Introduction
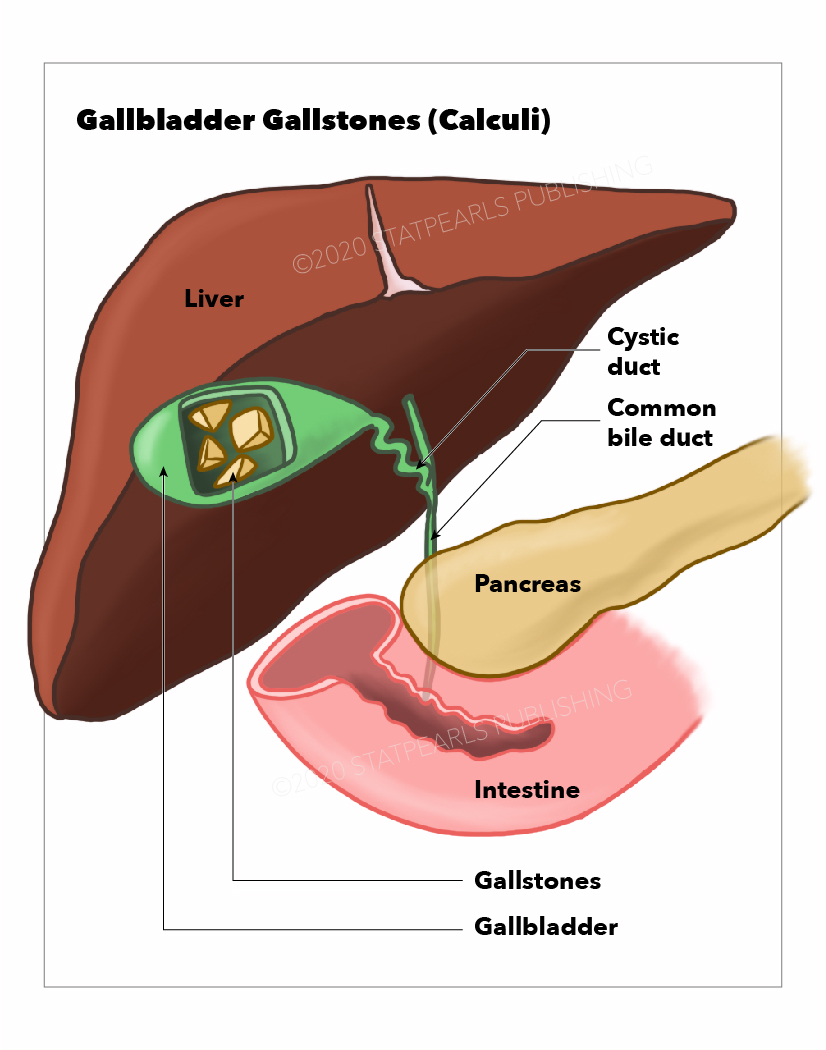
Gallstones are among the most common causes of gastrointestinal dysfunction in the United States and worldwide. Gallstones can cause both chronic pain and episodic discomfort. They also cause acute disorders affecting the pancreatic, biliary, hepatic, and gastrointestinal tract. In the United States, over 6.3 million men and 14.2 million women between the ages of 20 and 74 have gallstones. Although most individuals with gallstones are asymptomatic, about 10% may develop symptoms within 5 years and 20% within 20 years of diagnosis. The prevalence of gallstones increases with age. Over 25% of women older than 60 have gallstones.[1] See Image. Gallstones.
Gallstones arise from metabolic, environmental, and genetic factors, and their composition depends on the etiology. Gallstones can manifest as sand-like debris or form larger stones that can grow to several centimeters in diameter. Typically mobile within the gallbladder, they can also become fixed, either within the body of the gallbladder or at the opening of the cystic duct, disrupting the episodic gallbladder contractility, impairing perfusion, and promoting infection. When gallstones impede gallbladder function and block bile flow, biliary colic results as the gallbladder contracts against partially obstructing stones.
Biliary colic occurs as intermittent, post-prandial epigastric or right upper quadrant pain, and can also be associated with incomplete obstruction within the cystic or common bile duct. Colic may initially be self-limiting and does not typically require hospital admission, but can progress and become unrelenting, leading to inflammation and constant discomfort. Prolonged obstruction or complete impaction of a stone within the biliary tree can result in cholecystitis or cholangitis; at this point, the pain will be constant and progressive. If the cystic duct is obstructed for more than a few hours, the gallbladder becomes inflamed and is prone to infiltration of gut bacteria. Biliary obstruction leads to severe abdominal pain, cholangitis, and pancreatitis. Persons with chronic gallstones may develop progressive fibrosis and loss of gallbladder motility.[1]
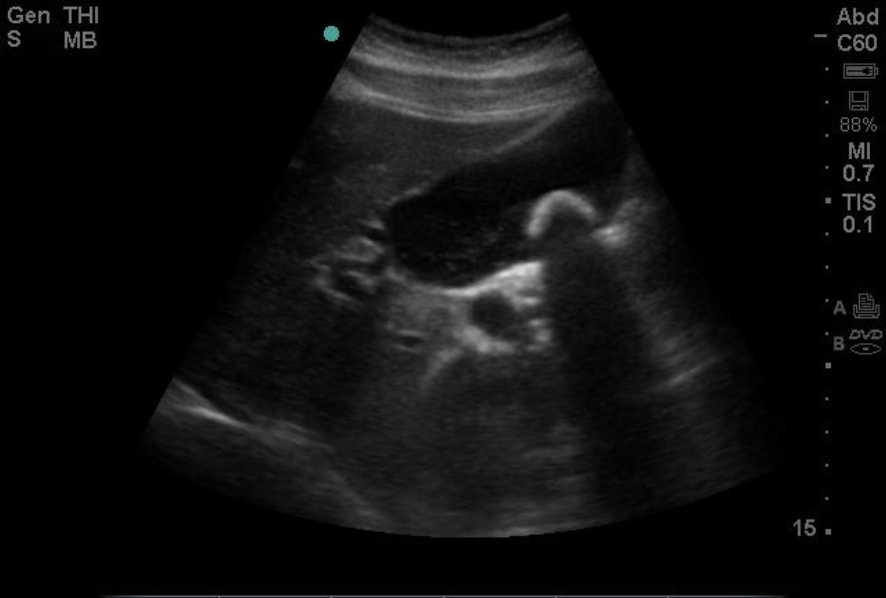
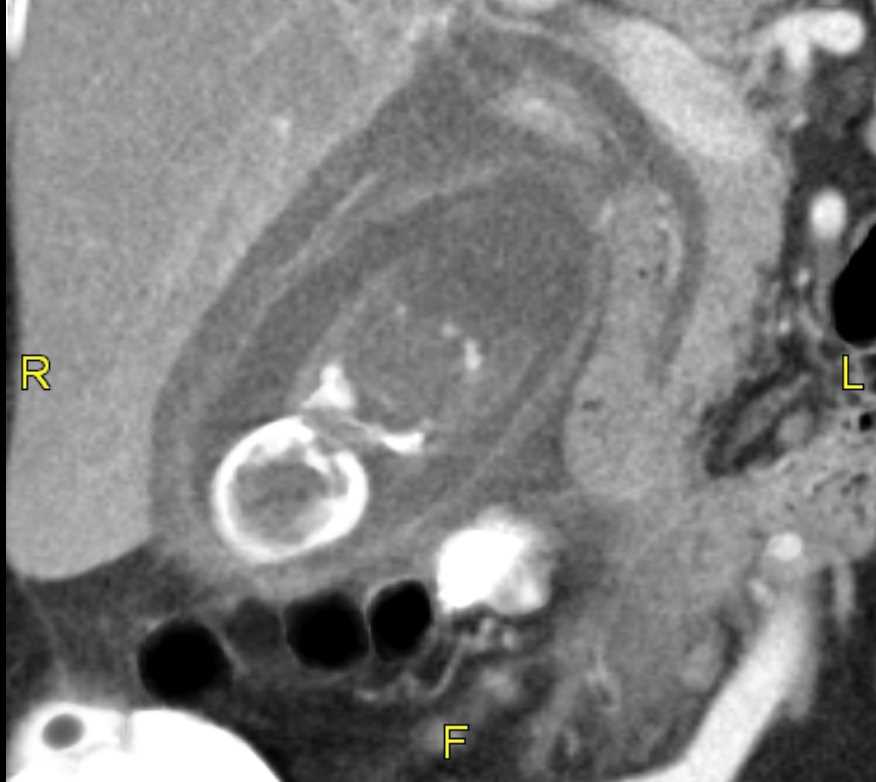
Ultrasound is the preferred diagnostic modality for detecting gallstones, but gallstones may be visualized on computed tomography (CT), magnetic resonance imaging (MRI), and, depending on calcium content, even on x-rays. See Image. Gallstone, Ultrasound Image. Treatment for gallstones depends on the clinical acuity and symptoms. See Image. Acute Cholecystitis Wall Thickening With Pericholecystic Fluid Gallstone, Computed Tomography. The standard of care for patients experiencing recurrent biliary colic or acute cholecystitis is laparoscopic cholecystectomy. One million cholecystectomies are performed annually in the United States, at least half of which are secondary to biliary colic and chronic cholecystitis.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Primary bile acids are synthesized from cholesterol in the liver. Vitamin C promotes the conversion of cholesterol into bile acids through hydroxylation. These primary bile acids are converted into secondary bile acids in the intestine. Tertiary bile acids are further modified from secondary bile acids by intestinal flora or hepatocytes. Bile acids are soluble, with a hydrophilic hydroxyl group, a glycine or taurine side chain, and a hydrophobic steroid ring.[3] Gallstones are formed from bile products that precipitate out of solution, including cholesterol, breakdown products of red blood cells, and a mixture of calcium bilirubinate, phosphate, carbonate, palmitate, and cholesterol. These products are suspended in a mucin glycoprotein matrix that acts as a nucleating factor for stone formation. Additional substances such as prostaglandins and arachidonyl lecithin promote stone crystallization.[4]
Cholesterol is the main component of the most common type of gallstone. Black stones, composed of calcium bilirubinate, result from the breakdown of hemoglobin. In contrast, brown stones form in the setting of bacterial or parasitic infection and are composed of a combination of calcium substrates, including calcium bilirubinate, calcium phosphate or palmitate, cholesterol, and bile.[4] Cholesterol stones are prevalent in individuals with diabetes and other metabolic dysfunctions. In contrast, black stones occur in those with an inflammatory disease, such as Crohn disease, or in those who undergo hemolysis, and brown-pigmented stones are observed in those with parasitic or bacterial infections and biliary strictures.[5] Primary gallbladder stones remain within the gallbladder or migrate into the cystic and common bile ducts, potentially causing pain and obstruction; gallstones may also form primarily within the ductal system.[6]
Epidemiology
In the United States, approximately 14 million women and 6 million men between the ages of 20 and 74 have gallstones. In 2023, symptomatic gallstones accounted for 2 million ambulatory care visits, 1 million emergency department visits, 605,000 outpatient and 280,000 inpatient laparoscopic cholecystectomies, and 49,000 inpatient open cholecystectomies. The prevalence of gallstones increases with age, and the need for intervention secondary to gallstones has been growing among older adults, Hispanic individuals, and women; indigenous Americans also have a high prevalence of gallstones, cited as 70% by some sources.[7] Cholesterol gallstones are increasing worldwide, particularly in Westernized nations, and are believed to impact 20% of the European population. In the developing world, stones derived from bilirubin are more common and are associated with hematologic disorders and biliary tree infections.[8]
