Anion Gap and Non-Anion Gap Metabolic Acidosis
Anion Gap and Non-Anion Gap Metabolic Acidosis
Introduction
Acidosis can be broadly classified into metabolic and respiratory etiologies, distinguished by their primary derangements. Taking a systematic approach to calculate each component of the acid-base physiology is crucial to arriving at the final diagnosis, especially in cases of mixed acid-base disorders.
Metabolic acidosis is characterized by a reduction in serum bicarbonate (HCO3) and a compensatory decrease in arterial pCO2, whereas respiratory acidosis results from elevated arterial pCO2 due to hypoventilation, with a secondary renal retention of bicarbonate to compensate.[1][2] Arterial blood gas (ABG) analysis, in conjunction with serum electrolytes, helps differentiate these disorders. A low pH with low bicarbonate (HCO3) suggests metabolic acidosis, while a low pH with elevated pCO2 indicates respiratory acidosis.[3] Acidemia is defined as a blood pH below 7.35.[4] Severe acidemia (pH <7.2) is associated with profound clinical consequences, including systemic vasodilation, reduced myocardial contractility and mean arterial pressure (MAP), diminished responsiveness to catecholamines and vasopressors, cardiac arrhythmias, hyperkalemia, insulin resistance, and altered mental status.[5] Given the invasiveness of ABGs, venous blood gases (VBGs) are sometimes used instead; the pH in VBGs is 0.02 to 0.05 lower than ABGs, and the PCO2 of VBG is about 4 to 6 mm Hg higher than that of ABG, but in severe hypoperfusion, VBG values can significantly vary.[6]
Metabolic acidosis can be further classified based on the anion gap, which is calculated as the anion gap = (Na + K) – (Cl + HCO3). The fundamental concept behind this is that to maintain homeostasis, the number of cations must equal the number of anions, so the anion gap measures the amount of unmeasured anions (which in normal physiological states is albumin). If the unmeasured anion/cation is paired with one of the components measured in the anion gap, the anion gap value will shift accordingly. Potassium is often left out of the equation, given its minimal contribution, simplifying the equation to anion gap = (Na) – (Cl + HCO3).[7] In high anion gap metabolic acidosis (HAGMA), abnormal or excess acids, eg, lactate, ketones, or toxic metabolites, accumulate, increasing unmeasured anions and thus widening the anion gap. A normal anion gap is 8 to 12 (if potassium is included, normal values are 12 to 16). Of note, venous HCO3 values are usually used to measure anion gap rather than values from the ABG, as venous values also account for carbonic acid and dissolved CO2. The venous values are typically 2 to 4 mEq/L higher than the arterial values.[8]
Conversely, in normal anion gap metabolic acidosis (NAGMA), also known as hyperchloremic acidosis, the increase in chloride balances the decrease in bicarbonate, maintaining electroneutrality with no net change in the anion gap.[9] Some etiologies (eg, renal insufficiency) can cause both gap and non–anion gap metabolic acidosis, depending on the clinical situation.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
High Anion Gap Metabolic Acidosis
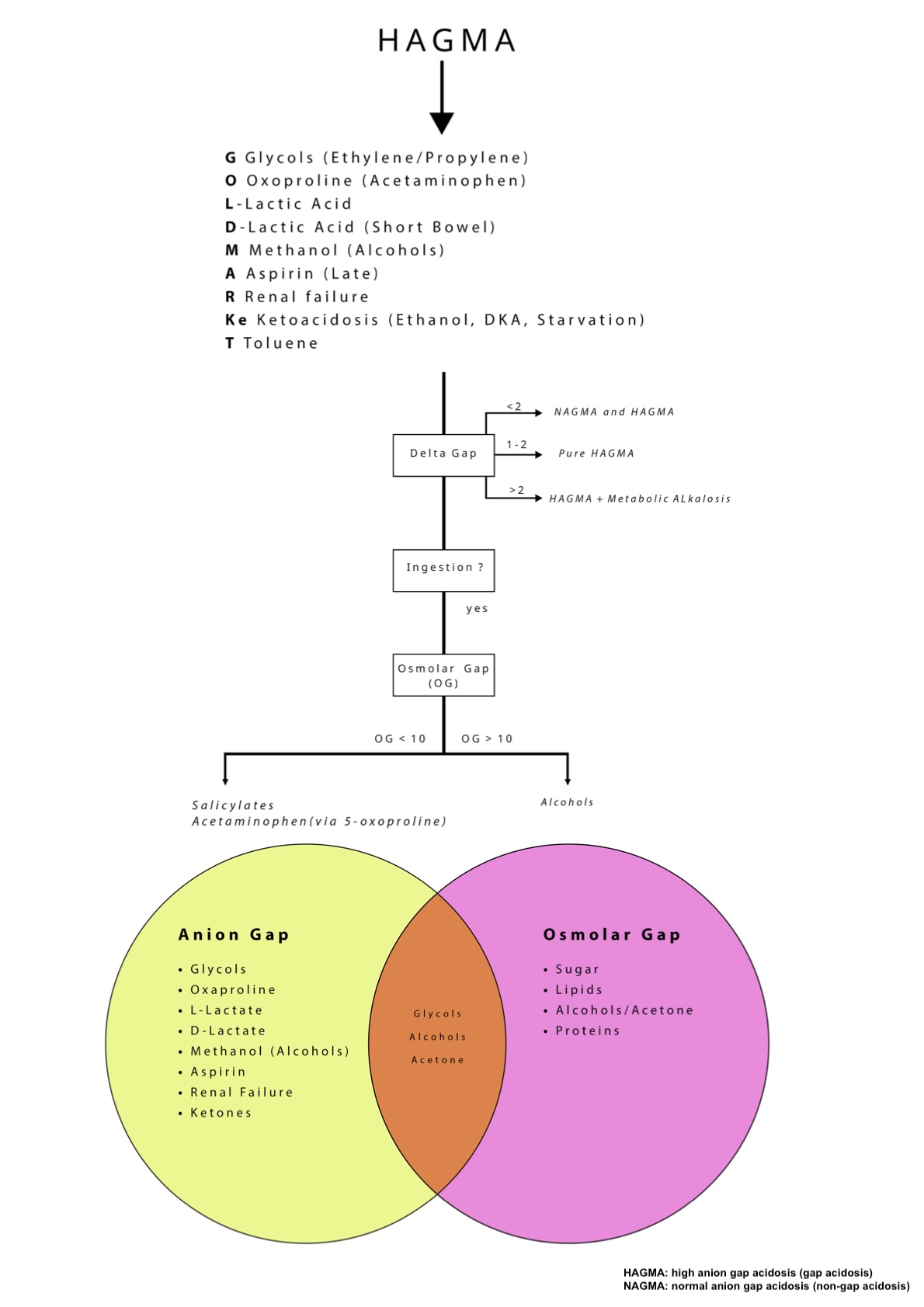
HAGMA can be a life-threatening acid-base disorder resulting from the accumulation of unmeasured anions in the plasma. These unmeasured anions arise from various exogenous toxins or endogenous metabolic products. Recognizing and addressing the underlying cause is critical for management. The following mnemonic, “GOLD MARKeT,” is widely used to categorize the major causes of HAGMA, each associated with distinct pathophysiological mechanisms:
- G – Glycols (ethylene glycol, propylene glycol): Ethylene glycol and propylene glycol are metabolized into organic acids, including glycolic acid, oxalic acid, and lactic acid. These acids increase the unmeasured anion load, resulting in HAGMA. Ethylene glycol toxicity additionally causes renal tubular injury through oxalate crystal deposition, exacerbating acid-base disturbances and renal dysfunction.[1]
