Introduction
Cytomegalovirus (CMV) is a double-stranded DNA virus that belongs to the family of Herpesviridae and is also known as herpesvirus-5 (HHV-5).[1] The CMV genome is the largest among human viruses, measuring approximately 230 kb and containing 200 genes that encode proteins.[2][3] Infection with CMV is common in both children and adults, with a worldwide seroprevalence ranging from 24% to 100%, which increases with age.[4] Transmission of CMV occurs through the exchange of bodily fluids, eg, saliva, fomites, transfusion of blood products, and by fluids during sexual contact. CMV can also be transmitted via solid organ transplantation (SOT) and hematopoietic stem cell transplantation (HSCT).[5] As with other herpesviruses, it establishes lifelong latency, remaining dormant in monocytes through specific mechanisms of transcriptional silencing.[1][6][7] CMV is a globally widespread virus that becomes latent after primary infection and can become reactivated in the setting of severe immunosuppression.
In immunocompetent patients, primary CMV typically causes asymptomatic disease or mild to moderate mononucleosis-like or flu-like syndromes, characterized by symptoms such as malaise, fever, lymphadenopathy, and arthralgia.[4] Infection with CMV is a leading cause of morbidity in immunosuppressed individuals, e.g., due to HIV/AIDS, after receiving SOT and HSCT. In the latter population, CMV can cause severe organ damage and even cause rejection of the transplanted organ.[8] Last but not least, mothers who become infected while they are pregnant may pass the virus to the embryo, and in some cases, this can cause congenital CMV.[4]
CMV infection is defined by the detection of active CMV replication in tissue specimens, peripheral blood, or other body fluids, irrespective of clinical manifestations. CMV disease refers to the presence of CMV infection in conjunction with clinically attributable symptoms and signs.[9] It is subclassified into CMV syndrome, characterized by non-specific constitutional symptoms, eg, fever, malaise, and lymphadenopathy, which are often accompanied by hematologic abnormalities such as leukopenia or thrombocytopenia, and tissue-invasive or end-organ CMV disease.[9] CMV syndrome involves direct viral-mediated injury to specific organs, including, but not limited to, the gastrointestinal tract, lungs, hepatobiliary system, renal parenchyma, myocardium, pancreas, central nervous system, and retina.[9]
Primary, but mostly reactivated CMV can cause viremia, infecting various organs, including the eyes, stomach, esophagus, and colon, especially in patients with profound immunosuppression.[8] CMV colitis and CMV esophagitis are the two most common gastrointestinal (GI) tract manifestations of CMV disease.[10]
Prevention of CMV viremia and CMV end-organ disease is critical, particularly in immunosuppressed patients. This can be achieved through assessment of CMV serostatus in both donor and recipient, along with regular monitoring for the emergence of viremia via frequent CMV DNA quantification. In high-risk populations, prophylactic strategies may involve universal antiviral administration to all at-risk individuals, targeted prophylaxis for those at highest risk, or a pre-emptive approach. The pre-emptive strategy involves initiating antiviral therapy upon early detection of CMV DNAemia, before the onset of clinical disease.[8]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
CMV is a ubiquitous, double-stranded DNA virus that belongs to the Herpesviridae family and is also known as Herpesvirus-5. Globally, the seroprevalence of CMV infection has been reported to range from 24% to 100%, although the data are heterogeneous.[5][11][12] Once CMV infection is acquired, the virus can persist in cells of the myeloid lineage, resulting in lifelong viral latency as it evades the immune system. In immunocompetent individuals, CMV is maintained in a state of balance; however, when immunosuppression occurs, CMV can begin to replicate.[8] Cytotoxic CD8 T-cells regularly control CMV reactivation; however, in immunosuppressed patients, such as those after SOT or HSCT, the failure of immune control and the stimulated CMV replication lead to reactivation. [13] This can occur locally at a specific organ and may be undetected by serum molecular viral load testing, or it can cause viremia, also known as CMV DNAemia.[8]
Epidemiology
Infection with CMV is common in both children and adults, and it is endemic worldwide. As with other herpesviruses, it establishes lifelong latency, remaining dormant in certain cells through specific mechanisms of transcriptional silencing.[1][6][7] Globally, the seroprevalence of CMV infection has been reported to range from 24% to 100%, although the data are heterogeneous and limited.[5][11][12] In the United States, age-adjusted CMV IgG seroprevalence was approximately 30% in children by the age of 5 and more than 50% in adults by the age of 40.[4] CMV seroprevalence is independently associated with female sex, lower socioeconomic status, older age, lower household education, and increased crowding in households.[4][11][14]
CMV infection can occur as a primary infection, reinfection, or reactivation. Transmission of CMV can occur in numerous ways, mostly via the exchange of bodily fluids, such as saliva, fomites, transfusion of blood products, SOT, HSCT, breastfeeding, viral shedding in close-contact settings, perinatally, and sexual transmission.[15][16] CMV represents the most common opportunistic viral infection in patients who have received HSCT and SOT and can cause life-threatening tissue-invasive disease, most commonly in the gastrointestinal tract, lungs, liver, eye, and central nervous system.[17] CMV is the most common viral opportunistic infection in patients with HIV/AIDS and can cause severe disease in patients, especially those with profound immunosuppression with a CD4 count <50 cells/µL.[18]
Reactivation is seen in patients who become immunocompromised and is associated with increased morbidity and mortality.[15][16]. The most common manifestations of CMV end-organ damage (EOD) are retinitis, followed by pneumonitis, colitis, esophagitis, encephalitis, polyneuropathy, and pneumonitis.[19][20] CMV colitis and esophagitis are the two most frequent CMV-related gastrointestinal tract CMV EOD.[10]
CMV in transplant patients
CMV infection and disease are important causes of morbidity and mortality in transplant recipients. CMV disease can present as CMV EOD, which is a tissue-invasive disease, and the CMV syndrome, characterized by fever, leukopenia, and malaise.[21] Tissue-invasive disease in this population manifests as EOD, including conditions such as colitis, esophagitis, pneumonitis, gastroenteritis, hepatitis, and rhinitis. Despite strategies to prevent CMV disease, CMV infection occurs in up to 50% of high-risk SOT patients, typically following organ transplantation from a CMV-seropositive donor to a CMV-seronegative recipient (D+/R−), and in 17% of CMV-seropositive recipients (R+).[17][22] Risk factors for CMV infection or disease include: a D+/R− serological status, seropositive recipients, induction with mycophenolic acid, steroid or polyclonal antibodies, advanced age of donors and recipients, and a history of acute organ rejection.[23] CMV infection following solid organ transplantation remains linked to a heightened risk of acute rejection and graft failure, increased mortality, and greater healthcare costs due to more frequent hospital readmissions, longer inpatient stays, and overall higher hospitalization costs.[24]
CMV infection in HSCT recipients
CMV reactivation is a common complication following allogeneic hematopoietic stem cell transplantation (Allo-HSCT), particularly during periods of immunosuppression. Positive CMV serology has been linked to reduced overall survival and increased non-relapse mortality, as reactivation can lead to severe end-organ disease, including pneumonia, colitis, and retinitis [25]. Beyond direct organ damage, CMV infection has also been linked to an increased risk of secondary bacterial and fungal infections, as well as a higher incidence of graft-versus-host disease (GVHD). This CMV section may be attributed to CMV's immunomodulatory effects, which can influence the dynamics of the immune system post-transplant. CMV serostatus is a critical determinant of post-transplant outcomes. Positive serology, particularly in the recipient, is associated with significant transplant-related morbidity and mortality.[26] The worst outcomes in terms of survival and non-relapse mortality occur in CMV seronegative donor/seropositive recipient (D−/R+) pairs, followed by seropositive donor/seropositive recipient (D+/R+) combinations [27].