Approximately 10% of individuals with gallstones develop symptoms within 5 years of diagnosis, 20% within 20 years, at a rate of 1% to 2% per year.[9] Of those with symptomatic gallstones, 1% to 2% experience complications, often due to common duct stones.[2] Common duct stones are discovered during 5% to 15% of cholecystectomies, increasing with age. The Swedish registry cited a prevalence of common duct stones detected during 11% of interoperative cholangiograms in those with symptomatic gallstones.[10]
There are multiple factors associated with an increased prevalence of gallstones. In Western nations, 75% of gallstones are cholesterol stones, associated with metabolic derangements such as dyslipidemia, diabetes, obesity, insulin resistance, and diets higher in saturated fats and sugar, and lower in fiber. Additional associations include lack of physical activity and conditions such as rapid weight loss or prolonged fasting that diminish gallbladder contractility and increase biliary secretion of cholesterol.[3][11][12] Genetic factors are believed to account for 25% to 30% of the risk of gallstone formation.[5] Estrogen levels have been shown to correlate with bile cholesterol and a decrease in gallbladder contractility. Women of reproductive age or taking estrogen-containing birth control medication have a 2-fold increase in gallstone formation compared with men.[13]
Pathophysiology
Gallstones occur when substances in bile exceed their solubility. As bile becomes supersaturated, small crystals precipitate and become stuck in gallbladder mucus, resulting in gallbladder sludge. Over time, these crystals coalesce and form large stones. The stones are often mobile and can migrate into the bile ducts. Cholangitis and pancreatitis may result if the biliary system becomes obstructed.[13] Stones composed of calcium bilirubinate may also form primarily in the ducts, whereas stones formed in the gallbladder are predominantly cholesterol stones.[10] Gallstones are associated with cirrhosis and form in the setting of biliary stasis from, for example, spinal cord injury and gastrectomy, and medications such as somatostatin and estrogen. Study results show that gallbladder innervation is diminished in those with gallstones.[11]
Cholesterol stones result from increased bile cholesterol due to hepatic secretion and excess triglycerides. These factors promote the secretion of gallbladder mucin, contribute to a larger fasting gallbladder volume, reduce postprandial emptying, and impair intestinal motility. Excess biliary cholesterol can also cause gallbladder wall proliferation and inflammation.[3][11][14] Medications, phospholipid homeostasis, gut microbiome, diet, metabolic syndrome, hormonal and bacterial milieu, genetics, and even high altitude contribute to cholesterol gallstone formation.[5] Fructose promotes gluconeogenesis and lipogenesis. Once glycogen accumulates in the liver, glycogen intermediates are used for triglyceride synthesis, and hypertriglyceridemia can cause decreased gallbladder emptying and impaired contractility due to decreased sensitivity to cholecystokinin.[3]
Cholesterol transporters can influence the amount of cholesterol in blood or bile. Lecithin increases cholesterol concentration in bile carried in lecithin vesicles that fuse and form cholesterol hydrate crystals, which become the nucleus of a cholesterol stone. Bile composition influences the rate of cholesterol crystallization, and a hypoactive gallbladder allows more time for crystals to form.[14] Phospholipid lamella also transports excess cholesterol and plays a role in cholesterol crystalization in conjunction with prostaglandins and arachidonyl lecithin. Granulocytes and neutrophils are attracted to the cholesterol crystals, and neutrophils shed deoxyribonucleic acid onto the crystals, which attract similar crystals, thus forming larger stones.[5][11][14]
Gallstones may form when there is an imbalance between the synthesis, dietary absorption, and recirculation of cholesterol. When deactivated, genes that promote hepatic cholesterol secretion into bile may increase sensitivity to dietary cholesterol intake, contributing to hypercholesterolemia and coronary artery disease, and when overexpressed, promote cholesterol secretion into the gallbladder, predisposing to stones.[14] Medications such as ezetimide and a state of insulin resistance inhibit cholesterol transport into the intestine and intestinal cholesterol absorption while promoting cholesterol synthesis, which is typically downregulated with increased dietary absorption.[11][14]
Gallstones are promoted by and contribute to metabolic imbalances and disease processes. Insulin resistance stimulates cholesterol synthesis by upregulating 3-hydroxy-3-methylglutaryl coenzyme A reductase, upregulating cholesterol secretion, and reducing intestinal absorption of cholesterol independent of obesity. Gallstones reduce insulin sensitivity and increase hepatic triglycerides. Obesity increases the size of hepatocytes, leading to a relative reduction in perfusion and activation of a transcription factor that may promote gallstone formation.[11]
Gallstones are associated with and may worsen cardiovascular disease. Study results show that individuals with gallstones have a higher risk of hypertension, diabetes mellitus, atrial fibrillation, hyperlipidemia, coronary artery disease, and cerebrovascular disease.[14] Autonomic neuropathy in individuals with diabetes is correlated with a greater number of gallstones.[11] Diminished gallbladder contractions may, in part, be due to a reduced amount of cholecystokinin released by the duodenum, leading to more gallstone formation. Lower fiber intake decreases colonic motility and increases the production of stone-forming secondary bile acids.[14]
