Introduction
Gilbert syndrome is a common genetic disorder affecting bilirubin metabolism in the liver. This condition, described in the early 1900s by Gilbert, Castaigne, and Lereboulette, is an autosomal recessive disorder that is a frequent cause of mild-to-moderate isolated unconjugated hyperbilirubinemia. The prevalence of Gilbert syndrome ranges from 2% to 20%, depending on an individual's ethnicity.[1][2] Reduced glucuronidation of bilirubin leads to unconjugated hyperbilirubinemia and recurrent episodes of jaundice.[1] Under normal circumstances, approximately 95% of bilirubin is unconjugated. Gilbert syndrome does not require treatment but must be distinguished from other disorders of unconjugated hyperbilirubinemia.[3]
Various diagnoses should be considered when evaluating patients with unconjugated hyperbilirubinemia, including disorders of bilirubin uptake, conjugation, and overproduction (see Image. Metabolic Pathway for Bilirubin in the Hepatocyte).[4] Disorders of hepatic uptake, storage, conjugation, and excretion can cause unconjugated and conjugated hyperbilirubinemia. Crigler-Najjar syndrome is characterized by marked unconjugated hyperbilirubinemia.[5] Moreover, hemolytic reactions, ineffective erythropoiesis, and resorbing hematomas induce bilirubin overproduction and subsequent unconjugated hyperbilirubinemia. Hemolytic reactions include but are not limited to hereditary enzyme deficiencies, hemoglobinopathies, red blood cell membrane defects, infections, medications, toxins, warm autoimmune hemolytic anemia, paroxysmal cold hemoglobinuria, and cold agglutinin disease that can lead to elevated unconjugated bilirubin levels.[6][7] Most patients with Gilbert syndrome are asymptomatic regarding liver disease, but they may express symptoms related to triggers. Triggers that can precipitate unconjugated hyperbilirubinemia of Gilbert syndrome include but are not limited to fasting, intercurrent illness, menstruation, and dehydration.[3]
Other acute and chronic liver diseases typically present with unconjugated and conjugated hyperbilirubinemia.[8] With hepatobiliary disorders, the proportion of conjugated bilirubin rises. Consequently, consideration of viral, metabolic, and autoimmune disorders of the liver is necessary when evaluating patients with hyperbilirubinemia and jaundice. Careful clinical assessment, targeted laboratory evaluation, and exclusion of other differential diagnoses associated with unconjugated hyperbilirubinemia, including other acute and chronic liver diseases, should be performed before diagnosing Gilbert syndrome. After diagnosing Gilbert syndrome, treatment is conservative with observation alone.[3] The prognosis of patients with Gilbert syndrome is excellent.[9]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
A variety of triggers can precipitate unconjugated hyperbilirubinemia and jaundice in patients with Gilbert syndrome. Fasting, hemolytic reactions, febrile illnesses, menstruation, and physical exertion are common precipitants.[3][10] Reducing the daily caloric intake to 400 kcal can lead to a 2- to 3-fold increase in bilirubin within 48 hours.[10][11] Moreover, a similar rise in bilirubin can present with an average-calorie diet without lipid supplementation.[12]
The bilirubin value typically returns to normal within 12 to 24 hours with a normal diet. Several theories have been proposed to explain unconjugated hyperbilirubinemia following dietary manipulation, including increased cycling of bilirubin by the enterohepatic circulation, decreased conjugation due to decreased levels of uridine 5'-diphospho-glucuronosyltransferase-glucuronic acid, a cosubstrate in glucuronidation, and release of bilirubin from fat cells.[13][14][15]
Epidemiology
Gilbert syndrome has a prevalence rate between 4% and 16%, but this can vary depending on ethnic ancestry.[16][17][18] In the White population, the prevalence of Gilbert syndrome has been estimated to be 2% to 10%. Japan and East Asia have a prevalence of about 2%, while India, Southern Asia, and the Middle East have rates of up to 20%.[19] Clinical manifestations are characteristically present during early adolescence and are more frequently found in men.[20] Most likely, differences in sex steroid concentrations and higher bilirubin production in males account for higher prevalence rates.[20] Most cases are diagnosed around puberty due to higher hemoglobin turnover and endogenous steroid hormone-induced inhibition of bilirubin glucuronidation.[20]
