Introduction
Blood is mandatory to maintain adequate circulation, provide nutrients, and remove waste from the brain, vital organs, and tissues.[1] A hemorrhage is the loss of blood components from the cardiovascular system. Hemorrhagic shock occurs when this blood loss leads to inadequate tissue oxygenation. Hemorrhage secondary to traumatic injury is the leading cause of death of Americans from one to 46 years of age.[2] The stop the bleed campaign was first launched in 2013. This educational program aims to reduce the morbidity and mortality associated with hemorrhage secondary to traumatic injury.
Significant improvements in clinical outcomes can be made by transferring knowledge from the military to civilian medicine. This article reviews some of the latest science and lessons learned to improve the care of a hemorrhaging patient.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
In 2000, the World health organization (WHO) estimated that injury resulted in 9% of the global mortality and 12% of the worldwide disease burden.[3] Worldwide, severe trauma results in the death of over 5 million persons annually and is projected to surpass 8 million annually by the year 2020.[4] Hemorrhage accounts for approximately 35% of the mortality from these traumatic injuries, second only to central nervous system (CNS) injury.[3]
Up to half of the deaths resulting from hemorrhage occur before reaching definitive care. Violence is the leading cause of these injuries, and traffic collisions are second. In 2015, the national trauma institute estimated that in the civilian arena, "severe bleeding accounts for greater than 35% of pre-hospital deaths and nearly 40% of deaths within the first 24 hours of injury."[2] Coagulopathy affects 25% of these injured patients. This results from "hemorrhage, hemodilution, hypothermia, and acidosis."[3]
Epidemiology
Ninety percent of the mortality caused by these injuries occurs in low and middle-income countries.[3] Half of those affected are between the ages of 15 and 44, and males are affected twice as often as females. In 2003, injuries were the third leading cause of death overall in the United States and the leading cause of death for those between the ages of 1 and 46. These injuries represented 30% of the years of potential life lost before age 65 and accounted for almost 10% of the national expenditure on healthcare. Despite advances in medicine and prehospital care, these numbers have not improved significantly. In 2008, the WHO estimated that over 16,000 people died daily from traumatic injuries.[5]
History and Physical
There are three main categories of bleeding. Lacerations, punctures, and amputations can result in arterial bleeding, which is characterized by spurting or pulsatile, bright red blood.[6] These wounds can also result in venous bleeding. Venous bleeding is characterized by flowing, dark red blood. This flow can be steady but lacks the force of the arterial system. Capillary bleeding is a much more common form resulting from injury to the integumentary system. Capillary bleeding is characterized by oozing blood from the damaged area. Capillary bleeding can be the most painful but the easiest to control due to its superficial nature.
A weak or absent radial pulse and a systolic blood pressure below 90 mm Hg are two indicators of shock in a hemorrhagic patient.[7] Similarly, pale or ashen skin and fast, thready central pulses are signs of hypovolemia. A capillary refill time of more than two seconds also indicates poor perfusion, which can indicate hypovolemia in the presence of significant trauma. The American College of Surgeons developed a classification system for advanced trauma life support (ATLS) to use some of these parameters to grade the progression of shock.[8]
They define class I shock as losing less than 15% of total blood volume and including mostly normal vital signs. Class II includes a blood loss of 15% to 30% and shows a slight deviation from normal vital signs. The vital sign deviation from normal increases in class III, where blood loss is between 31% and 40%. Finally, class IV includes patients with losses of greater than 40% of total blood volume, which can lead to altered mental status, hypotension, tachycardia, and tachypnea. In recent years several studies questioned the consistency of this classification system. These studies found base deficit (BD) to be a consistent measurement that could aid in classifying hemorrhage. ATLS was updated to include base deficit (BD) as a parameter of the existing hemorrhage classification system.[9]
- Class I: 0 to -2
- Class II: -2 to -6
- Class III: -6 to -10
- Class IV: -10 or less
Evaluation
The physical exam often provides the most valuable information regarding blood loss; however, many lab tests can assess the hemorrhaging patient. The lethal triad is comprised of coagulopathy, hypothermia, and acidosis.[10] Coagulopathy of trauma is a significant risk factor for hemorrhagic death. Viscoelastic whole blood assays such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM) quantify clotting kinetics and may be superior to conventional coagulation assays, given their potential for tailored treatment plans.[11][10]