Bile becomes more saturated with cholesterol during weight loss, but this has not been demonstrated following bariatric surgery. Following a Roux-en-Y gastric bypass, gallbladder emptying slows down, and its ejection fraction is reduced. Haal et al hypothesize that gallstone formation following bariatric surgery is on this basis, allowing time for gallstone crystallization, with an altered triglyceride composition that increases biliary cholesterol.[15] Individuals with decreased gallbladder emptying who have not undergone bariatric surgery, such as those on a low-calorie diet, have a greater concentration of bile acid and phospholipids but have cholesterol levels comparable to those with normal gallbladder emptying.[15]
Hormones can impact the formation of gallstones. Hormones such as vasoactive intestinal peptide and human fibroblast growth factor impact the gallbladder emptying and refilling phase in the postprandial period, released in response to increased acid in the duodenum. These hormones are activated when bile acids reach the terminal ileum, resulting in gallbladder relaxation and filling. Estrogen upregulates cholesterol synthesis and decreases bile acid synthesis by upregulating estrogen receptor alpha and a G-protein–coupled receptor.[11] In the liver, estrogen passively diffuses into cells and promotes cholesterol secretion into bile, increasing saturation.[14]
Bacterial infiltration of the gallbladder is promoted by bile stasis and impaired gallbladder motility. Organisms may enter the biliary system through the sphincter of Oddi or may invade the liver and bile through a hematogenous route, where they serve as stone nucleators. Mucus glycoprotein and phospholipid lamella initiate the crystallization of cholesterol stones, acting in conjunction with prostaglandins and arachidonyl lecithin in a milieu of cholesterol supersaturation, gallbladder hypomotility, and infection.[5][14]
Bacteria that produce biofilms are associated with stone formation. Results from a study comparing gut bacteria in individuals with and without gallstones found different subsets of bacteria dominant in each group.[16][17] Gram-positive anaerobes are linked to greater amounts of the stone-forming secondary bile acid deoxycholate.[11] An analysis of the genomes of bacteria within cholesterol versus pigmented stones found gram-positive bacteria within cholesterol stones but not pigmented stones. Microflora impact cholecystokinin secretion and immunomodulate mucin genes, which contribute to the nucleation of cholesterol gallstones.[14]
When cholesterol gallstones are colonized with microorganisms, the resulting leucocyte infiltration into the gallbladder mucosa in the presence of bilirubin leads to the formation of mixed stones. There are fewer beneficial bacteria, less bacterial diversity, and more pathogenic bacteria in individuals with gallstones. Gut bacteria impact the production of bile acids.[14] Bateria-Sharma et al isolated bacteria from gallstones and introduced them into synthetic bile-like environments containing varying amounts of cholesterol and calcium carbonate. After 20 days of incubation, they found that the slime activity of bacteria correlated with gallstone formation, and other bacterial properties hastened the nucleation of gallstones.[18]
The gut microbiome is altered by toxins such as pesticides and heavy metals, which may increase the risk of gallstones.[11] Polyfluoroalkyl substances (PFAS) are synthetic compounds that are commonly used in industry and consumer products. They are internalized through inhalation and ingestion and accumulate in plasma, liver, kidneys, and bile. PFAS are increasingly found in humans and animals, can incorporate into bile moving through the enterohepatic circulation, and can also directly disrupt the enterohepatic circulation. PFAS also interfere with lipid metabolism, hepatocyte function, and the regulation of androgens and estrogen.[19] Higher altitude may increase the risk of gallstone formation due to induced hepatic hypoxia, postulated from the upregulation of trimethylamine-N-oxide (TMAO), a monooxygenase that leads to cholesterol stone formation. Hypoxia promotes the expression of hepatic hypoxia-inducible factor 1 alpha, which upregulates TMAO, leading to the increased production of cholesterol gallstones.[20]
Certain genes are associated with an increased susceptibility to gallstones, including those involved with hepatic cholesterol secretion, apolipoprotein genes, mucin genes, and fibroblast growth receptor genes. Specifically, a polymorphism of the mucin-like protocadherin gene, rs3758650, is linked to a higher risk of developing symptomatic gallstones.[11] The influence of environmental factors such as insulin resistance on genes involving cholesterol transport and metabolism can result in epigenetic changes, resulting in increased biliary cholesterol secretion. Gene knockouts result in reduced biliary cholesterol secretion and increased hydrophilic bile acids.[11]
History and Physical
Gallstones may be asymptomatic and discovered incidentally or cause symptoms ranging from intermittent colicky pain to constant discomfort with nausea, vomiting, and fever, or even sepsis. Biliary colic is described as crampy post-prandial right upper quadrant or epigastric pain radiating to the back or scapula, especially following a high-fat meal, often accompanied by nausea and vomiting. If the gallbladder becomes acutely inflamed with bacterial infiltration, the pain is typically constant and progressive, often presenting as subcostal right upper quadrant pain elicited by palpation during inspiration. Pain may also present in the substernal area or left upper quadrant.[10][21]