Pathophysiology
Gilbert syndrome is inherited in an autosomal recessive manner. In the White population, Gilbert syndrome is most commonly associated with the homozygous polymorphism A(TA)7TAA in the promoter region of UGT1A1 (uridine diphosphoglucoronate-glucuronosyltransferase 1A1), which leads to a significant reduction in the glucuronidation of bilirubin. Homozygosity for a defect in the TATA box within the promoter region of UGT1A1 leads to a mutation of UGT1A1 called UGT1A1*28. The molecular defect inserts an additional dinucleotide sequence (TA) into the transcription initiation sequence: A(TA)6TAA to A(TA)7TAA.[1] UGT1A1 activity is only 30% to 50% normal in patients with Gilbert syndrome.[21]
Monoconjugated bile pigments are increased by 34% in this patient group.[21] Patients who are heterozygous for the mutation have serum bilirubin values higher than the normal population. Not all patients who are homozygous with a promoter mutation will develop Gilbert syndrome, as other factors (eg, male or female sex) may be important for clinical expression.[22][23][24] Individuals of Asian ancestry with Gilbert syndrome are much less likely to have the UG1A1*28 mutation. Rather, decreased expression of UGT1A1 is thought to be due to variants in the coding regions of the gene.
Gilbert syndrome represents a spectrum of disease activity, as represented by impaired UGT1A1 activity. More than 100 mutations have been associated with Gilbert Syndrome, with different frequencies noted in different ethnic groups. This is also why genetic testing should not be used to diagnose Gilbert syndrome definitively.
Histopathology
A liver biopsy is generally not required for patients suspected of having Gilbert syndrome unless other confounding diagnoses merit consideration. A liver biopsy, if done, will show normal histology. Nonspecific lipofuscin pigment found within the centrilobular region of the biopsy has also been described.[25][26]
Toxicokinetics
UGT1A1 is involved in the metabolism of estrogen and several other drugs through glucuronidation. Hence, individuals with Gilbert syndrome may be susceptible to toxicities from medications that require glucuronidation. Irinotecan is well-known to cause toxicity in patients with Gilbert syndrome.[27][28] The active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38) accumulates and can cause diarrhea and myelosuppression. Antiviral agents atazanavir and indinavir inhibit UGT1A1 and may cause hyperbilirubinemia.[29][29] Other drugs that suppress or compete with UGT1A1 activity include but are not limited to, acetaminophen, tyrosine kinase inhibitors, nonsteroidal anti-inflammatory drugs, statins, ezetimibe, oxazepam, lorazepam, lamotrigine, cyclosporin A, rifampin, ethinylestradiol, buprenorphine, menthol, and tocilizumab.[30][31][32][23]
History and Physical
Gilbert syndrome typically appears during adolescence.[20] Males are more commonly affected than females in a 3:1 ratio.[20] Except for mild jaundice, patients are usually asymptomatic from liver disease but may have complaints attributable to the triggers outlined above.[3][10] Patients with Gilbert syndrome have an increased incidence of pigmented gallstones.[33][34][35] Underlying hemolytic reactions result in bilirubin overproduction and may cause unconjugated hyperbilirubinemia.[6][7]
When evaluating a patient for Gilbert Syndrome, clinicians should obtain a detailed drug history to assess for drug-induced liver injury and to evaluate for drugs that suppress hepatic bilirubin metabolism, family history given the inheritance pattern of Gilbert syndrome, and obtain laboratory records, if available to identify patients with a history of prior intermittent episodes of isolated indirect hyperbilirubinemia. Clinicians should also consider other acute and chronic liver diseases based on the history and physical examination. Patients with elevated liver enzymes or incidental hepatic steatosis noted on imaging should be evaluated for metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH). Key factors that can predispose a patient to MASLD/MASH include diabetes, hypertension, obesity, and hyperlipidemia, as well as genetic risk factors such as genetic variants in PNPLA3, which is most commonly seen in Hispanic individuals.
