Definition/Introduction
High-altitude oxygenation refers to the process of improving oxygen delivery or enriching the body with additional oxygen under conditions of reduced atmospheric pressure at elevated altitudes.[1] The International Society for Mountain Medicine categorizes high-altitude environments into 3 regions based on elevation, as follows:
- High altitude: 1,500 to 3,500 m (4,900-11,500 ft) above sea level
- Very high altitude: 3,500 to 5,500 m (11,500-18,000 ft) above sea level
- Extreme altitude: At least 5,500 m (18,000 ft) above sea level (Source: International Society for Mountain Medicine, 2006)
These categories were originally established in the Society's guidelines and remain in use across major consensus statements and clinical references. Each altitude tier corresponds with progressively lower atmospheric oxygen availability.
Mount Everest, the tallest elevation on Earth, stands at 29,029 ft above sea level and falls within the extreme altitude category. Based on data from recent years, atmospheric pressure typically measures between 251 and 253 mm Hg (approximately 33 kPa) at the peak of this mountain (8,848 m) during the main climbing seasons.[2][3] Meanwhile, the standard atmospheric pressure at sea level is 760 mm Hg. Despite this marked difference in pressure, the fractional concentration of inspired oxygen remains constant across elevations.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Individuals at elevated risk for developing acute mountain sickness (AMS), in both mild and severe forms, include not only pilots, aircrew, and passengers of aircraft but also athletes, lowland travelers visiting ski resorts, participants in mountaineering expeditions, and people undertaking pilgrimages to monasteries, abbeys, shrines, or temples. If left untreated, AMS may progress to severe manifestations of high-altitude illness (HAI), specifically high-altitude pulmonary (HAPE) or cerebral (HACE) edema (see Image. High-Altitude Pulmonary Edema on Chest Radiography).
Rapid or passive ascent, such as by air travel, to elevations of 3,000 to 4,600 meters significantly increases the risk of AMS, with incidence rates as high as 30% to 40% among travelers flying to these altitudes compared to slower ascents by foot or vehicle. (Source: U.S. Centers for Disease Control and Prevention, 2025) A meta-analysis of over 11,000 individuals revealed a 4.5-fold higher AMS incidence following air travel compared to gradual ascent.[4] In a study of Nepali pilgrims rapidly ascending to nearly 4,380 meters, 29% to 34% developed AMS, and approximately 0.5% to 1% of the cases progressed to HACE.[5]
Clinical Significance
High altitudes can lead to reduced oxygen saturation levels or desaturation of arterial blood. The partial pressure of oxygen declines as elevation increases, serving as the principal factor in the development of HAI. Hypoxia, defined as diminished oxygen availability, decreases both the alveolar oxygen partial pressure and arterial partial pressure of oxygen (PaO2). For every 1,000-meter gain in altitude, PaO2 falls by approximately 12 mm Hg. Hypoxemia is defined as a PaO2 below 60 mm Hg or an arterial oxygen saturation less than 90%. In most individuals, hypoxemia begins to occur between 2,000 and 3,000 meters of altitude.[6]
Etiology
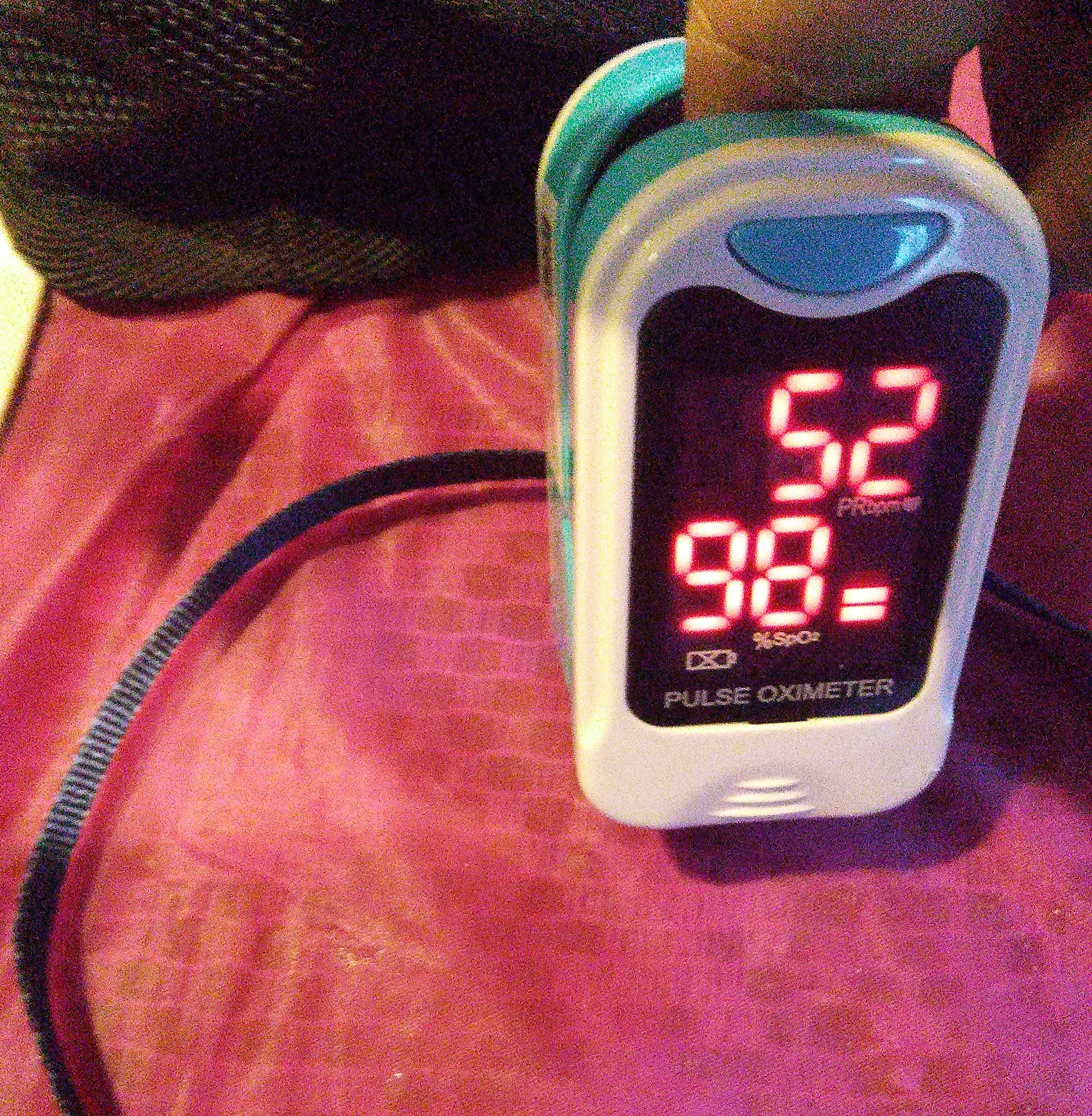
Low oxygen saturation at high altitudes results from reduced atmospheric pressure, which lowers the availability of oxygen for hemoglobin binding. This decrease in barometric pressure reduces the partial pressure of inspired oxygen, which in turn lowers the PaO2 and limits oxygen diffusion across the alveolar-capillary membrane.[7] "Oxygen saturation" refers to the proportion of hemoglobin molecules bound to oxygen in arterial blood. A pulse oximeter, a small noninvasive device typically applied to the index finger, measures both oxygen saturation and heart rate (see Image. Pulse Oximeter). Oxygen saturation normally ranges from 95% to 100%, while values below 90% are considered abnormal.
Several other conditions can cause low oxygen saturation in addition to high-altitude exposure. These conditions include alveolar hypoventilation due to obstructive sleep apnea or oversedation; ventilation-perfusion mismatch, as seen in pulmonary embolism or airway obstruction from chronic obstructive pulmonary disease and sleep apnea; diffusion impairments such as those caused by pneumonia, congestive heart failure, alveolar hemorrhage, emphysema, interstitial lung disease, or acute respiratory distress syndrome (ARDS); and anatomic or physiologic shunting, whether intrapulmonary or extrapulmonary.
