Definition/Introduction
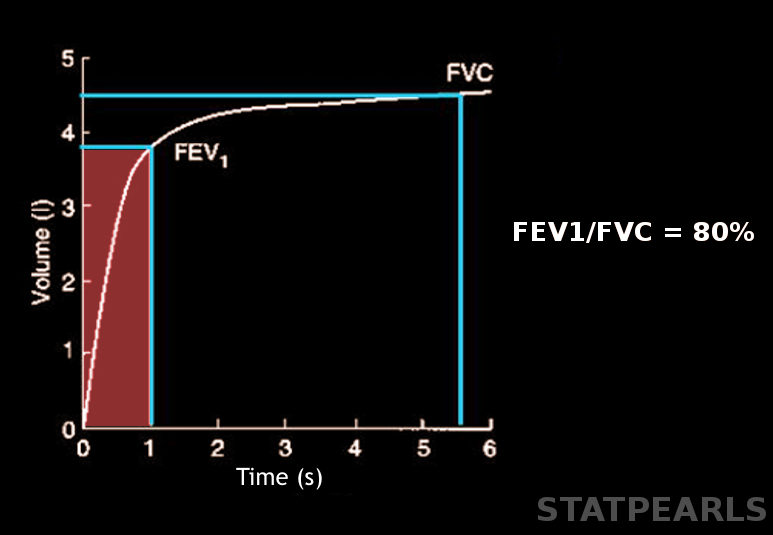
Forced expiratory volume (FEV) is a critical measurement in pulmonary function testing that quantifies the volume of air a person can forcibly exhale from the lungs within a specific time frame following maximal inhalation. FEV is denoted with a subscript indicating the measurement duration in seconds (eg, 1 and 6 seconds), with FEV1 and FEV6 being the most common parameters.[1][2] Please see StatPearls' companion resource, "Pulmonary Function Tests," for more information. This test is essential for diagnosing and monitoring respiratory conditions such as asthma, chronic obstructive pulmonary disease (COPD), and other obstructive and restrictive lung diseases (see Image. Forced Expiratory Volume).
The most commonly used parameter, FEV1, is the primary spirometric variable for assessing the severity of airflow obstruction and evaluating a patient's response to treatment. By reporting FEV1 as a Z-score instead of a percent predicted, healthcare professionals can eliminate biases related to age, sex, and height, enabling a more accurate assessment of lung function impairment and the development of tailored management strategies.
The American Thoracic Society's current guidelines recommend replacing race- and ethnicity-specific equations with "race-neutral" averaged reference equations, such as the Global Lung Function Initiative (GLI) reference equations, while recognizing the need for further research to assess the impact of these changes.[3] FEV6 can be a useful alternative to forced vital capacity (FVC).[2][4][5][6] Understanding and applying FEV measurements are essential for providing effective respiratory care and improving patient outcomes.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Spirometry is a test used to measure the ability of a person to inhale and exhale air over a specific period of time. Key parameters include FEV1 and either FVC or FEV6. Factors such as reduced patient effort, inadequate inhalation, difficulty following instructions, and certain medical conditions can impact the accuracy of spirometry results.[7] Technicians conducting the test must coach and motivate the patient to exert maximum effort to ensure accurate results. In the past, clinicians reported FEV as a percentage of predicted values based on age, gender, height, weight, and ethnicity.[8][9] A value of 80% of the predicted was traditionally considered the lower limit of normal (LLN).[10]
The American Thoracic Society and European Respiratory Society now recommend using the fifth percentile, corresponding to a Z-score of −1.645 or lower, as the LLN to eliminate biases related to age, height, and weight bias and to account for the natural age-related decline in the FEV1 to FVC ratio (FEV1/FVC).[11][12][13] The Z-score is a combination of the percent predicted value and the between-subject variability, which provides a single number that accounts for age- and height-related lung function variability related to healthy individuals.