Similar to TEG and ROTEM, prothrombin time (PT), international standardized ratio (INR), activated partial thromboplastin time (PTT), fibrinogen, and platelets can be used to detect coagulopathy. Furthermore, serum lactate and base deficit can be used to monitor the extent of tissue hypoperfusion. Single hematocrit measurements are not recommended for monitoring acute blood loss.[4]
Treatment / Management
Regarding hemorrhage control, the location of the bleeding is critically important. There are two major categories to consider: compressible and non-compressible. Any obvious bleeding in a compressible area should first be treated with direct digital pressure.[12] Should direct pressure fail, a tourniquet should be applied. Using specific pressure points to control bleeding is no longer recommended based on scientific literature, which has demonstrated collateral circulation defeating it. While the Stop The Bleed Campaign promotes placing all tourniquets as proximal as possible on a wounded extremity in a "high or die" mentality, this strategy is not universally recommended. The committee on tactical combat casualty care (CoTCCC) states that this should only be performed when the scene is still unsafe during care under fire.[12]
Once a scene has been secured, the CoTCCC recommends a new tourniquet be placed directly on the skin, two to three inches proximal to the wound, and the more proximal tourniquet loosened. If bleeding in any extremity continues after placing one tourniquet, a second should be placed side-by-side with the first.[7] A study of wounded service members and civilians from the recent wars in Iraq and Afghanistan demonstrated a 92% survival rate of casualties who had tourniquets applied to extremity injuries.[7] However, no casualties survived after suffering an uncontrolled extremity hemorrhage in which no tourniquet was used. Of the 308 limbs that had tourniquets applied, only ten nerve palsies were noted (3.2%), with four at the level of the tourniquet. Most of these reported palsies resolved within hours, and not a single amputation was performed due to tourniquet use. Other reports note that tourniquets have been left in place for up to six hours without loss of the affected limb.[4](A1)
Non-compressible hemorrhage often takes the form of truncal (thoraco-abdominopelvic) or junctional wounds. These wounds account for 21% of recent combat deaths.[13] Junctional areas, where an extremity meets the trunk, include the groin above the inguinal ligament, the gluteals and pelvis, the perineum, the axilla, and the base of the neck. Throughout history, several surgeons have made their own tourniquet devices and successfully used them on large arteries. However, these devices were used by experienced surgeons on patients who were not moving and did not need to be moved. In recent years, several truncal tourniquets have been developed for field use, including the combat-ready clamp (CRoC), the junctional emergency treatment tool (JETT), and the SAM junctional tourniquet (SJT).[14] (A1)
One recent study demonstrates their ease of deployment but shows the need for improvement when transporting patients who have a junctional tourniquet applied. Further research shows that a knee with significant force applied just superior to the umbilicus or just inferior and lateral to it may stop or slow hemorrhage from the aorta, iliac, or femoral arteries while these devices are applied.[13](B3)
In the early 2000s, hemostatic agents were being pressed into development. A chitosan-based dressing and a zeolite mineral powder were the first approved by the US military.[15] These hemostatic agents were most useful for non-compressible wounds. Chitosan-based dressings worked by adhering to hemorrhaging tissues and sealing vessels; however, their efficacy diminished over time. The zeolite mineral powder's mechanism of action is based on rapid water absorption, concentrating clotting factors and cells and increase clot formation. A dry fibrin sealant dressing was developed using concentrated fibrinogen and thrombin from donated human plasma. However, its expense and increased testing requirements prevented its widespread use. Similarly, a chitin-based dressing was created, but its cost far exceeds other equally effective agents.(B3)
One product, made of smectite minerals, was developed to both concentrate clotting elements by desiccation and activate the intrinsic coagulation pathway. While the products made of smectite minerals proved to be highly effective at hemostasis, studies showed that it caused thrombi to form that could migrate throughout the circulatory system, resulting in devastating complications. A surgical gauze impregnated with kaolin and aluminum silicate also proved to be effective at activating the intrinsic pathway and facilitating hemostasis without known side effects. Following these studies, the CoTCCC decided in 2015 to recommend surgical gauze impregnated with kaolin and aluminum silicate as the "hemostatic agent of choice."[12] Two chitosan-based dressings were recommended as alternatives to the surgical gauze impregnated with kaolin and aluminum silicate. Lastly, a syringe that injects expandable sponges into the wound tract to create a physical barrier and pressure inside the cavity proved highly effective against narrow but deep penetrating wounds in some regions.
