Introduction
Uterine leiomyomata, also known as uterine fibroids, are the most common benign gynecologic tumors, occurring in 50% to 70% of females by menopause, with rates reaching over 80% in Black women.[1][2] Leiomyomata originate from uterine smooth muscle cells in the myometrium, and their growth is primarily stimulated by circulating estrogen. Uterine fibroids are classically diagnosed on physical exam and ultrasound imaging, which is highly sensitive for this pathology.
While many leiomyomata are asymptomatic and discovered incidentally, 25% to 30% of women experience a spectrum of symptoms that increase morbidity and adversely affect their quality of life.[3] The most common symptoms include abnormal uterine bleeding (AUB), heavy menstrual bleeding (HMB), pelvic pain and pressure, low back pain, anemia, and bladder and bowel dysfunction. They continue to be the leading indication for hysterectomy.[3][4] Additionally, the presence of 1 or multiple leiomyomata may affect fertility, as the distortion of the uterus can prevent successful implantation and the continued survival of an intrauterine pregnancy. Uterine fibroids also increase the risk of certain obstetric complications, including recurrent pregnancy loss (RPL), preterm labor (PTL), abnormal placentation, the need for cesarean birth, and postpartum hemorrhage.[5][6][7]
With the current trend of increasing age at childbearing, understanding fertility-sparing management of uterine leiomyomata is critical; therefore, clinicians should have an in-depth understanding of the pathophysiology and evaluation of leiomyomata, as well as both fertility-sparing and nonfertility-sparing management options.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Uterine fibroids are benign monoclonal tumors originating from smooth muscle cells within the uterus.[8] The exact initiating factor of fibroid formation remains unclear. However, prevailing theories highlight the role of reproductive hormones, inherent abnormalities of the myometrium, and predisposing genetic variations that affect cell signaling pathways.[9][10][11]
Several molecular studies have identified specific gene mutations associated with leiomyomata, the most common of which is the MED12 gene located on chromosome X at position q13.[12][13][14] The MED12 gene codes for one of the key proteins in the mediator complex (subunit 12) that regulates RNA polymerase II; a mutation in this gene occurs in approximately 70% of uterine leiomyomas.[15] Other common mutations associated with leiomyomas include high mobility group AT-hook 1 and 2 (HMGA1 and HMGA2) and collagen type IV alpha 4 and 6 (COL4A4 and COL4A6).[16][14] Mutations in fumarate hydratase increase the risk for hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome, which often present with uterine leiomyomata; however, uterine leiomyomata associated with HLRCC are much less common than sporadic fibroids associated with MED12 mutations.
Additionally, uterine leiomyomata are hormonally sensitive tumors, and evidence has demonstrated that leiomyomas overexpress certain estrogen and progesterone receptors when compared to normal surrounding myometrium.[17][18] Studies indicate that the ovarian steroids estradiol and progesterone promote the growth of leiomyomas, and myometrial cells with high levels of estrogen and progesterone receptors can stimulate the growth of adjacent uterine leiomyomata stem cells in a paracrine fashion.[19] Additionally, the size of uterine leiomyomata typically declines during the postpartum period, with the use of hormone-suppressing gonadotropin-releasing hormone (GnRH) analogs, and after menopause, when levels of those hormones fall.[20]
Uterine Leiomyomata Risk Factors
Due to the pathology of uterine leiomyomata, the majority of significant risk factors include those that increase exposure to higher levels of endogenous estrogen over time. These risk factors include early menarche, nulliparity, late transition into menopause, obesity, diet, and a family history of uterine fibroids.[21] The most significant non-modifiable risk factor is African descent, which is associated with earlier age at diagnosis and an increased severity of symptoms. Hypertension is also associated with a 5-fold increased risk of developing leiomyomata compared to those without hypertension.[22]
A decreased risk for uterine leiomyomata has been noted with late menarche, multiparity, smoking, and the use of hormonal contraceptives.[23][24][4][12] The risk of uterine leiomyomata decreases with an increasing number of term pregnancies, such that women having more than 5 pregnancies have a quarter of the risk of nulliparous women.[25]
Epidemiology
Uterine fibroids may present at any age and in any community. However, prevalence varies significantly amongst different age groups and ethnicities.
Uterine leiomyomata are rare before puberty.[26] The incidence then rises significantly during the reproductive years, increasing with advancing age until menopause. The prevalence of leiomyomata in females of reproductive age is estimated to be between 20% and 40%, with the highest incidence found among women in their fifth and sixth decades of life.[27] Higher rates of uterine leiomyomata are found in premenopausal compared to postmenopausal individuals, likely due to the role of hormones in stimulating fibroid growth.
Studies have consistently shown a 3-fold increased risk of uterine leiomyomata among Black women compared to white women when controlling for other accepted risk factors, with the cumulative incidence of fibroids as high as 80% in Black women compared to 50% to 70% in White women.[1][2] In addition, black women tend to develop uterine leiomyomata at a younger age and present with larger uteri at the time of diagnosis. They are also more likely to suffer from blood-loss anemia compared to White women. Of note, this may be related, at least in part, to the fact that Black women generally tend to have earlier menarche compared to White women.[28] Observed racial disparities are likely due largely to social determinants of health, including but not limited to access to care, job security, and adequate health insurance.
In women presenting with infertility, 5% to 10% are found to have uterine leiomyomata. Leiomyomata are found to be the sole abnormality affecting fertility in 2% to 4% of the infertile female population.[29][30]
Histopathology
Histological Examination Leiomyomata
Uterine fibroids are composed of monoclonal smooth muscle and fibroblast cells that have transformed at the microscopic level under the influence of sex steroid hormones.[31] These abnormal cells feature upregulated expression of progesterone receptors (predominantly the PR-a isoform) and estrogen receptors (primarily ER-a).[32][33] Histologic samples of uterine leiomyomata demonstrate a significant extracellular matrix (ECM) composed of collagen, proteoglycan, and fibronectin adjacent to disordered myometrium cells.[34][35] Cells within a uterine leiomyomata typically have a low mitotic index.[20][36] A thin layer of areolar tissue and compressed muscle fibers characteristically encapsulates uterine leiomyomata.[36]
Histological Differentiation of Leiomyoma From Leiomyosarcoma
Benign leiomyomas are significantly more common than malignant leiomyosarcomas, and the conditions are differentiated based on their mitotic index, degree of cytologic atypia, and the presence of tumor cell necrosis. Leiomyomata demonstrate mild cytologic atypia, no tumor cell necrosis, and the mitotic activity is usually <5 mitotic figures per 10 high-power fields (hpf) in the most mitotically active areas. On the other hand, leiomyosarcomas demonstrate at least 2 of the following features: a mitotic index of at least 10 mitotic figures per 10 hpf, moderate to severe cytologic atypia, or the presence of tumor cell necrosis, also known as coagulative necrosis.[37]
Pathologic Classification
Uterine leiomyomata may be classified as subserosal, intramural, or submucosal. Uterine leiomyomata growing entirely within the myometrium are known as intramural. When fibroids extend superficially from just beneath the uterine serosa, they are known as subserosal leiomyomata. Subserosal leiomyomata may extend into the pelvis, lower abdomen, and, on occasion, the upper abdomen. Alternatively, submucosal uterine leiomyomata arise from the myometrium just deep to the endometrium and grow into the uterine cavity. Subserosal and submucosal fibroids may also be pedunculated.
The International Federation of Gynecology and Obstetrics (FIGO) classifies uterine leiomyomata as follows:
- Type 0: Pedunculated intracavitary
- Type 1: Submucosal with < 50% intramural
- Type 2: Submucosal with ≥ 50% intramural
- Type 3: Located 100% intramural, though directly contacts the endometrium
- Type 4: Located 100% intramural without direct contact with the endometrium
- Type 5: Subserosal with ≥ 50% intramural
- Type 6: Subserosal with < 50% intramural
- Type 7: Subserosal, pedunculated outside the uterine body
- Type 8: Other, including cervical and parasitic [38][39]
History and Physical
Clinical History
The presentation of uterine leiomyomata can vary significantly, depending on the number, size, and location of leiomyomas, and ranges from asymptomatic to intensely debilitating.[40] While a majority of patients with uterine leiomyomata are asymptomatic, approximately 25% to 30% of patients present with symptoms severe enough to warrant intervention.[8] Additionally, symptoms can evolve over time as uterine leiomyomata grow, degenerate, calcify, and hemorrhage. When symptomatic, the most common symptoms include AUB, pelvic pain or pressure (eg, dysmenorrhea, dyspareunia, and chronic pelvic pain), increased urinary frequency, constipation, infertility, and clinical features associated with blood-loss anemia.[41] Bleeding may be abnormal in volume, timing, or both.
Uterine leiomyomas that arise from the submucosa are more likely to disrupt the contraction of endometrial vasculature during menses and, as a result, may cause heavy bleeding, even when small.[42] Alternatively, large bulky uterine leiomyomata that extend from the serosal surface into the peritoneal cavity are more likely to cause bulk-related symptoms secondary to mass effect.[43] For example, large anterior uterine leiomyomata tend to manifest with pelvic pressure and bladder symptoms, while posterior leiomyomas can lead to constipation and low back pain.
Patients with leiomyomata may also present with infertility complaints. Adverse effects of leiomyomas on fertility are largely dependent on their location and ability to distort normal uterine and adnexal anatomy, thereby preventing normal gamete transport, embryo implantation, and the maintenance of early pregnancy.[44] Studies have shown evidence suggesting uterine leiomyomata can alter endometrial receptivity, impair local blood flow, and lead to chronic inflammation that creates a hostile environment for successful intrauterine pregnancy.[45][44]
Physical Examination
A speculum exam with a bimanual exam should be performed to assess the patient for vaginal and cervical pathology, as well as evaluate the size and shape of the female reproductive organs. On a bimanual exam, the examiner may palpate an enlarged, bulky uterus with an irregular contour.[12] If the leiomyomata are predominantly posterior, one may appreciate fullness in the posterior cul-de-sac with an anteriorly displaced cervix. However, an irregular uterine contour or increased uterine size may not be appreciated in patients with small intramural or submucosal leiomyoma. For this reason, a patient with symptoms suggestive of a leiomyoma on history warrants further evaluation, even when physical exam findings are unremarkable.
Clinicians should also consider evaluating for signs of anemia (eg, conjunctival pallor) and intracranial, thyroid, and intraabdominal pathology to identify potential secondary symptoms or alternative causes of abnormal bleeding or pain symptoms.
Evaluation
Imaging Studies
Transvaginal ultrasonography
Transvaginal ultrasonography (TVUS) imaging remains the best tool for initial evaluation when leiomyomata are suspected.[46] With a uterine size less than that of 10 weeks gestation, transvaginal ultrasound has 95% to 100% sensitivity in detecting uterine leiomyomas.[47] On TVUS, leiomyomas are typically described as well-circumscribed, concentric, hypoechoic masses with acoustic shadowing.[48] Calcifications or necrosis may distort the echogenicity (see Image. Calcified Fibroid).[48]
Saline infusion sonohysterography
Saline infusion sonohysterography (SIS) involves placing a catheter into the uterine cavity via the cervix and instilling the cavity with sterile saline. The saline distends the cavity under real-time transvaginal sonography, allowing for better assessment of intracavitary lesions. SIS is particularly useful when evaluating leiomyomata that distort the uterine cavity (ie, Type 0, 1, and 2 fibroids).[46][49]
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is the most effective imaging technique to detect the size and location of leiomyomata. However, given the cost and availability of this modality, MRI is not routinely used as a first-line test. MRI can be beneficial in some circumstances to assist in surgical planning, as this study can help clarify anatomic relationships within the pelvis and identify the extent of vascularization, fibroid degeneration, and depth of invasion into the myometrium.[3][50] MRI has not been shown to differentiate benign leiomyoma from leiomyosarcoma reliably.[51]
Laboratory Studies
For patients of reproductive age presenting with HMB or AUB, the initial evaluation should include a beta-human chorionic gonadotropin (hCG) test to rule out pregnancy, a complete blood count (CBC) to check for anemia, and thyroid stimulating hormone (TSH) and prolactin levels to evaluate for non-structural causes of AUB. Additionally, patients with risk factors for endometrial cancer (eg, obesity, chronic anovulation, and advanced age) should undergo an endometrial biopsy.[46]
Treatment / Management
While deciding on treatment options for leiomyomata, the size and location of the patient's leiomyomata, as well as her age, presenting symptoms, and desire for fertility preservation, all merit consideration.[4] The primary goal of treatment is to relieve bothersome signs and symptoms and, if desired, improve or maintain fertility.[40]
Management options include surveillance, medical management, traditional surgery, and several alternative procedural treatments. Surgical and procedural treatments are typically more effective at symptom improvement than medical management. However, due to the inherent risk associated with procedures, medical management is often an appropriate initial treatment choice.