- O – Oxoproline (5-oxoproline or pyroglutamic acid): Chronic acetaminophen use or certain antibiotic regimens can disrupt the gamma-glutamyl cycle, leading to the accumulation of 5-oxoproline. This organic acid accumulation is particularly pronounced in patients with malnutrition or chronic illness, contributing to metabolic acidosis with an elevated anion gap.[10] Possible exposures are through IV lorazepam and e-cigarett/e-vapor products.[11][12]
- L – Lactic Acid (L-lactic acidosis): Common in shock, sepsis, or mitochondrial dysfunction, L-lactic acidosis results from increased anaerobic metabolism. Lactic acid is a strong organic acid that contributes significantly to the anion gap.[13] Lactic acidosis can result from decreased oxygen delivery to organs (eg, sepsis, shock, emboli) or impaired oxygen utilization or lactate accumulation (eg, liver failure, kidney insufficiency, genetic abnormalities, metformin, linezolid, cocaine).[13] Notably, at physiologic pH, lactic acid is fully dissociated and exists in the deprotonated form of lactate. So when "lactic acid" levels are measured, the lactate anion is actually being measured. Please see StatPearls' companion reference, "Lactic Acidosis," for further information.
- D – D-lactic acid: D-lactic acidosis arises from abnormal bacterial fermentation of carbohydrates in the gastrointestinal tract, notably in patients with short bowel syndrome. D-lactate, an isomer not efficiently metabolized by humans, accumulates and leads to HAGMA.[14]
- M – Methanol: Methanol poisoning results in the hepatic metabolism of methanol to formic acid, a highly toxic metabolite. The accumulation of formic acid causes increased unmeasured anions, contributing to metabolic acidosis and clinical manifestations such as visual disturbances and central nervous system depression.[15]
- A – Aspirin (salicylates): Salicylates uncouple oxidative phosphorylation, leading to lactic acid and ketoacid accumulation, causing HAGMA. Early toxicity often presents with concurrent respiratory alkalosis due to respiratory center stimulation.[16]
- R – Renal failure and rhabdomyolysis: Impaired renal function diminishes the excretion of sulfate, phosphate, and other organic acids, causing retention of unmeasured anions and metabolic acidosis. Rhabdomyolysis releases intracellular acids and phosphate, often leading to acute kidney injury.[17]
- Ke – Ketones (diabetic, alcoholic, starvation ketoacidosis): In states of insulin deficiency or prolonged fasting, ketone bodies (acetoacetate and β-hydroxybutyrate) accumulate. These acidic metabolites increase the anion gap and contribute to metabolic acidosis in diabetic ketoacidosis, alcoholic ketoacidosis, and starvation.[18]
- T - Toluene: Toluene can cause gap or non–anion gap metabolic acidosis. Most commonly, toluene causes non–anion gap metabolic acidosis, but it can also cause anion gap metabolic acidosis less commonly. Toluene typically causes a distal renal tubular acidosis picture; however, related to hippurate accumulation, toluene can also produce an anion gap metabolic acidosis. Toluene toxicity can occur from occupational exposure to paint, paint thinner, and petrol, as well as from abuse of glue sniffing.[19][20]
Metformin presents a well-recognized cause of lactic acidosis. Used for over 60 years, metformin is one of the most commonly prescribed drugs for diabetes and prediabetes. Due to primarily being cleared by the kidneys, metformin is contraindicated when eGFR is lower than 30 mL/min/1.73 m2. Metformin blocks aerobic glycolysis in the mitochondria, thereby increasing anaerobic glycolysis and lactic acid accumulation.[21] The risk of this may be increased with concurrent use of sodium-glucose cotransporter 2 (SGLT2) inhibitors.[22] Please see StatPearls' companion reference, "Metformin-Associated Lactic Acidosis."