CMV in HIV/AIDS patients
CMV esophagitis is the second most common gastrointestinal manifestation of CMV in individuals with HIV/AIDS. CMV is the most frequent viral opportunistic infection in this population, particularly when CD4+ T lymphocyte counts fall below 50 cells/mm³.[28] Dysphagia and odynophagia are the hallmark symptoms of CMV esophagitis. Endoscopy with biopsy remains the gold standard for diagnosis. Endoscopic findings may include well-demarcated, vertical, single or multiple ulcers, or diffuse esophagitis, most commonly located in the mid-to-distal esophagus.[29]
CMV Colitis
CMV colitis occurs most commonly in immunocompromised hosts, including patients with HIV/AIDS and those who have undergone SOT, HSCT, and chemotherapy. CMV colitis accounts for up to 30% of CMV gastrointestinal disease in solid organ transplant (SOT) patients. The role of CMV in patients with inflammatory bowel disease (IBD) remains controversial. It is unclear whether CMV reactivation contributes to disease exacerbation in individuals with established IBD, occurs as a result of the disease or its treatment, or merely represents an incidental finding without pathogenic significance.[30][31] In addition, in patients with inflammatory bowel disease, studies have shown that immunosuppression from high-dose systemic corticosteroids is linked to the development of CMV colitis in patients with active ulcerative colitis.[32] Other immunomodulatory therapies used in ulcerative colitis, such as thiopurines and methotrexate, have also been associated with CMV infection, whereas anti-TNF agents have not shown a similar association.[33][34] Patients undergoing allo-HSCT are at increased risk for CMV infection or reactivation. However, distinguishing between gastrointestinal graft-versus-host disease (GVHD) and CMV colitis is crucial in this population. CMV reactivation in patients with severe ulcerative colitis is reported to have a prevalence of 4.5% to 16.6% and as high as 25% in patients requiring colectomy for severe colitis. The CMV infection rate in patients with severe corticosteroid-refractory ulcerative colitis ranges from 20% to 40% when infection was diagnosed using antigenemia and histological examination of tissue biopsies.[35][36]
CMV colitis can also occur in healthy immunocompetent patients, and there have been increasing reports, although the incidence is rare.[37] The prevalence of CMV infection in severe acute colitis ranges from 21% to 34% in healthy individuals.[38][39] These patients are usually older adults with underlying comorbidities, and reported cases have shown an association with underlying ulcerative colitis.[32] They usually are asymptomatic or have self-limited disease, usually presenting as gastroenteritis, duodenitis, ileitis, proctitis, or exacerbation of inflammatory bowel disease.[37] Colitis may result in a chronic infection or a lifelong carrier state with intermittent reactivation. Symptoms usually include diarrhea, abdominal pain, and hematochezia or melena. The outcomes are typically favorable, including resolution without antiviral treatment in nearly 25% of these patients.[40] CMV colitis should be a consideration in the differential diagnosis of hematochezia, not only in immunocompromised patients but also in immunocompetent patients, particularly in older adults with underlying comorbidities.[37]
CMV esophagitis
CMV esophagitis is the third leading cause of infectious esophagitis, after Candida and herpes simplex virus, mostly occurring in patients with profound immunosuppression, either from HIV/AIDS, treatment with immunosuppressants, including post-SOT nd HSCT. [41] Dysphagia and odynophagia are the most common presenting symptoms in CMV esophagitis, especially in the middle to distal esophagus. CMV esophagitis is associated with a poor prognosis, a risk of recurrence, and a high rate of morbidity and mortality at 12 months.[13]
Pathophysiology
Once CMV is transmitted, and the primary infection clears, the virus remains dormant in myeloid cells.[42] Vital replication and reactivation are contained primarily by cytotoxic T-cell immunity. However, when reactivation occurs, virions are released into the bloodstream and other body fluids, leading to the presence of symptoms, predominantly in immunocompromised patients.[43][44]
CMV can infect a large variety of cell types.[45] It can also remain latent in bone marrow progenitor cells after primary infection and replicate in various cells and tissues.[14] Highly conserved glycoproteins M and B (gM and gB, respectively) binding to the host cell’s heparin-sulfate proteoglycans is the first step in CMV infection.[45] Glycoprotein B is also crucial for the spread of CMV from cell to cell.[45]
In immunocompetent individuals, CMV replication is controlled by antibodies targeting key viral surface glycoproteins, as well as a robust cell-mediated immune response involving natural killer (NK) cells, CD4+ T cells, and CD8+ T cells. In immunocompromised patients, CMV disease may result from reactivation of the latent virus, primary infection, or reinfection with a different strain. Reactivation is primarily due to impaired cell-mediated immunity rather than a deficiency in humoral immunity, as these patients typically have sufficient levels of anti-CMV antibodies.[5] The inability to control CMV replication in these patients is mainly due to dysfunctional CD4+ and CD8+ T cell responses, including impaired mono- and polyfunctional cytokine production. Additionally, CMV encodes proteins that disrupt immune defenses by interfering with class I and class II HLA presentation, natural killer (NK) cell activity, the cell cycle, and key apoptotic and inflammatory pathways that generally help contain the virus[14]
Histopathology
Histopathological diagnosis of CMV infection in any tissue can be made by identifying cytomegalic cells and CMV inclusion bodies, which are visible when stained with hematoxylin and eosin (H&E). These cells measure 25 to 35 micrometers in size, with basophilic intranuclear inclusions that are occasionally surrounded by a clear halo; intracytoplasmic inclusions can also be observed.[5] Finding these cytomegalic cells can be tedious and requires an astute pathologist, as well as a sufficient number of biopsy samples for evaluation.[5] The advent of immunohistochemical staining using anti-CMV monoclonal antibodies is the gold standard for making a diagnosis.[5][46][47]
In gastrointestinal CMV infection, mucosal infection by CMV leads to inflammation and tissue necrosis, vascular endothelial involvement, and, ultimately, ischemic mucosal injury. The occlusion of vasculature contributes mainly to the cause of tissue injury and ulcer formation.[5] In CMV esophagitis, there are no specific endoscopic features that can confirm the diagnosis; therefore, the definitive diagnosis depends on biopsy findings. The diagnosis of CMV colitis requires histological examination of biopsy tissues taken from the base or edge of the ulcer. Patients with punched-out ulcers usually have a higher number of inclusion bodies on histology.[48] Histological examination can differentiate CMV colitis from other causes, eg, infectious colitis, ulcerative colitis, or drug-induced colitis.[49] Colonic mucosal biopsies stained with H&E may show viral inclusion bodies characteristic of CMV colitis, commonly referred to as "owl eye" inclusions, which are highly specific for CMV. However, H&E staining has limited sensitivity and is less reliable than immunohistochemistry, which is considered the gold standard for diagnosing CMV colitis.[47]
History and Physical
In healthy individuals, CMV infection is often asymptomatic and subclinical, typically resolving with minimal sequelae and a self-limiting course. When symptoms occur, the infection can present as a mononucleosis-like syndrome, including fever, fatigue, rash, and lymphadenopathy.[5] Rarely, symptoms can be prolonged, and on occasion, severe CMV infection may lead to sequelae, such as myocarditis, pneumonitis, and gastrointestinal involvement.[50]
CMV infection in immunosuppressed patients can result in viremia and/or EOD, affecting any organ. Clinical signs and symptoms are related to the organ affected by CMV. The affected organ may be the transplanted organ, resulting in impaired function and even graft rejection, or another organ that can exhibit dysfunction. Examples of these may be CMV pneumonitis in the case of a lung transplant who presented with respiratory symptoms or CMV nephritis in a patient who had undergone a lung transplant, who presented with hematuria.[51][52]
CMV infection can occur anywhere in the gastrointestinal tract in immunosuppressed patients.[5] In CMV esophagitis, the typical large, distal solitary or multiple esophageal ulcers usually produce symptoms such as odynophagia, dysphagia, and substernal chest pain.[5][53][41] Other symptoms include nausea, vomiting, abdominal pain, weight loss, and diarrhea.[10] Rare presentations include gastrointestinal bleeding and stricture formation.[5] Patients with CMV colitis present with non-specific symptoms, including diarrhea, abdominal pain, fever, rectal bleeding, and weight loss.[37] The symptoms can mimic an exacerbation of inflammatory bowel disease, and it is challenging to distinguish between ulcerative colitis and CMV colitis based solely on clinical presentation.[54] A high index of suspicion is necessary, and laboratory investigations are essential in diagnosing CMV colitis.
Evaluation
In immunocompetent patients with symptoms of CMV disease, including fever, malaise, and lymphadenopathy, a definitive diagnosis is rarely necessary. In patients who have received SOT or HSCT, serological testing for IgG of the donor and the recipient prior to the transplantation provides essential information regarding serostatus for risk-stratification of CMV infection. This should not be used for the diagnosis of acute infection, but for closely monitoring them and making decisions regarding CMV preventive strategies.[55][56]
In immunosuppressed patients or those for whom a diagnosis is necessary, serum viral DNA identification is the preferred initial test for suspected CMV infection. Quantitative CMV DNA PCR assays are the preferred method for viral detection and the most sensitive method for detecting CMV. High levels of DNA in blood in HSCT patients are a strong predictor of CMV disease.[55][56][57][56]
CMV pp65 antigenemia, which involves detecting the pp65 antigen in peripheral blood leukocytes, is a rapid and semi-quantitative method for diagnosing CMV infection. In transplant recipients, a positive pp65 assay is a strong predictor of progression to invasive CMV disease.[58][59]
For the diagnosis of tissue CMV disease, the gold standard for diagnosing CMV EOD is by visualizing pathognomonic CMV inclusion bodies on histopathological examination. CMV DNA PCR can also be used to diagnose CMV disease in tissues, such as the detection of CMV in bronchoalveolar lavage fluid or lung tissue samples through virus isolation, histopathologic testing, and immunohistochemical analysis.[52] .
CMV Gastrointestinal Disease
For the diagnosis of CMV gastrointestinal disease, the identification of CMV in biopsy specimens using standard histopathologic or immunohistochemical stains has traditionally been considered the gold standard for confirming proven disease. Quantitative PCR (qPCR) methods are increasingly being evaluated and utilized on tissue specimens to support the diagnosis of tissue-invasive CMV disease.[17]
CMV Esophagitis
The most reliable modality for diagnosing CMV esophagitis is endoscopy and biopsy.[13] It is essential to note that CMV can remain latent or be asymptomatic in immunocompetent hosts; therefore, the detection of CMV in tissue or body fluids does not necessarily indicate disease. Relying on serologic testing and anti-CMV antibodies is not helpful, as the disease correlates with defective cell-mediated immunity, rather than humoral immunity. Anti-CMV IgM indicates a recent infection, and anti-IgG can be present in most adults who have previously been infected with CMV. Blood and oropharyngeal viral cultures, even if obtained from patients with risk factors such as organ transplant recipients, can help identify those at high risk for developing the disease; however, they are not sensitive or specific for identifying active disease.[5]
The endoscopic appearance of CMV esophagitis ulcers is well-demarcated, vertical, or horizontal linear shallow ulcers at the mid to distal esophagus.[10] Ulcerations can be single or multiple, deep, and resemble cavitations, with associated features such as mucosal edema and nodularity.[13] The intervening mucosa between the ulcers is typically normal.[5][53] There are no pathognomonic endoscopic or pathological features for CMV disease; therefore, finding cytomegalic cells with CMV inclusion bodies is critical. Successful identification of CMV at a tissue level is associated with having an adequate number of samples and the expertise of the examining pathologist.[5] Immunohistochemistry to label specific CMV antigens is a useful adjunct modality when inclusion bodies are difficult to identify.[46] CMV esophagitis carries a poor prognosis, a high risk of recurrence, and a high rate of one-year morbidity and mortality.[13]
CMV Colitis
In healthy subjects, CMV colitis is usually asymptomatic or causes self-limited disease but may result in chronic infection or a life-long carrier state with intermittent reactivation. Patients with CMV colitis present with non-specific symptoms, including diarrhea, abdominal pain, fever, rectal bleeding, and weight loss. Hematochezia and diarrhea are the most frequently observed symptoms in these patients. Therefore, a high index of suspicion is necessary, and laboratory investigations are essential in diagnosing CMV colitis. Several methods are possible, including antigenemia, endoscopy, histological examination of biopsy tissues, CMV culture, and quantitative polymerase chain reaction of tissue samples.
The diagnosis of CMV colitis requires histological examination of biopsy tissues taken from the base or edge of the ulcer. One of the significant endoscopic findings in CMV colitis is the presence of ulcerations with a well-defined, punched-out appearance, usually found in 70% to 80% of patients.[54][60] However, ulcerations may be irregular and may also have a cobblestone-like appearance. An ulcer of the cecum involving the ileocecal valve is proposed as a specific finding in CMV colitis in patients with graft-versus-host disease.[61]
Patients with punched-out ulcers usually have a higher number of inclusion bodies on histology.[48] Histological examination can differentiate CMV colitis from other causes, eg, infectious colitis, ulcerative colitis, or drug-induced colitis.[49] Colonic mucosal biopsies stained with H&E may show viral inclusion bodies characteristic of CMV colitis, commonly referred to as "owl eye" inclusions, which are highly specific for CMV. However, H&E staining has limited sensitivity and is less reliable than immunohistochemistry, which is considered the gold standard for diagnosing CMV colitis.[47] Diagnosis of CMV colitis requires serologic testing and endoscopic biopsies to confirm the diagnosis.