Surveillance
Surveillance is generally preferred in individuals with asymptomatic leiomyomata. Current recommendations do not require serial imaging when following these patients.[4] However, repeat imaging is recommended when a patient experiences a change in clinical symptoms, eg, new or worsening bleeding, pain, signs or symptoms of anemia, or urinary or bowel dysfunction.[12]
Medical Management
Medical management is often used initially for patients with symptoms of AUB related to their leiomyomata. Some medical options are less effective at reducing bulk-related symptoms, so tailoring management based on clinical presentation and patient goals is essential.[40] Additionally, some agents, particularly those that suppress the hypothalamic-pituitary-ovarian (HPO) axis, can only be used for a limited time to prevent excessive loss of bone mineral density (BMD). While medical management preserves fertility and is typically associated with lower risks than surgical treatments, symptoms tend to recur when medications are stopped.
Hormonal contraceptives
Combined oral contraceptives work by preventing endometrial proliferation and have shown some benefits in improving leiomyomata-related HMB.[52][53][4] However, there is no evidence to support their use for the relief of bulk symptoms, and they do not improve fertility.[54][55] In a randomized trial comparing the use of the levonorgestrel intrauterine device (LNG-IUD) and a low-dose combined oral contraceptive (COC), COCs showed a decrease in menstrual blood loss. However, they were not as effective as the LNG-IUD.[56] (A1)
The use of progestin-only oral contraceptives is a reasonable option for patients with leiomyomata-associated HMB and contraindications to estrogen use. However, the direct evidence supporting their benefit for treating symptomatic fibroids is limited.
Levonorgestrel intrauterine device
Levonorgestrel intrauterine devices (LNG-IUDs) are commonly used in the treatment of HMB, including leiomyomata-related uterine bleeding.[55] The levonorgestrel in the LNG-IUD induces endometrial decidualization and glandular atrophy and desensitizes endometrial estrogen receptors, rendering them less responsive to estrogen, all of which reduces menstrual bleeding.[57][58] Around 20% of patients experience amenorrhea in the first year after insertion.[59] Multiple studies have demonstrated that despite reducing leiomyomata-related HMB, the LNG-IUD produces no significant reduction in fibroid volume among premenopausal individuals.[60][56] (A1)
Expulsion rates of IUDs may be affected by the size and location of the leiomyomata, with studies showing rates of expulsion among women with leiomyomas ranging as high as 15% to 20%.[61] A large retrospective cohort study from Getahun et. al involving over 228,000 nonpostpartum women found that, in general, rates of expulsion were significantly higher in individuals with a recent diagnosis of menorrhagia (from any cause) compared to those without, with an adjusted hazard ratio of 2.84 (95% CI 2.66-3.03).[62] Another study found that an initial uterine volume ≥200 cm3 is associated with increased expulsion rates compared to those with smaller uteri.[63] Caution also appears necessary when treating leiomyomata that distort the intrauterine cavity, as submucosal leiomyomas may lead to a higher expulsion rate.[3](A1)
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used as first-line agents for treating leiomyoma-related dysmenorrhea and HMB, given their affordability, availability, and limited adverse effect profile.[55] These medications decrease endometrial prostaglandin production, which subsequently reduces aberrant vascularization, menstrual blood loss, and menstrual pain.[40][12] They do not affect bulk symptoms or decrease the size of the leiomyomata.[64] A Cochrane review showed that NSAIDs reduce HMB compared to placebo, though they were less effective than tranexamic acid, danazol, or LNG-IUD.[65](A1)
Common recommendations include ibuprofen (600 to 1800 mg daily in divided doses) or naproxen (550 to 1100 mg daily) starting 1 to 2 days before the onset of bleeding and continuing throughout the duration of menstruation.[65](A1)
Tranexamic acid
Tranexamic acid (TXA) is a derivative of the amino acid lysine that can reversibly inhibit the lysine receptors on plasminogen. TXA prevents fibrin degradation and functionally stabilizes clot formation when bound to these receptors.[40] TXA has been approved for the treatment of HMB, with some evidence suggesting that TXA is superior to NSAID therapy for reducing menstrual blood flow.[12][64] However, TXA has not been approved or shown to decrease the tumor burden in uterine leiomyomata.[53][66](A1)
Theoretically, TXA should increase the risk of venous thromboembolism. Although clinical studies have found no significant evidence supporting this theoretical risk, TXA is usually prescribed as a single-agent therapy.[12][40]
Gonadotropin-releasing hormone agonists
Gonadotropin-releasing hormone (GnRH) agonists are peptide analogs of the GnRH molecule that act on the GnRH receptor in the pituitary gland. Initially, they cause a temporary increase in serum gonadotropins and downstream sex hormones, including estrogen and progesterone, leading to a flare in symptoms.[40][12] However, prolonged activation of the GnRH receptor, which typically occurs following several weeks of continuous therapy, leads to downregulation of the GnRH pituitary receptors, thereby suppressing pituitary release of gonadotropins and leading to a hypoestrogenic state.[67] While this leads to uterine atrophy and reductions in menstrual bleeding and leiomyomata size, GnRH agonists can also decrease bone mineral density (BMD) when used for longer durations of time. Additionally, uterine fibroids begin growing again once treatment is stopped, limiting their use over the long term.
GnRH agonists are used for the short-term management of AUB by reducing menstrual blood loss (and typically inducing a state of amenorrhea) and improving bulk symptoms related to uterine enlargement. A study by Friedman et al showed a decrease in uterine size by 45% after 24 weeks of treatment with a GnRH agonist. However, uterine size returned to pretreatment volumes 24 weeks after medication cessation.[68] (A1)
Given their adverse effect profile with long-term use and the fact that leiomyomata return following cessation of treatment, one of the primary uses of GnRH agonists is to provide short-term relief until the patient undergoes more definitive planned surgical treatment or natural menopause.[3][53] GnRH agonists are also used to decrease uterine volume before surgical therapy, which may ultimately allow for a minimally invasive surgical approach that would have been technically challenging with larger tumor burdens. In a recent Cochrane review, GnRH agonists were shown to reduce uterine volume by 175 mL and leiomyomata volume by 155 mL compared to placebo controls. The same review demonstrated that GnRH agonists also increased preoperative hemoglobin levels by an average of 0.88 g/dL, decreased intraoperative blood loss, and decreased postoperative complications.[69](A1)
With prolonged therapy, there is a risk of chemically induced menopause leading to vasomotor symptoms, vaginal atrophy/dryness, BMD loss, and unfavorable changes to lipid profiles.[3] To reduce the risks associated with decreases in BMD, the American College of Obstetricians and Gynecologists (ACOG) has recommended that the use of GnRH agonists as monotherapy be limited to 6 months or less.[70][71][72] If treatment is to exceed 6 months, add-back hormonal therapy with estrogen and progestin should be given, which can prolong the acceptable use of GnRH agonists by an additional 6 months (for a total of 12 months of therapy).[73](A1)
Leuprolide acetate, the most studied GnRH agonist in the management of leiomyomata, is given as an intramuscular depot injection, either once monthly at a dosage of 3.75 mg or once every 3 months at a dosage of 11.25 mg.
Gonadotropin-releasing hormone antagonists
Elagolix and relugolix are oral, nonpeptide, GnRH antagonists that function by reversible, dose-dependent suppression of GnRH, thereby inhibiting gonadotropin release and reducing ovarian sex hormone production, leading to a hypoestrogenic state.[74] Both medications are approved for the use of HMB related to leiomyomas. As pure GnRH antagonists, these medications do not produce an initial flare in symptoms, unlike the GnRH agonists.
Some preparations of these drugs are combined with hormone-add back therapy, simplifying therapy regimens. Elagolix is available as both a monotherapy pill and as a combination product. The combination product comes packaged with 2 capsules, the first containing elagolix 300 mg plus estradiol 1 mg plus norethindrone 0.5 mg and is taken in the morning; the second capsule containing elagolix 300 mg alone is taken at night.[75] Relugolix can also be taken as monotherapy or as a combination product consisting of relugolix, 40 mg of estradiol, 1mg, and norethindrone acetate 0.5 mg in a single capsule once daily.[76] Both combination products are approved in the United States for 24 months of use.
Elagolix effectively reduces leiomyoma-related bleeding. In 2 recent identical double-blinded, randomized, placebo-controlled, 6-month phase 3 trials (trials UF1 and UF2), 68.5% of 206 women in UF-1 and 76.5% of 189 women in UF-2 receiving elagolix plus add-back therapy achieved the primary endpoint of reduced menstrual blood loss to less than 80 mL during the final month of treatment and at least a 50% reduction in menstrual blood loss compared to baseline.[74] In these trials, for those who received elagolix alone (without add-back therapy), the same primary endpoint was met in 84.1% of 104 women in UF-1 and 77% of 95 women in UF-2. After 6 months of elagolix plus add-back therapy, no difference in BMD compared to placebo was noted, although women who received elagolix alone demonstrated BMD loss.[74]
In a subsequent extension phase 3 trial, when therapy was given for an additional 6 months (for a total of 12 months of treatment), more than half of the participants demonstrated amenorrhea and reported significant improvements in quality of life.[75] The most common adverse effects of elagolix are hot flashes (7%) and headaches (5.5%). After 12 months of combination therapy, BMD of the lumbar spine was reduced by 1.5%. However, patients regained some of the BMD loss so that average BMD was only 0.6% below baseline when reassessed 1 year following cessation of treatment.[75]
Following the elagolix trials, the protocol was replicated for 2 international, randomized, double-blind placebo-controlled, 24-week, phase 3 trials for the study of relugolix. The relugolix trials demonstrated a reduction of menstrual blood loss in 71% to 80% of participants compared to placebo and significant decreases in pain, anemia, bulk-related symptoms, and uterine volume.[76] Moreover, relugolix was shown to preserve bone mineral density. The most common adverse effects of relugolix combination therapy were hot flashes and hypertension.
Selective progesterone receptor modulators
Selective progesterone receptor modulators (SPRMs) are a class of synthetic steroids that bind to progesterone receptors and have tissue-specific antagonist effects of varying degrees.[77] The primary drug in this class used for the treatment of symptomatic leiomyomata is ulipristal acetate (UPA). At the endometrium level, UPA prevents endometrial proliferation and decreases uterine bleeding. UPA also binds progesterone receptors that are abundant within the fibroids to decrease cellular proliferation and selectively induce apoptosis, ultimately reducing the size of the fibroids. Of note, SPRMs lead to histologic changes in the endometrium, referred to as progesterone receptor modulator-associated endometrial changes (PAECs), which are reversible and are not associated with malignancy.[77][78] (A1)
Clinical efficacy in the reduction of leiomyoma-related AUB and fibroid volume has been demonstrated in the European-based PEARL trials.[79][80] Among women who received UPA for 13 weeks in the randomized, placebo-controlled PEARL I trial, 91% had normalization of bleeding, 73% became amenorrheic, and leiomyoma volume was reduced by an average of 21%.[79] UPA is currently not approved in the United States for treating leiomyomata. Outside the United States, its use is limited due to the rare adverse effect of severe liver injury.(A1)
Aromatase inhibitors
Letrozole is a reversible, nonsteroidal, competitive aromatase inhibitor that blocks the conversion of androgens into estrogens. Studies have demonstrated that uterine leiomyoma tissue over-expresses aromatase. Thus, aromatase inhibitors reduce the local production of estrogen from the leiomyoma itself, thereby reducing fibroid proliferation and assisting in fibroid volume reduction.[81][82] One trial of 70 participants demonstrated a reduction in leiomyoma volume by 46% after 12 weeks of treatment; however, no significant difference was noted compared to GnRH agonists.[83](A1)
Another study demonstrated a decrease in operative time and intraoperative blood loss when letrozole was used preoperatively for 12 weeks.[84] Letrozole is not currently approved in the United States. for the treatment of symptomatic leiomyomata, though this medication is occasionally used off-label for this purpose. The most reported adverse effects are hot flashes and BMD loss with prolonged use.[83](A1)
Letrozole can be administered orally at a dosage of 2.5 mg to 5 mg daily for 12 weeks.
Surgical Management
Procedural intervention remains the most successful long-term treatment for most symptomatic leiomyomas. Myomectomy is the surgical removal of uterine leiomyomata via hysteroscopic, laparoscopic, robotic, or open abdominal surgery. Indications for myomectomy include AUB with or without symptomatic anemia, pain, bulk-related symptoms (eg, urinary urgency, frequency, hydronephrosis, constipation), fertility challenges, and in some cases, for the evaluation of potential malignancy. Establishing the type (location), size, and quantity of fibroids using recommended evaluation modalities is crucial to help guide clinicians in the optimal surgical approach. (Please refer to the Evaluation section for more information on recommended diagnostic studies.) Successful myomectomy typically preserves future fertility while reducing the size and symptoms of leiomyomata; however, many women will require subsequent procedures for recurrent symptomatic leiomyomata. For this reason, hysterectomy is still typically considered definitive management.