Another mnemonic for anion gap metabolic acidosis differential is "CAT MUDPILES" which represents the following etiologies:
- C: Cyanide and carbon monoxide poisoning
- A: Arsenic
- T: Toluene
- M: Methanol, Metformin
- U: Uremia
- D: Diabetic ketoacidosis
- P: Paraldehyde
- I: Iron, INH
- L: Lactate
- E: Ethylene glycol
- S: Salicylates
Non–Anion Gap Metabolic Acidosis
Non–anion gap metabolic acidosis is primarily caused by the loss of bicarbonate or the gain of chloride, and the main causes of this condition are diarrhea and renal tubular acidosis. Additional and less common etiologies include Addison disease, ureterosigmoid or pancreatic fistulas, carbonic anhydrase use, and chronic laxative use.[23] Other exogenous causes include the addition of hydrochloric acid (or substances with HCl as a metabolite), which causes a compensatory loss of bicarbonate, TPN administration, or administration of normal saline as described below.[13] Rarely are genetic syndromes associated with renal tubular acidosis.[23]
Renal tubular acidosis primarily causes NAGMA by decreased ammonium (NH4) production, which is needed for bicarbonate regeneration. In the absence of sufficient NH4, anions produced from metabolism and dietary intake are excreted with Na or K, causing avid retention of Na and Cl by the kidney. The Cl replaces the lost HCO3, resulting in hyperchloremic metabolic acidosis. A similar mechanim occurs with the presence of toluene or ketoacidosis (metabolized to hippurate and ketoanions) where these anions are excreted with Na or K.[24]
Gastrointestinal causes of NAGMA include loss of pancreatic, duodenal, biliary, or intestinal fluids, which are high in HCO3. Of the gastrointestinal causes of NAGMA, diarrhea is by far the most common, causing a lack of HCO3 reabsorption and loss through the intestinal tract. Secretory diarrhea causes a higher incidence of NAGMA than inflammatory diarrhea, as it contains a higher concentration of HCO3. Diversion of the ureters to the ileum or sigmoid colon after bladder removal (eg, bladder cancer) can also cause NAGMA, as the intestine will reabsorb NH4 and Cl and secrete HCO3.[23][24]
A common cause of non–anion gap metabolic acidosis is the administration of large amounts of normal saline. Examples would be intraoperative administration, shock, or burn victims.[25][26][27] This is related in part to the chloride load (the concentration in normal saline being 154 mEq/L, while about 100 to 106 mEq/L in plasma), as well as a dilutional effect, since normal saline contains no bicarbonate. NAGMA is also common in children with diarrhea who receive large volumes of normal saline.[28] Normal saline is associated with hyperchloremic metabolic acidosis in both hospitalized and healthy individuals, as well as children and adults. In addition, hyperchloremia itself is associated with increased mortality.[29][30] Multiple studies have shown that infusion of high-chloride fluid reduces renal perfusion through vasoconstriction and also decreases GFR. Some evidence also suggests that chloride-rich fluids may impair coagulation. For these reasons, many experts now suggest using fluids with a more physiologic chloride concentration, eg, lactated Ringer's or plasmalyte.[26][31]
A rare cause of non–anion gap metabolic acidosis is cholestyramine. This medication is a bile acid sequestrant used for hypercholesterolemia. It exchanges chloride anions for bile acids in the ileum, resulting in the excretion of bile acids through the feces. This exchange results in the excretion of bicarbonate and the reabsorption of chloride through the duodenal apical brush border bicarbonate/chloride antiporter. Usually, the kidney can compensate for this by reabsorbing bicarbonate; however, in settings of renal insufficiency or aldosterone insensitivity (or antagonism), these mechanisms are not available, and metabolic acidosis will ensue.[32][33]
Total parenteral nutrition (TPN) can also cause non–anion gap metabolic acidosis. If TPN does not contain acetate as a buffer, no source of bicarbonate. In addition, some amino acids (particularly arginine and lysine) are metabolized to HCl.[23][24]
Addison disease, or medications that block the action of aldosterone, can cause hyperchloremic metabolic acidosis. Aldosterone acts on the epithelial Na channel, located on the apical side of the distal convoluted tubule and collecting duct of the nephron, and the basolateral Na/K ATPase pump. Through these mechanisms, sodium is reabsorbed from the tubular lumen, and potassium and hydrogen ions are excreted. The resulting pattern is similar to Type IV RTA. Acquired hypoaldosteronism may be more common than previously thought, especially in hospitalized patients aged older than 65 and patients on multiple medications.[34][35]
Epidemiology
HAGMA is a prevalent acid-base disturbance among critically ill patients. Studies show that HAGMA is commonly encountered in intensive care units and is often related to conditions, eg, lactic acidosis, ketoacidosis, renal failure, toxin ingestion, and many other causes. Although the majority of ICU patients present with metabolic alkalosis, those with metabolic acidosis had higher mortality. Lactic acidosis, sepsis, and multiorgan failure were contributing factors.[36]
The clinical significance of HAGMA is underscored by its association with increased mortality rates. Elevated anion gap levels correlate strongly with higher in-hospital, 30-day, and 90-day mortality in critically ill patients, highlighting their prognostic value.[37] The presence of unmeasured anions, eg, lactate and ketones, reflects underlying pathophysiological states (eg, sepsis and renal failure).[38] Chronic acetaminophen use or certain antibiotic regimens can disrupt the gamma-glutamyl cycle, leading to the accumulation of 5-oxoproline. This organic acid accumulation is particularly pronounced in patients with malnutrition or chronic illness, contributing to metabolic acidosis with an elevated anion gap.