Histopathology
The identification of CMV disease in H&E-stained tissue sections relies on the characteristic presence of viral inclusions, often described as having an "owl's eye" appearance. These inclusions are highly specific for CMV. However, CMV-specific immunohistochemistry is the gold standard for confirming CMV infection in tissue biopsies. Therefore, if H&E-stained sections are negative but there is a strong clinical suspicion of CMV colitis, IHC staining should be performed to enhance diagnostic accuracy.[46]
Real-time Polymerase Chain Reaction
Qualitative and quantitative PCR (qPCR) for CMV DNA in blood is the diagnostic test of choice due to its high sensitivity and specificity.[17] Efforts are being made to improve standardization and reduce variability across PCR testing platforms and assays. This variability arises due to differences in assays, sample size, extraction methods, amplicon size, and sample types, such as whole blood or plasma.[17]
Generally, one must be vigilant when testing for blood viral load by PCR, as CMV DNA may be compartmentalized in tissue, especially in CMV EOD, and absent from the blood. In these cases, clinical utility is limited, as CMV DNA may be present in patients with active disease in tissue but is not detectable in blood. In contrast, serial PCR testing may offer greater clinical value by enabling the monitoring of viral dynamics over time and assessing disease progression or response to therapy.[17]
CMV Culture
This test offers high sensitivity and specificity for diagnosing CMV colitis; however, its drawback is that culture is time-consuming, which can delay diagnosis and hinder timely treatment in vulnerable patients.[62]
Serologic Testing
The CMV IgG test helps determine prior exposure to the virus, understand the serostatus of patients, and risk-stratify them, especially immunosuppressed individuals. CMV IgM testing may indicate an acute systemic infection or reactivation, but it lacks specificity and sensitivity for diagnosing tissue-invasive disease.
pp65 Antigenemia Assay
The pp65 antigenemia assay is a laboratory test to detect CMV pp65 antigen, a structural viral protein, in peripheral blood leukocytes. It is primarily utilized to diagnose and monitor active CMV infection, particularly in immunocompromised patients. This test is valuable for the early detection of CMV viremia and for guiding preemptive antiviral therapy before the onset of clinical disease. It may aid in the early detection of CMV viremia and help predict clinical outcomes in patients, especially in tissue-invasive disease, such as suspected CMV colitis. Although many centers now prefer quantitative CMV PCR due to its higher sensitivity and ease of automation, the pp65 assay remains clinically useful, especially in settings with established expertise in its interpretation and in non-neutropenic patients, where adequate leukocytes are available for analysis.[63][64]
Table. CMV Diagnostic Tests
| Diagnostic Test | Purpose | Sample Type | Clinical Utility | Limitations |
| CMV IgM | Recent antibody response | Detect recent or current infection | Pregnancy screening, initial exposure | False positives may persist post-infection |
| CMV IgG | Past antibody response | Detect prior exposure & immunity | Pregnancy, transplant evaluation | Doesn't indicate current infection |
| IgG Avidity | Strength of IgG binding | Distinguish recent vs past infection | Pregnancy (timing of infection) | Only useful early in infection |
| CMV PCR (DNA test) | Viral DNA in blood (quantitative) | Detect & monitor active infection | Immunocompromised patients, treatment response | Doesn't show tissue involvement |
| pp65 antigenemia | Viral protein in leukocytes | Detect active infection | Transplant patients | Requires fresh blood; labor-intensive; cannot be neutropenic |
| Tissue Biopsy | CMV inclusions via histopathology | Confirm tissue-invasive disease | CMV colitis, esophagitis, pneumonitis | Invasive; localized disease may be missed |
Treatment / Management
Immunocompetent patients present with minimal or no symptoms and are self-limited and do not require specific therapy other than symptomatic management. However, antiviral therapy should be considered in severe cases of CMV mononucleosis, CMV infection, and CMV disease in immunocompromised patients. Toxicity is not uncommon when using these agents, and their risks must be weighed against the benefits of initiating treatment. Prophylaxis is considered for all high-risk patients. Treatment of CMV disease or CMV end-organ disease is typically with antivirals. Patients should be monitored for treatment failures, in which cases, the causes should be considered, such as the emergence of resistance to therapy. In such cases, testing for antiviral drug resistance should be performed.
Prophylaxis is administered to a patient to prevent primary, reactivation, or recurrence of CMV infection. In contrast, preemptive therapy is given to asymptomatic CMV-infected patients who have had CMV detected on screening tests.
Patients who are immunosuppressed, either by living with HIV/AIDS, on immunosuppressants, or who will be undergoing a SOT or HSCT, should be tested for CMV IgG serostatus, as they are at high risk for complications from CMV infection. Despite progress in the prevention and treatment of CMV in these populations, CMV infection continues to impact SOT and HSCT recipients negatively, and it is therefore important to prevent it in these patients. Pre-transplant CMV serology status is the most widely used indicator that predicts the occurrence of CMV infection. [65]CMV infection occurs in up to 50% of high-risk SOT patients, usually when the organ is transplanted from a CMV-seropositive donor to a CMV-seronegative recipient (D+/R−), and in 17% of CMV-seropositive recipients (R+).[17][22] Risk factors for CMV infection or disease include a D+/R− serological status, seropositive recipients, administration of anti-thymocyte globulin due to its potent T-cell-depleting effects, induction with mycophenolic acid, steroid therapy, or polyclonal antibodies, advanced age of both donors and recipients, and a history of acute organ rejection.[23] Other factors that influence the depth of CMV infection are whether it is primary, reactivation, or superinfection, the degree of immunosuppression, and whether it is an SOT or HSCT.[17](A1)
Prevention
Recipients of solid organ transplants from CMV-seropositive donors (D+) who are at risk for CMV infection should receive prophylactic antiviral therapy, typically with valganciclovir or intravenous ganciclovir, along with routine virologic surveillance. The recipient's risk stratification determines the duration of prophylaxis.
High-risk patients (D+/R−) should receive prophylaxis for a minimum of 6 months, whereas intermediate-risk recipients (R+) are typically treated for 3 months. In low-risk individuals (D−/R−), a prophylactic course of at least 3 months is also generally advised to mitigate the risk of late-onset disease.[65]
Even with prophylaxis, however, CMV infections can still occur, and treatment can be challenging, even with the administration of standard-of-care first- and second-line antivirals.[65] Valganciclovir is the drug of choice, primarily because it is oral, has good bioavailability, and established efficacy; intravenous gancyclovir can be used if vascular access is available.[65] Newer antivirals, such as letermovir, have shown non-inferiority to valganciclovir in preventing CMV disease. Letermovir is a first-in-class antiviral that has been explicitly approved for this indication.[66][67] Maribavir has also been used for prophylactic treatment and has less toxicity than valganciclovir.[67](A1)
Despite preventive measures, CMV infection following solid organ transplantation remains linked to a heightened risk of acute rejection and graft failure, increased mortality, and higher healthcare costs due to more frequent hospital readmissions, longer inpatient stays, and overall higher hospitalization costs.[24]
Currently, there are six FDA-approved antiviral agents used in the management of CMV infections. Maribavir and letermovir have been most recently added to the armamentarium for the prevention and treatment of CMV infection. Each antiviral targets different aspects of the viral replication cycle, thus inhibiting replication.[16][67][16][68][69][70](B2)
-
Ganciclovir – This is the treatment of choice, especially for severe cases of CMV infection. It is a guanosine nucleoside analog activated by the viral UL97 kinase, and it inhibits viral DNA synthesis.
-
Valganciclovir – An oral prodrug of ganciclovir. It can be administered orally in less severe CMV infections.
-
Cidofovir – A cytidine monophosphate analog that inhibits viral DNA polymerase independent of viral activation.
-
Foscarnet – A pyrophosphate analog that directly inhibits the viral UL54 DNA polymerase.
-
Maribavir – A selective inhibitor of the viral UL97 kinase, with a different binding site and resistance profile from ganciclovir.[70]
-
Letermovir – This is the newest antiviral approved in 2017 for prevention and treatment, which works by targeting the viral terminase complex (UL56-UL89), thereby inhibiting CMV DNA cleavage and packaging.
Treatment of CMV
Valganciclovir and ganciclovir are the first-line treatments for CMV infection. However, their use may be limited by hematologic toxicity or the development of antiviral resistance. Resistance to ganciclovir can occur through mutations in UL97, which impairs drug phosphorylation, or UL54, which encodes the viral DNA polymerase.[67]
Cidofovir and foscarnet are second-line agents, primarily used when resistance or toxicity limits the effectiveness of first-line therapy. Both drugs are administered intravenously and are associated with significant nephrotoxicity.[67][71][72] All of these antivirals ultimately target the viral DNA polymerase, and mutations in UL54 may confer cross-resistance to ganciclovir, cidofovir, and foscarnet.[67][70](A1)
Letermovir, in contrast, is a CMV-specific antiviral with a different mechanism of action. It targets the pUL56 subunit of the terminase complex, inhibiting the final stage of viral DNA processing and packaging. Letermovir is well-tolerated, lacks hematologic or renal toxicity, and is available in both oral and intravenous formulations.[67][73]
Refractory CMV
Failure to achieve clinical or virologic response after at least 14 days of appropriately dosed antiviral therapy is defined as refractory or resistant CMV infection.[74][75] While antiviral resistance is a recognized cause, refractory CMV can also occur in the absence of documented resistance, though data on such cases remain limited. Reported rates of antiviral-resistant CMV in SOT recipients range up to 18%, particularly among those receiving prolonged or subtherapeutic antiviral therapy.[74]
Therapeutic management of CMV infections in SOT recipients remains challenging due to the limited efficacy-to-toxicity ratio of conventional antivirals and the complexity of transplant-related immunosuppression. Agents such as ganciclovir, foscarnet, and cidofovir are associated with significant adverse effects, including myelosuppression and nephrotoxicity, which can limit their utility. The recent availability of novel anti-CMV therapies, including maribavir and letermovir, along with investigational approaches such as adoptive CMV-specific T cell therapy, offers promising alternatives that may enhance viral control and improve outcomes in patients with refractory or recurrent CMV infections.[74] Emerging antiviral agents, such as letermovir and maribavir, have shown promise in the prophylactic setting and the management of resistant or refractory CMV infections, respectively.[67][71][72](A1)
CMV infection in HSCT recipients
CMV reactivation is a common complication following allogeneic hematopoietic stem cell transplantation (Allo-HSCT), particularly during periods of immunosuppression. Positive CMV serology has been linked to reduced overall survival and increased non-relapse mortality, as reactivation can lead to severe end-organ disease, including pneumonia, colitis, and retinitis[25]. Beyond direct organ damage, CMV infection has also been linked to an increased risk of secondary bacterial and fungal infections, as well as a higher incidence of graft-versus-host disease (GVHD). This connection may be attributed to CMV's immunomodulatory effects, which can influence the immune system's dynamics post-transplant. CMV serostatus is a critical determinant of post-transplant outcomes. Positive serology, particularly in the recipient, is associated with significant transplant-related morbidity and mortality.[26] The worst outcomes in terms of survival and non-relapse mortality occur in CMV seronegative donor/seropositive recipient (D−/R+) pairs, followed by seropositive donor/seropositive recipient (D+/R+) combinations [27].