Patients should be questioned about a history of significant alcohol use, which can lead to alcohol-related hepatitis and metabolic-alcohol-associated liver disease. Additionally, a history of intravenous drug use, blood transfusions, incarceration, tattoos, country of birth, and high-risk sexual activity should be obtained, which may suggest either hepatitis B or C.[36][37] A careful history of autoimmune diseases in the patient or family may suggest a consideration of primary biliary cholangitis, primary sclerosing cholangitis, or autoimmune hepatitis.[38] Patients with isolated Gilbert syndrome should not have any evidence of clinically significant portal hypertension or hepatic decompensation, as manifested by varices, ascites, or hepatic encephalopathy.
Evaluation
Gilbert syndrome is associated with an isolated unconjugated hyperbilirubinemia with a serum total bilirubin below 4 mg/dL. However, the bilirubin level can fluctuate depending on exacerbating factors.[3] Characteristically, patients will have a normal complete blood count, reticulocyte count, lactate dehydrogenase, and peripheral smear supportive of the absence of hemolysis.[19] The aminotransferases and alkaline phosphatase are normal.[39][40][41]
Diagnostic imaging of the liver and biliary tree is generally not required unless the clinician is considering another diagnosis. As previously discussed, liver biopsy is rarely indicated unless the clinician suspects another acute or chronic liver disorder. Provocative testing with a 48-hour fast or with medications such as nicotinic acid, phenobarbital, or rifampin is no longer routinely used or recommended.
Genetic testing with assays of UGT1A1 activity and polymerase chain reaction to identify gene polymorphisms in the TATA box of UGT1A1 can be considered in cases of diagnostic uncertainty when starting drugs that may affect UGT1A1 activity (Please refer to the Toxicokinetics section for more information on these medications) or when family counseling is essential.[17] If the patient is found to have elevated serum liver biochemistries, studies should be sent to evaluate for chronic viral hepatitis, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, Wilson disease, hemochromatosis, celiac disease, thyroid abnormalities, and alpha-1 antitrypsin deficiency.[36][42][43][44]
Treatment / Management
Patients with Gilbert syndrome do not require treatment.[3] Therefore, management primarily consists of reassuring patients and their families that this is a benign condition that does not require further testing. However, patients, family members, and clinicians should be counseled that individuals with Gilbert syndrome may be at an increased risk of drug toxicity when exposed to medications that suppress or affect UGT1A1 activity (Please refer to the Toxicokinetics section for more information on these medications).[23]
Differential Diagnosis
Unconjugated Hyperbilirubinemia
Differential diagnoses that should be considered with various causes of unconjugated hyperbilirubinemia include:
- Increased bilirubin production: Extravascular and intravascular hemolysis, resorbing hematoma, dyseryrthopoiesis, Wilson disease
- Impaired hepatic bilirubin uptake: Heart failure, portosystemic shunts, Gilbert syndrome, medications
- Impaired bilirubin conjugation: Gilbert syndrome, Crigler-Najjar syndrome types I and II, advanced liver disease [45]
Conjugated Hyperbilirubinemia
Differential diagnoses that should be considered with various causes of conjugated hyperbilirubinemia include:
- Defects of canalicular organic anion transport: Dubin-Johnson syndrome
- Defects of sinusoidal reuptake of conjugated bilirubin: Rotor syndrome
- Extrahepatic cholestasis: Choledocholithiasis, pancreaticobiliary malignancy, primary sclerosing cholangitis, pancreatitis, parasitic infection