All three gauze-type hemostatic agents recommended by CoTCCC should be placed on or packed into wounds and followed by at least three minutes of direct pressure. During this time, junctional tourniquets should be applied to sites amenable to their use. The CoTCCC also recommends that limb and junctional tourniquets be converted to a hemostatic or pressure dressing if all three of the following criteria are met: "the casualty is not in shock; it is possible to monitor the wound closely for bleeding, and the tourniquet is not being used to control bleeding from an amputated extremity." This should be completed within two hours of tourniquet placement whenever possible. Furthermore, the CoTCCC recommends that tourniquets in place for more than six hours not be removed unless the patient is under close monitoring with lab capabilities.
Several other physical actions can be performed to save a patient's life from hemorrhaging from a non-compressible area. Bleeding control of the abdomen can be accomplished by packing, including hemostatic agents, but it requires surgical capabilities.[13][4] Pelvic fractures should be stabilized with a pelvic binder or pelvic C-Clamp to control bleeding until the patient reaches the operating room.[4] Definitive treatment of abdominopelvic bleeding often requires surgical intervention. If a patient continues to exsanguinate despite other efforts, a resuscitative thoracotomy (RT) with aortic cross-clamping can be performed.[13][16] Similarly, angiographic embolization through resuscitative endovascular balloon occlusion of the aorta (REBOA) can be used to stop uncontrolled bleeding not amenable to other means. Recent studies suggest no overall difference in mortality between RT and REBOA.[16] For both procedures, the ischemia caused by occluding the aorta is one of the most significant complications.(A1)
Patients can be given several medications to promote and enhance their ability to form clots. Tranexamic acid (TXA) is a lysine derivative that blocks the activation of plasmin.[11] Plasmin breaks down fibrin clots, thereby inhibiting hemostasis. TXA has demonstrated improved mortality and decreased blood product requirements among trauma patients when given in the first three hours after injury. However, the use of TXA in urban areas with advanced prehospital and trauma systems has been questioned by several studies showing decreased efficacy. Since fibrinogen is one of the first clotting factors depleted following hemorrhage, several studies have focused on its use in trauma patients.[10]
While it has shown decreased mortality in the short term, the 30-day mortality was not significantly different, and more studies have been encouraged. Recombinant factor VIIa is another possible treatment for severe bleeding; however, it is not considered a first-line treatment.[8] It is approved for pre-operatively in patients with factor VII deficiency or hemophilia.[10] Prothrombin complex concentrate (PCC) is another medication that deserves further study for hemorrhaging patients. Four-factor PCC consists of factors II, VII, IX, and X and is used to reverse vitamin K antagonists as well as treat congenital coagulation disorders.[10]
Differential Diagnosis
In patients presenting with hemorrhage from any site, the following differentials should be considered:
- Blunt trauma
- Penetrating trauma
- Coagulation disorders
- Platelet disorders
- Anticoagulant medications
- Antiplatelet medications
- Malignancies
- Liver disease
- Vitamin deficiency
In patients presenting with hemorrhage from the gastrointestinal tract, the following differentials should be considered:
- Blunt or penetrating trauma of the head, neck, chest, abdomen, and pelvis
- Mucosal tears
- Varices
- Ulcers
- Esophagitis, gastritis, duodenitis
- Aortic dissection
- GI neoplasms and malignancies
- Angiodysplasias
- Diverticula
- Hemorrhoids
Prognosis
Mortality ranged from 13% for Mallory-Weiss tear to 34% for gastritis & duodenitis, 38–41% for duodenal ulcer, gastric ulcer, and oesophagitis, 52% for varices, and 95% for upper GI malignancy. Mortality was also higher for bleeds that occurred as inpatients (54%) than for bleeds presenting at admission (36%). In the case of traumatic hemorrhage, prognosis depends on whether the hemorrhagic site is compressible or non-compressible, the time to medical treatment, and whether a tourniquet is applied or not.
