remains outside mainstream surgical practice. The posterior approach consists of foraminotomy, with or without discectomy. Both techniques demonstrate comparable effectiveness in appropriately selected patients. Surgical treatment remains reserved for individuals with failed nonsurgical management or acute neurologic deterioration. Regardless of the chosen approach, potential complications may arise, including risks associated with anesthesia and procedural events, eg, nerve palsy, vascular injury, or laryngeal nerve damage.
Spinal cord stimulation may offer pain relief and functional improvement in select patients with persistent symptoms following surgery or in those with multifocal disease, although supporting evidence remains limited. Ongoing reassessment of neurologic function at every stage of care remains essential. Timely escalation of treatment ensures optimal outcomes when clinical targets are not achieved.
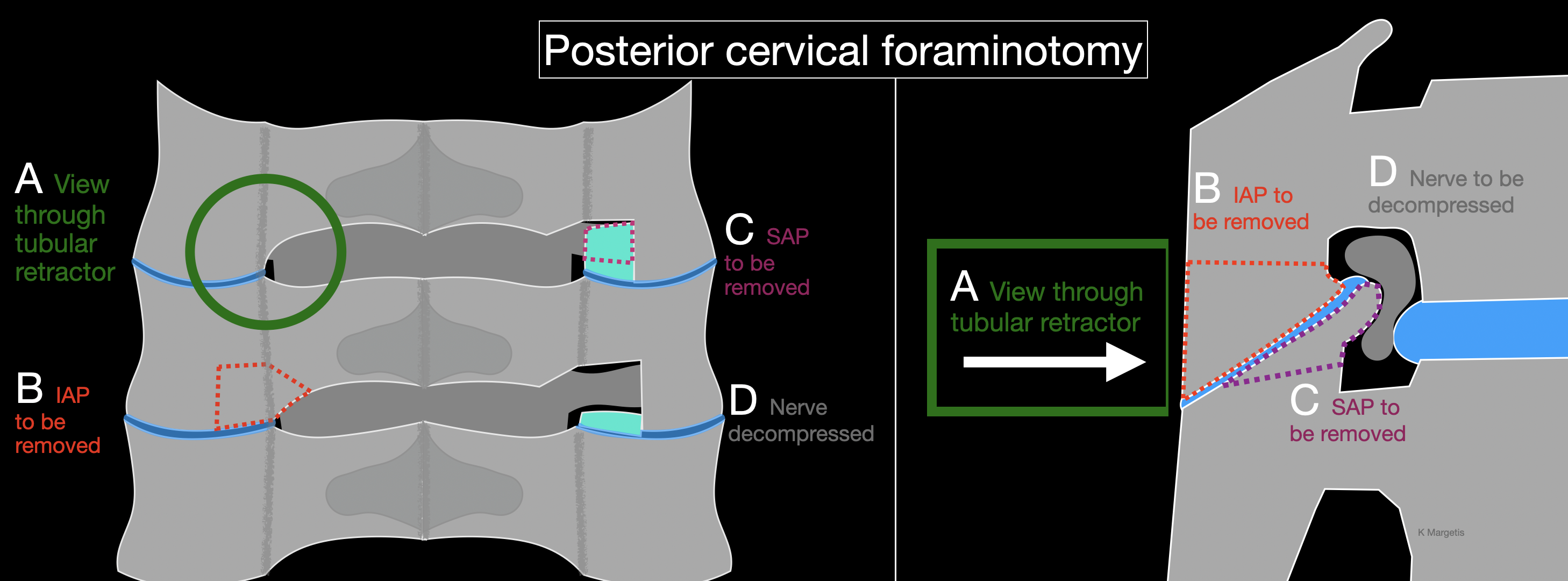
Operative technique of posterior cervical foraminotomy
Clinicians performing a posterior cervical foraminotomy commonly follow a standard operative technique (see Image. Posterior Cervical Foraminotomy). The patient is placed under general endotracheal anesthesia. Baseline somatosensory evoked potentials (SSEPs), motor evoked potentials (MEPs), and free-run electromyography (EMG) signals are recorded. Gardner-Wells tongs are applied. The patient is then flipped into the prone position on a Jackson table, taking care to leave the abdomen free and to pad all bony prominences. The head is supported on a face mask cushion and is placed under 5 to 10 lbs of longitudinal traction. An alternative is to use a Mayfield clamp.