Recent evidence implicates specific genetic risk factors, including mitochondrial DNA (mtDNA) haplogroups and point mutations, in susceptibility to HAI. In particular, the A4576G mtDNA mutation has been identified as a risk factor for AMS among young Han Chinese male individuals. Intolerance to high altitude in this population has also been associated with elevated neutrophil counts and an impaired ability to maintain oxygen saturation. In contrast, mtDNA mutations T11613C, A8923G, and T5543C appear to confer protective effects.[8] Additional findings highlight the role of inflammation and immune dysregulation in AMS susceptibility, with elevated circulating cytokines, including tumor necrosis factor α and interleukins 1, 6, and 8, observed in affected individuals.[9]
Other studies have reported additional risk factors for severe acute HAI among healthy young adults ascending to the Tibetan Plateau at elevations above 5,000 meters for the first time. These risk factors include residency at low altitudes and decreased lymphocyte counts.[10]
Beyond mitochondrial variants, genome-wide association studies have implicated nuclear genetic adaptations, particularly in EPAS1, EGLN1, and NOS3, in altitude tolerance. Tibetan populations exhibit increased expression of these genes, which support efficient oxygen delivery and attenuated erythropoietic response to hypoxia.[11][12] In contrast, Andean highlanders rely more on elevated hemoglobin concentrations as an adaptive mechanism.[13]
In a study of Andean children and adolescents residing in La Rinconada, Peru—the highest city in the world at 5,100 m—posterior cerebral blood flow (CBF) was found to be lower despite more severe hypoxemia. However, global CBF was comparable to that of Sherpa highlanders in the Khumbu Valley, Nepal (3,800 m) and lowland residents of Cardiff, Wales (44 m). As in adults, this regional disparity in CBF likely reflects differences in hemoglobin concentration at high altitude.[14] Posterior circulation may also be more susceptible to hypoxia due to impaired autoregulation, a phenomenon increasingly recognized as a contributor to the development of HACE.[15]
Diagnosis
Common manifestations of low blood oxygen levels include shortness of breath, cyanosis, profound fatigue and weakness, confusion, and headache. HAI is chiefly diagnosed on clinical grounds, relying on typical symptoms and physical examination findings. Measurement of vital signs, including blood pressure, respiratory rate, heart rate, and oxygen saturation, is essential. Diagnostic imaging, including chest radiography, computed tomography, and magnetic resonance imaging, may be employed to identify complications such as HAPE and HACE (see Images. Mild High-Altitude Cerebral Edema on Multimodal Imaging; Severe High-Altitude Cerebral Edema on Multimodal Imaging)
Recent advances have expanded diagnostic capabilities in both clinical and remote field settings. Point-of-care ultrasound (POCUS) has proven useful in detecting interstitial pulmonary edema by visualizing B-lines, offering a rapid, portable tool for diagnosing HAPE when radiography is unavailable.[16] Ultrasound-based determination of optic nerve sheath diameter (ONSD) is being investigated as a noninvasive method to screen for elevated intracranial pressure, potentially aiding in the early detection of HACE.[17]
Smartwatch-based wearable oximeters have shown reliability in detecting oxygen desaturation at high altitudes and may predict AMS risk.[18] In addition, wireless cerebral oximetry bands capable of measuring regional brain oxygen saturation have shown promise in identifying early cerebral hypoxia in expedition settings.[19]
Differential Diagnosis
Although the clinical manifestations of COVID-19-associated lung injury and HAPE share similarities, including hypoxemia, radiographic opacities, and impaired pulmonary function, their underlying pathophysiological mechanisms are distinct.[20] Accurate differentiation among COVID-19 pneumonia, HAPE, and ARDS is essential, as each condition requires a different therapeutic approach. Hypoxic pulmonary vasoconstriction (HPV) is a compensatory response in which pulmonary arteries constrict in regions of low oxygen tension to optimize ventilation-perfusion matching. The carotid body serves as the primary oxygen-sensing organ, detecting hypoxemia and triggering an increase in respiratory rate.