Physical exam during or following a bout of biliary colic is notable for abdominal tenderness, but persons are typically afebrile, with unremarkable labs and vital signs. Acute cholecystitis often involves more severe, at times unrelenting pain, and a mass may be palpated in the right upper quadrant, representing an edematous and thickened gallbladder. The affected person may be febrile and tachycardic. Those with common duct obstruction may be jaundiced. Gallstone obstruction in the proximity of the pancreatic duct may cause acute pancreatitis with symptoms of mid-epigastric pain and intractable vomiting. Ascending cholangitis, involving bacterial infiltration of the biliary system, presents with right upper quadrant pain, fever, and jaundice (Charcot triad). Persons may present with fever, hypo or hypertension, tachycardia. If left untreated, progressive decline includes neurologic changes and hypotension (Reynold pentad).[21]
Evaluation
Right upper quadrant ultrasound has a 90% specificity for gallstones and can detect stones as small as 2 mm, along with sludge and gallbladder polyps. Gallstones can also often be identified on computed tomography scans and magnetic resonance, and are frequently incidental findings on studies ordered for other reasons, but these modalities are not as sensitive for diagnosing acute cholecystitis. Approximately 10% of gallstones can be visualized on x-rays due to their calcium content. A hepatobiliary iminodiacetic acid scan uses a radiotracer to assess the function of the liver, gallbladder, and ductal system, and can demonstrate diminished gallbladder function. See Image. Gallbladder Gallstones (Calculi), Common Bile Duct. The study can help distinguish acute or chronic cholecystitis versus biliary dyskinesia, a chronically hypofunctioning gallbladder. Ultrasound findings of acute cholecystitis include gallbladder wall thickening greater than 3 mm, pericholecystic fluid, and a painful response to pressure from the ultrasound probe (Murphy sign).[22]
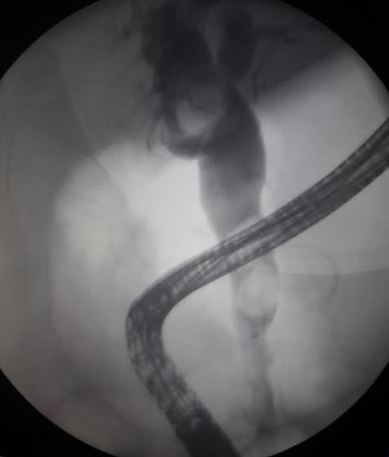
Dilation of the common bile duct observed on imaging may indicate the presence or recent passage of a common duct stone. Endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP) have high sensitivity and specificity for identifying common duct stones, but only ERCP can both diagnose and treat the blockage. See Image. Common Bile Duct Stones, Endoscopic Retrograde Cholangiopancreatography. MRCP is noninvasive but is limited by cost and availability, and ERCP is only available in centers with trained specialists.[10] The common duct may also be evaluated during a laparoscopic cholecystectomy using fluoroscopy with an intraoperative cholangiogram, or through a percutaneous transhepatic cholangiogram if ERCP is not an option. Some clinicians perform routine intraoperative cholangiograms during cholecystectomy, whereas others perform them selectively.[22]
The Japanese Society of Gastroenterology provides evidence-based guidelines, revised in 2021, for an evaluation protocol of suspected gallstones. According to these guidelines, once gallstones are identified on imaging, patients are divided into those with and without cholecystitis using criteria including pain, elevated white blood cell count, C-reactive protein, pericholecystic fluid, and gallbladder wall thickening.[9] Laboratory tests help evaluate the clinical impact of gallstones, such as in biliary obstruction and calculous cholecystitis, and include complete blood count, liver function tests, bilirubin, and alkaline phosphatase. Liver aminotransferase and alkaline phosphatase typically show mild-to-moderate elevation with acute cholecystitis and during and following biliary obstruction, while conjugated (direct) bilirubin levels are elevated. Asymptomatic stones within the gallbladder or passing through the biliary ducts typically do not produce any laboratory abnormalities.[23]
Treatment / Management
Treatment for gallstones ranges from reassurance to dietary and lifestyle recommendations to referral for operative management. Nonoperative management of biliary colic involves strict maintenance of a low-fat diet and supportive treatment with antiemetics and pain control, and counseling regarding risk for recurrence. Those with repeat episodes of colic are referred for an elective laparoscopic cholecystectomy. However, suppose the patient has severe abdominal pain or cannot tolerate oral intake. In that case, hospital admission and more urgent surgical management are warranted for symptomatic relief and to prevent further clinical sequela.
Impacted stones will cause progressive and unrelenting pain and are an indication for admission and timely surgery. Stones greater than 1 cm in size have a greater propensity to obstruct the cystic duct and may predispose to acute cholecystitis, so they should be treated with surgical management more urgently. Laparoscopic cholecystectomy is the standard of care for symptomatic gallstones. Open cholecystectomy is performed when the laparoscopic approach is not feasible or is found to be too difficult intraoperatively. In cases of acute cholecystitis in unstable patients or patients with poor surgical candidates, a cholecystostomy tube can be placed by interventional radiology as either a temporizing or palliative measure.[10]
Common bile duct stones can be removed with a preoperative, postoperative, or intraoperative ERCP, or intraoperatively with a laparoscopic or open common bile duct exploration. During a common bile duct exploration, the common duct is accessed through the cystic duct and visualized fluoroscopically or directly through a choledochotomy and visualized using a choledochoscope. The choledochoscope requires a second monitor, and fluoroscopy utilizes radiation, but both are effective approaches.[24] A common duct incision risks subsequent duct stenosis and requires skilled closure. Instead of primary closure, the common bile duct can be anastomosed to the bowel, in a side-to-side anastomosis or a Roux-en-Y choledochojejunostomy to avoid stenosis.