Growing evidence indicates that race- and ethnicity-specific equations underestimate the severity of pulmonary disease in Black populations. Current guidelines from the American Thoracic Society and European Respiratory Society recommend using race-neutral equations, such as the GLI reference equations. Although the GLI reference equations include data from individuals of various self-reported racial and ethnic backgrounds, race selection is not necessary for applying the formula.
Ongoing research is essential to assess the impact of adopting a race-neutral approach. A recent study indicates that race-based and race-neutral equations yield similar predictions for symptoms, healthcare utilization, and mortality. However, the prevalence of nonobstructive ventilatory impairments decreases among self-identified White and Hispanic individuals, from approximately 4.5% to 1.5%, while it increases among self-identified Black individuals, from 5.7% to 14%.[14] This reclassification could significantly affect standards for disqualification from certain jobs, such as firefighting, as well as eligibility for disability, prioritization for lung transplants, and disability compensation for veterans.
The FEV1/FVC represents the fraction of air a patient exhales in the first second. This value is the most critical parameter for detecting airflow obstruction. Traditionally, an FEV1/FVC of 0.7 has been considered normal. However, this fixed ratio can result in overdiagnosis of airflow obstruction in patients aged 70 or older and underdiagnosis in younger individuals. Reporting FEV1/FVC as a Z-score, with a threshold of −1.645 or lower as the LLN, helps reduce the incidence of misdiagnosis.
Spirometry requires substantial effort and can increase pressure in the chest, abdomen, head, and eyes. Patients who have experienced a myocardial infarction within the prior month or who have unstable angina should avoid spirometry. Additionally, patients who have undergone abdominal, intracranial, or eye surgery, or those with a pneumothorax in the prior 6 weeks, should not undergo spirometry testing.
Clinical Significance
Patients may undergo spirometry for various reasons, including chronic persistent cough, wheezing, dyspnea, and exertional cough or chest pain. Pulmonary function tests objectively measure a patient's response to bronchodilators, monitor disease progression, assess levels of respiratory impairment, and evaluate the effects of exposure to chemicals in the workplace. Technicians record the highest FVC, FEV1, and FEV6 from 3 attempts as the final result. A low FVC may result from poor patient effort, airflow limitations, or restrictions caused by diseases affecting the lung parenchyma, pleura, or thoracic cage. FEV1 measures the mechanical properties of the lungs; FEV1/FVC helps differentiate between obstructive and restrictive lung diseases. FEV1 accounts for approximately 75% to 85% of the FVC in healthy lungs.
Both restrictive and obstructive disorders cause a reduction in FEV1. In obstructive diseases, FEV1 is disproportionately reduced compared to FVC, resulting in FEV1/FVC falling below the LLN. While FEV1 is the most important measurement for assessing the severity of airflow obstruction, FEV1/FVC is essential for detecting airflow obstruction. However, this ratio is still not a reliable indicator of disease severity. Clinicians use FEV1 to monitor disease severity in patients with COPD and asthma.
FEV1, FVC, and total lung capacity are reduced in restrictive disorders, leading to a normal or elevated FEV1/FVC. Please see StatPearls' companion resources, "Emphysema" and "Chronic Emphysema," for more information.[15] Some authors suggest that FEV1/FEV6 may serve as a replacement for FEV1/FVC, as FEV6 offers improved accuracy, requires less physical effort, and is more reproducible.[16]
The most common causes of decreased FEV1 include airway obstruction from bronchospasm, airway inflammation, loss of lung elastic recoil, and increased airway secretions. The change in FEV1 after the administration of inhaled bronchodilators can help assess the reversibility of airway obstruction. An increase in FEV1 of 10% or more following bronchodilator use is considered a positive response.[17] A positive response is typically observed in reversible airway obstruction, such as asthma. However, clinicians should be aware that a lack of significant improvement in FEV1 alone does not necessarily indicate that the patient will not benefit clinically from bronchodilator therapy.[18]
Additional indicators of beneficial bronchodilator effects include significant increases in inspiratory capacity (IC) or vital capacity (VC) after treatment. In contrast, nonreversible obstruction, as seen in patients with COPD, will not show a significant positive response to bronchodilator administration. Please see StatPearls' companion resource, "Emphysema," for more information.[15][19]
Nursing, Allied Health, and Interprofessional Team Interventions
FEV is a crucial measurement in pulmonary function testing that quantifies the volume of air a person can forcibly exhale within a specific timeframe following maximal inhalation. This measurement is crucial for diagnosing and monitoring respiratory conditions such as asthma, COPD, and other obstructive or restrictive lung diseases.