- Intrahepatic cholestasis: Viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease, primary biliary cholangitis, drugs and toxins, sepsis, infiltrative diseases, parenteral nutrition, sickle cell disease, pregnancy, end-stage liver disease [45]
Prognosis
Patients with Gilbert syndrome have an excellent prognosis.[3] Outcomes of patients with Gilbert syndrome are similar to that of the general population. The possible beneficial effects of mild unconjugated hyperbilirubinemia include a lower incidence of atherosclerosis, increased insulin sensitivity, decreased risk of metabolic syndrome and obesity, as well as a lower incidence of autoimmune diseases, endometrial cancer, Hodgkin lymphoma, and cancer-related mortality.[3][23][3][30][46]
Complications
Gilbert syndrome is a benign, autosomal recessive inherited disorder of bilirubin metabolism.[3] Patients are at an increased risk for more severe drug interactions, the development of pigmented gallstones, and more severe jaundice during the neonatal age, as well as in individuals with coexisting hemolytic diseases.[23] Patients with this disease are not at risk for progressive liver disease, hepatic decompensation, or liver-related mortality.[3] Patients and their families should be informed of the disease's inherited and benign nature, and unnecessary testing should be avoided. If there is clinical suspicion of either acute or chronic liver disease based on the clinical presentation or laboratory studies, a more thorough evaluation should be undertaken for viral, metabolic, and autoimmune liver diseases.
Consultations
Primary care clinicians and associated healthcare professionals can diagnose and monitor patients with Gilbert syndrome.[47] If the diagnosis is disputed or patients present with findings suggestive of another liver disease, they should be referred to a liver disease specialist for additional evaluation and treatment.
Deterrence and Patient Education
Patients with Gilbert syndrome should be informed about potential triggers such as fasting, intercurrent illness, menstruation, overexertion, hemolytic reactions, and dehydration that may cause a rise in unconjugated bilirubin.[10][47] Avoidance of triggers may be advantageous to reduce anxiety about abnormal bilirubin values. Patients and their families should be aware of the benign nature of the disorder and its inheritance pattern and that no treatment is necessary; they should also be informed about the excellent prognosis of Gilbert syndrome.
Pearls and Other Issues
Gilbert syndrome is a benign, inherited disorder of bilirubin metabolism without the risk of progressive liver disease, hepatic decompensation, or increased mortality. However, these patients are at increased risk from drug toxicity when exposed to medications that suppress or affect UGT1A1 activity. Unnecessary testing should be avoided, and patients should be followed conservatively. Moreover, patients with this disorder may benefit from the enhanced cardioprotective and antineoplastic effects seen with mild elevations in plasma bilirubin levels.
Enhancing Healthcare Team Outcomes
Gilbert syndrome may be encountered by primary care clinicians, advanced practice clinicians, emergency department clinicians, and other healthcare professionals, including pediatric, gastroenterology, or hepatology clinicians.[47] Healthcare personnel should be informed about the benign nature of this disorder and its excellent prognosis. Appropriate and targeted diagnostic testing should be carried out, and inappropriate testing should be avoided.
Patients whose clinical examination or tests suggest another liver disease or hepatic decompensation should be referred to a gastroenterologist or hepatologist. As with any patient encounter, high-quality, patient-centered care is necessary. Careful communication among all clinicians to streamline care should be paramount to enhance patient satisfaction and outcomes.