CMV Esophagitis
The mainstay of CMV esophagitis treatment is ganciclovir and valganciclovir.[14] Treatment of active disease should begin with induction therapy using intravenous (IV) ganciclovir at a dose of 10-15 mg/kg per day, administered in 2-3 divided doses daily for 3 to 6 weeks, depending on clinical circumstances.[5][76] Maintenance therapy with IV ganciclovir at 5 mg/kg daily is indicated in cases with concurrent retinitis or recurrent gastrointestinal disease after discontinuation of induction therapy. Relapse is unfortunately common, as the patients have a baseline, severely persistent immune deficiency most of the time, and treatment only suppresses the infection but does not eliminate it.[5] If recurrence occurs, repeat induction therapy should be performed, followed by maintenance treatment. Foscarnet is an alternative to ganciclovir in cases where resistance to the first-line treatment occurs. Failure of monotherapy calls for an attempt at combination therapy with IV ganciclovir and IV foscarnet.[5] Current treatments still consist of antivirals, ganciclovir, and valganciclovir, but research into alternatives is ongoing.[5][76][77] (A1)
CMV Colitis
The majority of immunocompetent patients with CMV colitis may require no treatment with antiviral medications. Due to the severity of the side effects of antiviral drugs, such as ganciclovir, there is no evidence to suggest that treatment with antiviral medications is necessary in these patients. Due to the significant side effects associated with antiviral agents such as ganciclovir, including myelosuppression, hepatotoxicity, nephrotoxicity, and central nervous system toxicity, there is no substantial evidence that antiviral therapy significantly improves outcomes in this population. However, antiviral treatment may be considered in select cases, particularly in males over the age of 55 with severe disease and underlying conditions that compromise immune function, such as diabetes mellitus or chronic kidney disease. In these cases, ganciclovir or valganciclovir is the treatment of choice[47]
CMV reactivation, commonly observed in patients with severe or corticosteroid-resistant inflammatory bowel disease (IBD), also does not universally require antiviral therapy. This is because the virus is often non-pathogenic in these settings, and treatment may not provide clinical benefit. Antiviral therapy should be considered under the following circumstances:
- When CMV reactivation leads to CMV colitis and histological examination shows high-grade CMV density [78]
- Patients with low-grade CMV density who have corticosteroid-refractory disease or are corticosteroid-dependent
- A large endoscopically visible ulcer may indicate the need for antiviral therapy.
It is essential to note that there are insufficient publications with poor-quality data regarding the treatment of CMV colitis with antiviral agents and whether this approach improves colectomy and mortality outcomes. Further research, including large randomized trials, is necessary to define subgroups that can benefit from treatment. Concomitant use of anti-TNF therapy with antiviral therapy may be considered to treat CMV reactivation in ulcerative colitis patients. Ganciclovir is effective in treating and preventing cytomegalovirus (CMV) disease in patients undergoing bone marrow transplantation.[79] The question of identifying high-risk groups and the use of prophylactic therapy is currently a topic of research.[80](A1)
Differential Diagnosis
Mononucleosis-type CMV infection
- Early HIV infection
- Human Herpesvirus 6 infection
- Viral hepatitis
- Autoimmune hepatitis
- Epstein-Barr virus
- Infectious mononucleosis
CMV esophagitis
Cytomegalovirus esophagitis typically presents as a single, large, isolated ulcer in the distal esophagus, but can also manifest as diffuse esophagitis.[5] The diffuse presentation can be challenging to distinguish from other disorders, such as herpes simplex virus (HSV) esophagitis and gastroesophageal reflux disease (GERD). The differentiating factor is that HSV esophagitis typically presents as multiple, small, shallow ulcers, rather than extensive ulcers. HSV esophagitis also affects the entire esophagus, whereas CMV esophagitis normally affects the mid-to-distal esophagus.[53]
- Achalasia
- Acid peptic disease
- Aphthous ulcers
- Barrett's esophagus
- Candidiasis
- Cryptococcosis
- Drug-induced dysphagia
- Epstein-Barr virus infection
- Esophageal cancer
- Gastroesophageal reflux disease
- Herpes simplex esophagitis
- Histoplasmosis
- Tuberculosis
CMV colitis
The differential diagnosis for cytomegalovirus colitis includes:
- Viral/bacterial gastroenteritis
- Inflammatory bowel disease
- Colorectal cancer
- Toxic megacolon
- Diverticulitis
- Irritable bowel disease
- Celiac disease
- Graft-versus-host disease
Prognosis
CMV infection in immunosuppressed patients is associated with significant morbidity and mortality. Recent studies have highlighted the impact of CMV infection across various immunocompromised populations. In SOT patients, CMV infection has been linked to markedly increased mortality.[81] A study reported a 47% mortality rate in patients with CMV infection compared to 13% in those without.[81] After adjusting for confounding factors, CMV infection remained a strong independent predictor of mortality.[82] In HSCT recipients, CMV infection significantly contributes to mortality.[83] CMV infection often leads to prolonged hospitalization, a higher rate of medical complications, and significantly worse clinical outcomes. These risks highlight the importance of early detection, close monitoring, and, when indicated, timely antiviral therapy in high-risk patients.[84]
CMV Esophagitis
Upper gastrointestinal CMV disease in immunocompromised individuals is associated with significant morbidity and a high risk of mortality, with some studies reporting one-year mortality rates as high as 25%.[13]Risk factors for poor outcomes include profound immunosuppression eg in SOT or HSCT recipients, individuals with advanced HIV/AIDS, , delayed diagnosis, and lack of antiviral treatment.[41]Clinical manifestations can range from odynophagia and gastrointestinal bleeding to ulceration and perforation, particularly in the esophagus or stomach. Timely diagnosis through endoscopy with biopsy, followed by appropriate antiviral therapy, typically ganciclovir or valganciclovir, can significantly improve patient outcomes and reduce mortality. However, even with treatment, outcomes remain guarded in patients with severe underlying disease or multi-organ involvement.[41]
CMV Colitis
The overall prognosis of CMV colitis is generally excellent. However, certain risk factors have been associated with poorer outcomes and increased mortality. In immunocompetent individuals, prognosis is influenced by age, with those over 55 experiencing slightly higher mortality rates. The need for surgical intervention is also linked to a less favorable prognosis. Additionally, some studies have found an association between male gender and increased mortality in CMV colitis cases. In immunocompromised patients, early diagnosis and prompt treatment significantly improve outcomes. Patients with CMV colitis reactivation in the context of ulcerative colitis typically have a worse prognosis, although timely therapy can greatly enhance clinical outcomes in this group as well.[78]
Complications
Tissue-invasive CMV disease affects various organ systems and can be life-threatening. CMV colitis presents with diarrhea, abdominal pain, and gastrointestinal bleeding, and may progress to serious complications such as perforation or toxic megacolon. CMV pneumonitis is another severe manifestation, especially in transplant recipients, and presents with cough, dyspnea, and hypoxia, often with a high mortality rate.[85] Other forms of organ involvement include CMV hepatitis, causing liver dysfunction; esophagitis or gastritis, leading to odynophagia, nausea, and GI bleeding; and CMV retinitis, which causes painless vision loss and retinal necrosis, especially in individuals with advanced HIV/AIDS.[85]CMV encephalitis may also occur, resulting in confusion, seizures, and altered mental status.[85]
In solid organ transplant and bone marrow transplant recipients, CMV infection is strongly associated with graft dysfunction and rejection.[86] It can contribute to both acute and chronic graft rejection, including bronchiolitis obliterans in lung transplant patients and graft vasculopathy in heart transplant recipients.[86]
CMV infection also predisposes immunosuppressed individuals to secondary infections, as it suppresses overall immune function. This immunosuppressive effect can increase the risk of concurrent bacterial, fungal, and other viral infections, including Epstein-Barr virus (EBV)-associated post-transplant lymphoproliferative disorder (PTLD) and invasive fungal infections, such as aspergillosis.[87][88]
Additionally, CMV can cause bone marrow suppression, leading to anemia, leukopenia, and thrombocytopenia. These hematologic complications are particularly concerning in patients already dealing with drug-induced cytopenias from immunosuppressive therapy.[84]
CMV Esophagitis
Malnutrition can develop in the setting of dysphagia and odynophagia, which can be devastating in the setting of severe illnesses.[10] If the esophageal CMV ulcerations become extensive and deep enough, perforation and massive GI bleeding can occur spontaneously or as a complication during endoscopy.[5] [13] Stricture formation, while uncommon, has been reported to occur despite undergoing treatment with antivirals and even as the initial presentation of CMV esophagitis without prior known ulcers.[18] One case report noted the formation of a stricture severe enough to cause complete obliteration of the esophageal lumen in a patient who had completed antiviral treatment and whose post-treatment pathology samples were negative for CMV and HSV, demonstrating that strictures can form despite disease treatment and resolution.[18]
Complications of CMV include:[89][90]
- Chronic inflammation
- Large bowel perforation
- Toxic megacolon
- Pseudo-membrane formation
- Development of ischemic colitis
- Patients with CMV colitis complicating inflammatory bowel disease may develop severe hemorrhage and colon perforation.