FEV1, combined with FVC, is the most commonly used parameter for identifying airway obstruction. FEV1 is also a reliable indicator of disease severity and is used to monitor patients with COPD and asthma. However, comparing FEV1 to predicted values based on age, gender, height, and ethnicity is no longer recommended.
All spirometry values currently use the fifth percentile as the LLN, corresponding to a Z-score of −1.645 or lower, to eliminate age, sex, and height bias and support tailored management strategies.[20] in addition, race-neutral formulas are recommended for calculating predicted spirometry values to reduce racial bias in healthcare.
Accurate spirometry depends on good patient effort, proper inhalation, and the ability to follow instructions, as decreased effort can result in misdiagnosis and inappropriate treatment. In obstructive diseases, FEV1 is disproportionately reduced compared to FVC, leading to a low FEV1/FVC with a Z-score of −1.645 or lower. In restrictive disorders, both FEV1 and FVC are reduced, causing FEV1/FVC to remain normal or elevated. Some experts suggest using the FEV1/FEV6 instead of FEV1/FVC, as FEV6 offers greater accuracy, lower physical demands, and improved reproducibility.
The FEV1 response to inhaled bronchodilators can help assess the reversibility of airway obstruction. However, clinicians should be aware that a lack of significant improvement in FEV1 does not necessarily indicate that the patient will not benefit from bronchodilator therapy. Clinicians should consider changes in other values, such as IC and VC. Understanding and utilizing FEV measurements are fundamental in delivering effective respiratory care and improving patient outcomes.
Enhancing patient-centered care, outcomes, safety, and team performance related to FEV measurement requires a multidisciplinary approach involving physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals. Physicians and advanced practitioners must be proficient in conducting and interpreting spirometry tests, especially FEV1 and FVC, to effectively diagnose and monitor respiratory conditions such as asthma and COPD.
Healthcare team members must collaborate to prepare patients for spirometry, ensure accurate technique, and educate them on the importance of these tests and proper inhaler use. Adhering to standardized spirometry protocols and participating in quality improvement initiatives are essential for enhancing accuracy and effectiveness.
Effective interprofessional communication is crucial and can be improved through meticulous documentation and timely sharing of specialist input and test results. Collaborative care planning and patient education are essential strategies, enabling all healthcare team members to work together in creating and adjusting treatment plans based on spirometry results and patient responses. By integrating these skills, strategies, and communication practices, healthcare teams can significantly enhance patient-centered care, safety, and outcomes in managing respiratory conditions through FEV measurements.