In severe COVID-19 pneumonia, hypoxemia often appears disproportionately profound relative to the degree of dyspnea. This phenomenon is attributed to ARDS and a loss of effective HPV. Importantly, the behavior of HPV differs between HAPE and COVID-19, with significant therapeutic implications. In HAPE, pharmacologic inhibition of HPV using carbonic anhydrase inhibitors (CAIs), calcium channel blockers (CCBs), or phosphodiesterase-5 inhibitors improves oxygenation. In contrast, many patients with COVID-19 exhibit systemic hypertension, and the use of vasodilators, particularly CCBs, may impair residual HPV and worsen gas exchange. Preserving HPV in such patients is therefore a clinical priority.[21]
Prevention and Treatment
Blood oxygen saturation cannot exceed 100%, and achieving 100% saturation is not possible while breathing ambient air at sea level or higher elevations. Maximum saturation levels can only be attained through supplemental oxygen delivered via medical devices such as oxygen masks, Gamow bags, and oxygen-enriched tents. Specially constructed homes with oxygen-controlled rooms are used to simulate lower-altitude environments in some mountainous regions, including parts of Colorado. Portable hyperbaric chambers are also employed at high altitudes, particularly in emergency situations.[22]
High-altitude oxygenation can assist individuals living in or traveling to high elevations in acclimatizing, thereby preventing or reducing the severity of HAI and lowering the risk of progression to life-threatening complications. Altitude simulation generators are available for acclimatization and can be used in controlled indoor environments, such as bedrooms. These devices produce variable oxygen concentrations corresponding to the targeted elevation, ranging from 20.9% at sea level to approximately 9.5% at 20,000 feet (6,000 meters) above sea level.
Prolonged oxygen inhalation can produce adverse effects, including retinopathy and vision loss. Reports have documented changes in corneal thickness among patients with HAPE following systemic oxygen therapy.[23]
Recent epidemiological studies suggest that short-term intake of cocoa flavanols may enhance cerebral oxygenation. Specifically, supplementation for a week has been associated with increased oxygenation in the prefrontal cortex at rest and during moderate-intensity exercise under both normoxic and hypoxic conditions.[24]
Pharmacologic agents are also employed for the prevention and treatment of HAIs, with some improving oxygenation directly. Acetazolamide is the most widely used medication for preventing HAI and remains the 1st-line option in most protocols.[25] Benzolamide, another CAI, has been shown to improve oxygenation and reduce symptoms of AMS, with fewer reported side effects compared to acetazolamide.[26] Additional studies support the use of dexamethasone in improving pulmonary hemodynamics among patients with chronic obstructive pulmonary disease (COPD) who ascend to high altitudes.[27]
Nursing, Allied Health, and Interprofessional Team Interventions
Several interventions are available for the treatment and prevention of HAI. These therapies are broadly categorized into pharmacological and nonpharmacological approaches.
Pharmacological Treatment of High-Altitude Illness
Pharmacological interventions include the use of CAIs such as acetazolamide, which is primarily used for prophylaxis. A typical regimen consists of 125 mg twice daily, initiated at least 2 days before ascent. Dexamethasone is indicated for the treatment of severe AMS and HACE. A common dosing protocol involves a loading dose of 8 mg, administered orally, intramuscularly, or intravenously when available, followed by 4 mg every 6 hours. This regimen should be accompanied by descent of at least 500 to 1,000 meters and supplemental oxygen when accessible.
Mild AMS may be managed with symptomatic treatment. Analgesics such as acetaminophen (1,000 mg) or ibuprofen (400 mg), along with antiemetics like metoclopramide (10 mg), may be used for relief. Supportive measures include avoiding intense physical activity, halting further ascent, and preventing dehydration through adequate fluid intake.
Other pharmacologic agents, including CCBs such as nifedipine, have been employed in the management of pulmonary hypertension and HAPE. Dosing regimens include 20 mg or 30 mg every 8 or 12 hours. However, evidence for the efficacy of this agent is currently limited. In cases where ARDS is the underlying cause of HAPE, targeted management of ARDS is critical to prevent further complications.[28]
Systematic reviews evaluating the effects of phosphodiesterase-5 inhibitors, including sildenafil (40, 50, or 100 mg) and tadalafil (10 mg), have shown no significant improvement in clinical outcomes among individuals affected by HAPE.[29] These agents are also associated with side effects and adverse reactions, prompting ongoing investigation into the efficacy and safety of medicinal plants for the prevention and treatment of HAI.