Hysteroscopic myomectomy
Hysteroscopic myomectomy (HSC-M) is primarily used to resect submucosal leiomyomata. Complete resection rates are highest for type 0 and type 1 fibroids, with rates as high as 97% and 90%, respectively.[85] HSC-M can also be used for type 2 leiomyomata; however, multistage or repeat procedures are often required due to lower complete resection rates. Patients with leiomyomata >3 cm in size or with ≥2 fibroids also have a higher risk of needing a repeat procedure.[86] The overall rate of incomplete resection is approximately 5% to 17%.[87] Regarding improvements in quality of life (QoL) and symptomatic improvement, HSC-M has an overall success rate of 94.4%.[88][89](A1)
As a minimally invasive procedure that avoids entry into the peritoneal cavity, HSC-M is associated with minimal recovery time, minimal need for opioids, minimal postoperative restrictions, faster return to daily activity, and decreased perioperative morbidity.[90] Patients typically report a significant improvement in symptom severity and quality of life within 6 to 12 weeks of surgery.[90] (B2)
A 2019 consensus statement from the Global Congress on Hysteroscopy Scientific Committee (GCHSC) published in the Journal of Minimally Invasive Gynecology (JMIG) recommends hysteroscopic myomectomy in the presence of at least one submucous myoma even when asymptomatic, noting that expectant management is also acceptable in patients without desires for immediate fertility and myomas <15 mm.[91] This same consensus statement also recommends cold loop resectoscopic myomectomy, with energy on the pure "cut" setting, to minimize the risk of intrauterine adhesion formation. However, the committee notes that dissection, vaporization, and morcellation are all acceptable alternatives. Similarly, guidelines from the International Society for Gynecologic Endoscopy (ISGE) recommend resectoscopic techniques for types 0, 1, and 2, noting that morcellation can also be recommended for type 0 leiomyomas.[92](B3)
Leiomyoma type also affects the volume of distention fluid absorbed during the procedure. The deeper the fibroid penetrates the myometrium, the greater the volume of fluid absorbed, with a study showing patients with type 0, type 1, and type 2 fibroids absorbing an average of 450 mL, 957 mL, and 1682 mL, respectively.[93]
Overall, HSC-M is a very safe procedure with a complication rate of <1%.[94] The most common complication with hysteroscopy, in general, is uterine perforation, which complicates 0.76% of hysteroscopic procedures and most frequently occurs during entry into the uterine cavity. Uterine perforation is also the most common reason for excessive bleeding during the procedure. Other complications of HSC-M are associated with excess fluid absorption of the distention medium, which can occur during complicated resections with large or multiple leiomyomata that prolong the procedure.[95](B2)
Laparoscopic myomectomy
Laparoscopic myomectomy (LSC-M) is a minimally invasive surgical approach to remove symptomatic leiomyomata classified as types 2 through 7 and occasionally type 8. Location, size, and the number of leiomyomata are all crucial determinants for the success of the surgery and reducing recurrence.
LSC-M relieves symptoms in 80% of patients undergoing the procedure, similar to abdominal myomectomy. However, LSC-M is associated with significantly fewer complications and a shorter recovery period than open procedures.[96] In a study assessing outcomes in the immediate postoperative period, patients who underwent LSC-M had less pain at the 6- and 48-hour marks, a 50% lower chance of perioperative fever, and shorter hospitalizations than those who underwent abdominal myomectomy.[97] One study looking specifically at the short-term QoL measures following LSC-M demonstrated that patients returned to normal activity 1 week earlier and returned to work 20 days sooner than patients who underwent abdominal myomectomy.[90] (A1)
The risk of complications during LSC-M is increased in patients with leiomyomata >5 cm, with >3 total fibroids, and in those with intramural and intraligamentous fibroids.[98] A large multicenter study evaluating over 2000 patients found a total complication rate of 11.1% (n=225), of which 9.1% were minor complications and 2% were major complications.[98](B2)
The most common minor complications were fever of unexplained origin (5.1%) and urinary tract infections (3.4%); major complications included hemorrhage (0.7%), hematoma (0.48%), bowel injury (0.04%), and postoperative kidney failure (0.04%). Overall, conversion to laparotomy is a rare event. In a study of over 700 intended laparoscopic myomectomies, only 7 had to be converted to an open abdominal approach.[99] Risk factors for conversion to abdominal myomectomy include the size of leiomyomata (>5 cm on ultrasound), intramural type leiomyomata, anterior fibroids, preoperative use of GnRH agonists, and increased BMI.[100] Long-term complications of surgery include adhesive disease (36% to 66%) and recurrence of leiomyomata.[101][102][103](A1)
Experienced laparoscopists can typically perform myomectomies laparoscopically regardless of the leiomyoma size, number, or location.[104] Larger myomas can be placed inside Endo bags, the edges of which are brought extracorporeally through a port site. This allows for contained power morcellation or incisions, reducing the myoma's size to remove the leiomyoma without enlarging the incisions.[105](A1)
Robot-assisted laparoscopic myomectomy
The use of robot-assisted laparoscopic myomectomy (RLSC-M) has grown in popularity amongst many minimally invasive gynecologic surgeons in recent years. Preference for the use of either technique is based on the surgeon's skill level in using the conventional versus robotic approach in handling complex cases. Surgeons tend to prefer RLSC-M in cases that involve ≥10 leiomyomata that are ≤7 cm or in patients with obesity due to improved visibility.[106] The most significant difference between these techniques is the conversion rate to abdominal myomectomy. In a systematic review and meta-analysis of over 2000 patients, conventional LSC-M was associated with a 4.5-fold higher risk of conversion to abdominal myomectomy than RLSC-M.[107] (B2)
Regarding outcomes and complications, a meta-analysis of 8 retrospective trials comparing the 2 techniques demonstrated no significant difference in complications, operative time, length of hospital stays, and postoperative fertility.[108] Given these subtle differences and the increased costs associated with RLSC-M, there is no strong indication for choosing it over conventional LSC-M. With both approaches, the failure rate remains comparably low.[109](A1)
Abdominal myomectomy
Abdominal myomectomy (ABD-M) is a major surgical procedure typically reserved for complex cases where a minimally invasive approach is contraindicated or in failed laparoscopic or robotic cases. Similar to LSC-M, an ABD-M is best used for symptomatic intramural and subserosal fibroids.
Benefits of ABD-M include a shorter operative time of 126 minutes, compared to 155 and 181 minutes with LSC-M and RLSC-M, respectively.[110] Additionally, a lower risk of recurrence with ABD-M 8 years postprocedure compared with LSC-M (63.4% versus 76.2%) was noted, thought to be due to the improvements in the surgeon's ability to identify and remove subcentimeter-sized leiomyomata with an ABD-M approach.[111] In a retrospective cohort study looking at 30 patients who underwent ABD-M, overall symptoms improved in 90% of patients, and 79% were satisfied with their choice of the procedure.[96] Regarding fertility outcomes, a Cochrane review demonstrated no difference between LSC-M and ABD-M.[112](A1)
Despite the similar outcomes in symptom reduction, fertility, and benefits in operative time, ABD-M is associated with significantly higher rates of morbidity. Blood loss is considerably higher with ABD-M compared to minimally invasive alternatives, with an average of 200 mL of estimated blood loss during ABD-M compared to 150 mL and 100 mL in LSC-M and RLSC-M, respectively.[110] A systematic review and meta-analysis by Chen et al demonstrated that ABD-M caused a longer duration of postoperative ileus and increased length of hospital stay.[113] A Cochrane review concluded that pain was increased at the 6- and 48-hour marks after an ABD-M compared to LSC-M. This review also found a 50% increased risk of postoperative fever in patients who underwent ABD-M compared to LSC-M.[97](A1)
A prospective nationwide registry called Comparing Options For Management: Patient-centered Results for Uterine Fibroids (COMPARE-UF) allowed for the comparison of short-term effects of different routes of myomectomy in 1206 women. This registry found that regardless of the type of myomectomy, surgical approaches improved short-term health-related quality of life and symptom severity score improvement. Return to usual activity averaged 0 days in HSC-M compared to 21 and 28 days for laparoscopic and abdominal myomectomy, respectively.[90](B2)
Due to the higher rate of surgical complications, preoperative optimization is paramount before ABD-M.
Myomectomy for fertility improvement
Many studies have demonstrated a clear relationship between uterine leiomyomata and infertility; however, surgical management of leiomyomata for the sole purpose of improving fertility (ie, in otherwise asymptomatic patients) is less clear-cut and largely depends on the location of the leiomyomata.
Leiomyomata that distort the uterine cavity (ie, submucosal leiomyomata, FIGO types 0, 1, and 2) appear to influence reproductive outcomes significantly.[114] In a systematic review and meta-analysis of 23 RCTs evaluating pregnancy outcomes of infertile women with and without submucosal leiomyomata, the women with submucosal leiomyomata had significantly lower rates of implantation (RR=0.283), clinical pregnancy (RR=0.363), and ongoing pregnancy and live birth (RR=0.318). Furthermore, women with submucosal leiomyomata have increased rates of spontaneous abortion.[115][116](A1)
Evidence suggests that cavity-distorting myomas should be surgically removed to improve reproductive outcomes even if the patient is otherwise asymptomatic.[114] A prospective study of 181 infertile women with a solitary leiomyoma <4 cm compared pregnancy outcomes in those who underwent myomectomy versus those who underwent expectant management. In patients with SM fibroids (n=52), those who underwent myomectomy had a clinical pregnancy rate of 43% compared to 27% in the expectant management group after 1 year of enrollment (P < 0.05).[117] Another study investigating conception rates up to 3 years after HSC-M found a 49% conception rate after resection of type 0 leiomyomata and 33% after resection of type 1 leiomyomata.[118] At this time, there is insufficient evidence to suggest that HSC-M for submucosal leiomyomata reduces miscarriage rates.[114](B2)
At this time, the American Society for Reproductive Medicine (ASRM) states that "In asymptomatic women with cavity-distorting myomas (intramural with a submucosal component or submucosal), myomectomy (open, laparoscopic, or hysteroscopic) may be considered to improve pregnancy rates." [114] However, if intrauterine abnormalities are not seen on routine imaging, the European Society of Human Reproduction and Embryology (ESHRE) recommends against diagnostic hysteroscopy for the detection and possible correction of intracavitary lesions in individuals with unexplained infertility.
The negative impact of intramural leiomyomata on fertility outcomes has also been demonstrated in multiple studies. In general, women with IM fibroids have lower rates of implantation, clinical pregnancy, and ongoing pregnancy/live birth.[115][116] One of the largest prospective cohort studies investigating the success of in vitro fertilization (IVF) in women with and without leiomyomata showed that in women with intramural leiomyomata ≤5 cm (mean size 2.3 cm), the clinical pregnancy rate per embryo transfer was 23.3% compared to 34.1% in women without leiomyomata.[119] (A1)
However, there is limited data regarding whether or not the removal of these leiomyomata improves fertility outcomes. An RCT of 181 women with a solitary fibroid <4 cm and at least 1 year of infertility showed that myomectomy was not associated with an improved clinical pregnancy rate in women with IM fibroids (n=23, 56.5%, NS).[117] On the other hand, a prospective, non-randomized cohort study of 318 women with unexplained infertility or recurrent pregnancy loss showed that women who underwent LSC-M had higher birth rates than those with leiomyomata who did not undergo LSC-M (42% [44/106] vs. 11% [12/106], P <.001, respectively).[120] Overall, myomectomy for intramural leiomyomata does not necessarily guarantee the resolution of infertility, but there is fair evidence suggesting the procedure does not impair reproductive outcomes.[114] If a patient with large intramural leiomyomata has failed multiple IVF cycles, myomectomy should be strongly considered. Ultimately, the decision to undergo myomectomy for intramural leiomyomata should result from a shared decision-making process between the patient and her surgeon.(B2)
Studies looking at subserosal fibroids suggest they do not play a significant role in infertility and, thus, do not need to be removed to improve reproductive outcomes.[115][116](A1)
Hysterectomy
Hysterectomy is still considered to be the most definitive treatment of uterine leiomyomata.[12] Like myomectomy, a hysterectomy may be performed vaginally, laparoscopically, robotically, or through an open abdominal approach. The choice of surgical approach is highly dependent on the patient's clinical presentation and the individual surgeon's experience and preferences.[12] Like myomectomies, minimally invasive approaches are associated with lower blood loss, faster recovery time, and fewer complications.
Alternative Procedural Treatment Modalities
Uterine artery embolization
Uterine artery embolization (UAE) is a percutaneous transcatheter embolization technique that occludes the cervicovaginal branch of both uterine arteries to deprive leiomyomata of their blood supply, leading to their degeneration (see Image. Uterine Artery Embolization).[109] Ideal candidates for this approach are patients with HMB caused by intramural leiomyomata who are either resistant to or not ideal candidates for surgery.