Media
(Click Image to Enlarge)
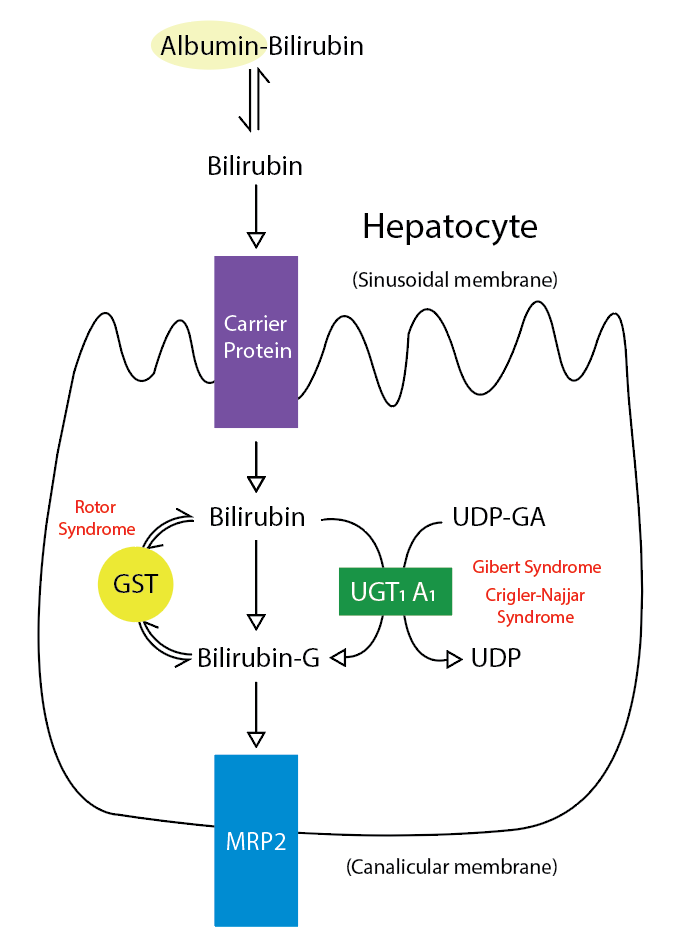
Metabolic Pathway for Bilirubin in the Hepatocyte. Bilirubin-G corresponds to bilirubin glucuronate; the donor is uridine diphosphate glucuronic acid (UDP-GA). This is catalyzed by the enzyme uridine diphosphate-glucuronyltransferase (UGT1A1). Gilbert and Crigler-Najjar syndrome is associated with decreases in UGT1A1 activity. Glutathione-S-transferase (GST) is a carrier protein that assists bilirubin uptake into the cytosol and may be implicated in Rotor syndrome.
Contributed by R Kabir, MD
References
Bosma PJ. Inherited disorders of bilirubin metabolism. Journal of hepatology. 2003 Jan:38(1):107-17 [PubMed PMID: 12480568]
Burchell B, Hume R. Molecular genetic basis of Gilbert's syndrome. Journal of gastroenterology and hepatology. 1999 Oct:14(10):960-6 [PubMed PMID: 10530490]
Fretzayas A, Moustaki M, Liapi O, Karpathios T. Gilbert syndrome. European journal of pediatrics. 2012 Jan:171(1):11-5. doi: 10.1007/s00431-011-1641-0. Epub 2011 Dec 9 [PubMed PMID: 22160004]
Erlinger S, Arias IM, Dhumeaux D. Inherited disorders of bilirubin transport and conjugation: new insights into molecular mechanisms and consequences. Gastroenterology. 2014 Jun:146(7):1625-38. doi: 10.1053/j.gastro.2014.03.047. Epub 2014 Apr 1 [PubMed PMID: 24704527]
Level 3 (low-level) evidenceStrauss KA, Ahlfors CE, Soltys K, Mazareigos GV, Young M, Bowser LE, Fox MD, Squires JE, McKiernan P, Brigatti KW, Puffenberger EG, Carson VJ, Vreman HJ. Crigler-Najjar Syndrome Type 1: Pathophysiology, Natural History, and Therapeutic Frontier. Hepatology (Baltimore, Md.). 2020 Jun:71(6):1923-1939. doi: 10.1002/hep.30959. Epub 2020 Feb 5 [PubMed PMID: 31553814]
Muraca M, Fevery J, Blanckaert N. Relationships between serum bilirubins and production and conjugation of bilirubin. Studies in Gilbert's syndrome, Crigler-Najjar disease, hemolytic disorders, and rat models. Gastroenterology. 1987 Feb:92(2):309-17 [PubMed PMID: 3792767]
Level 3 (low-level) evidenceMuraca M, Blanckaert N. Liquid-chromatographic assay and identification of mono- and diester conjugates of bilirubin in normal serum. Clinical chemistry. 1983 Oct:29(10):1767-71 [PubMed PMID: 6616822]
Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. The American journal of gastroenterology. 2017 Jan:112(1):18-35. doi: 10.1038/ajg.2016.517. Epub 2016 Dec 20 [PubMed PMID: 27995906]
Horsfall LJ, Nazareth I, Pereira SP, Petersen I. Gilbert's syndrome and the risk of death: a population-based cohort study. Journal of gastroenterology and hepatology. 2013 Oct:28(10):1643-7. doi: 10.1111/jgh.12279. Epub [PubMed PMID: 23701650]
Level 2 (mid-level) evidenceFelsher BF, Rickard D, Redeker AG. The reciprocal relation between caloric intake and the degree of hyperbilirubinemia in Gilbert's syndrome. The New England journal of medicine. 1970 Jul 23:283(4):170-2 [PubMed PMID: 5424007]