In terms of NAGMA, a comprehensive review found that 19% to 41% of patients in ICU settings demonstrated non–anion gap metabolic acidosis. In addition, 20% to 55% of people with chronic renal insufficiency had a NAGMA.[24]
Pathophysiology
High Anion Gap Metabolic Acidosis
Anion gap
The anion gap compares sodium concentration (and sometimes potassium) with the sum of chloride and bicarbonate concentrations. The main negatively charged anion causing the anion gap is albumin. Calculating the anion gap helps distinguish between HAGMA and NAGMA, thereby aiding in determining the etiologies. The change in anion gap should then be compared to the change in bicarbonate to evaluate for further acid-base disorders. Whenever possible, the change in anion gap should be measured against the patient's baseline anion gap, which is typically obtained when the patient is relatively healthy.[24]
Compensatory respiratory alkalosis
Metabolic acidosis causes a compensatory hyperventilation response within 12 to 24 hours, which can be calculated through Winter's formula: PaCO2 = (1.5 x HCO3 +8 ) ±2. The minimum PaCO2 level that can be reduced to is 8 to 12 mEq/L. Conventionally, the bicarbonate level used is from a venous blood sample, which is generally 2 to 4 mEq/L higher than an arterial sample.[8] An anion gap >18 almost always indicates the presence of an organic acid. Rarely, the accumulation of phosphates and sulfates in kidney failure can create a large anion gap, but the gap is rarely >18.[13]
Hypoalbuminemia
The albumin level must also be taken into account because albumin is a negatively charged protein; therefore, albumin loss leads to the retention of negatively charged ions, eg, bicarbonate and chloride, which can underestimates the severity of the anion gap.[39] For each 1 g/100 mL below the normal value of 4.5 g/100 mL, the anion gap falls by about 2.5 mEq/L. The following formula can estimate this correction:
[anion gap](corrected) = [anion gap](measured) + 2.5 × (4.0 - [albumin]) [8][40]
This correction ensures more accurate identification of HAGMA, particularly in patients with hypoalbuminemia due to conditions, eg, sepsis, malnutrition, liver disease, or chronic illness.[38] For example, a patient with a measured anion gap of 10 and an albumin of 2.0 g/dL would have a corrected anion gap of 15, indicating a true HAGMA that might otherwise be overlooked.
Delta/delta or delta gap
After calculating the anion gap and determining that the patient has HAGMA, the next critical step is to calculate the delta gap (also called the delta-delta) to evaluate for mixed acid-base disorders. The delta gap compares the change in the anion gap to the change in serum bicarbonate, based on the principle that in pure HAGMA, the increase in the anion gap should be approximately equal to the decrease in bicarbonate. The delta gap ratio is calculated as: Δanion gap / ΔHCO3 = (corrected anion gap − 12) / (24 − measured HCO3), where 12 mEq/L is the upper limit of the normal anion gap and 24 mEq/L is the normal bicarbonate level. The following interpretations of this ratio helps identify concurrent acid-base disturbances:
- A ratio between 1 and 2 generally indicates a pure HAGMA.
- A ratio <1 suggests a concurrent normal anion gap (non–anion gap) metabolic acidosis.
- A ratio >2 indicates an additional metabolic alkalosis or preexisting elevated bicarbonate (see Image. Algorithm for Assessing Anion Gap and Non–Anion Gap Acidosis).
Calculating the delta gap ratio is crucial, particularly in critical care settings where multiple disorders may coexist.[7][9][41]
Osmolar gap
The osmolar gap is a valuable tool in evaluating HAGMA, particularly in cases of suspected toxic alcohol ingestion. Osmolar gaps can also be caused by iatrogenic factors, eg, radiocontrast administration, sorbitol (from TPN), maltose, dextrose, or mannitol. Osmolar gap is calculated by subtracting the calculated serum osmolality from the measured serum osmolality, using the following formula:
Calculated osmolality = 2 × (Na + K) + (glucose ÷ 18) + (BUN ÷ 2.8) + (ethanol ÷ 4.6)
A normal osmolar gap is typically <10 mOsm/kg, while an elevated gap (>10 mOsm/kg) suggests the presence of exogenous osmoles, including methanol, ethylene glycol, or isopropanol.[8] The coexistence of HAGMA with an elevated osmolar gap is highly suggestive of toxic alcohol ingestion. Each toxic alcohol has characteristic metabolites and the following clinical manifestations:
- Methanol is metabolized to formic acid and presents with altered mental status (AMS), blurry vision, dilated pupils, and papilledema.