In a study assessing patients 10 years following UAE, 62% of patients reported their HMB had resolved at 2 years, with 83% reporting resolution of HMB at 5 years postprocedure.[121] Overall, 78% of patients reported they were very satisfied with the treatment at the 10-year mark. UAE is associated with a decreased risk for transfusion, shorter hospital stays, and quicker return to baseline functioning compared with surgical management.[122][123](A1)
Although the UAE can relieve many patients and avoid risks associated with surgery, studies have shown that as many as 19% to 38% of patients undergo reintervention for persistent symptoms.[124] Additionally, the rate of reintervention requiring hysterectomy was 28% over 5 years.[125] (A1)
Major complications occur in 1% to 12% of patients and include rehospitalization (often for fever or pain), ovarian failure, pulmonary embolism, and unplanned hysterectomy.[126] Minor complications include fever, pain, nausea, pelvic infection, postembolization syndrome, and vaginal discharge.(B3)
Conflicting data regarding reproductive outcomes after UAE compared to myomectomy have been reported. Overall, UAE is not the ideal treatment for patients who desire future fertility due to a potential postprocedure decline in ovarian reserve and increased risk of pregnancy-related complications. For example, UAE for uterine leiomyomata is associated with an increased rate of miscarriage (35.2% versus 16.5%; OR, 2.8; 95% CI, 2.0–3.8), cesarean delivery (66% versus 48.5%; OR, 2.1; 95% CI, 1.4–2.9), and postpartum hemorrhage (13.9% versus 2.5%; OR, 6.4; 95% CI, 3.5–11.7) compared to expectant management of fibroids.[127](A1)
Magnetic resonance-guided focused ultrasound surgery
Magnetic resonance-guided focused ultrasound surgery (MRgFUS) is a minimally invasive treatment that involves continuous waves of ultrasound energy over a 3- to 5-hour period under magnetic resonance (MR) guidance. Eventually, the goal is to heat the entire leiomyoma to >57 °C for at least 1 second to induce coagulative necrosis.[109][128] Leiomyomata with a high T2-weighted signal intensity are more likely to respond to the treatment.[129] The procedure is associated with very minimal recovery time and low radiation exposure.
A study performed by Funaki et al looking at 91 women over 24 months who underwent MRgFUS demonstrated a mean reduction in leiomyoma volume of 36.5% at 6 months and 39.5% at 24 months.[130] Another study showed reductions in leiomyoma volume of 86%, 93%, and 88% at 3, 6, and 12 months, respectively.[131] In patients with primarily intramural myomas >3 cm and uteri size <24 weeks gestation, MRgFUS decreased the percentage of patients reporting excessive bleeding from 69% to 31% and relieved pressure symptoms in 49% of patients.[132](B2)
Limited research has been performed regarding reproductive outcomes after MRgFUS, with most data coming from a few case reports and small case series. Existing data is reassuring, with a case series describing 54 pregnancies in 51 women after MRgFUS.[133] More studies are needed to determine whether MRgFUS should be recommended for patients seeking future fertility.(A1)
Potential complications of MRgFUS include skin burns, nerve damage, necrosis of nontargeted tissue, bone heating, and venous thrombosis due to prolonged immobility.[109][132][134] One study found an overall complication rate of 13.1% when evaluating 150 women who underwent the procedure; however, only one of these was a major complication (ie, deep vein thrombosis).[131] In the same study, 7.4% of patients required treatment for leiomyoma-related symptoms within 1 year of the procedure.(B2)
Radiofrequency ablation (RFA)
Radiofrequency ablation (RFA) is a treatment that involves either the laparoscopic or transcervical insertion of a conductive needle array into the leiomyoma using ultrasound guidance. Radiofrequency energy is then applied to the leiomyoma, heaving the tissue to >100 °C, leading to coagulative necrosis in the tissue.[135] Ultrasound is used to monitor the tissue throughout the procedure.
In a prospective international trial observing a cohort of 135 women with leiomyomata and HMB, menstrual blood loss decreased from baseline levels by 38.3 months, and fibroid volume decreased by 45.1% 1 year following the procedure.[136] Furthermore, symptom severity and quality of life scores both improved significantly, and 94% of women reported satisfaction with the procedure. Another study of 31 women showed an 82% improvement in symptom severity scores and a mean uterine volume reduction from 105.9 cm3 at baseline to 53.5 cm3 after 1 year.[137] (B2)
A meta-analysis found that rates of surgical reintervention after RFA were 4.2%, 8.2%, and 11.5% at 1, 2, and 3 years, respectively.[138] Included in the meta-analysis was the Halt trial, which looked at outcomes of RFA after 3 years in 104 patients. The authors reported a reintervention rate of 11% (14 patients), which included 11 hysterectomies, 2 laparoscopic myomectomies, and 1 UAE.[139] The incidence of complications and adverse effects remains inconsistent across studies.(A1)
Data is limited regarding fertility and pregnancy after RFA for uterine leiomyomata. One study reported 15 pregnancies in 13 patients, yielding 13 live births after RFA for leiomyomata.[135] In addition, a case series of 30 pregnancies by Berman et al reported 26 full-term live births and 4 pregnancy losses.[140] Current trials are beginning to include more patients desiring fertility, given that RFA causes minimal myometrial damage. However, no definitive conclusions can be drawn about pregnancy outcomes and pregnancy-related risks at this time.(B2)
Differential Diagnosis
The differential diagnosis for uterine leiomyomas includes both benign and malignant diseases that cause uterine enlargement, abnormal bleeding, pelvic pain (eg, dysmenorrhea, chronic pain, and dyspareunia), and infertility.
According to the International Federation of Gynecology and Obstetrics (FIGO), the differential for AUB in women of reproductive age includes the conditions that make up the PALM-COEIN classification system.[141][142] This system is a mnemonic that groups AUB etiologies into structural (polyps, adenomyosis, leiomyomata, malignancy [PALM]) and nonstructural (coagulopathy, ovulatory dysfunction, endometrial dysfunction, iatrogenic, and not yet classified [COEIN]) categories.
Adenomyosis, in particular, has been shown to have a high rate of coexistence with uterine leiomyomata.[143] Adenomyosis often presents with an enlarged uterus, HMB, and dysmenorrhea. Additionally, adenomyosis can present as a myometrial mass, known as an adenomyoma. Despite this, both conditions can typically be differentiated on imaging.[46] A leiomyoma is typically well-circumscribed, whereas adenomyomas tend to have indistinct borders. Additional sonographic features that support a diagnosis of adenomyosis include asymmetric myometrial thickness, heterogeneous myometrium, heterogenous cysts, and subendometrial echogenic linear striations.[144][145] Additionally, adenomyosis in isolation is much less likely to cause a mass effect or significant bulk symptoms.
Additionally, clinicians should realize that leiomyomas and leiomyosarcomas can have nearly identical presentations, as leiomyosarcomas typically present with AUB and a rapidly enlarging uterus. Brohl et al concluded that sarcomas are diagnosed in presumed leiomyomata after surgery in 1 in 340 women. That number increases to 1 in 98 women who are 75 to 79 years of age.[146] Although no reliable method to differentiate these pathologies without biopsy exists, clinicians should recognize factors suggestive of leiomyosarcoma, including postmenopausal status, a predominantly subserosal mass, solitary leiomyoma, rapid growth, and a T2-weighted signal heterogeneity on magnetic resonance imaging.[4][147]
Prognosis
The overall prognosis of uterine fibroids depends on the size, number, and location of fibroids, the initial presentation, and the treatment modality selected. Many patients remain asymptomatic indefinitely.
Some notable risk factors for fibroid recurrence after surgical or procedural treatment include age less than 35.5 years at treatment, 2 or fewer fibroids at the time of surgery, size of the uterus less than 13 weeks gestation, and no childbearing after surgery.[148] At 8 years postoperation, ABD-M has a 63.4% risk of fibroid recurrence compared to 76.2% after LSC-M.[111] Reintervention rates range from 19% to 38% after UAE and are as high as 54% after MRgFUS.[124][88]
For those patients who become pregnant following myomectomy, delivery mode, and timing depend on the number and locations of uterine incisions and whether any of the incisions transected the full thickness of the myometrium. The primary concern is the risk of uterine rupture. This risk is higher in patients with entry into the uterine cavity during myomectomy. Although published data is limited, the risk of uterine rupture after myomectomy is reportedly closer to 1%, similar to the risk of a trial of labor after cesarean (TOLAC), with successful vaginal delivery being achieved in about 90% of patients.[149] According to the Society of Maternal-Fetal Medicine (SMFM), patients with a prior myomectomy with entry into the uterine cavity should undergo a scheduled cesarean delivery between 37 and 38 weeks gestation. However, if extensive transfundal scarring is suggested from the myomectomy operative report, delivery should be considered between 36 and 37 weeks gestation, similar to patients with a history of a classical cesarean delivery.[150]
Complications
Nonobstetric Complications
Uterine fibroids can lead to any of the following complications:
- Chronic pelvic pain
- Heavy menstrual bleeding
- Anemia
- Infertility
- Sexual dysfunction
- Constipation
- Urinary tract infections or urinary incontinence
- Torsion of a pedunculated fibroid
- Degeneration with or without infection
Obstetric Complications
Much of the evidence regarding obstetric complications in the setting of uterine fibroids is conflicting. However, some evidence has supported the association of uterine fibroids with increased rates of preterm labor and birth, placental abruption, placenta previa, malpresentation, preterm prelabor rupture of membranes, abnormal labor, cesarean birth, and postpartum hemorrhage.[5][6][7]
Some studies suggest that an increased risk of preterm labor is most apparent when there are multiple fibroids, a fibroid is >5 cm in size, or the placenta is located near the fibroid.[151][7] The exact reason for this association is unclear. Theories include activation of inflammatory pathways that ultimately trigger preterm labor, localized increases in oxytocin levels, and mechanical changes in the uterine muscle around the fibroid leading to contractions as the uterus expands. On the other hand, a 2021 prospective cohort study involving over 4600 women with singleton pregnancies, 475 of whom also had at least 1 uterine fibroid, found no difference in preterm birth rates (10.2% versus 10.3%).[152] Currently, experts generally recommend against the treatment of leiomyomas during pregnancy.
Deterrence and Patient Education
In addition to reviewing the risks and benefits of each treatment option, patients should be informed that:
- Leiomyomas are extremely common, and in premenopausal women, over 99.7% are benign.
- Leiomyomas themselves, along with several treatment options, may impact future fertility.
- Genetics may play a role in leiomyoma development.
- Obesity is a risk factor for uterine leiomyomata; encouraging healthy lifestyle habits may reduce the risk of leiomyomata.
Additionally, although fibroids are considered a benign diagnosis, they can exert a negative impact on the patient's mental and physical health. Emphasis should be placed on proper patient education while considering how the patient perceives the disease. In describing leiomyomata, words, eg, tumor or neoplasm, should be avoided, in general, by all members of the healthcare team to ensure the patient has a good understanding of the disease.
Enhancing Healthcare Team Outcomes
The management of uterine leiomyomata requires a multifaceted approach that integrates skills, strategies, and responsibilities across various healthcare disciplines to ensure optimal patient-centered care. Physicians, advanced practitioners, nurses, pharmacists, and other health professionals must collaborate to provide comprehensive care tailored to the patient’s symptoms, treatment goals, and fertility considerations. Obstetricians and gynecologists must possess strong clinical and surgical skills while also recognizing when to consult fertility specialists, particularly for patients older than 35 who desire future pregnancy. Shared decision-making is essential, ensuring patients understand the risks, benefits, and long-term implications of various treatment options, including medical therapy, interventional procedures, and surgery. Effective patient education and counseling foster adherence to treatment plans and improve patient satisfaction, making interprofessional collaboration a key component of high-quality care.