Deterrence and Patient Education
Deterrence strategies are essential in preventing CMV infection among immunosuppressed individuals. One of the primary deterrence methods includes the use of antiviral prophylaxis to prevent CMV reactivation, particularly in high-risk groups such as HSCT recipients. Another approach is preemptive therapy, which involves frequent monitoring of CMV viral loads in patients at risk. Antiviral treatment is initiated as soon as viral replication is detected, thereby preventing the progression to symptomatic disease. Additionally, infection control measures, such as rigorous hand hygiene and proper handling of bodily fluids, are crucial, especially in healthcare settings where immunocompromised patients are present.
Patient education is equally important in minimizing the impact of CMV infection. Educating patients about their risk and the potential complications of CMV, such as end-organ damage and increased mortality, is vital, especially for those undergoing immunosuppressive treatments. Patients should be taught to recognize early symptoms of CMV infection, such as fever, malaise, and organ-specific complaints, which allows for timely medical intervention. Ensuring adherence to prescribed antiviral regimens is another key element; patients must understand that consistent medication use can prevent viral reactivation and severe complications. Furthermore, lifestyle counseling should be provided, emphasizing the importance of avoiding contact with individuals who are sick, practicing safe food handling, and maintaining proper hygiene.
Through a combination of medical deterrence strategies and comprehensive patient education, healthcare providers can significantly reduce the incidence, severity, and consequences of CMV infections in immunocompromised populations.
Enhancing Healthcare Team Outcomes
For patients who are immunocompromised, CMV can have significant morbidity and mortality.CMV can affect nearly any organ system, making an interprofessional approach essential for comprehensive care. Immunocompromised patients may be candidates for prophylactic therapy; however, careful consideration must be given to potential drug interactions, toxicity, and the risk of developing antiviral resistance.
Once CMV infection is diagnosed in an immunocompromised patient, long-term follow-up is critical. Ongoing monitoring ensures the timely detection of complications, effective management of medication side effects, and regular assessment for disease recurrence. In cases of CMV retinitis, regular follow-up with an ophthalmologist is necessary to prevent vision loss and monitor treatment response.[91]
The involvement of an interprofessional team is essential to improving outcomes in patients at risk for or diagnosed with CMV infections. Key members of this team include primary care providers, infectious disease specialists, transplant surgeons, transplant nurses, and clinical pharmacists and nurses.
This collaborative approach ensures comprehensive, patient-centered care, helping to optimize both prevention and treatment strategies for CMV-related diseases.
CMV esophagitis
The first step in improving outcomes for patients with CMV esophagitis is identifying patients at risk. Primary care physicians play a crucial role in educating immunocompromised patients about potential complications, including CMV esophagitis. This education should also extend to individuals who are about to become immunocompromised, such as patients with newly diagnosed cancer preparing for chemotherapy, or those starting immunosuppressive therapy for autoimmune diseases. PCPs should counsel patients on recognizing key symptoms of CMV esophagitis, including odynophagia, dysphagia, and retrosternal chest pain. For patients with HIV, emphasizing adherence to HAART is critical to prevent CD4 count decline, which significantly increases the risk for opportunistic infections such as CMV esophagitis. All patients at risk should also receive guidance on universal precautions, particularly when handling bodily fluids and other potentially infectious materials.
If CMV esophagitis is suspected, primary care physicians should conduct a thorough history and physical examination, paying particular attention to identifying relevant risk factors. Patients should be referred to a gastroenterologist for an upper endoscopy with biopsy, as histopathologic confirmation is essential for diagnosis. A pathologist should review biopsy samples. If challenges arise during treatment or complications occur, infectious disease specialists should be consulted for assistance in management.
CMV colitis
The diagnosis and management of CMV colitis are complex and require a coordinated interprofessional approach. Key team members may include gastroenterologists, internists, infectious disease specialists, pathologists, clinical pharmacists, specialized nurses, oncologists, and transplant surgeons. A collaborative strategy is crucial for identifying high-risk patients, facilitating early diagnosis, and the prompt initiation of treatment.
Specialty-trained nurses play a vital role by educating patients on symptom recognition and ensuring adherence to clinical and serologic monitoring protocols, particularly in patients with SOT, to facilitate early detection of infection. Clinical pharmacists and infectious disease specialists contribute by managing antiviral therapy, monitoring for toxicity, and identifying potential drug-drug interactions to prevent adverse events.
By working together, the interprofessional team can deliver optimal prophylaxis, monitoring, and treatment, ultimately improving clinical outcomes for patients at the highest risk of CMV colitis.
Media
(Click Image to Enlarge)
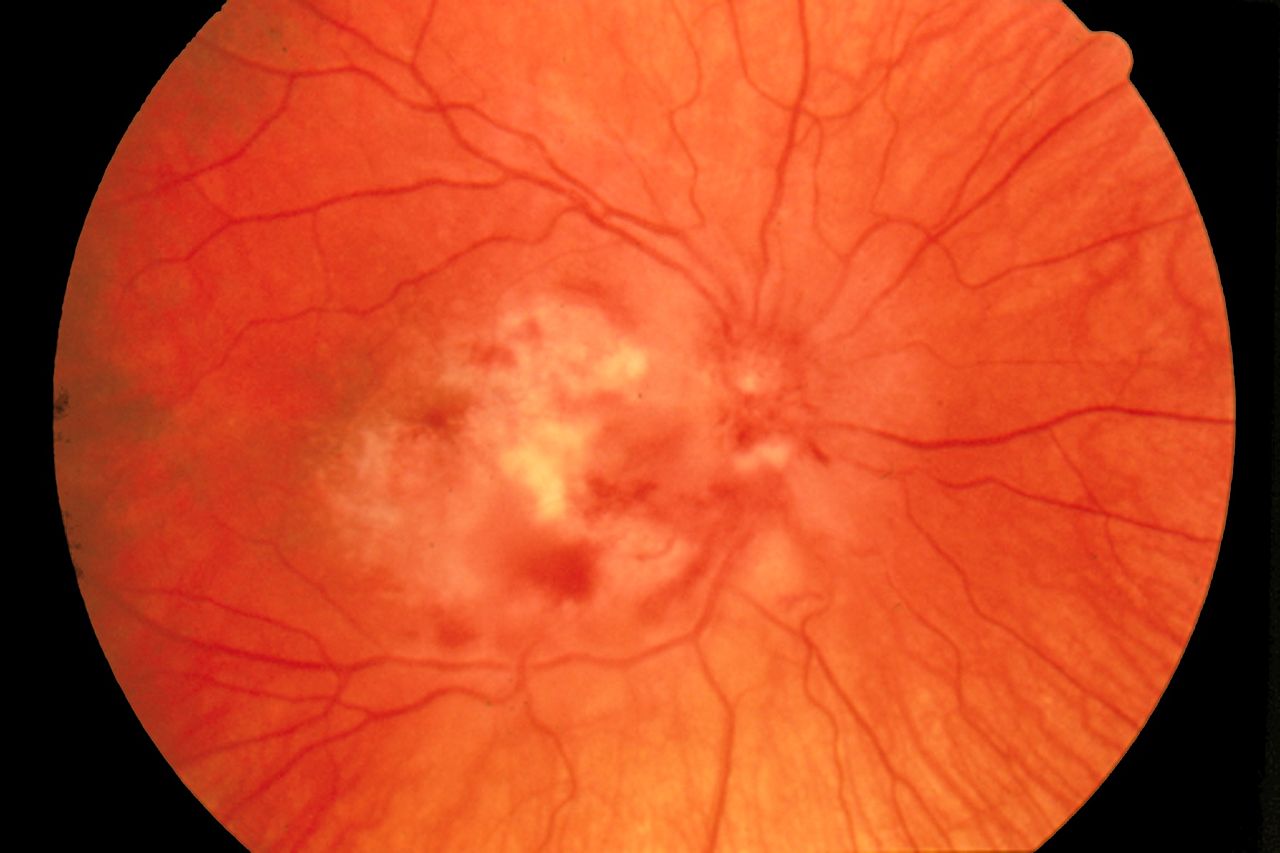
Cytomegalovirus Retinitis. The image depicts a view of a fundus affected by cytomegalovirus retinitis.