Animal studies have demonstrated that combining conventional pharmacologic agents such as acetazolamide with medicinal plants, including Rhodiola rosea L., may enhance the prevention and treatment of HAI. This combined approach has shown particular benefit among populations residing in the Qinghai–Tibet Plateau.[30]
Both human and animal studies have reported promising findings with Chinese herbal medicines such as Zanthoxylum armatum (Rutaceae), which is rich in flavonoids and polyphenols with antioxidant properties that may reduce oxidative stress at the cellular level. These phytochemicals have been investigated for their role in preventing and treating HAI, particularly high-altitude pulmonary hypertension. However, further in vitro and in vivo studies are necessary to fully establish the efficacy, safety, and adverse effect profiles of these natural compounds.[31]
Nonpharmacological Therapy of High-Altitude Illness
Nonpharmacological interventions primarily aim to relieve symptoms of HAI through measures such as supplemental oxygen or descent to lower elevations. However, these strategies present logistical challenges due to the weight and bulk of oxygen delivery equipment (eg, oxygen bottles, cylinders, or tanks), limited access to healthcare facilities, and the difficulty of transporting both patients and equipment in remote, high-altitude, or mountainous terrain.
Some studies have demonstrated that auto–positive end-expiratory pressure (auto-PEEP)—a technique similar to pursed-lip breathing—is not inferior to bottled oxygen therapy for the initial management of HAPE. A key advantage of this approach is its continuous availability without the need for external equipment.[32]
Other investigations have compared nonpharmacological strategies such as simulated short-term acclimatization and long-term physiological adaptation across different population groups residing on the Tibetan Plateau. These groups include native highlanders, native lowlanders, and recently acclimatized newcomers, highlighting variability in altitude tolerance and response to hypobaric hypoxia.[33]
Nursing, Allied Health, and Interprofessional Team Monitoring
Interprofessional collaboration is essential to ensure early detection, appropriate monitoring, and effective treatment of HAI. Technology-assisted approaches, such as telemedicine platforms, offer viable solutions for delivering emergency care in remote and extreme environments. An example is the e-Rés@MONT teleconsultation platform, which has been designed, developed, and implemented in 5 mountain resort huts and 3 remote outpatient clinics in the Valle d'Aosta region of the Mont Blanc massif in Italy.[34]
As previously mentioned, pulse oximeters, wearable oxygen sensors such as smartwatches, and wireless cerebral oximeters have all been developed for use in high-altitude settings. Beyond their diagnostic utility, these tools may be employed for continuous physiologic monitoring, particularly during sleep or prolonged exposure, providing real-time data on oxygen saturation and cerebral oxygenation. When integrated into remote care platforms, these technologies enhance the interprofessional team’s capacity to detect early deterioration and coordinate timely intervention.[35]
Air travel to high-altitude destinations presents significant challenges and increases the risk of HAI. A preflight medical evaluation is recommended, particularly for individuals with acute or chronic medical conditions. Patients and travelers should consult their healthcare provider before travel, while pilots and crew must obtain medical certification issued by an Aviation Medical Examiner (AME) prior to flight clearance. Preacclimatization strategies, including hypoxia stimulation testing and structured exercise training, should be incorporated along with other prophylactic measures outlined in this activity to mitigate the health risks associated with altitude exposure and support a safer, more comfortable travel experience.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
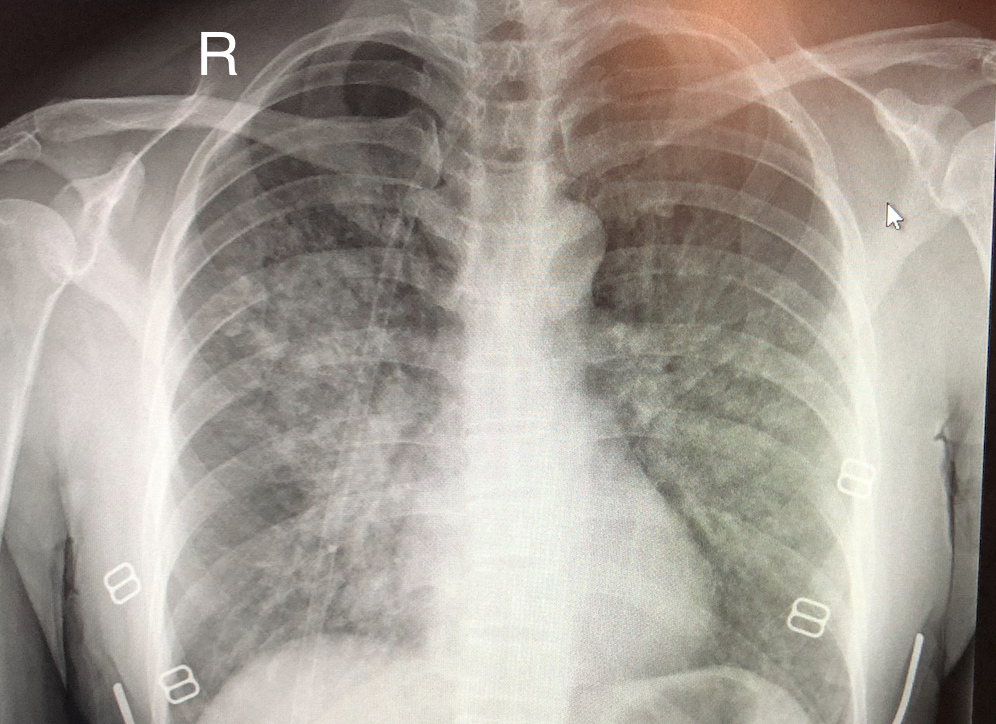
High-Altitude Pulmonary Edema on Chest Radiography. Chest x-ray demonstrates ill-defined alveolar infiltrates with a predilection for the right middle lung zone.