Care coordination is vital for optimizing patient safety, outcomes, and team performance in both medical and surgical management of leiomyomata. Pharmacists and nursing staff play a crucial role in guiding patients through medical treatment regimens, addressing concerns about adverse effects, and ensuring adherence. When procedural or surgical intervention is necessary, interprofessional teamwork between gynecologists, interventional radiologists, anesthesiologists, perioperative nurses, and administrative personnel ensures seamless transitions through the phases of care. Preoperative optimization enhances surgical outcomes, including hematology or primary care consultation for anemia or comorbidities. The implementation of Enhanced Recovery After Surgery (ERAS) protocols relies on clear communication between outpatient and inpatient nursing teams, as well as the surgeon, to ensure adherence to best practices. By fostering interprofessional cooperation and effective communication, healthcare teams can improve patient safety, reduce complications, and enhance overall outcomes for individuals with uterine leiomyomata.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG : an international journal of obstetrics and gynaecology. 2017 Sep:124(10):1501-1512. doi: 10.1111/1471-0528.14640. Epub 2017 May 13 [PubMed PMID: 28296146]
Level 1 (high-level) evidenceBaird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. American journal of obstetrics and gynecology. 2003 Jan:188(1):100-7 [PubMed PMID: 12548202]
. Management of Symptomatic Uterine Leiomyomas: ACOG Practice Bulletin, Number 228. Obstetrics and gynecology. 2021 Jun 1:137(6):e100-e115. doi: 10.1097/AOG.0000000000004401. Epub [PubMed PMID: 34011888]
De La Cruz MS, Buchanan EM. Uterine Fibroids: Diagnosis and Treatment. American family physician. 2017 Jan 15:95(2):100-107 [PubMed PMID: 28084714]
Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. American journal of obstetrics and gynecology. 2008 Apr:198(4):357-66. doi: 10.1016/j.ajog.2007.12.039. Epub [PubMed PMID: 18395031]
Level 1 (high-level) evidencePérez-Roncero GR, López-Baena MT, Ornat L, Cuerva MJ, Garcia-Casarrubios P, Chedraui P, Pérez-López FR. Uterine fibroids and preterm birth risk: A systematic review and meta-analysis. The journal of obstetrics and gynaecology research. 2020 Sep:46(9):1711-1727. doi: 10.1111/jog.14343. Epub 2020 Jul 6 [PubMed PMID: 32633025]
Level 1 (high-level) evidenceShavell VI, Thakur M, Sawant A, Kruger ML, Jones TB, Singh M, Puscheck EE, Diamond MP. Adverse obstetric outcomes associated with sonographically identified large uterine fibroids. Fertility and sterility. 2012 Jan:97(1):107-10. doi: 10.1016/j.fertnstert.2011.10.009. Epub 2011 Nov 17 [PubMed PMID: 22100166]
Giuliani E, As-Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2020 Apr:149(1):3-9. doi: 10.1002/ijgo.13102. Epub 2020 Feb 17 [PubMed PMID: 31960950]
Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environmental health perspectives. 2003 Jun:111(8):1037-54 [PubMed PMID: 12826476]
Level 3 (low-level) evidenceOkolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best practice & research. Clinical obstetrics & gynaecology. 2008 Aug:22(4):571-88. doi: 10.1016/j.bpobgyn.2008.04.002. Epub 2008 Jun 4 [PubMed PMID: 18534913]
Townsend DE, Sparkes RS, Baluda MC, McClelland G. Unicellular histogenesis of uterine leiomyomas as determined by electrophoresis by glucose-6-phosphate dehydrogenase. American journal of obstetrics and gynecology. 1970 Aug 15:107(8):1168-73 [PubMed PMID: 5458572]
Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nature reviews. Disease primers. 2016 Jun 23:2():16043. doi: 10.1038/nrdp.2016.43. Epub 2016 Jun 23 [PubMed PMID: 27335259]
Li Z, Maeda D, Kudo-Asabe Y, Tamura D, Nanjo H, Hayashi A, Ikemura M, Fukayama M, Goto A. MED12 is frequently mutated in ovarian and other adnexal leiomyomas. Human pathology. 2018 Nov:81():89-95. doi: 10.1016/j.humpath.2018.06.013. Epub 2018 Jun 23 [PubMed PMID: 29944972]
Bertsch E, Qiang W, Zhang Q, Espona-Fiedler M, Druschitz S, Liu Y, Mittal K, Kong B, Kurita T, Wei JJ. MED12 and HMGA2 mutations: two independent genetic events in uterine leiomyoma and leiomyosarcoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014 Aug:27(8):1144-53. doi: 10.1038/modpathol.2013.243. Epub 2014 Jan 3 [PubMed PMID: 24390224]
Level 2 (mid-level) evidenceMäkinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, Yan J, Enge M, Taipale M, Aavikko M, Katainen R, Virolainen E, Böhling T, Koski TA, Launonen V, Sjöberg J, Taipale J, Vahteristo P, Aaltonen LA. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science (New York, N.Y.). 2011 Oct 14:334(6053):252-5. doi: 10.1126/science.1208930. Epub 2011 Aug 25 [PubMed PMID: 21868628]
Mehine M, Kaasinen E, Mäkinen N, Katainen R, Kämpjärvi K, Pitkänen E, Heinonen HR, Bützow R, Kilpivaara O, Kuosmanen A, Ristolainen H, Gentile M, Sjöberg J, Vahteristo P, Aaltonen LA. Characterization of uterine leiomyomas by whole-genome sequencing. The New England journal of medicine. 2013 Jul 4:369(1):43-53. doi: 10.1056/NEJMoa1302736. Epub 2013 Jun 5 [PubMed PMID: 23738515]
Benassayag C, Leroy MJ, Rigourd V, Robert B, Honoré JC, Mignot TM, Vacher-Lavenu MC, Chapron C, Ferré F. Estrogen receptors (ERalpha/ERbeta) in normal and pathological growth of the human myometrium: pregnancy and leiomyoma. The American journal of physiology. 1999 Jun:276(6):E1112-8. doi: 10.1152/ajpendo.1999.276.6.E1112. Epub [PubMed PMID: 10362625]
Doherty L, Mutlu L, Sinclair D, Taylor H. Uterine fibroids: clinical manifestations and contemporary management. Reproductive sciences (Thousand Oaks, Calif.). 2014 Sep:21(9):1067-92. doi: 10.1177/1933719114533728. Epub 2014 May 12 [PubMed PMID: 24819877]
Level 3 (low-level) evidenceBulun SE. Uterine fibroids. The New England journal of medicine. 2013 Oct 3:369(14):1344-55. doi: 10.1056/NEJMra1209993. Epub [PubMed PMID: 24088094]
Level 3 (low-level) evidenceGhosh S, Naftalin J, Imrie R, Hoo WL. Natural History of Uterine Fibroids: A Radiological Perspective. Current obstetrics and gynecology reports. 2018:7(3):117-121. doi: 10.1007/s13669-018-0243-5. Epub 2018 May 8 [PubMed PMID: 30101039]
Level 3 (low-level) evidencePavone D, Clemenza S, Sorbi F, Fambrini M, Petraglia F. Epidemiology and Risk Factors of Uterine Fibroids. Best practice & research. Clinical obstetrics & gynaecology. 2018 Jan:46():3-11. doi: 10.1016/j.bpobgyn.2017.09.004. Epub 2017 Oct 1 [PubMed PMID: 29054502]
Takeda T, Sakata M, Isobe A, Miyake A, Nishimoto F, Ota Y, Kamiura S, Kimura T. Relationship between metabolic syndrome and uterine leiomyomas: a case-control study. Gynecologic and obstetric investigation. 2008:66(1):14-7. doi: 10.1159/000114250. Epub 2008 Jan 30 [PubMed PMID: 18230910]
Level 2 (mid-level) evidencePurohit P, Vigneswaran K. Fibroids and Infertility. Current obstetrics and gynecology reports. 2016:5():81-88 [PubMed PMID: 27217980]
Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. International journal of women's health. 2013:5():93-100. doi: 10.2147/IJWH.S38800. Epub 2013 Feb 27 [PubMed PMID: 23467803]
Level 2 (mid-level) evidenceRoss RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. British medical journal (Clinical research ed.). 1986 Aug 9:293(6543):359-62 [PubMed PMID: 3730804]
Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocrine reviews. 2013 Feb:34(1):130-62. doi: 10.1210/er.2012-1043. Epub 2013 Jan 9 [PubMed PMID: 23303565]
Level 3 (low-level) evidenceWallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstetrics and gynecology. 2004 Aug:104(2):393-406 [PubMed PMID: 15292018]
Level 3 (low-level) evidenceWise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, Rosenberg L. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. American journal of epidemiology. 2004 Jan 15:159(2):113-23 [PubMed PMID: 14718211]
Level 2 (mid-level) evidenceGuo XC, Segars JH. The impact and management of fibroids for fertility: an evidence-based approach. Obstetrics and gynecology clinics of North America. 2012 Dec:39(4):521-33. doi: 10.1016/j.ogc.2012.09.005. Epub [PubMed PMID: 23182558]
Buttram VC Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertility and sterility. 1981 Oct:36(4):433-45 [PubMed PMID: 7026295]
Stewart EA. Uterine fibroids. Lancet (London, England). 2001 Jan 27:357(9252):293-8 [PubMed PMID: 11214143]
Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010 Jun:151(6):2433-42. doi: 10.1210/en.2009-1225. Epub 2010 Apr 7 [PubMed PMID: 20375184]
Level 3 (low-level) evidenceBrandon DD, Erickson TE, Keenan EJ, Strawn EY, Novy MJ, Burry KA, Warner C, Clinton GM. Estrogen receptor gene expression in human uterine leiomyomata. The Journal of clinical endocrinology and metabolism. 1995 Jun:80(6):1876-81 [PubMed PMID: 7775635]
Islam MS, Ciavattini A, Petraglia F, Castellucci M, Ciarmela P. Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Human reproduction update. 2018 Jan 1:24(1):59-85. doi: 10.1093/humupd/dmx032. Epub [PubMed PMID: 29186429]
Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. The Journal of clinical endocrinology and metabolism. 1994 Sep:79(3):900-6 [PubMed PMID: 8077380]
Holdsworth-Carson SJ, Zaitseva M, Vollenhoven BJ, Rogers PA. Clonality of smooth muscle and fibroblast cell populations isolated from human fibroid and myometrial tissues. Molecular human reproduction. 2014 Mar:20(3):250-9. doi: 10.1093/molehr/gat083. Epub 2013 Nov 15 [PubMed PMID: 24243625]
Rubisz P, Ciebiera M, Hirnle L, Zgliczyńska M, Łoziński T, Dzięgiel P, Kobierzycki C. The Usefulness of Immunohistochemistry in the Differential Diagnosis of Lesions Originating from the Myometrium. International journal of molecular sciences. 2019 Mar 6:20(5):. doi: 10.3390/ijms20051136. Epub 2019 Mar 6 [PubMed PMID: 30845657]
Munro MG, Critchley HO, Broder MS, Fraser IS, FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2011 Apr:113(1):3-13. doi: 10.1016/j.ijgo.2010.11.011. Epub 2011 Feb 22 [PubMed PMID: 21345435]
Munro MG, Critchley HOD, Fraser IS, FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2018 Dec:143(3):393-408. doi: 10.1002/ijgo.12666. Epub 2018 Oct 10 [PubMed PMID: 30198563]