Alternatively, the duct opening can be controlled with a T-tube that is removed over time, but this may introduce bacteria into the biliary system, and the tube may become displaced, causing bile peritonitis.[10] One strategy for intraoperative ERCP involves a combination of laparoscopic and endoscopic approaches. In this approach, a guidewire is introduced into the cystic duct, passed through the Ampulla of Vater into the duodenum, and then grasped by a snare through the endoscope. A sphincterotome is passed over the guidewire to perform sphincterotomy and remove stones. This method provides for a shorter hospital stay, reduced cost, and a lesser amount of anesthesia than the need for postoperative intervention, but requires greater expertise and coordination of personnel.[10]
Ascending cholangitis must be urgently addressed by removing the obstruction, using endoscopic instrumentation, performing percutaneous transhepatic intervention and surgery, and administering early antibiotics. Pancreatitis from gallstone obstruction must be similarly treated promptly. Cholecystectomy should be performed in an interval fashion after the patient recovers from acute illness.[8][25]
Pharmacologic or mechanical treatment for gallstones is not commonly used due to low efficacy.[2] Lithotripsy does not impede subsequent stone formation, and the procedure requires trained personnel. However, lithotripsy has a limited role as part of a multi-modal approach for difficult stones. Mechanical lithotripsy can be a secondary maneuver following failed sphincterotomy for common duct stones, and electrohydraulic lithotripsy or laser lithotripsy can be used in an attempt to break up stones under fluoroscopy. Laser therapy is expensive and limited by its excessive heat generation. Extracorporeal shock-wave lithotripsy may be attempted to help treat challenging common duct stones, but efficacy is relatively low.[8] Most operators use compressible water-filled bags placed externally on the body during shock-wave lithotripsy, but the associated discomfort requires intraprocedural pain management. Extracorporeal shock-wave treatment is contraindicated in the setting of portal thrombus and umbilical varices and can cause arrhythmias, haemobilia, cholangitis, pancreatitis, ileus, and biliary colic.[10](A1)
Ursodeoxycholic acid, or ursodiol, is a bile acid used to dissolve bile stones and treat liver pathology such as biliary cirrhosis. Although it can be administered daily for gallstone dissolution, the dissolution rate is less than 50%, and the underlying etiology is not addressed. Pharmacologic therapy intended to mitigate the formation of gallstones includes statins such as ezetimibe. Statins block hepatic cholesterol synthesis by inhibiting the hydroxymethylglutaryl-coenzyme A reductase enzyme and activating peroxisome proliferator-activated receptor gamma.[5] Alternative therapies to treat gallstones include defatted walnut powder extract containing juglandis, attributed to phenolic acids, and a medley of Chinese herbs.[5](B3)
Differential Diagnosis
The signs and symptoms associated with gallstones may also be observed in the following conditions:
- Appendicitis
- Renal calculi
- Cholangiocarcinoma
- Pancreatitis
- Peptic ulcer disease
- Gastroesophageal reflux disease
- Myocardial infarction
- Aortic dissection
- Pneumonia
- Esophageal spasm
- Hepatitis
- Mesenteric ischemia
- Renal calculi
- Gastroenteritis
Pertinent Studies and Ongoing Trials
Shenoy et al reviewed the literature on treating symptomatic cholelithiasis in adults from 2000 to 2020, including operative and non-operative approaches. Variables measured included length of stay and hospital readmission. Interventions compared included cholecystectomy versus observation, cholecystectomy versus lithotripsy, elective versus urgent cholecystectomy, pharmacologic intervention to treat gallstones, and pain management with observation.[2] The findings indicated that individuals who underwent surgery experienced better pain control compared to those who received lithotripsy for gallstones.
In addition, for patients with uncontrolled pain, surgery was more effective compared to merely observing their condition without intervention. When comparing elective to urgent surgery, it was noted that 25% of individuals who underwent elective surgery required additional treatment before their surgery date. No significant differences were found in individuals treated with chenodeoxycholic or ursodeoxycholic acid versus placebo in pain or stone dissolution. A study compared acupuncture versus observation for gallstone clearance or dissolution and found no difference. In the pain management category, loxiglumide, a cholecystokinin-1 receptor blocker, was superior to hyoscine-N-butyl bromide, an anticholinergic.[2]
When recommending treatment, the authors recommended stratification of patients by symptoms, including time to symptom onset and severity of recurrent symptoms. They note that early intervention in those with symptomatic gallstones eliminates clinical decline and complications experienced during the waiting period and that waiting results in greater costs and increased morbidity. Patient stratification helps calculate the risk of complications from elective rather than urgent intervention.[2] The SECURE trial examined persons with symptomatic, uncomplicated cholelithiasis and compared treatment strategies against the eventual operation rate; it found that some strategies reduced the number of operative interventions by a small amount, but overall were not too effective at pain reduction or overall need for operation.[26]
Prognosis
Although gallstones are prognostically favorable, and less than 50% of those with gallstones develop symptoms, in the United States, they are associated with increased mortality from cardiovascular disease and cancer.[27] Individuals who undergo cholecystectomy may experience mild bloating and other gastrointestinal symptoms, such as post-prandial diarrhea, exacerbated by fattier foods, but generally enjoy longevity and quality of life comparable to those without gallstones. The mortality rate following laparoscopic cholecystectomy is less than 0.5%, but is greater for emergency cases. Results from a recent study based in India found a 30-day morbidity for cholecystectomy of 11% and mortality of 0.2%. Factors associated with increased morbidity included greater body mass index, history of previous abdominal surgery, intraabdominal adhesions, conversion to an open procedure, and gangrenous gallbladder. The study's conversion from laparoscopic to open was 1.3%, and the bile duct injury rate was 0.3%.[28]
Ahmad H M Nassar et al identified individuals with body mass index (BMI) greater than 35 among 4699 laparoscopic cholecystectomies performed over 19 years and found no significant difference in operative times, morbidity, operative difficulty, readmission, or mortality compared with a leaner control group.[29] Fagenson et al found a direct correlation between frailty as measured by the Modified Frailty Index and postoperative morbidity and mortality following laparoscopic cholecystectomy for acute cholecystitis.[30] Fugazzola et al developed a preoperative risk score for individuals undergoing cholecystectomy for acute calculous cholecystitis through a multicenter observational study from September 2021 to September 2022, including 1253 individuals in 79 centers. The findings revealed a 30-day morbidity of 6.6% and a 30-day mortality of 1%.[31]
Complications
Gallstones may impede gallbladder motility and cause inflammation, leading to acute or chronic cholecystitis, empyema, gangrenous cholecystitis, and emphysematous cholecystitis. Stones stuck within the common bile duct cause obstruction, leading to jaundice, proximal dilation of the biliary tree, pancreatitis, and cholangitis.[32][33][34] Gallstones may also externally compress a bile duct, such as the common bile duct or hepatic duct, from within the cystic duct or gallbladder neck, a condition known as Mirizzi syndrome. Gallstones may become impacted within the gallbladder and erode the wall, resulting in a cholecystoenteric fistula between the gallbladder and the bowel, most commonly the duodenum, also called Bouveret syndrome. See Image. Multiple Biliary Hamartoma, Ultrasound.
A gallstone can become lodged within the distal small bowel and cause an ileus, occurring in 0.3% to 0.5% of individuals with gallstones.[9][10][35] There are procedural complications from ERCP, including injury to bile ducts and duodenum, haemobilia, and pancreatitis, and there are rare case reports of pulmonary bile embolism after ERCP for gallstone pancreatitis.[10] Complications encountered during or after cholecystectomy include injury to a bile duct or bowel, retained stones in the common bile duct, incisional hernia, and chronic right upper quadrant pain.[36]
Postoperative and Rehabilitation Care
Postoperative care may range from same-day discharge for biliary colic or uncomplicated cholecystitis to prolonged intensive care unit admission for ascending cholangitis or severe pancreatitis. Following hospital discharge, dietary modification, including lower-fat and smaller portions, is important for an uneventful recovery.