A lateral fluoroscopic image is obtained to confirm that the spine is aligned properly and that the correct level is targeted. The operative site is prepped and draped. Time out is performed. Local anesthetic is infiltrated into the wound.
A 2 cm paramedian skin incision is performed. After incising the fascia, serial dilation is performed. Caution is needed to prevent any instrument from entering the intralaminar space and risking spinal cord injury. Therefore, we do not use a localizing needle. Instead, the first dilator is advanced while constantly moved 1 cm up and down (in a cranial-to-caudal direction), which aids soft-tissue dissection and makes it more likely to encounter bone rather than slide into the intralaminar interval. After progressively larger dilators have been placed, a 14 mm tubular retractor is docked over the final dilator to maintain the working corridor. An alternative is to perform a mini-open exposure, involving a midline incision and elevation of the paraspinal muscles ipsilaterally only.
Anterior-posterior and lateral fluoroscopic images confirm both the correct spinal level and the accurate placement of the tubular retractor. Under microscopic visualization, soft tissue is carefully cleared from the bony surface to expose the lateral edge of the laminae and the medial portion of the facet joint. A 3 mm “matchstick” burr removes the medial third of the inferior-medial aspect of the inferior articular process of the rostral vertebra. Preservation of at least 50% of the facet joint remains essential to prevent postoperative instability.
Next, a 2- or 3-mm diamond burr is used to remove the medial third of the superior-medial aspect of the superior articular process of the caudal vertebra. Compared to other tools, the diamond burr presents a lower risk of nerve injury if it breaches the bone, though it generates more heat and requires constant irrigation to minimize thermal damage. An eggshell-thin layer of bone remains after burring and is removed using upward-curved curettes. Once the nerve root is largely decompressed, 1 mm Kerrison rongeurs are used to assist with the final bone removal. Early use of Kerrison instruments may pose a higher risk of nerve injury, especially in severely stenotic foramina, where the footplate must be inserted with caution. If an extruded disc fragment is present, a nerve hook may be employed at this stage to retrieve the fragment while maneuvering around the nerve.
Epidural venous oozing is controlled with hemostatic agents as needed. The surgical field undergoes thorough irrigation. A surgical drain is rarely required. Closure begins with the fascia, sutured using 0 Vicryl on a UR-6 needle. The deep dermal layer is approximated with 3-0 Vicryl sutures, followed by a running 3-0 Monocryl subcuticular stitch to close the skin. A sterile dressing is applied, and a soft cervical collar is placed to support postoperative comfort.
Differential Diagnosis
Accurate diagnosis of cervical radiculopathy requires systematic exclusion of several disorders that produce neck-related arm pain, sensory loss, or weakness. Each competing condition displays distinctive clinical, electrodiagnostic, or imaging features that direct evaluation and management.
Shoulder Pathology
Rotator-cuff tears, impingement, and glenohumeral arthritis often present with lateral shoulder pain and abduction weakness. Shoulder tenderness to palpation and painful passive range of motion favor intrinsic shoulder disease. Pain usually eases when the arm rests at the side, whereas the Spurling maneuver is negative.
Median Nerve Entrapment
Carpal tunnel syndrome produces nocturnal paresthesias that worsen with hand activity and spare the neck. Neck pain is uncommon. Electrodiagnostic studies reveal slowed median sensory conduction across the wrist, without evidence of paraspinal denervation. When median blockade relieves more than half of the symptoms, attention shifts to the wrist, rather than the cervical roots.
Ulnar Neuropathy at the Elbow
Cubital tunnel compression causes numbness of the fourth and fifth digits and intrinsic hand weakness. Sensation in the ulnar forearm remains intact because the medial antebrachial cutaneous nerve supplies it. Strength of abductor pollicis brevis, flexor pollicis brevis, opponens pollicis, and the lateral 2 lumbricals is preserved, helping to separate ulnar neuropathy from C8 to T1 radiculopathy.
Neuralgic Amyotrophy (Parsonage–Turner syndrome)
Acute, severe shoulder pain followed by focal paresis suggests brachial neuritis.
Thoracic Outlet Syndrome
Positional paresthesias exacerbated by overhead activity, associated vascular signs, or diminished radial pulse differentiate thoracic outlet syndrome from root compression. Provocative elevation tests reproduce symptoms, whereas cervical traction does not relieve them.