Maryrosegrant, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
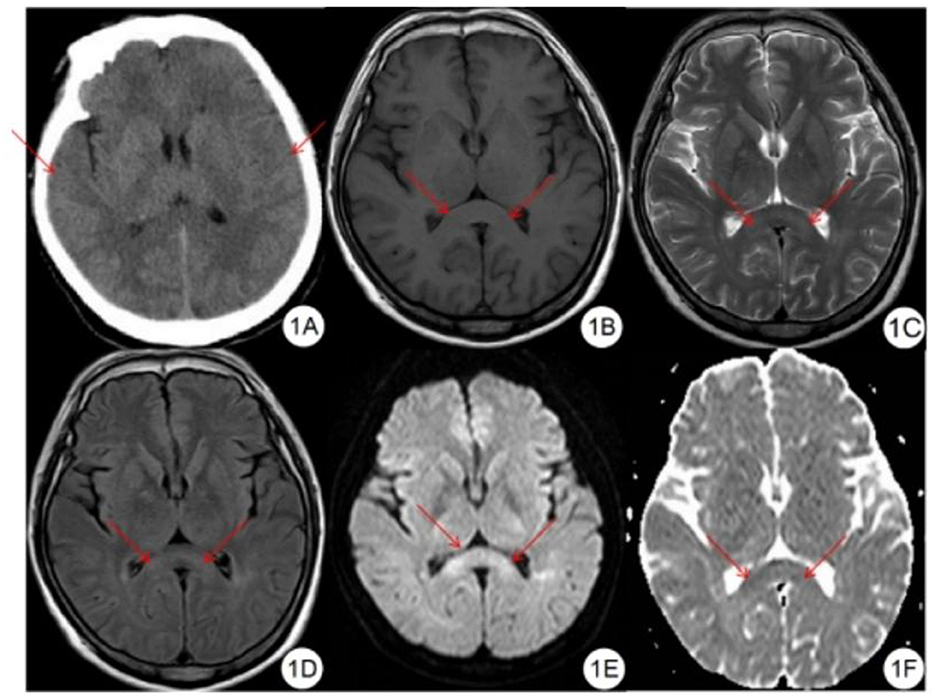
Mild High-Altitude Cerebral Edema on Multimodal Imaging. (A) Noncontrast-enhanced computed tomography shows mildly effaced sulci and cerebral fissures. (B) T1-weighted imaging demonstrates symmetrical slight hypointensity in the splenium of the corpus callosum. (C) T2-weighted imaging reveals symmetrical slight hyperintensity in the same region. (D) Fluid-attenuated inversion recovery sequence shows similar symmetrical slight hyperintensity in the splenium. (E) Diffusion-weighted imaging demonstrates symmetrical hyperintensity. (F) Apparent diffusion coefficient mapping shows corresponding symmetrical hypointensity in the splenium of the corpus callosum.
Long C, Bao H. Study of high-altitude cerebral edema using multimodal imaging. Front Neurol. 2023;13:1041280.
doi: 10.3389/fneur.2022.1041280.
(Click Image to Enlarge)
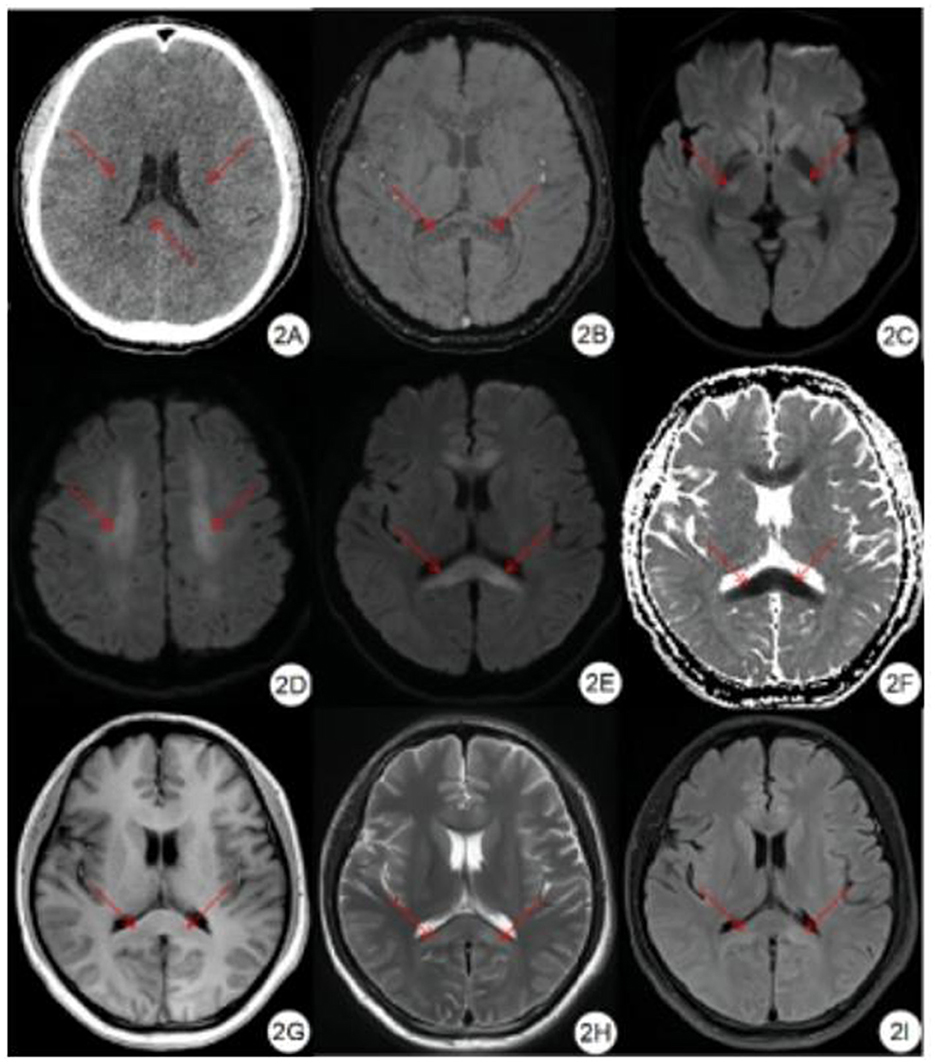
Severe High-Altitude Cerebral Edema on Multimodal Imaging. (A) Noncontrast-enhanced computed tomography reveals reduced attenuation in the bilateral corona radiata and corpus callosum, with mild sulcal and fissural effacement. (B) Susceptibility-weighted imaging shows symmetrical punctate hypointense signals in the brain parenchyma, notably in the rostral (genu) and caudal (splenium) corpus callosum. (C-E) Diffusion-weighted imaging shows bilateral hyperintensity in the corticospinal tracts, centrum semiovale, and corpus callosum. (F) Apparent diffusion coefficient maps show corresponding symmetrical low signal, suggesting restricted diffusion. (G-I) T1-weighted, T2-weighted, and fluid-attenuated inversion recovery sequences demonstrate symmetrical signal changes in the corpus callosum.
Long C, Bao H. Study of high-altitude cerebral edema using multimodal imaging. Front Neurol. 2023;13:1041280.
doi: 10.3389/fneur.2022.1041280.
References
West JB. Improving oxygenation at high altitude: acclimatization and O2 enrichment. High altitude medicine & biology. 2003 Fall:4(3):389-98 [PubMed PMID: 14561244]
Szymczak RK, Marosz M, Grzywacz T, Sawicka M, Naczyk M. Death Zone Weather Extremes Mountaineers Have Experienced in Successful Ascents. Frontiers in physiology. 2021:12():696335. doi: 10.3389/fphys.2021.696335. Epub 2021 Jul 5 [PubMed PMID: 34290622]
Matthews T, Perry LB, Lane TP, Elmore AC, Khadka A, Aryal D, Shrestha D, Tuladhar S, Baidya SK, Gajurel A, Potocki M, Mayewski PA. Into Thick(er) Air? Oxygen Availability at Humans' Physiological Frontier on Mount Everest. iScience. 2020 Dec 18:23(12):101718. doi: 10.1016/j.isci.2020.101718. Epub 2020 Nov 20 [PubMed PMID: 33376965]
Burtscher J, Swenson ER, Hackett PH, Millet GP, Burtscher M. Flying to high-altitude destinations: Is the risk of acute mountain sickness greater? Journal of travel medicine. 2023 Jun 23:30(4):. doi: 10.1093/jtm/taad011. Epub [PubMed PMID: 36694981]
Zafren K, Pun M, Regmi N, Bashyal G, Acharya B, Gautam S, Jamarkattel S, Lamichhane SR, Acharya S, Basnyat B. High altitude illness in pilgrims after rapid ascent to 4380 M. Travel medicine and infectious disease. 2017 Mar-Apr:16():31-34. doi: 10.1016/j.tmaid.2017.03.002. Epub 2017 Mar 9 [PubMed PMID: 28285976]