National Eye Institute, Public Domain, via Wikimedia Commons
References
Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, Griffiths P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Reviews in medical virology. 2019 May:29(3):e2034. doi: 10.1002/rmv.2034. Epub 2019 Jan 31 [PubMed PMID: 30706584]
Level 1 (high-level) evidenceCharles OJ, Venturini C, Gantt S, Atkinson C, Griffiths P, Goldstein RA, Breuer J. Genomic and geographical structure of human cytomegalovirus. Proceedings of the National Academy of Sciences of the United States of America. 2023 Jul 25:120(30):e2221797120. doi: 10.1073/pnas.2221797120. Epub 2023 Jul 17 [PubMed PMID: 37459519]
Boeckh M, Geballe AP. Cytomegalovirus: pathogen, paradigm, and puzzle. The Journal of clinical investigation. 2011 May:121(5):1673-80. doi: 10.1172/JCI45449. Epub [PubMed PMID: 21659716]
Fowler K, Mucha J, Neumann M, Lewandowski W, Kaczanowska M, Grys M, Schmidt E, Natenshon A, Talarico C, Buck PO, Diaz-Decaro J. A systematic literature review of the global seroprevalence of cytomegalovirus: possible implications for treatment, screening, and vaccine development. BMC public health. 2022 Sep 1:22(1):1659. doi: 10.1186/s12889-022-13971-7. Epub 2022 Sep 1 [PubMed PMID: 36050659]
Level 1 (high-level) evidenceGoodgame RW. Gastrointestinal cytomegalovirus disease. Annals of internal medicine. 1993 Nov 1:119(9):924-35 [PubMed PMID: 8215005]
Forte E, Zhang Z, Thorp EB, Hummel M. Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response. Frontiers in cellular and infection microbiology. 2020:10():130. doi: 10.3389/fcimb.2020.00130. Epub 2020 Mar 31 [PubMed PMID: 32296651]
Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Reviews in medical virology. 2010 Jul:20(4):202-13. doi: 10.1002/rmv.655. Epub [PubMed PMID: 20564615]
Kotton CN, Torre-Cisneros J, International CMV Symposium Faculty, Aguado JM, Alain S, Baldanti F, Baumann G, Boeken U, de la Calle M, Carbone J, Ciceri F, Comoli P, Couzi L, Danziger-Isakov L, Fernández-Ruiz M, Girmenia C, Grossi PA, Hirsch HH, Humar A, Kamar N, Kotton C, Ljungman P, Malagola M, Mira E, Mueller N, Sester M, Teng CJ, Torre-Cisneros J, Ussetti P, Westall G, Wolf D, Zamora M. Cytomegalovirus in the transplant setting: Where are we now and what happens next? A report from the International CMV Symposium 2021. Transplant infectious disease : an official journal of the Transplantation Society. 2022 Dec:24(6):e13977. doi: 10.1111/tid.13977. Epub 2022 Nov 11 [PubMed PMID: 36271650]
Ljungman P, Chemaly RF, Khawaya F, Alain S, Avery R, Badshah C, Boeckh M, Fournier M, Hodowanec A, Komatsu T, Limaye AP, Manuel O, Natori Y, Navarro D, Pikis A, Razonable RR, Westman G, Miller V, Griffiths PD, Kotton CN, CMV Definitions Working Group of the Transplant Associated Virus Infections Forum. Consensus Definitions of Cytomegalovirus (CMV) Infection and Disease in Transplant Patients Including Resistant and Refractory CMV for Use in Clinical Trials: 2024 Update From the Transplant Associated Virus Infections Forum. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2024 Sep 26:79(3):787-794. doi: 10.1093/cid/ciae321. Epub [PubMed PMID: 39041385]
Level 3 (low-level) evidenceWang HW, Kuo CJ, Lin WR, Hsu CM, Ho YP, Lin CJ, Su MY, Chiu CT, Wang CL, Chen KH. The clinical characteristics and manifestations of cytomegalovirus esophagitis. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus. 2016 May:29(4):392-9. doi: 10.1111/dote.12340. Epub 2015 Feb 26 [PubMed PMID: 25715747]
Level 2 (mid-level) evidenceBate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010 Jun 1:50(11):1439-47. doi: 10.1086/652438. Epub [PubMed PMID: 20426575]
Level 3 (low-level) evidenceBanerjee D, Deb R, Dar L, Mirdha BR, Pati SK, Thareja S, Falodia S, Ahuja V. High frequency of parasitic and viral stool pathogens in patients with active ulcerative colitis: report from a tropical country. Scandinavian journal of gastroenterology. 2009:44(3):325-31. doi: 10.1080/00365520802556809. Epub [PubMed PMID: 19040190]
Level 2 (mid-level) evidenceMarques S, Carmo J, Pinto D, Bispo M, Ramos S, Chagas C. Cytomegalovirus Disease of the Upper Gastrointestinal Tract: A 10-Year Retrospective Study. GE Portuguese journal of gastroenterology. 2017 Nov:24(6):262-268. doi: 10.1159/000479232. Epub 2017 Sep 2 [PubMed PMID: 29255766]
Level 2 (mid-level) evidenceEmery VC. Cytomegalovirus: recent progress in understanding pathogenesis and control. QJM : monthly journal of the Association of Physicians. 2012 May:105(5):401-5. doi: 10.1093/qjmed/hcr262. Epub 2011 Dec 22 [PubMed PMID: 22198913]
Level 3 (low-level) evidenceZheng QY, Huynh KT, van Zuylen WJ, Craig ME, Rawlinson WD. Cytomegalovirus infection in day care centres: A systematic review and meta-analysis of prevalence of infection in children. Reviews in medical virology. 2019 Jan:29(1):e2011. doi: 10.1002/rmv.2011. Epub 2018 Oct 10 [PubMed PMID: 30306730]
Level 1 (high-level) evidenceBartlett AW, Hall BM, Palasanthiran P, McMullan B, Shand AW, Rawlinson WD. Recognition, treatment, and sequelae of congenital cytomegalovirus in Australia: An observational study. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2018 Nov:108():121-125. doi: 10.1016/j.jcv.2018.09.017. Epub 2018 Sep 27 [PubMed PMID: 30300787]
Level 2 (mid-level) evidenceLimaye AP, Babu TM, Boeckh M. Progress and Challenges in the Prevention, Diagnosis, and Management of Cytomegalovirus Infection in Transplantation. Clinical microbiology reviews. 2020 Dec 16:34(1):. doi: 10.1128/CMR.00043-19. Epub 2020 Oct 28 [PubMed PMID: 33115722]
Sheth A, Boktor M, Diamond K, Lavu K, Sangster G. Complete esophageal obliteration secondary to cytomegalovirus in AIDS patient. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus. 2010 Aug:23(6):E32-4. doi: 10.1111/j.1442-2050.2010.01095.x. Epub 2010 Jul 23 [PubMed PMID: 20659143]
Level 3 (low-level) evidenceAnders HJ, Goebel FD. Neurological manifestations of cytomegalovirus infection in the acquired immunodeficiency syndrome. International journal of STD & AIDS. 1999 Mar:10(3):151-9; quiz 160-1 [PubMed PMID: 10340195]
Dieterich DT, Rahmin M. Cytomegalovirus colitis in AIDS: presentation in 44 patients and a review of the literature. Journal of acquired immune deficiency syndromes. 1991:4 Suppl 1():S29-35 [PubMed PMID: 1848619]
Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, Pikis A, Razonable RR, Miller V, Griffiths PD, Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Jan 1:64(1):87-91 [PubMed PMID: 27682069]
Natori Y, Alghamdi A, Tazari M, Miller V, Husain S, Komatsu T, Griffiths P, Ljungman P, Orchanian-Cheff A, Kumar D, Humar A, CMV Consensus Forum. Use of Viral Load as a Surrogate Marker in Clinical Studies of Cytomegalovirus in Solid Organ Transplantation: A Systematic Review and Meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018 Feb 1:66(4):617-631. doi: 10.1093/cid/cix793. Epub [PubMed PMID: 29020339]
Level 1 (high-level) evidenceTang Y, Guo J, Li J, Zhou J, Mao X, Qiu T. Risk factors for cytomegalovirus infection and disease after kidney transplantation: A meta-analysis. Transplant immunology. 2022 Oct:74():101677. doi: 10.1016/j.trim.2022.101677. Epub 2022 Jul 25 [PubMed PMID: 35901951]
Level 1 (high-level) evidenceHakimi Z, Aballéa S, Ferchichi S, Scharn M, Odeyemi IA, Toumi M, Saliba F. Burden of cytomegalovirus disease in solid organ transplant recipients: a national matched cohort study in an inpatient setting. Transplant infectious disease : an official journal of the Transplantation Society. 2017 Oct:19(5):. doi: 10.1111/tid.12732. Epub 2017 Jul 21 [PubMed PMID: 28599091]
Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, Green JS, Saad A, Antin JH, Savani BN, Lazarus HM, Seftel M, Saber W, Marks D, Aljurf M, Norkin M, Wingard JR, Lindemans CA, Boeckh M, Riches ML, Auletta JJ. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016 May 19:127(20):2427-38. doi: 10.1182/blood-2015-11-679639. Epub 2016 Feb 16 [PubMed PMID: 26884374]
Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. The Journal of infectious diseases. 2002 Feb 1:185(3):273-82 [PubMed PMID: 11807708]
Schmidt-Hieber M, Tridello G, Ljungman P, Mikulska M, Knelange N, Blaise D, Socié G, Volin L, Blijlevens N, Fegueux N, Yakoub-Agha I, Forcade E, Maertens J, Chevallier P, Passweg J, Cornelissen J, Russell N, Craddock C, Bourhis JH, Marchand T, Reményi P, Cahn JY, Michallet M, Montoto S, Kröger N, Glaß B, Styczynski J. The prognostic impact of the cytomegalovirus serostatus in patients with chronic hematological malignancies after allogeneic hematopoietic stem cell transplantation: a report from the Infectious Diseases Working Party of EBMT. Annals of hematology. 2019 Jul:98(7):1755-1763. doi: 10.1007/s00277-019-03669-z. Epub 2019 Apr 16 [PubMed PMID: 30993417]
Jakharia N, Howard D, Riedel DJ. CMV Infection in Hematopoietic Stem Cell Transplantation: Prevention and Treatment Strategies. Current treatment options in infectious diseases. 2021:13(3):123-140. doi: 10.1007/s40506-021-00253-w. Epub 2021 Jul 21 [PubMed PMID: 34305463]
Moliya P, Singh A, Singh N, Kumar V, Sohal A. Insights into gastrointestinal manifestation of human immunodeficiency virus: A narrative review. World journal of virology. 2025 Mar 25:14(1):99249. doi: 10.5501/wjv.v14.i1.99249. Epub [PubMed PMID: 40134843]