Kashani BN, Centini G, Morelli SS, Weiss G, Petraglia F. Role of Medical Management for Uterine Leiomyomas. Best practice & research. Clinical obstetrics & gynaecology. 2016 Jul:34():85-103. doi: 10.1016/j.bpobgyn.2015.11.016. Epub 2015 Nov 25 [PubMed PMID: 26796059]
Stewart EA. Clinical practice. Uterine fibroids. The New England journal of medicine. 2015 Apr 23:372(17):1646-55. doi: 10.1056/NEJMcp1411029. Epub [PubMed PMID: 25901428]
Nishino M, Togashi K, Nakai A, Hayakawa K, Kanao S, Iwasaku K, Fujii S. Uterine contractions evaluated on cine MR imaging in patients with uterine leiomyomas. European journal of radiology. 2005 Jan:53(1):142-6 [PubMed PMID: 15607866]
Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. American journal of obstetrics and gynecology. 2013 Oct:209(4):319.e1-319.e20. doi: 10.1016/j.ajog.2013.07.017. Epub 2013 Jul 24 [PubMed PMID: 23891629]
Level 2 (mid-level) evidencePractice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons. Myomas and reproductive function. Fertility and sterility. 2008 Nov:90(5 Suppl):S125-30. doi: 10.1016/j.fertnstert.2008.09.012. Epub [PubMed PMID: 19007608]
Cook H, Ezzati M, Segars JH, McCarthy K. The impact of uterine leiomyomas on reproductive outcomes. Minerva ginecologica. 2010 Jun:62(3):225-36 [PubMed PMID: 20595947]
Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstetrics and gynecology. 2012 Jul:120(1):197-206. doi: 10.1097/AOG.0b013e318262e320. Epub [PubMed PMID: 22914421]
Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. American journal of obstetrics and gynecology. 2002 Mar:186(3):409-15 [PubMed PMID: 11904599]
Woźniak A, Woźniak S. Ultrasonography of uterine leiomyomas. Przeglad menopauzalny = Menopause review. 2017 Dec:16(4):113-117. doi: 10.5114/pm.2017.72754. Epub 2017 Dec 30 [PubMed PMID: 29483851]
Cicinelli E, Romano F, Anastasio PS, Blasi N, Parisi C, Galantino P. Transabdominal sonohysterography, transvaginal sonography, and hysteroscopy in the evaluation of submucous myomas. Obstetrics and gynecology. 1995 Jan:85(1):42-7 [PubMed PMID: 7800322]
Level 1 (high-level) evidenceOmary RA, Vasireddy S, Chrisman HB, Ryu RK, Pereles FS, Carr JC, Resnick SA, Nemcek AA Jr, Vogelzang RL. The effect of pelvic MR imaging on the diagnosis and treatment of women with presumed symptomatic uterine fibroids. Journal of vascular and interventional radiology : JVIR. 2002 Nov:13(11):1149-53 [PubMed PMID: 12427815]
Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Human reproduction update. 2016 Nov:22(6):665-686 [PubMed PMID: 27466209]
Venkatachalam S, Bagratee JS, Moodley J. Medical management of uterine fibroids with medroxyprogesterone acetate (Depo Provera): a pilot study. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2004 Oct:24(7):798-800 [PubMed PMID: 15763792]
Level 3 (low-level) evidenceLewis TD, Malik M, Britten J, San Pablo AM, Catherino WH. A Comprehensive Review of the Pharmacologic Management of Uterine Leiomyoma. BioMed research international. 2018:2018():2414609. doi: 10.1155/2018/2414609. Epub 2018 Jan 28 [PubMed PMID: 29780819]
Lethaby A, Wise MR, Weterings MA, Bofill Rodriguez M, Brown J. Combined hormonal contraceptives for heavy menstrual bleeding. The Cochrane database of systematic reviews. 2019 Feb 11:2(2):CD000154. doi: 10.1002/14651858.CD000154.pub3. Epub 2019 Feb 11 [PubMed PMID: 30742315]
Level 1 (high-level) evidenceSohn GS, Cho S, Kim YM, Cho CH, Kim MR, Lee SR, Working Group of Society of Uterine Leiomyoma. Current medical treatment of uterine fibroids. Obstetrics & gynecology science. 2018 Mar:61(2):192-201. doi: 10.5468/ogs.2018.61.2.192. Epub 2018 Feb 13 [PubMed PMID: 29564309]
Sayed GH, Zakherah MS, El-Nashar SA, Shaaban MM. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2011 Feb:112(2):126-30. doi: 10.1016/j.ijgo.2010.08.009. Epub 2010 Nov 19 [PubMed PMID: 21092958]
Level 1 (high-level) evidenceBahamondes L, Fernandes A, Monteiro I, Bahamondes MV. Long-acting reversible contraceptive (LARCs) methods. Best practice & research. Clinical obstetrics & gynaecology. 2020 Jul:66():28-40. doi: 10.1016/j.bpobgyn.2019.12.002. Epub 2019 Dec 20 [PubMed PMID: 32014434]
Kokolina VF, Iakunina LN, Dubovaia TN. [The system of regulation of blood coagulation in girls with juvenile uterine hemorrhage]. Pediatriia. 1990:(5):68-70 [PubMed PMID: 2399071]
Sergison JE, Maldonado LY, Gao X, Hubacher D. Levonorgestrel intrauterine system associated amenorrhea: a systematic review and metaanalysis. American journal of obstetrics and gynecology. 2019 May:220(5):440-448.e8. doi: 10.1016/j.ajog.2018.12.008. Epub 2018 Dec 7 [PubMed PMID: 30527945]
Level 1 (high-level) evidenceJiang W, Shen Q, Chen M, Wang Y, Zhou Q, Zhu X, Zhu X. Levonorgestrel-releasing intrauterine system use in premenopausal women with symptomatic uterine leiomyoma: a systematic review. Steroids. 2014 Aug:86():69-78. doi: 10.1016/j.steroids.2014.05.002. Epub 2014 May 14 [PubMed PMID: 24832215]
Level 1 (high-level) evidenceZapata LB, Whiteman MK, Tepper NK, Jamieson DJ, Marchbanks PA, Curtis KM. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010 Jul:82(1):41-55. doi: 10.1016/j.contraception.2010.02.011. Epub 2010 Mar 29 [PubMed PMID: 20682142]
Level 1 (high-level) evidenceGetahun D, Fassett MJ, Gatz J, Armstrong MA, Peipert JF, Raine-Bennett T, Reed SD, Zhou X, Schoendorf J, Postlethwaite D, Shi JM, Saltus CW, Wang J, Xie F, Chiu VY, Merchant M, Alabaster A, Ichikawa LE, Hunter S, Im TM, Takhar HS, Ritchey ME, Chillemi G, Pisa F, Asiimwe A, Anthony MS. Association between menorrhagia and risk of intrauterine device-related uterine perforation and device expulsion: results from the Association of Uterine Perforation and Expulsion of Intrauterine Device study. American journal of obstetrics and gynecology. 2022 Jul:227(1):59.e1-59.e9. doi: 10.1016/j.ajog.2022.03.025. Epub 2022 Mar 12 [PubMed PMID: 35292234]
Magalhaes J, Ferreira-Filho ES, Soares-Junior JM, Baracat EC. Uterine volume, menstrual patterns, and contraceptive outcomes in users of the levonorgestrel-releasing intrauterine system: A cohort study with a five-year follow-up. European journal of obstetrics, gynecology, and reproductive biology. 2022 Sep:276():56-62. doi: 10.1016/j.ejogrb.2022.06.029. Epub 2022 Jul 2 [PubMed PMID: 35809459]
Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. The Cochrane database of systematic reviews. 2013 Jan 31:(1):CD000400. doi: 10.1002/14651858.CD000400.pub3. Epub 2013 Jan 31 [PubMed PMID: 23440779]
Level 1 (high-level) evidenceBofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. The Cochrane database of systematic reviews. 2019 Sep 19:9(9):CD000400. doi: 10.1002/14651858.CD000400.pub4. Epub 2019 Sep 19 [PubMed PMID: 31535715]
Level 1 (high-level) evidenceWellington K, Wagstaff AJ. Tranexamic acid: a review of its use in the management of menorrhagia. Drugs. 2003:63(13):1417-33 [PubMed PMID: 12825966]
Newton CL, Riekert C, Millar RP. Gonadotropin-releasing hormone analog therapeutics. Minerva ginecologica. 2018 Oct:70(5):497-515. doi: 10.23736/S0026-4784.18.04316-2. Epub 2018 Sep 26 [PubMed PMID: 30264955]
Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstetrics and gynecology. 1991 May:77(5):720-5 [PubMed PMID: 1901638]
Level 1 (high-level) evidenceLethaby A, Puscasiu L, Vollenhoven B. Preoperative medical therapy before surgery for uterine fibroids. The Cochrane database of systematic reviews. 2017 Nov 15:11(11):CD000547. doi: 10.1002/14651858.CD000547.pub2. Epub 2017 Nov 15 [PubMed PMID: 29139105]
Level 1 (high-level) evidenceAmerican College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstetrics and gynecology. 2008 Aug:112(2 Pt 1):387-400. doi: 10.1097/AOG.0b013e318183fbab. Epub [PubMed PMID: 18669742]
Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: long-term follow-up. Obstetrics and gynecology. 2002 May:99(5 Pt 1):709-19 [PubMed PMID: 11978277]
Level 1 (high-level) evidenceGonzalez-Barcena D, Alvarez RB, Ochoa EP, Cornejo IC, Comaru-Schally AM, Schally AV, Engel J, Reissmann T, Riethmüller-Winzen H. Treatment of uterine leiomyomas with luteinizing hormone-releasing hormone antagonist Cetrorelix. Human reproduction (Oxford, England). 1997 Sep:12(9):2028-35 [PubMed PMID: 9363724]
Bradley LD, Gueye NA. The medical management of abnormal uterine bleeding in reproductive-aged women. American journal of obstetrics and gynecology. 2016 Jan:214(1):31-44. doi: 10.1016/j.ajog.2015.07.044. Epub 2015 Aug 5 [PubMed PMID: 26254516]
Schlaff WD, Ackerman RT, Al-Hendy A, Archer DF, Barnhart KT, Bradley LD, Carr BR, Feinberg EC, Hurtado SM, Kim J, Liu R, Mabey RG Jr, Owens CD, Poindexter A, Puscheck EE, Rodriguez-Ginorio H, Simon JA, Soliman AM, Stewart EA, Watts NB, Muneyyirci-Delale O. Elagolix for Heavy Menstrual Bleeding in Women with Uterine Fibroids. The New England journal of medicine. 2020 Jan 23:382(4):328-340. doi: 10.1056/NEJMoa1904351. Epub [PubMed PMID: 31971678]
Simon JA, Al-Hendy A, Archer DF, Barnhart KT, Bradley LD, Carr BR, Dayspring T, Feinberg EC, Gillispie V, Hurtado S, Kim J, Liu R, Owens CD, Muneyyirci-Delale O, Wang A, Watts NB, Schlaff WD. Elagolix Treatment for Up to 12 Months in Women With Heavy Menstrual Bleeding and Uterine Leiomyomas. Obstetrics and gynecology. 2020 Jun:135(6):1313-1326. doi: 10.1097/AOG.0000000000003869. Epub [PubMed PMID: 32459423]
Al-Hendy A, Lukes AS, Poindexter AN 3rd, Venturella R, Villarroel C, Critchley HOD, Li Y, McKain L, Arjona Ferreira JC, Langenberg AGM, Wagman RB, Stewart EA. Treatment of Uterine Fibroid Symptoms with Relugolix Combination Therapy. The New England journal of medicine. 2021 Feb 18:384(7):630-642. doi: 10.1056/NEJMoa2008283. Epub [PubMed PMID: 33596357]
Ali M, Al-Hendy A. Selective progesterone receptor modulators for fertility preservation in women with symptomatic uterine fibroids. Biology of reproduction. 2017 Sep 1:97(3):337-352. doi: 10.1093/biolre/iox094. Epub [PubMed PMID: 29025038]
Murji A, Whitaker L, Chow TL, Sobel ML. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. The Cochrane database of systematic reviews. 2017 Apr 26:4(4):CD010770. doi: 10.1002/14651858.CD010770.pub2. Epub 2017 Apr 26 [PubMed PMID: 28444736]