Media
(Click Image to Enlarge)
References
Matarese A, Sardu C, Shu J, Santulli G. Why is chronic obstructive pulmonary disease linked to atrial fibrillation? A systematic overview of the underlying mechanisms. International journal of cardiology. 2019 Feb 1:276():149-151. doi: 10.1016/j.ijcard.2018.10.075. Epub 2018 Oct 25 [PubMed PMID: 30446289]
Level 3 (low-level) evidencePan MM, Zhang HS, Sun TY. [Value of forced expiratory volume in 6 seconds (FEV(6)) in the evaluation of pulmonary function in Chinese elderly males]. Zhonghua yi xue za zhi. 2017 May 30:97(20):1556-1561. doi: 10.3760/cma.j.issn.0376-2491.2017.20.011. Epub [PubMed PMID: 28592061]
Bhakta NR, Bime C, Kaminsky DA, McCormack MC, Thakur N, Stanojevic S, Baugh AD, Braun L, Lovinsky-Desir S, Adamson R, Witonsky J, Wise RA, Levy SD, Brown R, Forno E, Cohen RT, Johnson M, Balmes J, Mageto Y, Lee CT, Masekela R, Weiner DJ, Irvin CG, Swenson ER, Rosenfeld M, Schwartzstein RM, Agrawal A, Neptune E, Wisnivesky JP, Ortega VE, Burney P. Race and Ethnicity in Pulmonary Function Test Interpretation: An Official American Thoracic Society Statement. American journal of respiratory and critical care medicine. 2023 Apr 15:207(8):978-995. doi: 10.1164/rccm.202302-0310ST. Epub [PubMed PMID: 36973004]
Chen G, Jiang L, Wang L, Zhang W, Castillo C, Fang X. The accuracy of a handheld "disposable pneumotachograph device" in the spirometric diagnosis of airway obstruction in a Chinese population. International journal of chronic obstructive pulmonary disease. 2018:13():2351-2360. doi: 10.2147/COPD.S168583. Epub 2018 Aug 2 [PubMed PMID: 30122915]
Reyes-García A, Torre-Bouscoulet L, Pérez-Padilla R. CONTROVERSIES AND LIMITATIONS IN THE DIAGNOSIS OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. 2019:71(1):28-35. doi: 10.24875/RIC.18002626. Epub [PubMed PMID: 30810541]
Alotaibi N, Borg BM, Abramson MJ, Paul E, Zwar N, Russell G, Wilson S, Holland AE, Bonevski B, Mahal A, George J. Different Case Finding Approaches to Optimise COPD Diagnosis: Evidence from the RADICALS Trial. International journal of chronic obstructive pulmonary disease. 2023:18():1543-1554. doi: 10.2147/COPD.S371371. Epub 2023 Jul 20 [PubMed PMID: 37492489]
Level 3 (low-level) evidenceDerom E, van Weel C, Liistro G, Buffels J, Schermer T, Lammers E, Wouters E, Decramer M. Primary care spirometry. The European respiratory journal. 2008 Jan:31(1):197-203. doi: 10.1183/09031936.00066607. Epub [PubMed PMID: 18166597]
Zakaria R, Harif N, Al-Rahbi B, Aziz CBA, Ahmad AH. Gender Differences and Obesity Influence on Pulmonary Function Parameters. Oman medical journal. 2019 Jan:34(1):44-48. doi: 10.5001/omj.2019.07. Epub [PubMed PMID: 30671183]
Gao C, Zhang X, Wang D, Wang Z, Li J, Li Z. Reference values for lung function screening in 10- to 81-year-old, healthy, never-smoking residents of Southeast China. Medicine. 2018 Aug:97(34):e11904. doi: 10.1097/MD.0000000000011904. Epub [PubMed PMID: 30142794]
Shapira U, Krubiner M, Ehrenwald M, Shapira I, Zeltser D, Berliner S, Rogowski O, Shenhar-Tsarfaty S, Bar-Shai A. Eosinophil levels predict lung function deterioration in apparently healthy individuals. International journal of chronic obstructive pulmonary disease. 2019:14():597-603. doi: 10.2147/COPD.S192594. Epub 2019 Mar 7 [PubMed PMID: 30880949]
Sharma M, Joshi S, Banjade P, Ghamande SA, Surani S. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 Guidelines Reviewed. The open respiratory medicine journal. 2024:18():e18743064279064. doi: 10.2174/0118743064279064231227070344. Epub 2024 Jan 10 [PubMed PMID: 38660684]