Complications
Uncontrolled hemorrhage is detrimental and can cause death. Additional complications can include:
- Ischemia from hemorrhagic shock
- Coagulopathies
- Multiorgan failure
- Sepsis
Deterrence and Patient Education
Following the success of the surviving sepsis campaign, the Stop The Bleeding Campaign was launched in Europe in 2013.[5] The campaign aims to "reduce the number of patients who die within 24 hours after arrival in hospital due to exsanguination by at least 20% within five years." Knowing that over 5 million people die annually from traumatic injuries and that a third of trauma patients present to the hospital with coagulopathy, they aim to support hemostatic resuscitation measures. The campaign focuses on providing guidelines to facilitate the early recognition and treatment of hemorrhage and coagulopathy. The acronym STOP stands for search, treat, observe and prevent. In 2015, the White House launched the stop the bleed campaign in the United States. This campaign has similar objectives to its European predecessor and also provides layperson medical training. The US Department of homeland security hosts the stop the bleed website.
Enhancing Healthcare Team Outcomes
Improving outcomes in patients with hemorrhage starts the moment the patient is injured. Stop the bleeding campaigns, layperson aid, proper dispatch, and timely response followed by rapid treatment and transport by EMS with early notification to the receiving hospital are paramount.[17][18] [Level 4] The goal of creating trauma teams was to mobilize adequate resources, distribute the workload of assessments and procedures, and improve patient outcomes. While there are significant differences among what constitutes a trauma team, it is essential to have a surgeon and/or an emergency medicine provider and an anesthetist available. Furthermore, having a designated trauma team leader has been found to improve patient outcomes.[19] [Level 4]
ATLS training and adherence to its recommendations and checklists have also led to decreased mortality in trauma patients.[20][21][22][21][20] All interprofessional healthcare team members should at least be familiar with basic measures to control bleeding, as it can be the difference between life and death for the patient. [Level 4]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
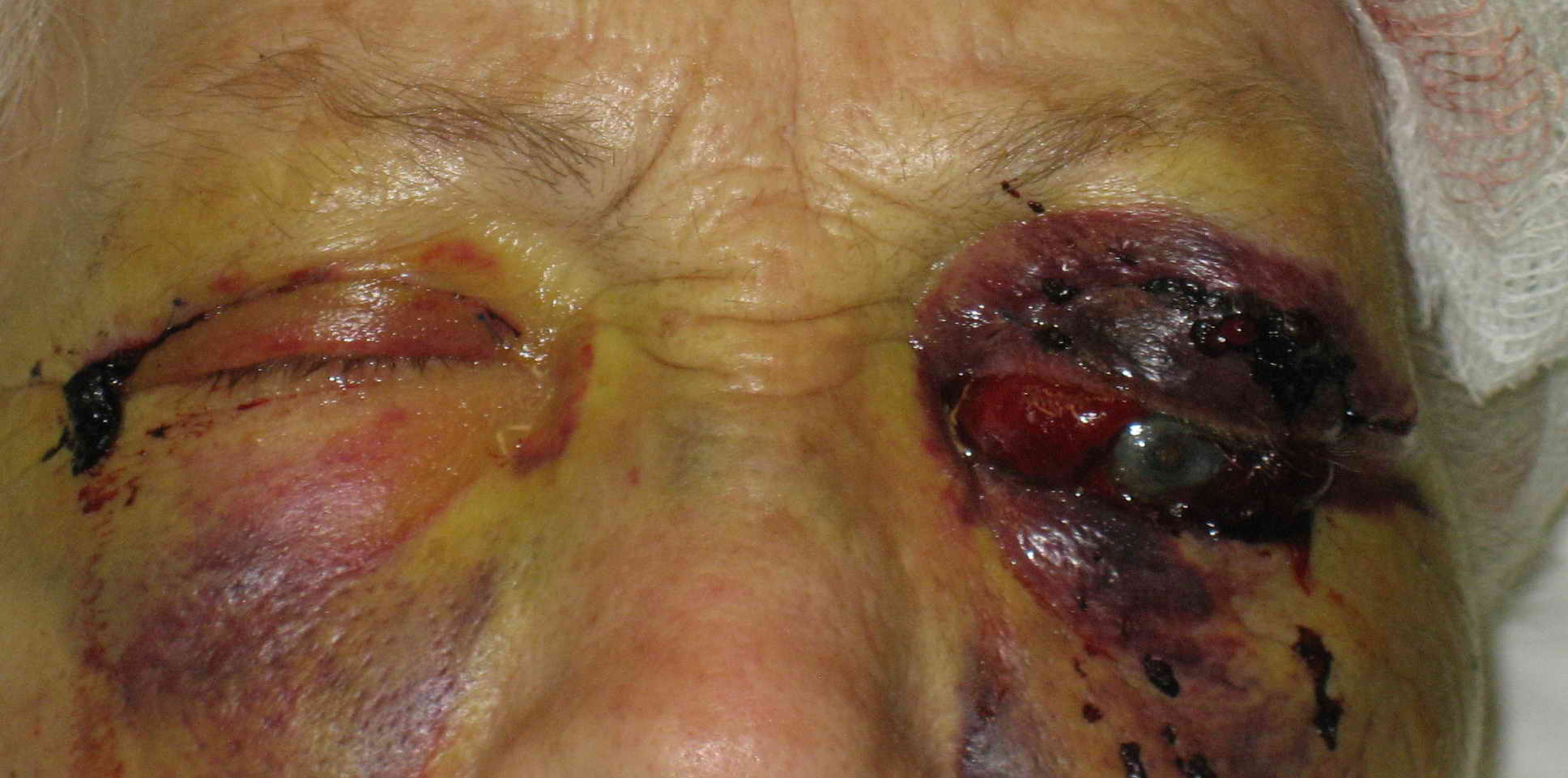
Retrobulbar Hematoma. The patient developed severe pain in the left eye and orbit after an uneventful upper blepharoplasty. The patient was not seen and examined until the following morning when this photograph was taken. The patient has no perception of light in the left eye. A canthotomy, cantholysis, and evacuation of orbital hemorrhage did not help this patient, as this should have been performed much earlier.
Contributed by Prof. BCK Patel MD, FRCS
(Click Video to Play)
References
Planas JH, Waseem M, Sigmon DF. Trauma Primary Survey. StatPearls. 2025 Jan:(): [PubMed PMID: 28613551]