Peripheral Entrapments and Plexopathies
Posterior interosseous nerve syndrome, long thoracic nerve palsy, and idiopathic brachial plexitis can mimic segmental weakness. Detailed motor mapping and nerve conduction studies localize the lesion distal to the foramen.
Myofascial and Musculoskeletal Pain Syndromes
Cervical facet arthropathy, myofascial trigger points, and sprain-strain injuries present with localized tenderness, normal neurologic examination, and imaging that lacks root compression.
Serious Cervical Spine Disorders
Spinal cord compression, infection, or neoplasm must be suspected when bilateral symptoms, gait disturbance, or systemic signs accompany radicular complaints. Magnetic resonance imaging is mandatory in these scenarios.
Prognosis
Most patients recover without surgery. A systematic review showed that at 3 years, 83% regain satisfactory function.[27]
A systematic review of randomized controlled trials has demonstrated that surgery results in faster reductions in pain and disability during the first year. After 12 months, the functional differences between surgical and nonsurgical care narrow.[28] A study modelled outcomes after anterior surgical techniques for cervical degenerative radiculopathy. Unsuccessful cases correlated with heavy work, low education, litigation, prior surgery, symptoms longer than 3 months, high baseline disability, and anxiety. Smoking and a foreign mother tongue further predicted persistent arm pain.[29]
Postoperative arm pain and disability improve less in C8 radiculopathy compared with C5 to C7. Surgery can relieve arm pain more effectively than numbness, which can often persist.[30]
Complications
Adverse events related to cervical radiculopathy arise from the natural history of nerve root compression, conservative treatment, spinal injections, and operative interventions.
Most patients improve without surgery, yet medications and rehabilitative modalities are not risk-free. Nonsteroidal anti-inflammatory drugs can provoke gastrointestinal bleeding and renal dysfunction, while prolonged opioid use is associated with dependence and delayed return to work. Evidence for gabapentin, pregabalin, and tricyclic antidepressants is limited; dizziness, imbalance, and sedation are common and may hinder rehabilitation. Short oral corticosteroid tapers occasionally shorten pain duration but can worsen glycemic control and mood. Physical therapy and exercise carry minimal medical risk, although transient neck soreness is frequent. However, neck manipulation performed with excessive force can exacerbate neurologic symptoms.
Epidural injections deliver anti-inflammatory medication directly to the inflamed nerve root. Severe mishaps are rare, but they are well documented. Reported major events include epidural hematoma, abscess, chemical or bacterial meningitis, spinal cord or brain-stem infarction from inadvertent arterial injection of particulate steroid, and direct spinal cord trauma. Minor adverse effects include transient flushing and postdural puncture headache.[31]
The overall postoperative morbidity after anterior cervical discectomy and fusion is 16%, based on an aggregation of 50,000 patients.[32] Excessive neck swelling, pseudarthrosis, dysphagia, and cage or graft subsidence each approach 10%, and their likelihood increases with multilevel fusion or advanced age.
Postoperative and Rehabilitation Care
Manual-based treatments, including traction, mobilization, and manipulation, are utilized for the rehabilitation of cervical radiculopathy. Traction is considered the main cornerstone based on available literature. In addition, manual therapy can include various forms of massage and exercises, including stretching, strengthening, and neurodynamic exercises.[33][34] Mechanical traction yields improved outcomes compared to manual traction, based on the limited research comparing them; however, further research is needed.[35]
Therapeutic efforts are cited as having multiple benefits, including reducing pain, improving functional outcomes, and improving the timeliness of outcomes depending on the onset of treatment and how aggressive the treatment plan is. It is worth noting that the effectiveness of individual treatments has not been demonstrated in the literature. Combining these treatments has been shown to reduce symptoms of radiculopathy. Further research is needed for a more comprehensive understanding.[33][34]
Deterrence and Patient Education
Clinicians should educate patients that modifiable exposures often precede the onset of symptoms associated with cervical radiculopathy. Activities such as repetitive overhead work, heavy lifting, prolonged driving, and the use of vibrating tools that affect the upper limbs contribute to nerve root irritation. Smoking intensifies these risks and prolongs recovery. Early intervention targeting workplace ergonomics, incorporating scheduled micro-breaks, utilizing mechanical aids, and promoting smoking cessation helps reduce cumulative cervical load and lower symptom burden.