Level 1 (high-level) evidenceDonnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E, Terrill P, Osterloh I, Loumaye E, PEARL I Study Group. Ulipristal acetate versus placebo for fibroid treatment before surgery. The New England journal of medicine. 2012 Feb 2:366(5):409-20. doi: 10.1056/NEJMoa1103182. Epub [PubMed PMID: 22296075]
Level 1 (high-level) evidenceDonnez J, Vázquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC, Barlow DH, Palacios S, Donnez O, Bestel E, Osterloh I, Loumaye E, PEARL III and PEARL III Extension Study Group. Long-term treatment of uterine fibroids with ulipristal acetate ☆. Fertility and sterility. 2014 Jun:101(6):1565-73.e1-18. doi: 10.1016/j.fertnstert.2014.02.008. Epub 2014 Mar 12 [PubMed PMID: 24630081]
Level 1 (high-level) evidenceGurates B, Parmaksiz C, Kilic G, Celik H, Kumru S, Simsek M. Treatment of symptomatic uterine leiomyoma with letrozole. Reproductive biomedicine online. 2008 Oct:17(4):569-74 [PubMed PMID: 18854113]
Level 3 (low-level) evidenceBulun SE, Imir G, Utsunomiya H, Thung S, Gurates B, Tamura M, Lin Z. Aromatase in endometriosis and uterine leiomyomata. The Journal of steroid biochemistry and molecular biology. 2005 May:95(1-5):57-62 [PubMed PMID: 16024248]
Song H, Lu D, Navaratnam K, Shi G. Aromatase inhibitors for uterine fibroids. The Cochrane database of systematic reviews. 2013 Oct 23:2013(10):CD009505. doi: 10.1002/14651858.CD009505.pub2. Epub 2013 Oct 23 [PubMed PMID: 24151065]
Level 1 (high-level) evidenceLeone Roberti Maggiore U, Scala C, Venturini PL, Ferrero S. Preoperative treatment with letrozole in patients undergoing laparoscopic myomectomy of large uterine myomas: a prospective non-randomized study. European journal of obstetrics, gynecology, and reproductive biology. 2014 Oct:181():157-62. doi: 10.1016/j.ejogrb.2014.07.040. Epub 2014 Aug 11 [PubMed PMID: 25150954]
Level 1 (high-level) evidenceWamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstetrics and gynecology. 1993 Nov:82(5):736-40 [PubMed PMID: 8414318]
Hart R, Molnár BG, Magos A. Long term follow up of hysteroscopic myomectomy assessed by survival analysis. British journal of obstetrics and gynaecology. 1999 Jul:106(7):700-5 [PubMed PMID: 10428527]
Van Dongen H, Emanuel MH, Smeets MJ, Trimbos B, Jansen FW. Follow-up after incomplete hysteroscopic removal of uterine fibroids. Acta obstetricia et gynecologica Scandinavica. 2006:85(12):1463-7 [PubMed PMID: 17260223]
Sandberg EM, Tummers FHMP, Cohen SL, van den Haak L, Dekkers OM, Jansen FW. Reintervention risk and quality of life outcomes after uterine-sparing interventions for fibroids: a systematic review and meta-analysis. Fertility and sterility. 2018 Apr:109(4):698-707.e1. doi: 10.1016/j.fertnstert.2017.11.033. Epub [PubMed PMID: 29653718]
Level 1 (high-level) evidencePolena V, Mergui JL, Perrot N, Poncelet C, Barranger E, Uzan S. Long-term results of hysteroscopic myomectomy in 235 patients. European journal of obstetrics, gynecology, and reproductive biology. 2007 Feb:130(2):232-7 [PubMed PMID: 16530319]
Level 2 (mid-level) evidenceLaughlin-Tommaso SK, Lu D, Thomas L, Diamond MP, Wallace K, Wegienka G, Vines AI, Anchan RM, Wang T, Maxwell GL, Jacoby V, Marsh EE, Spies JB, Nicholson WK, Stewart EA, Myers ER. Short-term quality of life after myomectomy for uterine fibroids from the COMPARE-UF Fibroid Registry. American journal of obstetrics and gynecology. 2020 Apr:222(4):345.e1-345.e22. doi: 10.1016/j.ajog.2019.09.052. Epub 2019 Oct 31 [PubMed PMID: 31678093]
Level 2 (mid-level) evidenceLaganà AS, Alonso Pacheco L, Tinelli A, Haimovich S, Carugno J, Ghezzi F, Mazzon I, Bettocchi S. Management of Asymptomatic Submucous Myomas in Women of Reproductive Age: A Consensus Statement from the Global Congress on Hysteroscopy Scientific Committee. Journal of minimally invasive gynecology. 2019 Mar-Apr:26(3):381-383. doi: 10.1016/j.jmig.2018.06.020. Epub 2018 Aug 18 [PubMed PMID: 30012468]
Level 3 (low-level) evidenceLoddo A, Djokovic D, Drizi A, De Vree BP, Sedrati A, van Herendael BJ. Hysteroscopic myomectomy: The guidelines of the International Society for Gynecologic Endoscopy (ISGE). European journal of obstetrics, gynecology, and reproductive biology. 2022 Jan:268():121-128. doi: 10.1016/j.ejogrb.2021.11.434. Epub 2021 Dec 1 [PubMed PMID: 34902749]
Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertility and sterility. 1997 Nov:68(5):881-6 [PubMed PMID: 9389820]
Jansen FW, Vredevoogd CB, van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstetrics and gynecology. 2000 Aug:96(2):266-70 [PubMed PMID: 10908775]
Level 2 (mid-level) evidence. The Use of Hysteroscopy for the Diagnosis and Treatment of Intrauterine Pathology: ACOG Committee Opinion, Number 800. Obstetrics and gynecology. 2020 Mar:135(3):e138-e148. doi: 10.1097/AOG.0000000000003712. Epub [PubMed PMID: 32080054]
Level 3 (low-level) evidenceBroder MS, Goodwin S, Chen G, Tang LJ, Costantino MM, Nguyen MH, Yegul TN, Erberich H. Comparison of long-term outcomes of myomectomy and uterine artery embolization. Obstetrics and gynecology. 2002 Nov:100(5 Pt 1):864-8 [PubMed PMID: 12423842]
Level 2 (mid-level) evidenceBhave Chittawar P, Franik S, Pouwer AW, Farquhar C. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. The Cochrane database of systematic reviews. 2014 Oct 21:2014(10):CD004638. doi: 10.1002/14651858.CD004638.pub3. Epub 2014 Oct 21 [PubMed PMID: 25331441]
Level 1 (high-level) evidenceSizzi O, Rossetti A, Malzoni M, Minelli L, La Grotta F, Soranna L, Panunzi S, Spagnolo R, Imperato F, Landi S, Fiaccamento A, Stola E. Italian multicenter study on complications of laparoscopic myomectomy. Journal of minimally invasive gynecology. 2007 Jul-Aug:14(4):453-62 [PubMed PMID: 17630163]
Level 2 (mid-level) evidenceSandberg EM, Cohen SL, Jansen FW, Einarsson JI. Analysis of Risk Factors for Intraoperative Conversion of Laparoscopic Myomectomy. Journal of minimally invasive gynecology. 2016 Mar-Apr:23(3):352-7. doi: 10.1016/j.jmig.2015.10.017. Epub 2015 Nov 9 [PubMed PMID: 26546180]
Dubuisson JB, Fauconnier A, Fourchotte V, Babaki-Fard K, Coste J, Chapron C. Laparoscopic myomectomy: predicting the risk of conversion to an open procedure. Human reproduction (Oxford, England). 2001 Aug:16(8):1726-31 [PubMed PMID: 11473973]
Dubuisson JB, Fauconnier A, Chapron C, Kreiker G, Nörgaard C. Second look after laparoscopic myomectomy. Human reproduction (Oxford, England). 1998 Aug:13(8):2102-6 [PubMed PMID: 9756277]
Mais V, Ajossa S, Piras B, Guerriero S, Marongiu D, Melis GB. Prevention of de-novo adhesion formation after laparoscopic myomectomy: a randomized trial to evaluate the effectiveness of an oxidized regenerated cellulose absorbable barrier. Human reproduction (Oxford, England). 1995 Dec:10(12):3133-5 [PubMed PMID: 8822429]
Level 1 (high-level) evidenceHasson HM, Rotman C, Rana N, Sistos F, Dmowski WP. Laparoscopic myomectomy. Obstetrics and gynecology. 1992 Nov:80(5):884-8 [PubMed PMID: 1407934]
Paredes JS, Lee CL, Chua PT. Myomectomy: Choosing the Surgical Approach - A Systematic Review. Gynecology and minimally invasive therapy. 2024 Jul-Sep:13(3):146-153. doi: 10.4103/gmit.gmit_152_23. Epub 2024 Jul 18 [PubMed PMID: 39184254]
Level 1 (high-level) evidenceGargiulo AR, Lewis EI, Kaser DJ, Srouji SS. Robotic single-site myomectomy: a step-by-step tutorial. Fertility and sterility. 2015 Nov:104(5):e13. doi: 10.1016/j.fertnstert.2015.07.1159. Epub 2015 Aug 20 [PubMed PMID: 26300020]
Vargas MV, Moawad GN, Sievers C, Opoku-Anane J, Marfori CQ, Tyan P, Robinson JK. Feasibility, Safety, and Prediction of Complications for Minimally Invasive Myomectomy in Women With Large and Numerous Myomata. Journal of minimally invasive gynecology. 2017 Feb:24(2):315-322. doi: 10.1016/j.jmig.2016.11.014. Epub 2016 Dec 7 [PubMed PMID: 27939896]
Level 2 (mid-level) evidenceGkegkes ID, Iatrakis G, Iavazzo PE, Bakalianou K, Iavazzo C. Robotic Management of Fibroids: Discussion of Use, Criteria and Advantages. Acta medica (Hradec Kralove). 2020:63(2):63-66. doi: 10.14712/18059694.2020.18. Epub [PubMed PMID: 32771070]
Iavazzo C, Mamais I, Gkegkes ID. Robotic assisted vs laparoscopic and/or open myomectomy: systematic review and meta-analysis of the clinical evidence. Archives of gynecology and obstetrics. 2016 Jul:294(1):5-17. doi: 10.1007/s00404-016-4061-6. Epub 2016 Mar 11 [PubMed PMID: 26969650]
Level 1 (high-level) evidenceGingold JA, Gueye NA, Falcone T. Minimally Invasive Approaches to Myoma Management. Journal of minimally invasive gynecology. 2018 Feb:25(2):237-250. doi: 10.1016/j.jmig.2017.07.007. Epub 2017 Jul 19 [PubMed PMID: 28734973]
Barakat EE, Bedaiwy MA, Zimberg S, Nutter B, Nosseir M, Falcone T. Robotic-assisted, laparoscopic, and abdominal myomectomy: a comparison of surgical outcomes. Obstetrics and gynecology. 2011 Feb:117(2 Pt 1):256-266. doi: 10.1097/AOG.0b013e318207854f. Epub [PubMed PMID: 21252737]
Level 2 (mid-level) evidenceKotani Y, Tobiume T, Fujishima R, Shigeta M, Takaya H, Nakai H, Suzuki A, Tsuji I, Mandai M, Matsumura N. Recurrence of uterine myoma after myomectomy: Open myomectomy versus laparoscopic myomectomy. The journal of obstetrics and gynaecology research. 2018 Feb:44(2):298-302. doi: 10.1111/jog.13519. Epub 2017 Dec 11 [PubMed PMID: 29227004]
Metwally M, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. The Cochrane database of systematic reviews. 2012 Nov 14:11():CD003857. doi: 10.1002/14651858.CD003857.pub3. Epub 2012 Nov 14 [PubMed PMID: 23152222]
Level 1 (high-level) evidenceChen R, Su Z, Yang L, Xin L, Yuan X, Wang Y. The effects and costs of laparoscopic versus abdominal myomectomy in patients with uterine fibroids: a systematic review and meta-analysis. BMC surgery. 2020 Mar 20:20(1):55. doi: 10.1186/s12893-020-00703-0. Epub 2020 Mar 20 [PubMed PMID: 32192462]
Level 1 (high-level) evidencePractice Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org, Practice Committee of the American Society for Reproductive Medicine. Removal of myomas in asymptomatic patients to improve fertility and/or reduce miscarriage rate: a guideline. Fertility and sterility. 2017 Sep:108(3):416-425. doi: 10.1016/j.fertnstert.2017.06.034. Epub [PubMed PMID: 28865538]