Burtscher J, Gatterer H, Niederseer D, Vonbank K, Burtscher M. Flying to high-altitude destinations. Minerva medica. 2025 Feb:116(1):43-61. doi: 10.23736/S0026-4806.24.09286-3. Epub 2024 Aug 5 [PubMed PMID: 39101381]
West JB. High-altitude medicine. American journal of respiratory and critical care medicine. 2012 Dec 15:186(12):1229-37. doi: 10.1164/rccm.201207-1323CI. Epub 2012 Oct 26 [PubMed PMID: 23103737]
Li Z, Liu C, Guo J, Shi Y, Li Y, Wang J, Zhou S, Chen Y. Mitochondrial DNA Variation Correlated With the High Altitude Intolerance in Chinese Young Han Males. Frontiers in cardiovascular medicine. 2022:9():832136. doi: 10.3389/fcvm.2022.832136. Epub 2022 Feb 25 [PubMed PMID: 35282372]
Steele AR, Howe CA, Gibbons TD, Foster K, Williams AM, Caldwell HG, Brewster LM, Duffy J, Monteleone JA, Subedi P, Anholm JD, Stembridge M, Ainslie PN, Tremblay JC. Hemorheological, cardiorespiratory, and cerebrovascular effects of pentoxifylline following acclimatization to 3,800 m. American journal of physiology. Heart and circulatory physiology. 2024 Mar 1:326(3):H705-H714. doi: 10.1152/ajpheart.00783.2023. Epub 2024 Jan 19 [PubMed PMID: 38241007]
Gao C, Qi GD, Wang D, Zhang ZH, Liu ZX, Ge RD, Yong Z, Yan LE. Incidence and risk factors of severe acute high-altitude illness in healthy adults first entering the northern Tibetan Plateau of over 5,000 m. Frontiers in public health. 2024:12():1400236. doi: 10.3389/fpubh.2024.1400236. Epub 2024 Sep 9 [PubMed PMID: 39319295]
Level 2 (mid-level) evidenceZheng W, He Y, Guo Y, Yue T, Zhang H, Li J, Zhou B, Zeng X, Li L, Wang B, Cao J, Chen L, Li C, Li H, Cui C, Bai C, Baimakangzhuo, Qi X, Ouzhuluobu, Su B. Large-scale genome sequencing redefines the genetic footprints of high-altitude adaptation in Tibetans. Genome biology. 2023 Apr 13:24(1):73. doi: 10.1186/s13059-023-02912-1. Epub 2023 Apr 13 [PubMed PMID: 37055782]
Level 2 (mid-level) evidenceWu D, Liu Y, Chen W, Shao J, Zhuoma P, Zhao D, Yu Y, Liu T, Yu R, Gan Y, Yuzheng B, Huang Y, Zhang H, Bi X, Tao C, Lai S, Luo Q, Zhang D, Wang H, Zhaxi P, Zhang J, Qiao J, Zeng C. How Placenta Promotes the Successful Reproduction in High-Altitude Populations: A Transcriptome Comparison between Adaptation and Acclimatization. Molecular biology and evolution. 2022 Jun 2:39(6):. doi: 10.1093/molbev/msac120. Epub [PubMed PMID: 35642306]
Level 2 (mid-level) evidenceAmaru R, Song J, Reading NS, Gordeuk VR, Prchal JT. "What We Know and What We Do Not Know about Evolutionary Genetic Adaptation to High Altitude Hypoxia in Andean Aymaras". Genes. 2023 Mar 3:14(3):. doi: 10.3390/genes14030640. Epub 2023 Mar 3 [PubMed PMID: 36980912]
Howe CA, Verges S, Nowak-Flück D, Talbot JS, Champigneulle B, Stauffer E, Brugniaux JV, Doutreleau S, Hancco I, Niroula S, Pichon A, McManus AM, Stembridge M, Ainslie PN. Cerebral blood flow in Andean children and adolescents living above 5,000 m. Journal of neurophysiology. 2025 Apr 1:133(4):1138-1145. doi: 10.1152/jn.00513.2024. Epub 2025 Mar 6 [PubMed PMID: 40049741]