Maintaining proper cervical posture during daily activities plays a crucial role in symptom management. Patients benefit from maintaining proper alignment by keeping their ears positioned directly over their shoulders, avoiding sustained neck flexion during screen use, and rotating their trunk instead of twisting their neck when lifting objects. An ergonomic chair with adjustable armrests and a monitor positioned at eye level helps alleviate foraminal stress and supports spinal health. Regular aerobic activity and targeted muscle strengthening reduce the risk of recurrence by improving paraspinal endurance and spinal stability. A supervised rehabilitation program that includes neck-specific exercises in combination with general conditioning typically offers the greatest benefit.
Patients should understand that most acute cases resolve without the need for surgical intervention. Up to 85% improvement within 12 weeks supports the recommendation for an initial non-operative management plan in motivated individuals. Clinicians must reinforce appropriate medication use. Nonsteroidal anti-inflammatory drugs (NSAIDs) and short oral corticosteroid tapers can effectively reduce inflammation and pain. Opioid analgesics, however, delay recovery and should be reserved only for refractory episodes that do not respond to first-line therapies.
Self-management strategies also contribute to symptom control. Intermittent cervical traction, the use of a soft collar for brief periods during the day, and sleeping with a contoured pillow can relieve radicular discomfort. Indirect osteopathic or physiotherapy techniques are generally safe and helpful. In contrast, high-velocity cervical manipulation may worsen neurological deficits and should be avoided.
Clinicians must highlight red flag symptoms that necessitate immediate reassessment. Progressive arm weakness, new-onset gait imbalance, changes in bladder or bowel function, or severe, unrelenting pain despite guideline-directed treatment signal the need for prompt imaging and specialist referral to prevent irreversible neurologic damage. Furthermore, patients should receive clear guidance on the importance of adherence and maintaining realistic expectations. Long-term control depends on consistent lifestyle modification, regular exercise, and scheduled follow-up. This proactive approach empowers patients to stay active, manage symptoms effectively, and reduce the likelihood of recurrence.
Enhancing Healthcare Team Outcomes
Effective management of cervical radiculopathy requires seamless collaboration among physicians, advanced practitioners, nurses, pharmacists, physical therapists, and other allied health professionals. Neurologists and primary care clinicians often serve as the first point of contact, guiding initial assessments, coordinating imaging, and ruling out red flag conditions. Nurse practitioners play a crucial role in monitoring symptom progression, reinforcing education, and ensuring timely follow-up care. Pharmacists contribute by evaluating medication regimens, advising on safe use of anti-inflammatory agents, and counseling patients on minimizing opioid exposure. Physical therapists implement individualized exercise programs to enhance cervical stability and reduce pain, supporting conservative management efforts. Throughout this process, interprofessional communication ensures that each team member remains informed and aligned in therapeutic strategy, contributing to a unified plan that prioritizes patient safety and functional recovery.
A stepwise approach underpins the strategy for cervical radiculopathy management. Conservative interventions—such as NSAIDs, physical therapy, and translaminar epidural steroid injections—form the first tier of care. Most acute cases resolve without surgery, and little evidence supports surgical superiority in the absence of progressive neurological deficits. However, delaying surgery when deficits develop can result in poorer outcomes. Physicians and surgeons must therefore coordinate closely to determine the optimal timing for referral or operative planning. Nurses and advanced practitioners should remain vigilant in identifying signs of worsening conditions and escalate care promptly when necessary. Interprofessional teamwork reduces fragmentation, avoids overtreatment, and prevents undertreatment. By aligning goals across specialties, the care team ensures not only better outcomes and reduced complications but also enhanced patient-centered care and overall team performance.