Desai P, Patel P. Fibroids, infertility and laparoscopic myomectomy. Journal of gynecological endoscopy and surgery. 2011 Jan:2(1):36-42. doi: 10.4103/0974-1216.85280. Epub [PubMed PMID: 22442534]
Level 2 (mid-level) evidencePritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertility and sterility. 2009 Apr:91(4):1215-23. doi: 10.1016/j.fertnstert.2008.01.051. Epub 2008 Mar 12 [PubMed PMID: 18339376]
Level 1 (high-level) evidenceCasini ML, Rossi F, Agostini R, Unfer V. Effects of the position of fibroids on fertility. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2006 Feb:22(2):106-9 [PubMed PMID: 16603437]
Level 2 (mid-level) evidenceVercellini P, Zàina B, Yaylayan L, Pisacreta A, De Giorgi O, Crosignani PG. Hysteroscopic myomectomy: long-term effects on menstrual pattern and fertility. Obstetrics and gynecology. 1999 Sep:94(3):341-7 [PubMed PMID: 10472856]
Hart R, Khalaf Y, Yeong CT, Seed P, Taylor A, Braude P. A prospective controlled study of the effect of intramural uterine fibroids on the outcome of assisted conception. Human reproduction (Oxford, England). 2001 Nov:16(11):2411-7 [PubMed PMID: 11679530]
Level 1 (high-level) evidenceBulletti C, De Ziegler D, Polli V, Flamigni C. The role of leiomyomas in infertility. The Journal of the American Association of Gynecologic Laparoscopists. 1999 Nov:6(4):441-5 [PubMed PMID: 10548702]
de Bruijn AM, Ankum WM, Reekers JA, Birnie E, van der Kooij SM, Volkers NA, Hehenkamp WJ. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 10-year outcomes from the randomized EMMY trial. American journal of obstetrics and gynecology. 2016 Dec:215(6):745.e1-745.e12. doi: 10.1016/j.ajog.2016.06.051. Epub 2016 Jul 5 [PubMed PMID: 27393268]
Level 1 (high-level) evidenceGupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. The Cochrane database of systematic reviews. 2014 Dec 26:2014(12):CD005073. doi: 10.1002/14651858.CD005073.pub4. Epub 2014 Dec 26 [PubMed PMID: 25541260]
Level 1 (high-level) evidenceMara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovascular and interventional radiology. 2008 Jan-Feb:31(1):73-85 [PubMed PMID: 17943348]
Level 1 (high-level) evidenceHartmann KE, Fonnesbeck C, Surawicz T, Krishnaswami S, Andrews JC, Wilson JE, Velez-Edwards D, Kugley S, Sathe NA. Management of Uterine Fibroids. 2017 Dec:(): [PubMed PMID: 30789683]
van der Kooij SM, Hehenkamp WJ, Volkers NA, Birnie E, Ankum WM, Reekers JA. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 5-year outcome from the randomized EMMY trial. American journal of obstetrics and gynecology. 2010 Aug:203(2):105.e1-13. doi: 10.1016/j.ajog.2010.01.049. Epub 2010 Jul 1 [PubMed PMID: 20579960]
Level 1 (high-level) evidenceCarrillo TC. Uterine Artery Embolization in the Management of Symptomatic Uterine Fibroids: An Overview of Complications and Follow-up. Seminars in interventional radiology. 2008 Dec:25(4):378-86. doi: 10.1055/s-0028-1102997. Epub [PubMed PMID: 21326579]
Level 3 (low-level) evidenceHomer H, Saridogan E. Uterine artery embolization for fibroids is associated with an increased risk of miscarriage. Fertility and sterility. 2010 Jun:94(1):324-30. doi: 10.1016/j.fertnstert.2009.02.069. Epub 2009 Apr 9 [PubMed PMID: 19361799]
Level 1 (high-level) evidenceHesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovascular and interventional radiology. 2013 Feb:36(1):5-13. doi: 10.1007/s00270-012-0367-3. Epub 2012 Mar 28 [PubMed PMID: 22453202]
Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. American journal of obstetrics and gynecology. 2007 Feb:196(2):184.e1-6 [PubMed PMID: 17306674]
Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2009 Nov:34(5):584-9. doi: 10.1002/uog.7455. Epub [PubMed PMID: 19852041]
Level 2 (mid-level) evidenceGorny KR, Woodrum DA, Brown DL, Henrichsen TL, Weaver AL, Amrami KK, Hangiandreou NJ, Edmonson HA, Bouwsma EV, Stewart EA, Gostout BS, Ehman DA, Hesley GK. Magnetic resonance-guided focused ultrasound of uterine leiomyomas: review of a 12-month outcome of 130 clinical patients. Journal of vascular and interventional radiology : JVIR. 2011 Jun:22(6):857-64. doi: 10.1016/j.jvir.2011.01.458. Epub 2011 Apr 8 [PubMed PMID: 21482137]
Level 2 (mid-level) evidenceHesley GK, Felmlee JP, Gebhart JB, Dunagan KT, Gorny KR, Kesler JB, Brandt KR, Glantz JN, Gostout BS. Noninvasive treatment of uterine fibroids: early Mayo Clinic experience with magnetic resonance imaging-guided focused ultrasound. Mayo Clinic proceedings. 2006 Jul:81(7):936-42 [PubMed PMID: 16835973]
Clark NA, Mumford SL, Segars JH. Reproductive impact of MRI-guided focused ultrasound surgery for fibroids: a systematic review of the evidence. Current opinion in obstetrics & gynecology. 2014 Jun:26(3):151-61. doi: 10.1097/GCO.0000000000000070. Epub [PubMed PMID: 24751998]
Level 1 (high-level) evidenceStewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gostout B, Hesley G, Kim HS, Hengst S, Gedroyc WM. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertility and sterility. 2006 Jan:85(1):22-9 [PubMed PMID: 16412721]
Level 2 (mid-level) evidenceLee BB, Yu SP. Radiofrequency Ablation of Uterine Fibroids: a Review. Current obstetrics and gynecology reports. 2016:5(4):318-324 [PubMed PMID: 27917310]
Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstetrics and gynecology. 2013 May:121(5):1075-1082. doi: 10.1097/AOG.0b013e31828b7962. Epub [PubMed PMID: 23635746]
Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 Ablation System. Journal of minimally invasive gynecology. 2011 May-Jun:18(3):364-71. doi: 10.1016/j.jmig.2011.02.006. Epub [PubMed PMID: 21545960]
Level 2 (mid-level) evidenceBradley LD, Pasic RP, Miller LE. Clinical Performance of Radiofrequency Ablation for Treatment of Uterine Fibroids: Systematic Review and Meta-Analysis of Prospective Studies. Journal of laparoendoscopic & advanced surgical techniques. Part A. 2019 Dec:29(12):1507-1517. doi: 10.1089/lap.2019.0550. Epub 2019 Nov 8 [PubMed PMID: 31702440]
Level 1 (high-level) evidenceBerman JM, Guido RS, Garza Leal JG, Pemueller RR, Whaley FS, Chudnoff SG, Halt Study Group. Three-year outcome of the Halt trial: a prospective analysis of radiofrequency volumetric thermal ablation of myomas. Journal of minimally invasive gynecology. 2014 Sep-Oct:21(5):767-74. doi: 10.1016/j.jmig.2014.02.015. Epub 2014 Mar 5 [PubMed PMID: 24613404]
Berman JM, Shashoua A, Olson C, Brucker S, Thiel JA, Bhagavath B. Case Series of Reproductive Outcomes after Laparoscopic Radiofrequency Ablation of Symptomatic Myomas. Journal of minimally invasive gynecology. 2020 Mar-Apr:27(3):639-645. doi: 10.1016/j.jmig.2019.06.009. Epub 2019 Jun 23 [PubMed PMID: 31238151]
Level 2 (mid-level) evidenceMarnach ML, Laughlin-Tommaso SK. Evaluation and Management of Abnormal Uterine Bleeding. Mayo Clinic proceedings. 2019 Feb:94(2):326-335. doi: 10.1016/j.mayocp.2018.12.012. Epub [PubMed PMID: 30711128]
Benetti-Pinto CL, Rosa-E-Silva ACJS, Yela DA, Soares Júnior JM. Abnormal Uterine Bleeding. Revista brasileira de ginecologia e obstetricia : revista da Federacao Brasileira das Sociedades de Ginecologia e Obstetricia. 2017 Jul:39(7):358-368. doi: 10.1055/s-0037-1603807. Epub 2017 Jun 12 [PubMed PMID: 28605821]
Struble J, Reid S, Bedaiwy MA. Adenomyosis: A Clinical Review of a Challenging Gynecologic Condition. Journal of minimally invasive gynecology. 2016 Feb 1:23(2):164-85. doi: 10.1016/j.jmig.2015.09.018. Epub 2015 Sep 30 [PubMed PMID: 26427702]
Kepkep K, Tuncay YA, Göynümer G, Tutal E. Transvaginal sonography in the diagnosis of adenomyosis: which findings are most accurate? Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2007 Sep:30(3):341-5 [PubMed PMID: 17659649]
Level 2 (mid-level) evidenceAtri M, Reinhold C, Mehio AR, Chapman WB, Bret PM. Adenomyosis: US features with histologic correlation in an in-vitro study. Radiology. 2000 Jun:215(3):783-90 [PubMed PMID: 10831700]
Level 2 (mid-level) evidenceBrohl AS, Li L, Andikyan V, Običan SG, Cioffi A, Hao K, Dudley JT, Ascher-Walsh C, Kasarskis A, Maki RG. Age-stratified risk of unexpected uterine sarcoma following surgery for presumed benign leiomyoma. The oncologist. 2015 Apr:20(4):433-9. doi: 10.1634/theoncologist.2014-0361. Epub 2015 Mar 12 [PubMed PMID: 25765878]
Level 2 (mid-level) evidenceChen I, Firth B, Hopkins L, Bougie O, Xie RH, Singh S. Clinical Characteristics Differentiating Uterine Sarcoma and Fibroids. JSLS : Journal of the Society of Laparoendoscopic Surgeons. 2018 Jan-Mar:22(1):. doi: 10.4293/JSLS.2017.00066. Epub [PubMed PMID: 29398899]
Yoo EH, Lee PI, Huh CY, Kim DH, Lee BS, Lee JK, Kim D. Predictors of leiomyoma recurrence after laparoscopic myomectomy. Journal of minimally invasive gynecology. 2007 Nov-Dec:14(6):690-7 [PubMed PMID: 17980328]
Level 2 (mid-level) evidenceOdejinmi F, Strong S, Sideris M, Mallick R. Caesarean section in women following an abdominal myomectomy: a choice or a need? Facts, views & vision in ObGyn. 2020 May 7:12(1):57-60 [PubMed PMID: 32696025]
. ACOG Practice Bulletin No. 205: Vaginal Birth After Cesarean Delivery. Obstetrics and gynecology. 2019 Feb:133(2):e110-e127. doi: 10.1097/AOG.0000000000003078. Epub [PubMed PMID: 30681543]
Gulersen M, Krantz D, Rochelson B, Berghella V, Blitz MJ. The association between uterine fibroid number and size and risk of preterm birth. American journal of obstetrics & gynecology MFM. 2024 Aug:6(8):101415. doi: 10.1016/j.ajogmf.2024.101415. Epub 2024 Jun 27 [PubMed PMID: 38944115]
Sundermann AC, Aldridge TD, Hartmann KE, Jones SH, Torstenson ES, Edwards DRV. Uterine fibroids and risk of preterm birth by clinical subtypes: a prospective cohort study. BMC pregnancy and childbirth. 2021 Aug 17:21(1):560. doi: 10.1186/s12884-021-03968-2. Epub 2021 Aug 17 [PubMed PMID: 34404387]
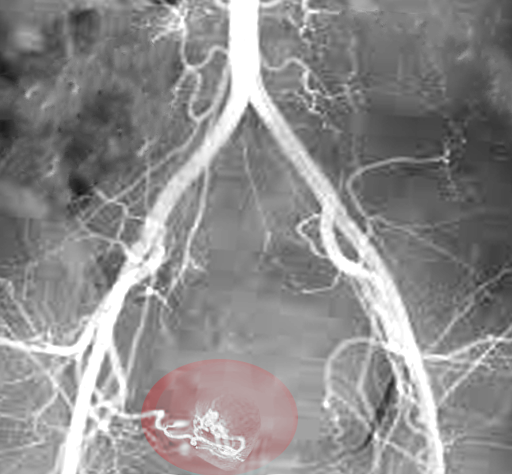
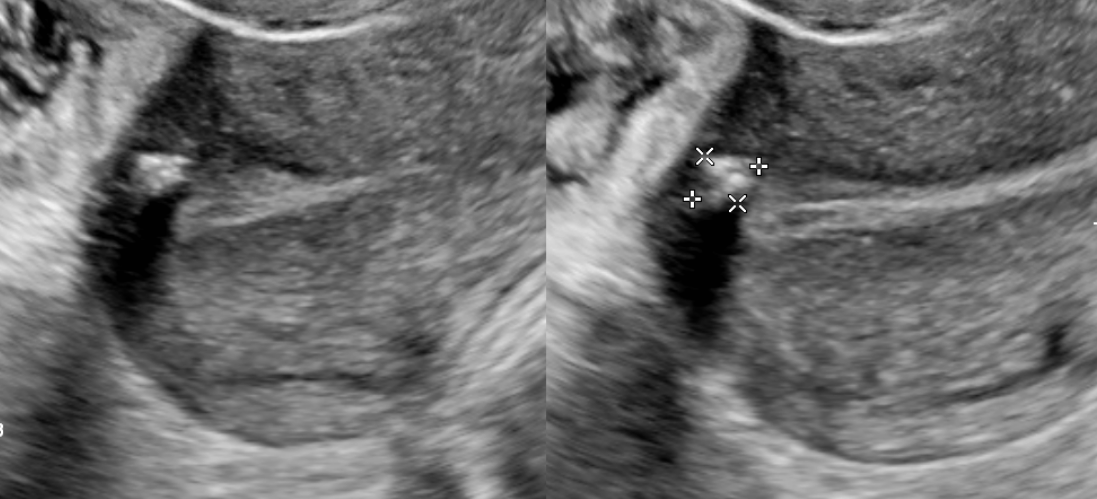
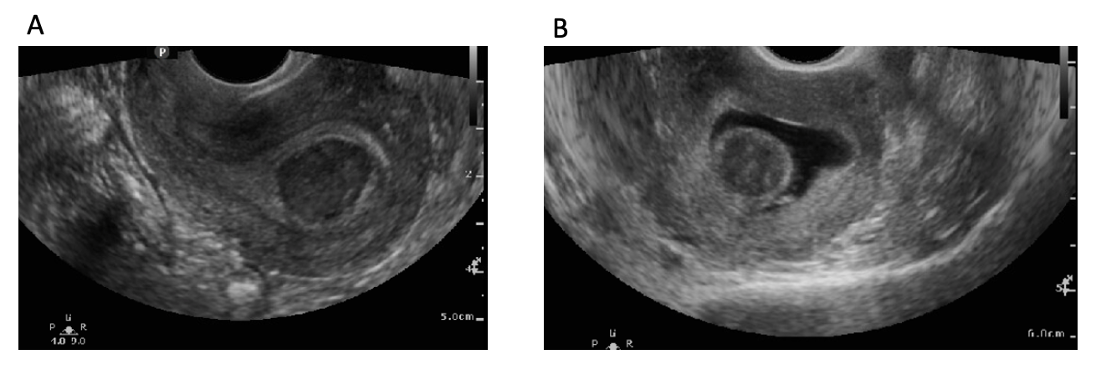
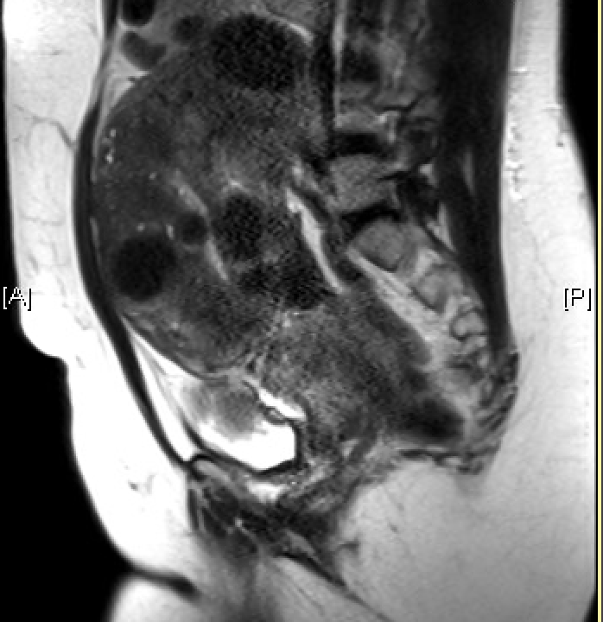
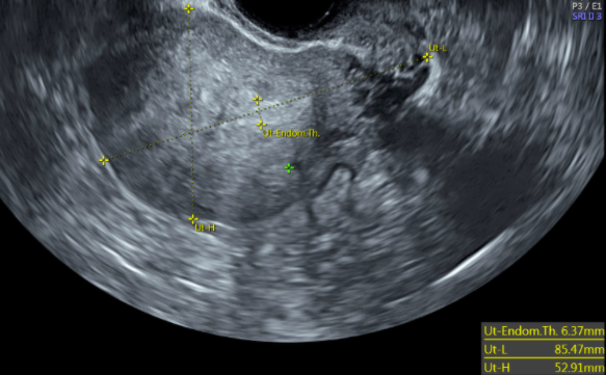
![FIGO Leiomyoma Subclassification System, 2018 [30609040]](/pictures/getimagecontent//18370)