- Ethylene glycol forms oxalic acid and can cause AMS, cranial nerve palsies, flank pain, hematuria, hypocalcemia with tetany, and calcium oxalate crystals (envelope shaped crystals) leading to acute kidney injury (AKI).
- Propylene glycol is converted to lactic acid and may cause AKI and liver injury.
- Diethylene glycol is metabolized to diglycolic acid and presents with AKI, nausea/vomiting, hepatitis, pancreatitis, neuropathy, AMS, and elevated lactate.
- Isopropyl alcohol is converted to acetone and typically causes AMS, fruity breath, pancreatitis, and elevated lactate with a normal or mildly elevated anion gap.
- Ethanol is metabolized to acetaldehyde and may result in ketoacidosis, lactic acidosis, and metabolic alkalosis due to vomiting.
Despite its utility, the osmolar gap has limitations—it may be normal early in intoxication, unreliable in rapidly evolving cases, and confounded by rare toxins like bromide.[42][43] Accurate lab interpretation and clinical context are essential, as other conditions like diabetic ketoacidosis (DKA) or sepsis can also affect the osmolar and anion gaps.
Nonanion Gap Metabolic Acidosis
In nonanion gap metabolic acidosis, gastrointestinal and renal losses of bicarbonate can be distinguished via urine anion gap analysis:
- Urine anion gap = Urine Na + Urine K – Urine Cl, if urine pH is <6.5
- Urine anion gap = Urine Na + Urine K - (Urine Cl + Urine HCO3), if urine pH is >6.5 [44]
The urine anion gap can help estimate the urinary ammonium. The urinary anion concentration decreases and can become negative if ammonium (a cation) is elevated as part of a normal adaptive renal response to an extrarenal etiology of metabolic acidosis, eg, diarrhea or another gastrointestinal source. When the urine anion gap is positive, this suggests decreased ammonium excretion, which, in the presence of metabolic acidosis, suggests renal impairment such as distal renal tubular acidosis or chronic kidney disease.[44] The urine pH is often not a reliable indicator of urine acidification, as the body will produce more ammonia to buffer the excess hydrogen in the setting of prolonged metabolic acidosis.[44]
Histopathology
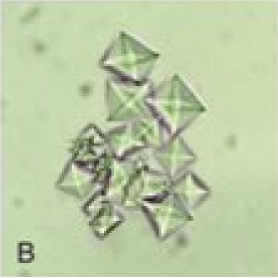
Microscopy has limited utility in the evaluation of metabolic acidosis; however, an important exception is the assessment for ethylene glycol ingestion. Ethylene glycol is metabolized into toxic compounds, including oxalate, which can bind calcium to form calcium oxalate crystals. At sufficiently high toxic doses, calcium oxalate monohydrate crystals can deposit in tissues such as the kidneys and brain, and precipitate in the urine.[42]
On urine microscopy, these calcium oxalate monohydrate crystals appear as dumbbells, spindles, ovals, or “picket fence” shapes.[45] These can be distinguished from calcium oxalate dihydrate crystals, which are classically octahedral and described as “envelope-shaped”.[3] Identifying these crystals in the urine can support the diagnosis of ethylene glycol poisoning, particularly when combined with clinical and laboratory findings, eg, elevated anion and osmolar gaps (see Image. Calcium Oxalate Dihydrate Crystals).[46]
History and Physical
Clinical History
A detailed patient history plays a crucial role in diagnosing metabolic acidosis in both hospitalized and nonhospitalized individuals. Clinicians must inquire thoroughly about medication use, including over-the-counter and ingested substances. Identifying a history of diabetes, chronic alcohol use, attempted self-harm, and gastrointestinal disturbances such as diarrhea remains essential. For hospitalized patients, documentation should include whether large volumes of normal saline or other medications were administered in the emergency department.
Comprehensive symptom assessment contributes significantly to diagnostic accuracy. Although often nonspecific, symptoms of acidosis, including anorexia, fatigue, headache, nausea, and vomiting, warrant attention. Clinicians should specifically ask about gastrointestinal symptoms, eg, diarrhea or vomiting. Signs of renal insufficiency, including nocturia, pruritus, or oliguria, should be explored.[18][47] Additionally, clinicians must consider symptoms suggestive of diabetes, eg, polyuria or polydipsia.
Physical Examination
Physical examination should include a careful review of vital signs. Hypotension may suggest volume depletion from gastrointestinal losses or a hypoaldosterone state. The presence of Kussmaul respirations—deep, labored breathing—often indicates a compensatory response to metabolic acidosis. Findings, eg, dry mucous membranes and a characteristic ketotic, chemical odor on the breath, may suggest diabetic ketoacidosis. Asterixis and changes in mental status can reflect renal insufficiency.[48] In advanced stages, metabolic acidosis may present with respiratory failure, altered consciousness, arrhythmias, and hemodynamic instability.