Media
(Click Image to Enlarge)
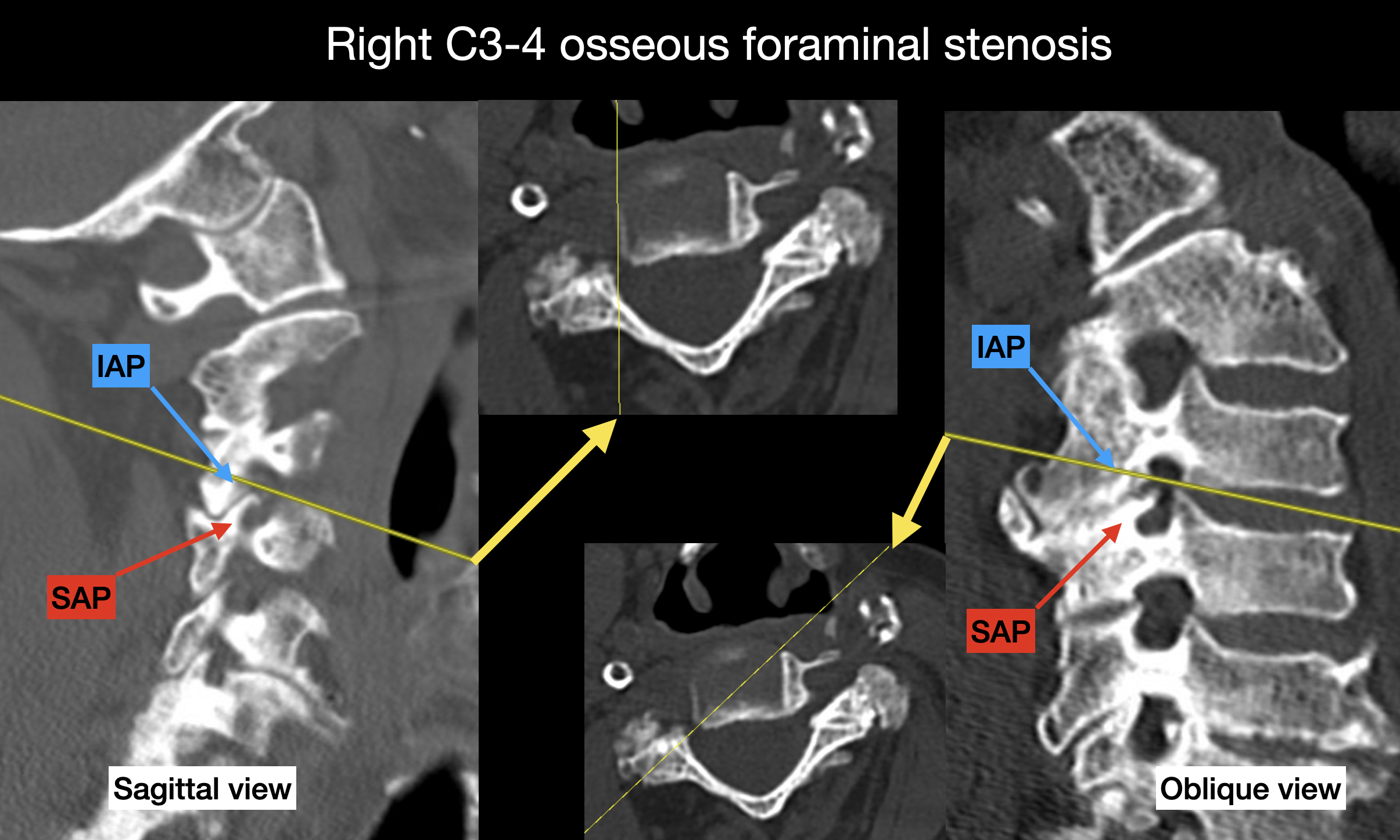
Osseous Foraminal Stenosis. CT image (bone window) demonstrating marked narrowing of the right C3–4 neural foramen. Note that osteophytes arising from the superior articular process (SAP) project into the foramen to a greater extent than those originating from the inferior articular process (IAP). Note that the oblique views (right image) offer a better delineation of the foraminal stenosis than the sagittal view (left image).