Level 3 (low-level) evidenceChambers JA, Seastedt K, Krell R, Caterson E, Levy M, Turner N. "Stop the Bleed": A U.S. Military Installation's Model for Implementation of a Rapid Hemorrhage Control Program. Military medicine. 2019 Mar 1:184(3-4):67-71. doi: 10.1093/milmed/usy185. Epub [PubMed PMID: 30085214]
Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. The Journal of trauma. 2006 Jun:60(6 Suppl):S3-11 [PubMed PMID: 16763478]
Level 3 (low-level) evidenceSpahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Vincent JL, Rossaint R. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Critical care (London, England). 2013 Apr 19:17(2):R76. doi: 10.1186/cc12685. Epub 2013 Apr 19 [PubMed PMID: 23601765]
Level 1 (high-level) evidenceRossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Filipescu D, Hunt BJ, Komadina R, Maegele M, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Vincent JL, Spahn DR, STOP Bleeding Campaign. The STOP the Bleeding Campaign. Critical care (London, England). 2013 Apr 26:17(2):136. doi: 10.1186/cc12579. Epub 2013 Apr 26 [PubMed PMID: 23635083]
Hoogenboom BJ, Smith D. Management of bleeding and open wounds in athletes. International journal of sports physical therapy. 2012 Jun:7(3):350-5 [PubMed PMID: 22666650]
Kragh JF Jr, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, Holcomb JB. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Annals of surgery. 2009 Jan:249(1):1-7. doi: 10.1097/SLA.0b013e31818842ba. Epub [PubMed PMID: 19106667]
Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Hunt BJ, Komadina R, Nardi G, Neugebauer E, Ozier Y, Riddez L, Schultz A, Stahel PF, Vincent JL, Spahn DR, Task Force for Advanced Bleeding Care in Trauma. Management of bleeding following major trauma: an updated European guideline. Critical care (London, England). 2010:14(2):R52. doi: 10.1186/cc8943. Epub 2010 Apr 6 [PubMed PMID: 20370902]
Mutschler M, Nienaber U, Brockamp T, Wafaisade A, Fabian T, Paffrath T, Bouillon B, Maegele M, TraumaRegister DGU. Renaissance of base deficit for the initial assessment of trauma patients: a base deficit-based classification for hypovolemic shock developed on data from 16,305 patients derived from the TraumaRegister DGU®. Critical care (London, England). 2013 Mar 6:17(2):R42. doi: 10.1186/cc12555. Epub 2013 Mar 6 [PubMed PMID: 23497602]
Johansson PI, Stensballe J, Ostrowski SR. Current management of massive hemorrhage in trauma. Scandinavian journal of trauma, resuscitation and emergency medicine. 2012 Jul 9:20():47. doi: 10.1186/1757-7241-20-47. Epub 2012 Jul 9 [PubMed PMID: 22776724]
Bardes JM, Palmer A, Con J, Wilson A, Schaefer G. Antifibrinolytics in a rural trauma state: assessing the opportunities. Trauma surgery & acute care open. 2017:2(1):e000107. doi: 10.1136/tsaco-2017-000107. Epub 2017 Oct 5 [PubMed PMID: 29766102]
Bennett BL. Bleeding Control Using Hemostatic Dressings: Lessons Learned. Wilderness & environmental medicine. 2017 Jun:28(2S):S39-S49. doi: 10.1016/j.wem.2016.12.005. Epub 2017 Mar 17 [PubMed PMID: 28318991]