Barrett PV. Hyperbilirubinemia of fasting. JAMA. 1971 Sep 6:217(10):1349-53 [PubMed PMID: 5109641]
Gollan JL, Bateman C, Billing BH. Effect of dietary composition on the unconjugated hyperbilirubinaemia of Gilbert's syndrome. Gut. 1976 May:17(5):335-40 [PubMed PMID: 1278716]
Felsher BF, Carpio NM, VanCouvering K. Effect of fasting and phenobarbital on hepatic UDP-glucuronic acid formation in the rat. The Journal of laboratory and clinical medicine. 1979 Mar:93(3):414-27 [PubMed PMID: 107254]
Level 3 (low-level) evidenceKotal P, Vítek L, Fevery J. Fasting-related hyperbilirubinemia in rats: the effect of decreased intestinal motility. Gastroenterology. 1996 Jul:111(1):217-23 [PubMed PMID: 8698202]
Level 3 (low-level) evidenceBrink MA, Méndez-Sánchez N, Carey MC. Bilirubin cycles enterohepatically after ileal resection in the rat. Gastroenterology. 1996 Jun:110(6):1945-57 [PubMed PMID: 8964422]
Level 3 (low-level) evidenceRaijmakers MT, Jansen PL, Steegers EA, Peters WH. Association of human liver bilirubin UDP-glucuronyltransferase activity with a polymorphism in the promoter region of the UGT1A1 gene. Journal of hepatology. 2000 Sep:33(3):348-51 [PubMed PMID: 11019988]
Borlak J, Thum T, Landt O, Erb K, Hermann R. Molecular diagnosis of a familial nonhemolytic hyperbilirubinemia (Gilbert's syndrome) in healthy subjects. Hepatology (Baltimore, Md.). 2000 Oct:32(4 Pt 1):792-5 [PubMed PMID: 11003624]
Roy-Chowdhury N, Deocharan B, Bejjanki HR, Roy-Chowdhury J, Koliopoulos C, Petmezaki S, Valaes T. Presence of the genetic marker for Gilbert syndrome is associated with increased level and duration of neonatal jaundice. Acta paediatrica (Oslo, Norway : 1992). 2002:91(1):100-1 [PubMed PMID: 11883809]
Level 3 (low-level) evidenceWagner KH, Shiels RG, Lang CA, Seyed Khoei N, Bulmer AC. Diagnostic criteria and contributors to Gilbert's syndrome. Critical reviews in clinical laboratory sciences. 2018 Mar:55(2):129-139. doi: 10.1080/10408363.2018.1428526. Epub 2018 Feb 1 [PubMed PMID: 29390925]
Muraca M, Fevery J. Influence of sex and sex steroids on bilirubin uridine diphosphate-glucuronosyltransferase activity of rat liver. Gastroenterology. 1984 Aug:87(2):308-13 [PubMed PMID: 6428963]
Level 3 (low-level) evidenceKaplan M. Gilbert's syndrome and jaundice in glucose-6-phosphate dehydrogenase deficient neonates. Haematologica. 2000:85(E-letters):E01 [PubMed PMID: 11114816]
Level 3 (low-level) evidenceBosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. The New England journal of medicine. 1995 Nov 2:333(18):1171-5 [PubMed PMID: 7565971]
Level 2 (mid-level) evidenceVítek L, Tiribelli C. Gilbert's syndrome revisited. Journal of hepatology. 2023 Oct:79(4):1049-1055. doi: 10.1016/j.jhep.2023.06.004. Epub 2023 Jun 28 [PubMed PMID: 37390966]