Contributed by K Margetis, MD, PhD
(Click Image to Enlarge)
References
Peolsson A, Peterson G, Hermansen A, Ludvigsson ML, Dedering Å, Löfgren H. Physiotherapy after anterior cervical spine surgery for cervical disc disease: study protocol of a prospective randomised study to compare internet-based neck-specific exercise with prescribed physical activity. BMJ open. 2019 Feb 19:9(2):e027387. doi: 10.1136/bmjopen-2018-027387. Epub 2019 Feb 19 [PubMed PMID: 30782952]
Level 1 (high-level) evidenceHassan KZ, Sherman AL. Epidural Steroids. StatPearls. 2025 Jan:(): [PubMed PMID: 30726005]
Doughty CT, Bowley MP. Entrapment Neuropathies of the Upper Extremity. The Medical clinics of North America. 2019 Mar:103(2):357-370. doi: 10.1016/j.mcna.2018.10.012. Epub [PubMed PMID: 30704687]
Ament JD, Karnati T, Kulubya E, Kim KD, Johnson JP. Treatment of cervical radiculopathy: A review of the evolution and economics. Surgical neurology international. 2018:9():35. doi: 10.4103/sni.sni_441_17. Epub 2018 Feb 14 [PubMed PMID: 29527393]
Kuo DT, Tadi P. Cervical Spondylosis. StatPearls. 2025 Jan:(): [PubMed PMID: 31855384]
Sharrak S, Al Khalili Y. Cervical Disc Herniation. StatPearls. 2025 Jan:(): [PubMed PMID: 31536225]
Battié MC, Videman T, Kaprio J, Gibbons LE, Gill K, Manninen H, Saarela J, Peltonen L. The Twin Spine Study: contributions to a changing view of disc degeneration. The spine journal : official journal of the North American Spine Society. 2009 Jan-Feb:9(1):47-59. doi: 10.1016/j.spinee.2008.11.011. Epub [PubMed PMID: 19111259]
Rajesh N, Moudgil-Joshi J, Kaliaperumal C. Smoking and degenerative spinal disease: A systematic review. Brain & spine. 2022:2():100916. doi: 10.1016/j.bas.2022.100916. Epub 2022 Aug 7 [PubMed PMID: 36248118]
Level 1 (high-level) evidenceJackson JA, Liv P, Sayed-Noor AS, Punnett L, Wahlström J. Risk factors for surgically treated cervical spondylosis in male construction workers: a 20-year prospective study. The spine journal : official journal of the North American Spine Society. 2023 Jan:23(1):136-145. doi: 10.1016/j.spinee.2022.08.009. Epub 2022 Aug 24 [PubMed PMID: 36028215]
Sun Y, Jin M, Yu T, Zhang J. Cardiovascular risk factors mediating the protective effect of education on cervical spondylosis risk. Scientific reports. 2023 Jan 17:13(1):936. doi: 10.1038/s41598-023-28153-7. Epub 2023 Jan 17 [PubMed PMID: 36650225]
Mansfield M, Smith T, Spahr N, Thacker M. Cervical spine radiculopathy epidemiology: A systematic review. Musculoskeletal care. 2020 Dec:18(4):555-567. doi: 10.1002/msc.1498. Epub 2020 Jul 25 [PubMed PMID: 32710604]
Level 1 (high-level) evidenceLin LH, Lin TY, Chang KV, Tzang CC, Wu WT, Özçakar L. Diagnostic Performance of Spurling's Test for the Assessment Subacute and Chronic Cervical Radiculopathy: A Systematic Review and Meta-analysis. American journal of physical medicine & rehabilitation. 2025 Aug 1:104(8):717-723. doi: 10.1097/PHM.0000000000002707. Epub 2025 Feb 4 [PubMed PMID: 39938056]
Level 1 (high-level) evidenceDonnally III CJ, Hanna A, Odom CK. Cervical Myelopathy. StatPearls. 2025 Jan:(): [PubMed PMID: 29493937]
Munro CF, Yurac R, Moritz ZC, Fehlings MG, Rodrigues-Pinto R, Milligan J, Margetis K, Kotter MRN, Davies BM. Targeting earlier diagnosis: What symptoms come first in Degenerative Cervical Myelopathy? PloS one. 2023:18(3):e0281856. doi: 10.1371/journal.pone.0281856. Epub 2023 Mar 31 [PubMed PMID: 37000805]