Kragh JF Jr, Murphy C, Dubick MA, Baer DG, Johnson J, Blackbourne LH. New tourniquet device concepts for battlefield hemorrhage control. U.S. Army Medical Department journal. 2011 Apr-Jun:():38-48 [PubMed PMID: 21607905]
Level 3 (low-level) evidenceGaspary MJ, Zarow GJ, Barry MJ, Walchak AC, Conley SP, Roszko PJD. Comparison of Three Junctional Tourniquets Using a Randomized Trial Design. Prehospital emergency care. 2019 Mar-Apr:23(2):187-194. doi: 10.1080/10903127.2018.1484968. Epub 2018 Aug 17 [PubMed PMID: 30118363]
Level 1 (high-level) evidenceKheirabadi B. Evaluation of topical hemostatic agents for combat wound treatment. U.S. Army Medical Department journal. 2011 Apr-Jun:():25-37 [PubMed PMID: 21607904]
Level 3 (low-level) evidenceRibeiro Junior MAF, Feng CYD, Nguyen ATM, Rodrigues VC, Bechara GEK, de-Moura RR, Brenner M. The complications associated with Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). World journal of emergency surgery : WJES. 2018:13():20. doi: 10.1186/s13017-018-0181-6. Epub 2018 May 11 [PubMed PMID: 29774048]
Mahama MN, Kenu E, Bandoh DA, Zakariah AN. Emergency response time and pre-hospital trauma survival rate of the national ambulance service, Greater Accra (January - December 2014). BMC emergency medicine. 2018 Oct 3:18(1):33. doi: 10.1186/s12873-018-0184-3. Epub 2018 Oct 3 [PubMed PMID: 30285650]
Hung YC, Bababekov YJ, Stapleton SM, Mukhopadhyay S, Huang SL, Briggs SM, Chang DC. Reducing road traffic deaths: where should we focus global health initiatives? The Journal of surgical research. 2018 Sep:229():337-344. doi: 10.1016/j.jss.2018.04.036. Epub 2018 May 10 [PubMed PMID: 29937011]
Tsang B, McKee J, Engels PT, Paton-Gay D, Widder SL. Compliance to advanced trauma life support protocols in adult trauma patients in the acute setting. World journal of emergency surgery : WJES. 2013 Oct 2:8(1):39. doi: 10.1186/1749-7922-8-39. Epub 2013 Oct 2 [PubMed PMID: 24088362]
Hashmi ZG, Haider AH, Zafar SN, Kisat M, Moosa A, Siddiqui F, Pardhan A, Latif A, Zafar H. Hospital-based trauma quality improvement initiatives: first step toward improving trauma outcomes in the developing world. The journal of trauma and acute care surgery. 2013 Jul:75(1):60-8; discussion 68. doi: 10.1097/TA.0b013e31829880a0. Epub [PubMed PMID: 23778440]
Level 2 (mid-level) evidenceHedges JR, Adams AL, Gunnels MD. ATLS practices and survival at rural level III trauma hospitals, 1995-1999. Prehospital emergency care. 2002 Jul-Sep:6(3):299-305 [PubMed PMID: 12109572]
Level 2 (mid-level) evidenceParsons SE, Carter EA, Waterhouse LJ, Fritzeen J, Kelleher DC, Oʼconnell KJ, Sarcevic A, Baker KM, Nelson E, Werner NE, Boehm-Davis DA, Burd RS. Improving ATLS performance in simulated pediatric trauma resuscitation using a checklist. Annals of surgery. 2014 Apr:259(4):807-13. doi: 10.1097/SLA.0000000000000259. Epub [PubMed PMID: 24096751]
Level 1 (high-level) evidence