Level 3 (low-level) evidencePark SC, Jeen YM, Jeen YT. Approach to cytomegalovirus infections in patients with ulcerative colitis. The Korean journal of internal medicine. 2017 May:32(3):383-392. doi: 10.3904/kjim.2017.087. Epub 2017 Apr 20 [PubMed PMID: 28490715]
Papadakis KA, Tung JK, Binder SW, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. The American journal of gastroenterology. 2001 Jul:96(7):2137-42 [PubMed PMID: 11467645]
Level 2 (mid-level) evidenceLee HS, Park SH, Kim SH, Kim J, Choi J, Lee HJ, Kim WS, Lee JM, Kwak MS, Hwang SW, Yang DH, Kim KJ, Ye BD, Byeon JS, Myung SJ, Yoon YS, Yu CS, Kim JH, Yang SK. Risk Factors and Clinical Outcomes Associated with Cytomegalovirus Colitis in Patients with Acute Severe Ulcerative Colitis. Inflammatory bowel diseases. 2016 Apr:22(4):912-8. doi: 10.1097/MIB.0000000000000675. Epub [PubMed PMID: 26829410]
Level 2 (mid-level) evidenceShukla T, Singh S, Tandon P, McCurdy JD. Corticosteroids and Thiopurines, But Not Tumor Necrosis Factor Antagonists, are Associated With Cytomegalovirus Reactivation in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Journal of clinical gastroenterology. 2017 May/Jun:51(5):394-401. doi: 10.1097/MCG.0000000000000758. Epub [PubMed PMID: 27875356]
Level 1 (high-level) evidenceMcCurdy JD, Jones A, Enders FT, Killian JM, Loftus EV Jr, Smyrk TC, Bruining DH. A model for identifying cytomegalovirus in patients with inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015 Jan:13(1):131-7; quiz e7. doi: 10.1016/j.cgh.2014.05.026. Epub 2014 Jun 30 [PubMed PMID: 24993369]
Level 2 (mid-level) evidenceDomènech E, Vega R, Ojanguren I, Hernández A, Garcia-Planella E, Bernal I, Rosinach M, Boix J, Cabré E, Gassull MA. Cytomegalovirus infection in ulcerative colitis: a prospective, comparative study on prevalence and diagnostic strategy. Inflammatory bowel diseases. 2008 Oct:14(10):1373-9. doi: 10.1002/ibd.20498. Epub [PubMed PMID: 18452205]
Level 2 (mid-level) evidenceMaconi G, Lombardini M, Furfaro F, Bezzio C, Zerbi P, Ardizzone S. Long-term outcome of inflammatory bowel diseases with cytomegalovirus colitis: effect of antiviral treatment. European journal of gastroenterology & hepatology. 2014 Oct:26(10):1146-51. doi: 10.1097/MEG.0000000000000175. Epub [PubMed PMID: 25089547]
Level 2 (mid-level) evidenceInayat F, Hussain Q, Shafique K, Tasleem SH, Hurairah A. Cytomegalovirus Colitis in Immunocompetent Patients. Cureus. 2016 Nov 8:8(11):e869 [PubMed PMID: 27980888]
Wada Y, Matsui T, Matake H, Sakurai T, Yamamoto J, Kikuchi Y, Yorioka M, Tsuda S, Yao T, Yao S, Haraoka S, Iwashita A. Intractable ulcerative colitis caused by cytomegalovirus infection: a prospective study on prevalence, diagnosis, and treatment. Diseases of the colon and rectum. 2003 Oct:46(10 Suppl):S59-65 [PubMed PMID: 14530660]
Criscuoli V, Casà A, Orlando A, Pecoraro G, Oliva L, Traina M, Rizzo A, Cottone M. Severe acute colitis associated with CMV: a prevalence study. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2004 Dec:36(12):818-20 [PubMed PMID: 15646428]
Karigane D, Takaya S, Seki Y, Mastumoto Y, Onose A, Kosakai A, Sugaya N, Mori T. Cytomegalovirus enteritis in immunocompetent subjects: a case report and review of the literature. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2014 May:20(5):325-9. doi: 10.1016/j.jiac.2013.12.004. Epub 2014 Jan 24 [PubMed PMID: 24751234]
Level 3 (low-level) evidenceHoversten P, Kamboj AK, Wu TT, Katzka DA. Risk Factors, Endoscopic Features, and Clinical Outcomes of Cytomegalovirus Esophagitis Based on a 10-year Analysis at a Single Center. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2020 Mar:18(3):736-738. doi: 10.1016/j.cgh.2019.04.066. Epub 2019 May 8 [PubMed PMID: 31077832]
Level 2 (mid-level) evidenceReeves MB, Breidenstein A, Compton T. Human cytomegalovirus activation of ERK and myeloid cell leukemia-1 protein correlates with survival of latently infected cells. Proceedings of the National Academy of Sciences of the United States of America. 2012 Jan 10:109(2):588-93. doi: 10.1073/pnas.1114966108. Epub 2011 Dec 27 [PubMed PMID: 22203987]
Nosotti M, Tarsia P, Morlacchi LC. Infections after lung transplantation. Journal of thoracic disease. 2018 Jun:10(6):3849-3868. doi: 10.21037/jtd.2018.05.204. Epub [PubMed PMID: 30069386]
Faith SC, Durrani AF, Jhanji V. Cytomegalovirus keratitis. Current opinion in ophthalmology. 2018 Jul:29(4):373-377. doi: 10.1097/ICU.0000000000000481. Epub [PubMed PMID: 29708927]
Level 3 (low-level) evidenceIsaacson MK, Compton T. Human cytomegalovirus glycoprotein B is required for virus entry and cell-to-cell spread but not for virion attachment, assembly, or egress. Journal of virology. 2009 Apr:83(8):3891-903. doi: 10.1128/JVI.01251-08. Epub 2009 Feb 4 [PubMed PMID: 19193805]
Juric-Sekhar G, Upton MP, Swanson PE, Westerhoff M. Cytomegalovirus (CMV) in gastrointestinal mucosal biopsies: should a pathologist perform CMV immunohistochemistry if the clinician requests it? Human pathology. 2017 Feb:60():11-15. doi: 10.1016/j.humpath.2016.09.009. Epub 2016 Sep 22 [PubMed PMID: 27666768]
Yerushalmy-Feler A, Padlipsky J, Cohen S. Diagnosis and Management of CMV Colitis. Current infectious disease reports. 2019 Feb 15:21(2):5. doi: 10.1007/s11908-019-0664-y. Epub 2019 Feb 15 [PubMed PMID: 30771028]
Yang H, Zhou W, Lv H, Wu D, Feng Y, Shu H, Jin M, Hu L, Wang Q, Wu D, Chen J, Qian J. The Association Between CMV Viremia or Endoscopic Features and Histopathological Characteristics of CMV Colitis in Patients with Underlying Ulcerative Colitis. Inflammatory bowel diseases. 2017 May:23(5):814-821. doi: 10.1097/MIB.0000000000001095. Epub [PubMed PMID: 28426459]
Level 2 (mid-level) evidenceChidlovskii E, Deroux A, Bernard S, Couturier P. Cytomegalovirus colitis mimicking rectal carcinoma in an immunocompetent elderly woman. BMJ case reports. 2016 May 10:2016():. doi: 10.1136/bcr-2016-214694. Epub 2016 May 10 [PubMed PMID: 27166009]
Level 3 (low-level) evidenceRafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virology journal. 2008 Mar 27:5():47. doi: 10.1186/1743-422X-5-47. Epub 2008 Mar 27 [PubMed PMID: 18371229]
Level 1 (high-level) evidenceCarlier FM, Evrard P, Dumonceaux M. Cytomegalovirus nephritis in a lung transplant recipient: A case report. JHLT open. 2023 Oct:1():100003. doi: 10.1016/j.jhlto.2023.100003. Epub 2023 Aug 10 [PubMed PMID: 40144580]
Level 3 (low-level) evidenceLee HY, Rhee CK, Choi JY, Lee HY, Lee JW, Lee DG. Diagnosis of cytomegalovirus pneumonia by quantitative polymerase chain reaction using bronchial washing fluid from patients with hematologic malignancies. Oncotarget. 2017 Jun 13:8(24):39736-39745. doi: 10.18632/oncotarget.14504. Epub [PubMed PMID: 28061469]
Wilcox CM, Straub RF, Schwartz DA. Prospective endoscopic characterization of cytomegalovirus esophagitis in AIDS. Gastrointestinal endoscopy. 1994 Jul-Aug:40(4):481-4 [PubMed PMID: 7926541]
Levin A, Yaari S, Stoff R, Caplan O, Wolf DG, Israeli E. Diagnosis of Cytomegalovirus Infection during Exacerbation of Ulcerative Colitis. Digestion. 2017:96(3):142-148. doi: 10.1159/000479865. Epub 2017 Aug 26 [PubMed PMID: 28848127]
Duraisamy SK, Mammen S, Lakshminarayan SKR, Verghese S, Moorthy M, George B, Kannangai R, Varghese S, Srivastava A, Abraham AM. Performance of an in-house real-time PCR assay for detecting Cytomegalovirus infection among transplant patients from a tertiary care centre. Indian journal of medical microbiology. 2018 Apr-Jun:36(2):241-246. doi: 10.4103/ijmm.IJMM_18_126. Epub [PubMed PMID: 30084418]
Moresco BL, Svoboda MD, Ng YT. A Quiet Disease With Loud Manifestations. Seminars in pediatric neurology. 2018 Jul:26():88-91. doi: 10.1016/j.spen.2017.03.014. Epub 2017 Apr 2 [PubMed PMID: 29961530]
Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, Stevens-Ayers T, Flowers ME, Cunningham T, Corey L. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003 Jan 15:101(2):407-14 [PubMed PMID: 12393659]
Boeckh M, Bowden RA, Goodrich JM, Pettinger M, Meyers JD. Cytomegalovirus antigen detection in peripheral blood leukocytes after allogeneic marrow transplantation. Blood. 1992 Sep 1:80(5):1358-64 [PubMed PMID: 1325214]
Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematology/oncology clinics of North America. 2011 Feb:25(1):151-69. doi: 10.1016/j.hoc.2010.11.011. Epub [PubMed PMID: 21236396]
Suzuki H, Kato J, Kuriyama M, Hiraoka S, Kuwaki K, Yamamoto K. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection. World journal of gastroenterology. 2010 Mar 14:16(10):1245-51 [PubMed PMID: 20222169]
Level 2 (mid-level) evidenceMatsuda K, Ono S, Ishikawa M, Miyamoto S, Abiko S, Tsuda M, Yamamoto K, Kudo T, Shimizu Y, Hayase E, Hashimoto D, Teshima T, Matsuno Y, Sakamoto N. Cecum ulcer is a reliable endoscopic finding in cytomegalovirus colitis concomitant with graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Annals of hematology. 2018 May:97(5):877-883. doi: 10.1007/s00277-018-3241-9. Epub 2018 Jan 16 [PubMed PMID: 29340759]
Landry ML, Ferguson D. Comparison of quantitative cytomegalovirus antigenemia assay with culture methods and correlation with clinical disease. Journal of clinical microbiology. 1993 Nov:31(11):2851-6 [PubMed PMID: 8263166]
Moses S, Malathi J, Singha NR, Bagyalakshmi R, Madhavan HN. Determination of human cytomegalovirus pp65 antigenemia among renal transplant patients. Indian journal of nephrology. 2012 Sep:22(5):347-52. doi: 10.4103/0971-4065.103909. Epub [PubMed PMID: 23326044]