Evaluation
Metabolic acidosis is primarily diagnosed through a thorough history and laboratory values in plasma and urine. Initial studies that are conducted include the following:
- Basic metabolic panel (BMP)
- ABG (of VBG if the patient is not septic or severely hypoperfused)
- Lactate level
- Plasma and urine osmolarity
- Urine sodium, potassium, chloride, and bicarbonate
Evaluation of metabolic acidosis begins with assessing the pH on either a VBG or ABG analysis. A low pCO2 level in the setting of low pH indicates the presence of metabolic acidosis. Although the body initiates compensatory mechanisms, these responses rarely normalize the serum pH. Following this initial assessment, clinicians should calculate the anion gap, regardless of whether the serum pH appears close to normal. This calculation must be adjusted for the patient’s albumin level to ensure accuracy.
An elevated corrected anion gap requires further analysis, beginning with evaluation of the delta gap to assess for the presence of concurrent metabolic processes. Additionally, the osmolar gap should be measured to identify potential toxic ingestions or unmeasured solutes.
When the corrected anion gap remains within the normal range, additional urine studies become necessary. Assessment should include the urine anion gap, urine pH, and urine electrolyte concentrations. These values help differentiate between renal and gastrointestinal causes of NGMA. If the patient reports urinary, abdominal, or groin pain suggestive of renal colic, abdominal computed tomography imaging may be indicated, as nephrolithiasis may underlie or contribute to the acid-base disturbance.
Treatment / Management
Metabolic Acidosis Management
Management of metabolic acidosis begins with prompt identification and treatment of the underlying cause, eg, sepsis, while providing supportive measures including intravenous fluid resuscitation and respiratory support.[49] In cases of severe acidosis with pH below 7.1, the use of intravenous sodium bicarbonate may be considered, although its routine administration remains controversial. Findings from the BICAR-ICU multicenter randomized controlled trial demonstrated no significant mortality benefit from bicarbonate therapy in patients with severe metabolic acidosis (pH <7.2), except in those with concurrent acute kidney injury.[50](A1)
The cautious use of sodium bicarbonate stems from its potential adverse effects, including hypernatremia, hypocalcemia, intracellular acidification, increased lactate production, and reduced myocardial contractility. Continuous renal replacement therapy (CRRT) also provides limited effectiveness in clearing lactic acid, reinforcing the importance of supportive care and targeted treatment of the underlying condition in cases of lactic acidosis.[13]
Etiology-specific management
Treatment strategies must be tailored to the specific etiology of metabolic acidosis.
Thiamine is commonly administered in patients with lactic acidosis. When metformin-induced lactic acidosis occurs, hemodialysis may be required. Methanol undergoes hepatic metabolism to form formaldehyde, a toxic compound. Effective treatments for methanol toxicity include fomepizole—or ethanol if fomepizole is unavailable—and hemodialysis when methanol levels exceed 500 mg/L, or when severe acidemia, visual disturbances, obtundation, kidney failure, or hemodynamic instability are present. Ethylene glycol poisoning requires the same therapeutic approach.[13][51]
Management of diabetic ketoacidosis focuses on insulin therapy, continued until anion gap closure occurs. Treatment does not begin with bicarbonate, even when acidosis is present. Potassium repletion and volume restoration remain essential components of therapy. Euglycemia must be achieved before correction is considered complete. Alcoholic ketoacidosis requires thiamine supplementation and correction of electrolyte imbalances, along with volume repletion. Studies have shown no improvement in outcomes with intravenous bicarbonate administration in diabetic ketoacidosis.[13] Current guidelines from the American Diabetes Association recommend IV bicarbonate only when serum pH falls below 6.9.[52](B2)
Salicylate toxicity, which typically presents with a combination of respiratory alkalosis and metabolic acidosis, requires rapid gastrointestinal decontamination with activated charcoal and alkalinization of the serum and urine using intravenous bicarbonate. This approach helps reduce central nervous system penetration and enhances renal excretion. Blood pH must be monitored closely during treatment to avoid alkalemia (pH >7.6). Hemodialysis becomes necessary when salicylate concentrations surpass 100 mg/dL or when patients present with obtundation or pulmonary edema.[13]
Differential Diagnosis
Differential diagnoses that should also be considered in patients with metabolic acidosis:
- Compensation for respiratory alkalosis
- Hyperkalemia
- Anion gap acidosis uncorrected for albumin level
- Hemolysis
- Laboratory errors
Prognosis
The prognosis of metabolic acidosis largely depends on the underlying cause, the severity of acidemia, and the timeliness of diagnosis and treatment. When the primary etiology is promptly identified and effectively managed—eg, in diabetic ketoacidosis, sepsis, or toxic ingestions—patients often recover fully with minimal long-term sequelae. However, delayed treatment or severe acidemia (pH <7.1) significantly increases the risk of complications, including organ failure, cardiac arrhythmias, hemodynamic instability, inflammation, and death.[27]
Acute metabolic acidosis with a pH around 7.20 has been associated with mortality rates exceeding 50% if not promptly corrected.[38] Patients with comorbidities such as chronic kidney disease (CKD) or advanced liver disease typically have a worse prognosis. In particular, studies have shown that an increased anion gap in CKD patients correlates with higher all-cause mortality and faster progression to kidney failure.[53] Overall, early recognition and coordinated interprofessional care are critical to improving outcomes in metabolic acidosis.[54]
Complications
If not promptly recognized and treated, metabolic acidosis can lead to life-threatening complications. Severe acidemia (pH <7.1) may impair myocardial contractility, cause hypotension, and significantly increase the risk of cardiac arrhythmias. Respiratory muscle fatigue can occur due to sustained compensatory hyperventilation.