Bhakta NR, McGowan A, Ramsey KA, Borg B, Kivastik J, Knight SL, Sylvester K, Burgos F, Swenson ER, McCarthy K, Cooper BG, García-Río F, Skloot G, McCormack M, Mottram C, Irvin CG, Steenbruggen I, Coates AL, Kaminsky DA. European Respiratory Society/American Thoracic Society technical statement: standardisation of the measurement of lung volumes, 2023 update. The European respiratory journal. 2023 Oct:62(4):. pii: 2201519. doi: 10.1183/13993003.01519-2022. Epub 2023 Oct 12 [PubMed PMID: 37500112]
Al Sa'idi L, Berton DC, Neder JA. The 2022 ERS/ATS z-score classification to grade airflow obstruction: relationship with exercise outcomes across the spectrum of COPD severity. The European respiratory journal. 2024 Aug:64(2):. pii: 2301960. doi: 10.1183/13993003.01960-2023. Epub 2024 Aug 8 [PubMed PMID: 38936965]
Diao JA, He Y, Khazanchi R, Nguemeni Tiako MJ, Witonsky JI, Pierson E, Rajpurkar P, Elhawary JR, Melas-Kyriazi L, Yen A, Martin AR, Levy S, Patel CJ, Farhat M, Borrell LN, Cho MH, Silverman EK, Burchard EG, Manrai AK. Implications of Race Adjustment in Lung-Function Equations. The New England journal of medicine. 2024 Jun 13:390(22):2083-2097. doi: 10.1056/NEJMsa2311809. Epub 2024 May 19 [PubMed PMID: 38767252]
Gallucci M, Carbonara P, Pacilli AMG, di Palmo E, Ricci G, Nava S. Use of Symptoms Scores, Spirometry, and Other Pulmonary Function Testing for Asthma Monitoring. Frontiers in pediatrics. 2019:7():54. doi: 10.3389/fped.2019.00054. Epub 2019 Mar 5 [PubMed PMID: 30891435]
Bhatt SP, Kim YI, Wells JM, Bailey WC, Ramsdell JW, Foreman MG, Jensen RL, Stinson DS, Wilson CG, Lynch DA, Make BJ, Dransfield MT. FEV(1)/FEV(6) to diagnose airflow obstruction. Comparisons with computed tomography and morbidity indices. Annals of the American Thoracic Society. 2014 Mar:11(3):335-41. doi: 10.1513/AnnalsATS.201308-251OC. Epub [PubMed PMID: 24450777]
Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, Cooper BG, Culver B, Derom E, Hall GL, Hallstrand TS, Leuppi JD, MacIntyre N, McCormack M, Rosenfeld M, Swenson ER. ERS/ATS technical standard on interpretive strategies for routine lung function tests. The European respiratory journal. 2022 Jul:60(1):. pii: 2101499. doi: 10.1183/13993003.01499-2021. Epub 2022 Jul 13 [PubMed PMID: 34949706]
Beasley R, Hughes R, Agusti A, Calverley P, Chipps B, Del Olmo R, Papi A, Price D, Reddel H, Müllerová H, Rapsomaniki E. Prevalence, Diagnostic Utility and Associated Characteristics of Bronchodilator Responsiveness. American journal of respiratory and critical care medicine. 2024 Feb 15:209(4):390-401. doi: 10.1164/rccm.202308-1436OC. Epub [PubMed PMID: 38029294]
Fortis S, Comellas A, Make BJ, Hersh CP, Bodduluri S, Georgopoulos D, Kim V, Criner GJ, Dransfield MT, Bhatt SP, COPDGene Investigators–Core Units: <italic>Administrative Center</italic>, COPDGene Investigators–Clinical Centers: <italic>Ann Arbor VA</italic>. Combined Forced Expiratory Volume in 1 Second and Forced Vital Capacity Bronchodilator Response, Exacerbations, and Mortality in Chronic Obstructive Pulmonary Disease. Annals of the American Thoracic Society. 2019 Jul:16(7):826-835. doi: 10.1513/AnnalsATS.201809-601OC. Epub [PubMed PMID: 30908927]
Schiavi E, Ryu MH, Martini L, Balasubramanian A, McCormack MC, Fortis S, Regan EA, Bonini M, Hersh CP. Application of the ERS/ATS Spirometry Standards and Race-Neutral Equations in the COPDGene Study. American journal of respiratory and critical care medicine. 2024 Apr 12:():. doi: 10.1164/rccm.202311-2145OC. Epub 2024 Apr 12 [PubMed PMID: 38607551]