Marchetti S, Santangelo R, Manzara S, D'onghia S, Fadda G, Cattani P. Comparison of real-time PCR and pp65 antigen assays for monitoring the development of Cytomegalovirus disease in recipients of solid organ and bone marrow transplants. The new microbiologica. 2011 Apr:34(2):157-64 [PubMed PMID: 21617827]
Huh K, Lee SO, Kim J, Lee SJ, Choe PG, Kang JM, Yang J, Sung H, Kim SH, Moon C, Seok H, Shi HJ, Wi YM, Jeong SJ, Park WB, Kim YJ, Kim J, Ahn HJ, Kim NJ, Peck KR, Kim MS, Kim SI. Prevention of Cytomegalovirus Infection in Solid Organ Transplant Recipients: Guidelines by the Korean Society of Infectious Diseases and the Korean Society for Transplantation. Infection & chemotherapy. 2024 Mar:56(1):101-121. doi: 10.3947/ic.2024.0016. Epub 2024 Mar 12 [PubMed PMID: 38527780]
Limaye AP, Budde K, Humar A, Vincenti F, Kuypers DRJ, Carroll RP, Stauffer N, Murata Y, Strizki JM, Teal VL, Gilbert CL, Haber BA. Letermovir vs Valganciclovir for Prophylaxis of Cytomegalovirus in High-Risk Kidney Transplant Recipients: A Randomized Clinical Trial. JAMA. 2023 Jul 3:330(1):33-42. doi: 10.1001/jama.2023.9106. Epub [PubMed PMID: 37279999]
Level 1 (high-level) evidenceImlay HN, Kaul DR. Letermovir and Maribavir for the Treatment and Prevention of Cytomegalovirus Infection in Solid Organ and Stem Cell Transplant Recipients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 Jul 1:73(1):156-160. doi: 10.1093/cid/ciaa1713. Epub [PubMed PMID: 33197929]
Ngai JJ, Chong KL, Oli Mohamed S. Cytomegalovirus Retinitis in Primary Immune Deficiency Disease. Case reports in ophthalmological medicine. 2018:2018():8125806. doi: 10.1155/2018/8125806. Epub 2018 Sep 19 [PubMed PMID: 30327738]
Level 3 (low-level) evidenceMozaffar M, Shahidi S, Mansourian M, Badri S. Optimal Use of Ganciclovir and Valganciclovir in Transplanted Patients: How Does It Relate to the Outcome? Journal of transplantation. 2018:2018():8414385. doi: 10.1155/2018/8414385. Epub 2018 Sep 17 [PubMed PMID: 30319817]
Frange P, Leruez-Ville M. Maribavir, brincidofovir and letermovir: Efficacy and safety of new antiviral drugs for treating cytomegalovirus infections. Medecine et maladies infectieuses. 2018 Dec:48(8):495-502. doi: 10.1016/j.medmal.2018.03.006. Epub 2018 Apr 9 [PubMed PMID: 29650261]
Reusser P, Einsele H, Lee J, Volin L, Rovira M, Engelhard D, Finke J, Cordonnier C, Link H, Ljungman P, Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood. 2002 Feb 15:99(4):1159-64 [PubMed PMID: 11830461]
Level 1 (high-level) evidenceMattes FM, Hainsworth EG, Geretti AM, Nebbia G, Prentice G, Potter M, Burroughs AK, Sweny P, Hassan-Walker AF, Okwuadi S, Sabin C, Amooty G, Brown VS, Grace SC, Emery VC, Griffiths PD. A randomized, controlled trial comparing ganciclovir to ganciclovir plus foscarnet (each at half dose) for preemptive therapy of cytomegalovirus infection in transplant recipients. The Journal of infectious diseases. 2004 Apr 15:189(8):1355-61 [PubMed PMID: 15073671]
Level 1 (high-level) evidenceMarschall M, Stamminger T, Urban A, Wildum S, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. In vitro evaluation of the activities of the novel anticytomegalovirus compound AIC246 (letermovir) against herpesviruses and other human pathogenic viruses. Antimicrobial agents and chemotherapy. 2012 Feb:56(2):1135-7. doi: 10.1128/AAC.05908-11. Epub 2011 Nov 21 [PubMed PMID: 22106211]
Walti CS, Khanna N, Avery RK, Helanterä I. New Treatment Options for Refractory/Resistant CMV Infection. Transplant international : official journal of the European Society for Organ Transplantation. 2023:36():11785. doi: 10.3389/ti.2023.11785. Epub 2023 Oct 12 [PubMed PMID: 37901297]
Karantoni E, Zavras PD, Su Y, Fang J, Tamari R, Cho C, Perales MA, Stern A, Papanicolaou GA. Outcomes of Refractory Cytomegalovirus Infection in the First Year after Allogeneic Hematopoietic Cell Transplantation. Transplantation and cellular therapy. 2022 Jul:28(7):403.e1-403.e7. doi: 10.1016/j.jtct.2022.04.016. Epub 2022 Apr 25 [PubMed PMID: 35476955]
Whitley RJ, Jacobson MA, Friedberg DN, Holland GN, Jabs DA, Dieterich DT, Hardy WD, Polis MA, Deutsch TA, Feinberg J, Spector SA, Walmsley S, Drew WL, Powderly WG, Griffiths PD, Benson CA, Kessler HA. Guidelines for the treatment of cytomegalovirus diseases in patients with AIDS in the era of potent antiretroviral therapy: recommendations of an international panel. International AIDS Society-USA. Archives of internal medicine. 1998 May 11:158(9):957-69 [PubMed PMID: 9588429]
Level 1 (high-level) evidenceMeesing A, Razonable RR. New Developments in the Management of Cytomegalovirus Infection After Transplantation. Drugs. 2018 Jul:78(11):1085-1103. doi: 10.1007/s40265-018-0943-1. Epub [PubMed PMID: 29961185]
Beswick L, Ye B, van Langenberg DR. Toward an Algorithm for the Diagnosis and Management of CMV in Patients with Colitis. Inflammatory bowel diseases. 2016 Dec:22(12):2966-2976 [PubMed PMID: 27763950]
Goodrich JM, Mori M, Gleaves CA, Du Mond C, Cays M, Ebeling DF, Buhles WC, DeArmond B, Meyers JD. Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. The New England journal of medicine. 1991 Dec 5:325(23):1601-7 [PubMed PMID: 1658652]
Level 1 (high-level) evidenceBhutani D, Dyson G, Manasa R, Deol A, Ratanatharathorn V, Ayash L, Abidi M, Lum LG, Al-Kadhimi Z, Uberti JP. Incidence, risk factors, and outcome of cytomegalovirus viremia and gastroenteritis in patients with gastrointestinal graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015 Jan:21(1):159-64. doi: 10.1016/j.bbmt.2014.10.004. Epub 2014 Oct 16 [PubMed PMID: 25445637]
Level 2 (mid-level) evidenceLinares L, Sanclemente G, Cervera C, Hoyo I, Cofán F, Ricart MJ, Pérez-Villa F, Navasa M, Marcos MA, Antón A, Pumarola T, Moreno A. Influence of cytomegalovirus disease in outcome of solid organ transplant patients. Transplantation proceedings. 2011 Jul-Aug:43(6):2145-8. doi: 10.1016/j.transproceed.2011.05.007. Epub [PubMed PMID: 21839217]
Gardiner BJ, Bailey JP, Percival MA, Morgan BA, Warner VM, Lee SJ, Morrissey CO, Kaye DM, Peleg AY, Taylor AJ. Incidence and severity of cytomegalovirus infection in seropositive heart transplant recipients. Clinical transplantation. 2023 Jun:37(6):e14982. doi: 10.1111/ctr.14982. Epub 2023 Mar 29 [PubMed PMID: 36988473]
Meng XY, Fu HX, Zhu XL, Wang JZ, Liu X, Yan CH, Zhang YY, Mo XD, Wang Y, Han W, Chen YH, Chen DB, Liu HX, Chang YJ, Xu LP, Liu KY, Huang XJ, Zhang XH. Comparison of different cytomegalovirus diseases following haploidentical hematopoietic stem cell transplantation. Annals of hematology. 2020 Nov:99(11):2659-2670. doi: 10.1007/s00277-020-04201-4. Epub 2020 Jul 30 [PubMed PMID: 32734550]
Fernández S, Grafia I, Peyrony O, Canet E, Vigneron C, Monet C, Issa N, Decavele M, Moreau AS, Lautrette A, Lacave G, Morel G, Cadoz C, Argaud L, Statlender L, Azem K, Quenot JP, Lesieur O, Fernández J, Farrero M, Marcos MÁ, Lemiale V, Castro P, Azoulay É. Clinical characteristics and outcomes of immunocompromised critically ill patients with cytomegalovirus end-organ disease: a multicenter retrospective cohort study. Critical care (London, England). 2024 Jul 16:28(1):243. doi: 10.1186/s13054-024-05029-4. Epub 2024 Jul 16 [PubMed PMID: 39014504]
Level 2 (mid-level) evidenceMena Lora A, Khine J, Khosrodad N, Yeldandi V. Unusual Manifestations of Acute Cytomegalovirus Infection in Solid Organ Transplant Hosts: A Report of Two Cases. Case reports in transplantation. 2017:2017():4916973. doi: 10.1155/2017/4916973. Epub 2017 Sep 11 [PubMed PMID: 29085699]
Level 3 (low-level) evidenceRazonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clinical transplantation. 2019 Sep:33(9):e13512. doi: 10.1111/ctr.13512. Epub 2019 Mar 28 [PubMed PMID: 30817026]
Lam JKP, Azzi T, Hui KF, Wong AMG, McHugh D, Caduff N, Chan KH, Münz C, Chiang AKS. Co-infection of Cytomegalovirus and Epstein-Barr Virus Diminishes the Frequency of CD56(dim)NKG2A(+)KIR(-) NK Cells and Contributes to Suboptimal Control of EBV in Immunosuppressed Children With Post-transplant Lymphoproliferative Disorder. Frontiers in immunology. 2020:11():1231. doi: 10.3389/fimmu.2020.01231. Epub 2020 Jun 17 [PubMed PMID: 32625211]
Agrawal A, Ison MG, Danziger-Isakov L. Long-Term Infectious Complications of Kidney Transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2022 Feb:17(2):286-295. doi: 10.2215/CJN.15971020. Epub 2021 Apr 20 [PubMed PMID: 33879502]
Barling DR, Tucker S, Varia H, Isaacs P. Large bowel perforation secondary to CMV colitis: an unusual primary presentation of HIV infection. BMJ case reports. 2016 Dec 21:2016():. doi: 10.1136/bcr-2016-217221. Epub 2016 Dec 21 [PubMed PMID: 28003231]
Level 3 (low-level) evidenceHasegawa T, Aomatsu K, Nakamura M, Aomatsu N, Aomatsu K. Cytomegalovirus colitis followed by ischemic colitis in a non-immunocompromised adult: a case report. World journal of gastroenterology. 2015 Mar 28:21(12):3750-4. doi: 10.3748/wjg.v21.i12.3750. Epub [PubMed PMID: 25834346]
Level 3 (low-level) evidenceKotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A, The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018 Jun:102(6):900-931. doi: 10.1097/TP.0000000000002191. Epub [PubMed PMID: 29596116]
Level 3 (low-level) evidence