In cases involving toxic ingestions (eg, methanol or ethylene glycol), complications may include irreversible organ damage, eg, blindness, acute kidney injury, or neurologic impairment. Persistent or recurrent metabolic acidosis may worsen outcomes in patients with diabetes or chronic kidney disease. Ultimately, death is a potential complication, particularly in critically ill patients or those with delayed treatment.[55]
Consultations
Consultations that may also be involved in the management of high anion gap metabolic acidosis include:
- Emergency room physicians
- Intensivists
- Internists
- Toxicologists
- Nephrologists
Deterrence and Patient Education
Preventing metabolic acidosis requires addressing modifiable risk factors and educating patients on early recognition and avoidance of triggers. Individuals with diabetes should maintain glycemic control to prevent ketoacidosis. Patients must be warned about the dangers of ingesting toxins, eg, methanol or salicylates, and must ensure proper storage. Individuals with kidney disease require regular follow-up and adherence to their treatment regimen. Medication risks (eg, metformin, isoniazid) should be reviewed, especially in renal or hepatic impairment. Patients should be taught to recognize early signs, including nausea, confusion, or rapid breathing, and to seek timely medical care.
Enhancing Healthcare Team Outcomes
Optimal management of high anion gap metabolic acidosis relies on a cohesive interprofessional strategy that brings together the expertise of physicians, advanced practitioners, nurses, pharmacists, and laboratory staff. Physicians and nurse practitioners are responsible for synthesizing clinical presentations with laboratory results—including arterial blood gas analyses, electrolyte panels, and anion gap calculations—to identify the underlying cause. Timely recognition of life-threatening etiologies such as diabetic ketoacidosis, lactic acidosis, renal failure, or toxic ingestions directs immediate and appropriate interventions. Pharmacists play a critical role in reviewing medications, identifying toxic agents like salicylates or metformin, and recommending antidotes such as fomepizole or supportive therapies. Nurses continuously monitor patient status, assess fluid balance, report signs of deterioration, and implement treatment plans with precision and accuracy. Laboratory staff provide accurate and timely data essential to clinical decision-making, allowing rapid calculation of corrected anion gaps and osmolar gaps that guide further evaluation and therapy.
Effective communication and coordinated care workflows form the foundation of safe and patient-centered management. Structured team huddles and shared clinical pathways facilitate the early identification of metabolic disturbances, thereby reducing delays in care. Defining team roles helps prevent errors, especially during critical transitions such as initiating dialysis or managing patients with multi-organ dysfunction. Advanced practitioners and physicians lead interprofessional discussions, particularly when decisions must be made about escalating care, initiating renal replacement therapy, or limiting invasive interventions. Nurses advocate for patients’ needs and preferences, while pharmacists ensure medication safety and adjust therapies based on renal or hepatic function. Involving patients and families in discussions about prognosis and treatment options ensures ethical care and aligns clinical decisions with individual goals. Collaboration based on mutual respect strengthens team performance and directly contributes to improved patient safety, clinical outcomes, and satisfaction in managing high anion gap metabolic acidosis.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Barceloux DG, Krenzelok EP, Olson K, Watson W. American Academy of Clinical Toxicology Practice Guidelines on the Treatment of Ethylene Glycol Poisoning. Ad Hoc Committee. Journal of toxicology. Clinical toxicology. 1999:37(5):537-60 [PubMed PMID: 10497633]
Level 1 (high-level) evidenceAdrogué HJ, Madias NE. Management of life-threatening acid-base disorders. First of two parts. The New England journal of medicine. 1998 Jan 1:338(1):26-34 [PubMed PMID: 9414329]
Bear RA. A clinical approach to the diagnosis of Acid-base disorders. Canadian family physician Medecin de famille canadien. 1986 Apr:32():823-7 [PubMed PMID: 21267134]
Chan JC. Acid-base disorders and the kidney. Advances in pediatrics. 1983:30():401-71 [PubMed PMID: 6424418]
Level 3 (low-level) evidenceKellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Critical care (London, England). 2013 Feb 4:17(1):204. doi: 10.1186/cc11454. Epub 2013 Feb 4 [PubMed PMID: 23394211]