Deterrence and Patient Education
A higher intake of monounsaturated fats, fiber, olive oil, omega-3 fatty acids, and vegetable protein is protective against gallstones. Specifically, polyunsaturated fatty acids may assist with gallbladder emptying, and vegetable protein, vitamin C-rich fruits and vegetables, and coffee may increase gallbladder motility. In addition, exercise may help mitigate gallstone formation.[8] A diet higher in refined sugars, fructose, and fast food with insufficient fiber and vitamin C, as well as a general setting of increased insulin requirement, increases the risk of gallstones.[12] A person with biliary colic who chooses nonoperative management must be counseled regarding the signs of cholecystitis and advised to present to the hospital with persistent or progressive abdominal pain, with or without an accompanying fever. These patients should be informed of the risk for stone impaction and obstruction that can lead to infectious processes such as cholecystitis or cholangitis.[37]
Pearls and Other Issues
Much like asymptomatic cholelithiasis, asymptomatic choledocholithiasis can be managed conservatively, depending on symptoms and complications.[38] Gallstones are often identified on imaging for other reasons, and asymptomatic gallstones do not require intervention. However, healthcare professionals should be aware of the potentially severe clinical consequences of obstructing gallstones, including cholangitis and pancreatitis.
Impacted gallstones within the gallbladder may rarely give rise to gangrenous cholecystitis, and gallstones may erode through the gallbladder or ductal system, causing, for example, choledochoduodenal or cholecystocholedochal fistulae.[39][40][41] Gallstones in pregnancy are associated with worse maternal and neonatal outcomes, including preterm birth. Cholecystectomy can be performed safely during the early and mid stages of pregnancy.[11]
Enhancing Healthcare Team Outcomes
Gallstones may be asymptomatic, cause intermittent symptoms, or result in severe, debilitating illness. Gallstones incidentally identified on imaging do not warrant urgent intervention. If symptomatic, an interprofessional team, including primary care, radiology, gastroenterology, and general surgery, collaborates on the nature and timing of any planned intervention. Early consultation with a general surgeon in advance of urgent need is beneficial. If gallstone symptoms are recurrent, early elective intervention reduces the need for emergency department care and the risk of complications.[42]
Not all gallstones require surgery. Lifestyle modification, including a low-fat diet, regular exercise, and abstaining from prolonged fasting, may help minimize symptoms. The diagnosis and management of gallstone-related disorders require an interprofessional team, including emergency department personnel, gastroenterologists, specialty care nurses, and dietitians. Following cholecystectomy, patients require education regarding diet and activity and may require follow-up for any digestive difficulties. Close communication between team members is vital to lower the morbidity of gallstones.[42][43][44]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
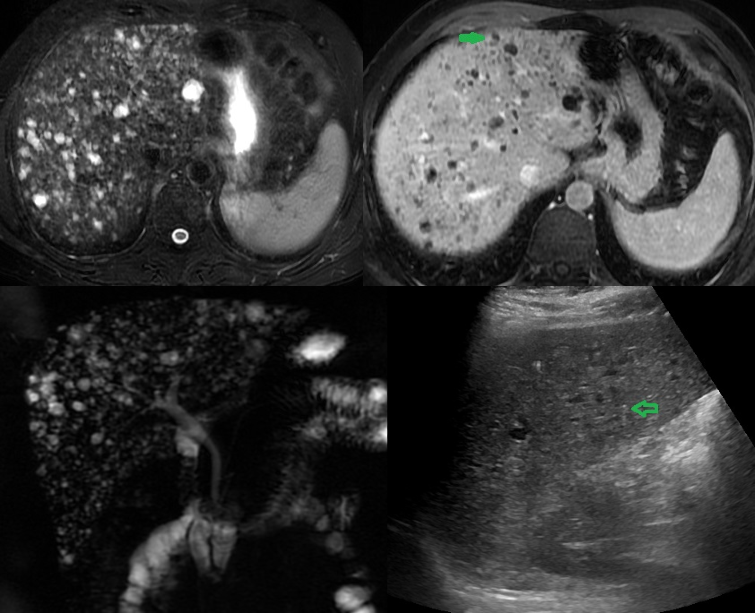
Multiple Biliary Hamartoma, Ultrasound. This is an ultrasound (US) of a 37-year-old woman diagnosed with gallstones and acute cholecystitis. The incidental findings: (A) US image shows multiple hypoechoic lesions, some of which have comet-tail artifacts, raising the possibility of numerous biliary hamartomas. (B) A T2-weighted magnetic resonance image (MRI) shows numerous cystic lesions with a signal similar to that of cerebrospinal fluid. (C) Post-contrast T2-weighted MRI shows some of these lesions with an enhancing mural nodule, which is highly specific for biliary hamartoma. (D) Magnetic resonance cholangiopancreatography 3D projection image has more lesions and demonstrates no communication with a normal caliber biliary duct.
Contributed by A Borhani, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Tsai TJ, Chan HH, Lai KH, Shih CA, Kao SS, Sun WC, Wang EM, Tsai WL, Lin KH, Yu HC, Chen WC, Wang HM, Tsay FW, Lin HS, Cheng JS, Hsu PI. Gallbladder function predicts subsequent biliary complications in patients with common bile duct stones after endoscopic treatment? BMC gastroenterology. 2018 Feb 27:18(1):32. doi: 10.1186/s12876-018-0762-6. Epub 2018 Feb 27 [PubMed PMID: 29486713]
Shenoy R, Kirkland P, Hadaya JE, Tranfield MW, DeVirgilio M, Russell MM, Maggard-Gibbons M. Management of symptomatic cholelithiasis: a systematic review. Systematic reviews. 2022 Dec 12:11(1):267. doi: 10.1186/s13643-022-02135-8. Epub 2022 Dec 12 [PubMed PMID: 36510302]
Level 1 (high-level) evidenceDi Ciaula A, Garruti G, Frühbeck G, De Angelis M, de Bari O, Wang DQ, Lammert F, Portincasa P. The Role of Diet in the Pathogenesis of Cholesterol Gallstones. Current medicinal chemistry. 2019:26(19):3620-3638. doi: 10.2174/0929867324666170530080636. Epub [PubMed PMID: 28554328]
Rebholz C, Krawczyk M, Lammert F. Genetics of gallstone disease. European journal of clinical investigation. 2018 Jul:48(7):e12935. doi: 10.1111/eci.12935. Epub 2018 May 9 [PubMed PMID: 29635711]
E S, Srikanth MS, Shreyas A, Desai S, Mehdi S, Gangadharappa HV, Suman, Krishna KL. Recent advances, novel targets and treatments for cholelithiasis; a narrative review. European journal of pharmacology. 2021 Oct 5:908():174376. doi: 10.1016/j.ejphar.2021.174376. Epub 2021 Jul 22 [PubMed PMID: 34303667]
Level 3 (low-level) evidenceHapca S, Ramsay G, Murchie P, Ahmed I. Biliary colic. BMJ (Clinical research ed.). 2021 Sep 13:374():n2085. doi: 10.1136/bmj.n2085. Epub 2021 Sep 13 [PubMed PMID: 34518165]