Memon N, Weinberger BI, Hegyi T, Aleksunes LM. Inherited disorders of bilirubin clearance. Pediatric research. 2016 Mar:79(3):378-86. doi: 10.1038/pr.2015.247. Epub 2015 Nov 23 [PubMed PMID: 26595536]
SAGILD U, DALGAARD OZ, TYGSTRUP N. Constitutional hyperbilirubinemia with unconjugated bilirubin in the serum and lipochrome-like pigment granules in the liver. Annals of internal medicine. 1962 Feb:56():308-14 [PubMed PMID: 14496018]
Strassburg CP. Hyperbilirubinemia syndromes (Gilbert-Meulengracht, Crigler-Najjar, Dubin-Johnson, and Rotor syndrome). Best practice & research. Clinical gastroenterology. 2010 Oct:24(5):555-71. doi: 10.1016/j.bpg.2010.07.007. Epub [PubMed PMID: 20955959]
Level 3 (low-level) evidenceIyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR, Coffman BL, Ratain MJ. Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. The Journal of clinical investigation. 1998 Feb 15:101(4):847-54 [PubMed PMID: 9466980]
Level 3 (low-level) evidenceBurchell B, Soars M, Monaghan G, Cassidy A, Smith D, Ethell B. Drug-mediated toxicity caused by genetic deficiency of UDP-glucuronosyltransferases. Toxicology letters. 2000 Mar 15:112-113():333-40 [PubMed PMID: 10720749]
Level 3 (low-level) evidenceLankisch TO, Moebius U, Wehmeier M, Behrens G, Manns MP, Schmidt RE, Strassburg CP. Gilbert's disease and atazanavir: from phenotype to UDP-glucuronosyltransferase haplotype. Hepatology (Baltimore, Md.). 2006 Nov:44(5):1324-32 [PubMed PMID: 17058217]
de Morais SM, Uetrecht JP, Wells PG. Decreased glucuronidation and increased bioactivation of acetaminophen in Gilbert's syndrome. Gastroenterology. 1992 Feb:102(2):577-86 [PubMed PMID: 1732127]
Carulli N, Ponz de Leon M, Mauro E, Manenti F, Ferrari A. Alteration of drug metabolism in Gilbert's syndrome. Gut. 1976 Aug:17(8):581-7 [PubMed PMID: 976795]
Level 3 (low-level) evidenceMiners JO, McKinnon RA, Mackenzie PI. Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology. 2002 Dec 27:181-182():453-6 [PubMed PMID: 12505351]
Level 3 (low-level) evidencedel Giudice EM, Perrotta S, Nobili B, Specchia C, d'Urzo G, Iolascon A. Coinheritance of Gilbert syndrome increases the risk for developing gallstones in patients with hereditary spherocytosis. Blood. 1999 Oct 1:94(7):2259-62 [PubMed PMID: 10498597]
Level 2 (mid-level) evidenceOriga R, Galanello R, Perseu L, Tavazzi D, Domenica Cappellini M, Terenzani L, Forni GL, Quarta G, Boetti T, Piga A. Cholelithiasis in thalassemia major. European journal of haematology. 2009 Jan:82(1):22-5. doi: 10.1111/j.1600-0609.2008.01162.x. Epub 2008 Oct 31 [PubMed PMID: 19021734]
Haverfield EV, McKenzie CA, Forrester T, Bouzekri N, Harding R, Serjeant G, Walker T, Peto TE, Ward R, Weatherall DJ. UGT1A1 variation and gallstone formation in sickle cell disease. Blood. 2005 Feb 1:105(3):968-72 [PubMed PMID: 15388579]