Feddersen B, Neupane P, Thanbichler F, Hadolt I, Sattelmeyer V, Pfefferkorn T, Waanders R, Noachtar S, Ausserer H. Regional differences in the cerebral blood flow velocity response to hypobaric hypoxia at high altitudes. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 Nov:35(11):1846-51. doi: 10.1038/jcbfm.2015.142. Epub 2015 Jun 17 [PubMed PMID: 26082017]
Lahham S, Moeller J, Choi H, Fischetti C, Myatt T, Bove N, Saadat S, Mazumder P, Algaze Gonzalez IM, Kurzweil A, Fox JC. Application of Point-of-care Ultrasound for Screening Climbers at High Altitude for Pulmonary B-lines. The western journal of emergency medicine. 2023 Feb 9:24(2):359-362. doi: 10.5811/westjem.2022.11.54300. Epub 2023 Feb 9 [PubMed PMID: 36976605]
Wang TW, Huang MK, Hsu CC, Jo SY, Lin YK, How CK, Tseng SF, Chung K, Chien DK, Chang WH, Chiu YH. High myopia at high altitudes. Frontiers in physiology. 2024:15():1350051. doi: 10.3389/fphys.2024.1350051. Epub 2024 Mar 8 [PubMed PMID: 38523807]
Zeng Z, Li L, Hu L, Wang K, Li L. Smartwatch measurement of blood oxygen saturation for predicting acute mountain sickness: Diagnostic accuracy and reliability. Digital health. 2024 Jan-Dec:10():20552076241284910. doi: 10.1177/20552076241284910. Epub 2024 Sep 27 [PubMed PMID: 39351311]
Si J, He Y, Yao J, Yu J, Jing R, He Q, Zhang X, Xiao L. High-altitude cerebral oxygen saturation detection using wireless wearable cerebral oximeter. Frontiers in neurology. 2024:15():1445563. doi: 10.3389/fneur.2024.1445563. Epub 2024 Sep 30 [PubMed PMID: 39403268]
Luks AM, Swenson ER. COVID-19 Lung Injury and High-Altitude Pulmonary Edema. A False Equation with Dangerous Implications. Annals of the American Thoracic Society. 2020 Aug:17(8):918-921. doi: 10.1513/AnnalsATS.202004-327CME. Epub [PubMed PMID: 32735170]
Archer SL, Sharp WW, Weir EK. Differentiating COVID-19 Pneumonia From Acute Respiratory Distress Syndrome and High Altitude Pulmonary Edema: Therapeutic Implications. Circulation. 2020 Jul 14:142(2):101-104. doi: 10.1161/CIRCULATIONAHA.120.047915. Epub 2020 May 5 [PubMed PMID: 32369390]
Flaherty GT. Under pressure: facilitating the emergency use of portable hyperbaric chambers at altitude. Travel medicine and infectious disease. 2014 Sep-Oct:12(5):420-1. doi: 10.1016/j.tmaid.2014.09.001. Epub 2014 Sep 8 [PubMed PMID: 25246227]
Patyal S, Yadav AK, Kotwal A. Changes in corneal thickness in patients with high-altitude pulmonary edema after systemic oxygen therapy. Indian journal of ophthalmology. 2018 Nov:66(11):1554-1557. doi: 10.4103/ijo.IJO_642_18. Epub [PubMed PMID: 30355859]
Decroix L, Tonoli C, Lespagnol E, Balestra C, Descat A, Drittij-Reijnders MJ, Blackwell JR, Stahl W, Jones AM, Weseler AR, Bast A, Meeusen R, Heyman E. One-week cocoa flavanol intake increases prefrontal cortex oxygenation at rest and during moderate-intensity exercise in normoxia and hypoxia. Journal of applied physiology (Bethesda, Md. : 1985). 2018 Jul 1:125(1):8-18. doi: 10.1152/japplphysiol.00055.2018. Epub 2018 Mar 15 [PubMed PMID: 29543135]