Unalp-Arida A, Ruhl CE. Burden of gallstone disease in the United States population: Prepandemic rates and trends. World journal of gastrointestinal surgery. 2024 Apr 27:16(4):1130-1148. doi: 10.4240/wjgs.v16.i4.1130. Epub [PubMed PMID: 38690054]
Gutt C, Schläfer S, Lammert F. The Treatment of Gallstone Disease. Deutsches Arzteblatt international. 2020 Feb 28:117(9):148-158. doi: 10.3238/arztebl.2020.0148. Epub [PubMed PMID: 32234195]
Fujita N, Yasuda I, Endo I, Isayama H, Iwashita T, Ueki T, Uemura K, Umezawa A, Katanuma A, Katayose Y, Suzuki Y, Shoda J, Tsuyuguchi T, Wakai T, Inui K, Unno M, Takeyama Y, Itoi T, Koike K, Mochida S. Evidence-based clinical practice guidelines for cholelithiasis 2021. Journal of gastroenterology. 2023 Sep:58(9):801-833. doi: 10.1007/s00535-023-02014-6. Epub 2023 Jul 15 [PubMed PMID: 37452855]
Level 1 (high-level) evidenceCianci P, Restini E. Management of cholelithiasis with choledocholithiasis: Endoscopic and surgical approaches. World journal of gastroenterology. 2021 Jul 28:27(28):4536-4554. doi: 10.3748/wjg.v27.i28.4536. Epub [PubMed PMID: 34366622]
Di Ciaula A, Wang DQ, Portincasa P. An update on the pathogenesis of cholesterol gallstone disease. Current opinion in gastroenterology. 2018 Mar:34(2):71-80. doi: 10.1097/MOG.0000000000000423. Epub [PubMed PMID: 29283909]
Level 3 (low-level) evidenceKotrotsios A, Tasis N, Angelis S, Apostolopoulos AP, Vlasis K, Papadopoulos V, Filippou DK. Dietary Intake and Cholelithiasis: A Review. Journal of long-term effects of medical implants. 2019:29(4):317-326. doi: 10.1615/JLongTermEffMedImplants.2020034732. Epub [PubMed PMID: 32749137]
Shabanzadeh DM. New determinants for gallstone disease? . Danish medical journal. 2018 Feb:65(2):. pii: B5438. Epub [PubMed PMID: 29393043]
Sun H, Warren J, Yip J, Ji Y, Hao S, Han W, Ding Y. Factors Influencing Gallstone Formation: A Review of the Literature. Biomolecules. 2022 Apr 6:12(4):. doi: 10.3390/biom12040550. Epub 2022 Apr 6 [PubMed PMID: 35454138]
Haal S, Guman MSS, Acherman YIZ, Jansen JPG, van Weeghel M, van Lenthe H, Wever EJM, Gerdes VEA, Voermans RP, Groen AK. Gallstone Formation Follows a Different Trajectory in Bariatric Patients Compared to Nonbariatric Patients. Metabolites. 2021 Oct 5:11(10):. doi: 10.3390/metabo11100682. Epub 2021 Oct 5 [PubMed PMID: 34677397]
Boyang H, Yanjun Y, Jing Z, Chenxin Y, Ying M, Shuwen H, Qiang Y. Investigating the influence of the gut microbiome on cholelithiasis: unveiling insights through sequencing and predictive modeling. Journal of applied microbiology. 2024 May 1:135(5):. pii: lxae096. doi: 10.1093/jambio/lxae096. Epub [PubMed PMID: 38614959]
Level 2 (mid-level) evidenceHu H, Shao W, Liu Q, Liu N, Wang Q, Xu J, Zhang X, Weng Z, Lu Q, Jiao L, Chen C, Sun H, Jiang Z, Zhang X, Gu A. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nature communications. 2022 Jan 11:13(1):252. doi: 10.1038/s41467-021-27758-8. Epub 2022 Jan 11 [PubMed PMID: 35017486]
Sharma R, Sachan SG, Sharma SR. In vitro analysis of gallstone formation in the presence of bacteria. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology. 2020 Oct:39(5):473-480. doi: 10.1007/s12664-020-01055-6. Epub 2020 Nov 17 [PubMed PMID: 33201443]
Shi T, Li D, Li D, Sun J, Xie P, Wang T, Li R, Li Z, Zou Z, Ren X. Individual and joint associations of per- and polyfluoroalkyl substances (PFAS) with gallstone disease in adults: A cross-sectional study. Chemosphere. 2024 Jun:358():142168. doi: 10.1016/j.chemosphere.2024.142168. Epub 2024 Apr 27 [PubMed PMID: 38685323]
Level 2 (mid-level) evidenceLuo M, Chen P, Tian Y, Rigzin N, Sonam J, Shang F, Tai C, Li T, Sang H. Hif-1α expression targets the TMA/Fmo3/TMAO axis to participate in gallbladder cholesterol stone formation in individuals living in plateau regions. Biochimica et biophysica acta. Molecular basis of disease. 2024 Jun:1870(5):167188. doi: 10.1016/j.bbadis.2024.167188. Epub 2024 Apr 22 [PubMed PMID: 38657913]
Wilkins T, Agabin E, Varghese J, Talukder A. Gallbladder Dysfunction: Cholecystitis, Choledocholithiasis, Cholangitis, and Biliary Dyskinesia. Primary care. 2017 Dec:44(4):575-597. doi: 10.1016/j.pop.2017.07.002. Epub 2017 Oct 5 [PubMed PMID: 29132521]
Hiwatashi K, Okumura H, Setoyama T, Ando K, Ogura Y, Aridome K, Maenohara S, Natsugoe S. Evaluation of laparoscopic cholecystectomy using indocyanine green cholangiography including cholecystitis: A retrospective study. Medicine. 2018 Jul:97(30):e11654. doi: 10.1097/MD.0000000000011654. Epub [PubMed PMID: 30045318]
Level 2 (mid-level) evidenceGallaher JR, Charles A. Acute Cholecystitis: A Review. JAMA. 2022 Mar 8:327(10):965-975. doi: 10.1001/jama.2022.2350. Epub [PubMed PMID: 35258527]
Han JH, So H, Bang SJ, Nah YW. [Surgical Removal of a Huge Common Bile Duct Stone]. The Korean journal of gastroenterology = Taehan Sohwagi Hakhoe chi. 2024 May 25:83(5):200-204. doi: 10.4166/kjg.2023.148. Epub [PubMed PMID: 38783622]
Hirajima S, Koh T, Sakai T, Imamura T, Kato S, Nishimura Y, Soga K, Nishio M, Oguro A, Nakagawa N. Utility of Laparoscopic Subtotal Cholecystectomy with or without Cystic Duct Ligation for Severe Cholecystitis. The American surgeon. 2017 Nov 1:83(11):1209-1213 [PubMed PMID: 29183521]
Comes DJ, Wennmacker SZ, Latenstein CSS, van der Bilt J, Buyne O, Donkervoort SC, Heisterkamp J, In't Hof K, Jansen J, Nieuwenhuijs VB, Steenvoorde P, Stockmann HBAC, Boerma D, Drenth JPH, van Laarhoven CJHM, Boermeester MA, Dijkgraaf MGW, de Reuver PR. Restrictive Strategy vs Usual Care for Cholecystectomy in Patients With Abdominal Pain and Gallstones: 5-Year Follow-Up of the SECURE Randomized Clinical Trial. JAMA surgery. 2024 Nov 1:159(11):1235-1243. doi: 10.1001/jamasurg.2024.3080. Epub [PubMed PMID: 39167382]