Jiang Z, Davies B, Zipser C, Margetis K, Martin A, Matsoukas S, Zipser-Mohammadzada F, Kheram N, Boraschi A, Zakin E, Obadaseraye OR, Fehlings MG, Wilson J, Yurac R, Cook CE, Milligan J, Tabrah J, Widdop S, Wood L, Roberts EA, Rujeedawa T, Tetreault L, AO Spine RECODE-DCM Diagnostic Criteria Incubator. The Frequency of Symptoms in Patients With a Diagnosis of Degenerative Cervical Myelopathy: Results of a Scoping Review. Global spine journal. 2024 May:14(4):1395-1421. doi: 10.1177/21925682231210468. Epub 2023 Nov 2 [PubMed PMID: 37917661]
Level 2 (mid-level) evidenceJiang Z, Davies B, Zipser C, Margetis K, Martin A, Matsoukas S, Zipser-Mohammadzada F, Kheram N, Boraschi A, Zakin E, Obadaseraye OR, Fehlings MG, Wilson J, Yurac R, Cook CE, Milligan J, Tabrah J, Widdop S, Wood L, Roberts EA, Rujeedawa T, Tetreault L, AO Spine RECODE-DCM Diagnostic Criteria Incubator. The value of Clinical signs in the diagnosis of Degenerative Cervical Myelopathy - A Systematic review and Meta-analysis. Global spine journal. 2024 May:14(4):1369-1394. doi: 10.1177/21925682231209869. Epub 2023 Oct 30 [PubMed PMID: 37903098]
Level 1 (high-level) evidenceLukies MW, Teoh WW, Clements W. Safety of CT-guided cervical nerve root corticosteroid injections. Journal of medical imaging and radiation oncology. 2019 Jun:63(3):300-306. doi: 10.1111/1754-9485.12870. Epub 2019 Mar 12 [PubMed PMID: 30859711]
Miyoshi K. [Dissociation of Anatomical (Neurological) Diagnosis and Imaging Diagnosis]. Brain and nerve = Shinkei kenkyu no shinpo. 2019 Mar:71(3):249-256. doi: 10.11477/mf.1416201250. Epub [PubMed PMID: 30827958]
Jenkins HJ, Downie AS, Moore CS, French SD. Current evidence for spinal X-ray use in the chiropractic profession: a narrative review. Chiropractic & manual therapies. 2018:26():48. doi: 10.1186/s12998-018-0217-8. Epub 2018 Nov 21 [PubMed PMID: 30479744]
Level 3 (low-level) evidenceBise S, Pesquer L, Feldis M, Bou Antoun M, Silvestre A, Hocquelet A, Dallaudière B. Comparison of three CT-guided epidural steroid injection approaches in 104 patients with cervical radicular pain: transforaminal anterolateral, posterolateral, and transfacet indirect. Skeletal radiology. 2018 Dec:47(12):1625-1633. doi: 10.1007/s00256-018-3027-0. Epub 2018 Jul 22 [PubMed PMID: 30032466]
Fakhoury J, Dowling TJ. Cervical Degenerative Disc Disease. StatPearls. 2025 Jan:(): [PubMed PMID: 32809607]
Nordin M, Randhawa K, Torres P, Yu H, Haldeman S, Brady O, Côté P, Torres C, Modic M, Mullerpatan R, Cedraschi C, Chou R, Acaroğlu E, Hurwitz EL, Lemeunier N, Dudler J, Taylor-Vaisey A, Sönmez E. The Global Spine Care Initiative: a systematic review for the assessment of spine-related complaints in populations with limited resources and in low- and middle-income communities. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2018 Sep:27(Suppl 6):816-827. doi: 10.1007/s00586-017-5446-3. Epub 2018 Feb 28 [PubMed PMID: 29492717]
Level 1 (high-level) evidenceFehlings MG, Tetreault LA, Riew KD, Middleton JW, Aarabi B, Arnold PM, Brodke DS, Burns AS, Carette S, Chen R, Chiba K, Dettori JR, Furlan JC, Harrop JS, Holly LT, Kalsi-Ryan S, Kotter M, Kwon BK, Martin AR, Milligan J, Nakashima H, Nagoshi N, Rhee J, Singh A, Skelly AC, Sodhi S, Wilson JR, Yee A, Wang JC. A Clinical Practice Guideline for the Management of Patients With Degenerative Cervical Myelopathy: Recommendations for Patients With Mild, Moderate, and Severe Disease and Nonmyelopathic Patients With Evidence of Cord Compression. Global spine journal. 2017 Sep:7(3 Suppl):70S-83S. doi: 10.1177/2192568217701914. Epub 2017 Sep 5 [PubMed PMID: 29164035]