Shlim DR. The use of acetazolamide for the prevention of high-altitude illness. Journal of travel medicine. 2020 Sep 26:27(6):. pii: taz106. doi: 10.1093/jtm/taz106. Epub [PubMed PMID: 31897486]
Collier DJ, Wolff CB, Hedges AM, Nathan J, Flower RJ, Milledge JS, Swenson ER. Benzolamide improves oxygenation and reduces acute mountain sickness during a high-altitude trek and has fewer side effects than acetazolamide at sea level. Pharmacology research & perspectives. 2016 Jun:4(3):e00203. doi: 10.1002/prp2.203. Epub 2016 May 19 [PubMed PMID: 27433337]
Lichtblau M, Furian M, Aeschbacher SS, Bisang M, Ulrich S, Saxer S, Sheraliev U, Marazhapov NH, Osmonov B, Estebesova B, Sooronbaev T, Bloch KE, Ulrich S. Dexamethasone improves pulmonary hemodynamics in COPD-patients going to altitude: A randomized trial. International journal of cardiology. 2019 May 15:283():159-164. doi: 10.1016/j.ijcard.2018.12.052. Epub 2018 Dec 28 [PubMed PMID: 30638985]
Level 1 (high-level) evidenceGuo L, Sun J, He Z, Shi Q, Ma S. Understanding Acute Respiratory Distress Syndrome in High-Altitude Environments: A Comprehensive Review of Diagnosis and Treatment. Medical science monitor : international medical journal of experimental and clinical research. 2023 Jul 20:29():e939935. doi: 10.12659/MSM.939935. Epub 2023 Jul 20 [PubMed PMID: 37469139]
Level 3 (low-level) evidenceBliss A, Mahajan S, Boehm KM. Systematic Review of the Effects of Phosphodiesterase-5 Inhibitors and Dexamethasone on High Altitude Pulmonary Edema (HAPE). Spartan medical research journal. 2019 Mar 4:3(3):7111 [PubMed PMID: 33655150]
Level 1 (high-level) evidenceCao C, Zhang H, Huang Y, Mao Y, Ma L, Zhang S, Zhang W. The combined use of acetazolamide and Rhodiola in the prevention and treatment of altitude sickness. Annals of translational medicine. 2022 May:10(10):541. doi: 10.21037/atm-22-2111. Epub [PubMed PMID: 35722398]
Batool Z, Amjad Kamal M, Shen B. Advanced treatment strategies for high-altitude pulmonary hypertension employing natural medicines: A review. Journal of pharmaceutical analysis. 2025 Mar:15(3):101129. doi: 10.1016/j.jpha.2024.101129. Epub 2024 Oct 25 [PubMed PMID: 40161446]
Tannheimer M, Lechner R. Initial Treatment of High-Altitude Pulmonary Edema: Comparison of Oxygen and Auto-PEEP. International journal of environmental research and public health. 2022 Dec 3:19(23):. doi: 10.3390/ijerph192316185. Epub 2022 Dec 3 [PubMed PMID: 36498257]
Cheng F, Shen RJ, Zheng Z, Chen ZJ, Huang PJ, Feng ZK, Li X, Lin N, Zheng M, Liang Y, Qu J, Lu F, Jin ZB, Yang J. Distinct methylomic signatures of high-altitude acclimatization and adaptation in the Tibetan Plateau. Cell discovery. 2025 May 6:11(1):45. doi: 10.1038/s41421-025-00795-z. Epub 2025 May 6 [PubMed PMID: 40328746]
Martinelli M, Moroni D, Bastiani L, Mrakic-Sposta S, Giardini G, Pratali L. High-altitude mountain telemedicine. Journal of telemedicine and telecare. 2022 Feb:28(2):135-145. doi: 10.1177/1357633X20921020. Epub 2020 Jun 15 [PubMed PMID: 32539486]
Prince TS, Thurman J, Huebner K. Acute Mountain Sickness. StatPearls. 2025 Jan:(): [PubMed PMID: 28613467]