Murphy EL, Bryzman SM, Glynn SA, Ameti DI, Thomson RA, Williams AE, Nass CC, Ownby HE, Schreiber GB, Kong F, Neal KR, Nemo GJ. Risk factors for hepatitis C virus infection in United States blood donors. NHLBI Retrovirus Epidemiology Donor Study (REDS). Hepatology (Baltimore, Md.). 2000 Mar:31(3):756-62 [PubMed PMID: 10706569]
Level 2 (mid-level) evidenceOtt JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012 Mar 9:30(12):2212-9. doi: 10.1016/j.vaccine.2011.12.116. Epub 2012 Jan 24 [PubMed PMID: 22273662]
Level 2 (mid-level) evidenceFischer HP, Goltz D. [Autoimmune liver diseases]. Der Pathologe. 2020 Sep:41(5):444-456. doi: 10.1007/s00292-020-00807-7. Epub [PubMed PMID: 32749523]
Vierling JM, Berk PD, Hofmann AF, Martin JF, Wolkoff AW, Scharschmidt BF. Normal fasting-state levels of serum cholyl-conjugated bile acids in Gilbert's syndrome: an aid to the diagnosis. Hepatology (Baltimore, Md.). 1982 May-Jun:2(3):340-3 [PubMed PMID: 7076117]
Douglas JG, Beckett GJ, Nimmo IA, Finlayson ND, Percy-Robb IW. Bile salt measurements in Gilbert's syndrome. European journal of clinical investigation. 1981 Dec:11(6):421-3 [PubMed PMID: 6800816]
Okolicsanyi L, Fevery J, Billing B, Berthelot P, Thompson RP, Schmid R, Berk PD. How should mild, isolated unconjugated hyperbilirubinemia be investigated? Seminars in liver disease. 1983 Feb:3(1):36-41 [PubMed PMID: 6340206]
Klevens RM, Miller JT, Iqbal K, Thomas A, Rizzo EM, Hanson H, Sweet K, Phan Q, Cronquist A, Khudyakov Y, Xia GL, Spradling P. The evolving epidemiology of hepatitis a in the United States: incidence and molecular epidemiology from population-based surveillance, 2005-2007. Archives of internal medicine. 2010 Nov 8:170(20):1811-8. doi: 10.1001/archinternmed.2010.401. Epub [PubMed PMID: 21059974]
Level 2 (mid-level) evidenceRodriguez-Castro KI, Hevia-Urrutia FJ, Sturniolo GC. Wilson's disease: A review of what we have learned. World journal of hepatology. 2015 Dec 18:7(29):2859-70. doi: 10.4254/wjh.v7.i29.2859. Epub [PubMed PMID: 26692151]
Patel D, McAllister SL, Teckman JH. Alpha-1 antitrypsin deficiency liver disease. Translational gastroenterology and hepatology. 2021:6():23. doi: 10.21037/tgh.2020.02.23. Epub 2021 Apr 5 [PubMed PMID: 33824927]
Fargo MV, Grogan SP, Saguil A. Evaluation of Jaundice in Adults. American family physician. 2017 Feb 1:95(3):164-168 [PubMed PMID: 28145671]
Ullrich D, Sieg A, Blume R, Bock KW, Schröter W, Bircher J. Normal pathways for glucuronidation, sulphation and oxidation of paracetamol in Gilbert's syndrome. European journal of clinical investigation. 1987 Jun:17(3):237-40 [PubMed PMID: 3113968]
VanWagner LB, Green RM. Evaluating elevated bilirubin levels in asymptomatic adults. JAMA. 2015 Feb 3:313(5):516-7. doi: 10.1001/jama.2014.12835. Epub [PubMed PMID: 25647209]