Level 1 (high-level) evidenceGuan Q, Xing F, Long Y, Xiang Z. Cervical intradural disc herniation: A systematic review. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2018 Feb:48():1-6. doi: 10.1016/j.jocn.2017.10.024. Epub 2017 Nov 20 [PubMed PMID: 29162303]
Level 1 (high-level) evidenceMattozzi I. [Conservative treatment of cervical radiculopathy with 5% lidocaine medicated plaster]. Minerva medica. 2015 Feb:106(1):1-7 [PubMed PMID: 25582970]
Level 2 (mid-level) evidenceCarlesso LC, Macdermid JC, Gross AR, Walton DM, Santaguida PL. Treatment preferences amongst physical therapists and chiropractors for the management of neck pain: results of an international survey. Chiropractic & manual therapies. 2014 Mar 24:22(1):11. doi: 10.1186/2045-709X-22-11. Epub 2014 Mar 24 [PubMed PMID: 24661461]
Level 3 (low-level) evidenceWong JJ, Côté P, Quesnele JJ, Stern PJ, Mior SA. The course and prognostic factors of symptomatic cervical disc herniation with radiculopathy: a systematic review of the literature. The spine journal : official journal of the North American Spine Society. 2014 Aug 1:14(8):1781-9. doi: 10.1016/j.spinee.2014.02.032. Epub 2014 Mar 12 [PubMed PMID: 24614255]
Level 1 (high-level) evidenceLuyao H, Xiaoxiao Y, Tianxiao F, Yuandong L, Ping Wang. Management of Cervical Spondylotic Radiculopathy: A Systematic review. Global spine journal. 2022 Oct:12(8):1912-1924. doi: 10.1177/21925682221075290. Epub 2022 Mar 24 [PubMed PMID: 35324370]
Level 1 (high-level) evidenceMjåset C, Solberg TK, Zwart JA, Småstuen MC, Kolstad F, Grotle M. Anterior surgical treatment for cervical degenerative radiculopathy: a prediction model for non-success. Acta neurochirurgica. 2023 Jan:165(1):145-157. doi: 10.1007/s00701-022-05440-2. Epub 2022 Dec 8 [PubMed PMID: 36481873]
Oshina M, Kawamura N, Tachibana N, Higashikawa A, Ono T, Takeshita Y, Okazaki R, Fukushima M, Iwai H, Kato S, Matsubayashi Y, Taniguchi Y, Tanaka S, Oshima Y. Comparison of surgical outcomes for cervical radiculopathy by nerve root level. Scientific reports. 2024 Aug 14:14(1):18891. doi: 10.1038/s41598-024-69843-0. Epub 2024 Aug 14 [PubMed PMID: 39143150]
Peene L, Cohen SP, Brouwer B, James R, Wolff A, Van Boxem K, Van Zundert J. 2. Cervical radicular pain. Pain practice : the official journal of World Institute of Pain. 2023 Sep:23(7):800-817. doi: 10.1111/papr.13252. Epub 2023 Jun 4 [PubMed PMID: 37272250]
Tavanaei R, Ansari A, Hatami A, Heidari MJ, Dehghani M, Hajiloo A, Khorasanizadeh M, Margetis K. Postoperative complications of anterior cervical discectomy and fusion: A comprehensive systematic review and meta-analysis. North American Spine Society journal. 2025 Mar:21():100596. doi: 10.1016/j.xnsj.2025.100596. Epub 2025 Feb 8 [PubMed PMID: 40145067]
Level 1 (high-level) evidenceKuligowski T, Skrzek A, Cieślik B. Manual Therapy in Cervical and Lumbar Radiculopathy: A Systematic Review of the Literature. International journal of environmental research and public health. 2021 Jun 7:18(11):. doi: 10.3390/ijerph18116176. Epub 2021 Jun 7 [PubMed PMID: 34200510]
Level 1 (high-level) evidenceCaridi JM, Pumberger M, Hughes AP. Cervical radiculopathy: a review. HSS journal : the musculoskeletal journal of Hospital for Special Surgery. 2011 Oct:7(3):265-72 [PubMed PMID: 23024624]
Bukhari SR, Shakil-Ur-Rehman S, Ahmad S, Naeem A. Comparison between effectiveness of Mechanical and Manual Traction combined with mobilization and exercise therapy in Patients with Cervical Radiculopathy. Pakistan journal of medical sciences. 2016 Jan-Feb:32(1):31-4. doi: 10.12669/pjms.321.8923. Epub [PubMed PMID: 27022340]