Level 1 (high-level) evidenceRuhl CE, Everhart JE. Gallstone disease is associated with increased mortality in the United States. Gastroenterology. 2011 Feb:140(2):508-16. doi: 10.1053/j.gastro.2010.10.060. Epub 2010 Nov 11 [PubMed PMID: 21075109]
Thapar VB, Thapar PM, Goel R, Agarwalla R, Salvi PH, Nasta AM, Mahawar K, IAGES Research Collaborative Group. Evaluation of 30-day morbidity and mortality of laparoscopic cholecystectomy: a multicenter prospective observational Indian Association of Gastrointestinal Endoscopic Surgeons (IAGES) Study. Surgical endoscopy. 2023 Apr:37(4):2611-2625. doi: 10.1007/s00464-022-09659-z. Epub 2022 Nov 10 [PubMed PMID: 36357547]
Nassar AHM, Khan KS, Ng HJ, Sallam M. Operative Difficulty, Morbidity and Mortality Are Unrelated to Obesity in Elective or Emergency Laparoscopic Cholecystectomy and Bile Duct Exploration. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2022 Sep:26(9):1863-1872. doi: 10.1007/s11605-022-05344-7. Epub 2022 May 31 [PubMed PMID: 35641812]
Fagenson AM, Powers BD, Zorbas KA, Karhadkar S, Karachristos A, Di Carlo A, Lau KN. Frailty Predicts Morbidity and Mortality After Laparoscopic Cholecystectomy for Acute Cholecystitis: An ACS-NSQIP Cohort Analysis. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2021 Apr:25(4):932-940. doi: 10.1007/s11605-020-04570-1. Epub 2020 Mar 24 [PubMed PMID: 32212087]
Level 2 (mid-level) evidenceFugazzola P, Cobianchi L, Di Martino M, Tomasoni M, Dal Mas F, Abu-Zidan FM, Agnoletti V, Ceresoli M, Coccolini F, Di Saverio S, Dominioni T, Farè CN, Frassini S, Gambini G, Leppäniemi A, Maestri M, Martín-Pérez E, Moore EE, Musella V, Peitzman AB, de la Hoz Rodríguez Á, Sargenti B, Sartelli M, Viganò J, Anderloni A, Biffl W, Catena F, Ansaloni L, S.P.Ri.M.A.C.C. Collaborative Group. Prediction of morbidity and mortality after early cholecystectomy for acute calculous cholecystitis: results of the S.P.Ri.M.A.C.C. study. World journal of emergency surgery : WJES. 2023 Mar 18:18(1):20. doi: 10.1186/s13017-023-00488-6. Epub 2023 Mar 18 [PubMed PMID: 36934276]
Del Vecchio Blanco G, Gesuale C, Varanese M, Monteleone G, Paoluzi OA. Idiopathic acute pancreatitis: a review on etiology and diagnostic work-up. Clinical journal of gastroenterology. 2019 Dec:12(6):511-524. doi: 10.1007/s12328-019-00987-7. Epub 2019 Apr 30 [PubMed PMID: 31041651]
Brägelmann J, Barahona Ponce C, Marcelain K, Roessler S, Goeppert B, Gallegos I, Colombo A, Sanhueza V, Morales E, Rivera MT, de Toro G, Ortega A, Müller B, Gabler F, Scherer D, Waldenberger M, Reischl E, Boekstegers F, Garate-Calderon V, Umu SU, Rounge TB, Popanda O, Lorenzo Bermejo J. Epigenome-Wide Analysis of Methylation Changes in the Sequence of Gallstone Disease, Dysplasia, and Gallbladder Cancer. Hepatology (Baltimore, Md.). 2021 Jun:73(6):2293-2310. doi: 10.1002/hep.31585. Epub 2021 Jun 15 [PubMed PMID: 33020926]
Sohail Z, Shaikh H, Iqbal N, Parkash O. Acute pancreatitis: A narrative review. JPMA. The Journal of the Pakistan Medical Association. 2024 May:74(5):953-958. doi: 10.47391/JPMA.9280. Epub [PubMed PMID: 38783446]
Level 3 (low-level) evidenceZaher EA, Ebrahim MA, Al Salman O, Patel P, Alchalabi M. Bigger Than a Hen's Egg: A Case of Bouveret Syndrome. Cureus. 2024 Apr:16(4):e58742. doi: 10.7759/cureus.58742. Epub 2024 Apr 22 [PubMed PMID: 38779279]
Level 3 (low-level) evidenceKnapik M, Okoń K, Ulatowska-Białas M. Fatal pulmonary bile embolism associated with acute pancreatitis - a case report and review of the literature. Polish journal of pathology : official journal of the Polish Society of Pathologists. 2024:75(1):54-57. doi: 10.5114/pjp.2024.135762. Epub [PubMed PMID: 38741429]
Level 3 (low-level) evidenceGross AR, Bacaj PJ, Williams HJ. Educational Case: Gallstones, Cholelithiasis, and Cholecystitis. Academic pathology. 2020 Jan-Dec:7():2374289520951902. doi: 10.1177/2374289520951902. Epub 2020 Sep 11 [PubMed PMID: 32964112]
Level 3 (low-level) evidenceField X, Tong C, Cox S, Crichton J, Goodwin B, Welsh F, Cha R. Outcomes of asymptomatic common bile duct stones detected at intraoperative cholangiography. The New Zealand medical journal. 2024 May 17:137(1595):73-79. doi: 10.26635/6965.6491. Epub 2024 May 17 [PubMed PMID: 38754115]
Katta T, Tavakoli K. Necrotizing Cholecystitis in the Gallbladder: A Case Report. Cureus. 2022 Jan:14(1):e21368. doi: 10.7759/cureus.21368. Epub 2022 Jan 18 [PubMed PMID: 35070585]
Level 3 (low-level) evidencePark JM, Kang CD, Kim JH, Lee SH, Nam SJ, Park SC, Lee SJ, Lee S. Cholecystoduodenal fistula presenting with upper gastrointestinal bleeding: A case report. World journal of clinical cases. 2021 Jan 16:9(2):410-415. doi: 10.12998/wjcc.v9.i2.410. Epub [PubMed PMID: 33521109]
Level 3 (low-level) evidenceB S B, Kar A, Dutta M, Mandal A, De Bakshi S. A case of choledochoduodenal fistula - an unusual case report. Clinical case reports. 2017 Sep:5(9):1462-1464. doi: 10.1002/ccr3.991. Epub 2017 Jul 20 [PubMed PMID: 28878904]
Level 3 (low-level) evidencePatel SS, Kohli DR, Savas J, Mutha PR, Zfass A, Shah TU. Surgery Reduces Risk of Complications Even in High-Risk Veterans After Endoscopic Therapy for Biliary Stone Disease. Digestive diseases and sciences. 2018 Mar:63(3):781-786. doi: 10.1007/s10620-018-4940-8. Epub 2018 Jan 29 [PubMed PMID: 29380173]
Genser L, Vons C. Can abdominal surgical emergencies be treated in an ambulatory setting? Journal of visceral surgery. 2015 Dec:152(6 Suppl):S81-9. doi: 10.1016/j.jviscsurg.2015.09.015. Epub 2015 Oct 27 [PubMed PMID: 26522504]
Coleman J. Bile duct injuries in laparoscopic cholecystectomy: nursing perspective. AACN clinical issues. 1999 Nov:10(4):442-54 [PubMed PMID: 10865529]
Level 3 (low-level) evidence