Chong WH, Saha BK, Medarov BI. Comparing Central Venous Blood Gas to Arterial Blood Gas and Determining Its Utility in Critically Ill Patients: Narrative Review. Anesthesia and analgesia. 2021 Aug 1:133(2):374-378. doi: 10.1213/ANE.0000000000005501. Epub [PubMed PMID: 33780397]
Level 3 (low-level) evidenceKraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clinical journal of the American Society of Nephrology : CJASN. 2007 Jan:2(1):162-74 [PubMed PMID: 17699401]
Fenves AZ, Emmett M. Approach to Patients With High Anion Gap Metabolic Acidosis: Core Curriculum 2021. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2021 Oct:78(4):590-600. doi: 10.1053/j.ajkd.2021.02.341. Epub 2021 Aug 13 [PubMed PMID: 34400023]
Berend K. Diagnostic Use of Base Excess in Acid-Base Disorders. The New England journal of medicine. 2018 Apr 12:378(15):1419-1428. doi: 10.1056/NEJMra1711860. Epub [PubMed PMID: 29641969]
Romero JE, Htyte N. An unusual cause of high anion gap metabolic acidosis: pyroglutamic acidemia. A case report. American journal of therapeutics. 2013 Sep-Oct:20(5):581-4. doi: 10.1097/MJT.0b013e318209dfdd. Epub [PubMed PMID: 21519223]
Level 3 (low-level) evidenceBelkoniene M, Socquet J, Njemba-Freiburghaus D, Pellaton C. Near fatal intoxication by nicotine and propylene glycol injection: a case report of an e-liquid poisoning. BMC pharmacology & toxicology. 2019 May 10:20(1):28. doi: 10.1186/s40360-019-0296-8. Epub 2019 May 10 [PubMed PMID: 31077262]
Level 3 (low-level) evidenceBielik N, Correia D, Rodrigues Crespo K, Goujon-Ginglinger C, Mitova MI. Pitfalls in the Detection of Volatiles Associated with Heated Tobacco and e-Vapor Products When Using PTR-TOF-MS. Journal of the American Society for Mass Spectrometry. 2024 Jun 5:35(6):1261-1271. doi: 10.1021/jasms.4c00062. Epub 2024 May 23 [PubMed PMID: 38780179]
Achanti A, Szerlip HM. Acid-Base Disorders in the Critically Ill Patient. Clinical journal of the American Society of Nephrology : CJASN. 2023 Jan 1:18(1):102-112. doi: 10.2215/CJN.04500422. Epub 2022 Aug 23 [PubMed PMID: 35998977]
Remund B, Yilmaz B, Sokollik C. D-Lactate: Implications for Gastrointestinal Diseases. Children (Basel, Switzerland). 2023 May 26:10(6):. doi: 10.3390/children10060945. Epub 2023 May 26 [PubMed PMID: 37371177]
Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA, American Academy of Clinical Toxicology Ad Hoc Committee on the Treatment Guidelines for Methanol Poisoning. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. Journal of toxicology. Clinical toxicology. 2002:40(4):415-46 [PubMed PMID: 12216995]
Level 1 (high-level) evidenceTemple AR. Acute and chronic effects of aspirin toxicity and their treatment. Archives of internal medicine. 1981 Feb 23:141(3 Spec No):364-9 [PubMed PMID: 7469627]
Khan FY. Rhabdomyolysis: a review of the literature. The Netherlands journal of medicine. 2009 Oct:67(9):272-83 [PubMed PMID: 19841484]
Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes care. 2009 Jul:32(7):1335-43. doi: 10.2337/dc09-9032. Epub [PubMed PMID: 19564476]
Yoshida N, Takahashi S, Hitaka M, Ohashi Y, Ichibayashi R. Role of Renal Replacement Therapy in Managing Toluene-Induced Acidosis. The American journal of case reports. 2024 Dec 28:25():e945657. doi: 10.12659/AJCR.945657. Epub 2024 Dec 28 [PubMed PMID: 39731755]
Level 3 (low-level) evidenceTuchscherer J, Rehman H. Metabolic acidosis in toluene sniffing. CJEM. 2013 Jul:15(4):249-52 [PubMed PMID: 23778000]
Di Mauro S, Filippello A, Scamporrino A, Purrello F, Piro S, Malaguarnera R. Metformin: When Should We Fear Lactic Acidosis? International journal of molecular sciences. 2022 Jul 28:23(15):. doi: 10.3390/ijms23158320. Epub 2022 Jul 28 [PubMed PMID: 35955455]
Tomita D, Bamba Y, Kuwabara N, Akakabe S, Matsubayashi Y, Nakagawa S, Sone H, Nishiyama K. A Case of Concurrent Metformin-Associated Lactic Acidosis and Euglycemic Diabetic Ketoacidosis. The Journal of emergency medicine. 2025 Aug:75():222-226. doi: 10.1016/j.jemermed.2025.02.018. Epub 2025 Feb 17 [PubMed PMID: 40537294]
Level 3 (low-level) evidenceAlexander RT, Bitzan M. Renal Tubular Acidosis. Pediatric clinics of North America. 2019 Feb:66(1):135-157. doi: 10.1016/j.pcl.2018.08.011. Epub [PubMed PMID: 30454739]
Kraut JA, Madias NE. Differential diagnosis of nongap metabolic acidosis: value of a systematic approach. Clinical journal of the American Society of Nephrology : CJASN. 2012 Apr:7(4):671-9. doi: 10.2215/CJN.09450911. Epub 2012 Mar 8 [PubMed PMID: 22403272]
Level 1 (high-level) evidenceAbutalib RA, Alamri AJ, Aqel SA, Alhumaidi IM, Almohini IA. Acute Respiratory Distress and Hyperchloremic Metabolic Acidosis as a Result of Massive Irrigation Fluid Extravasation After Arthroscopic Shoulder Surgery: A Case Report and Recommendations for Preventable Complications. The American journal of case reports. 2020 Nov 13:21():e926357. doi: 10.12659/AJCR.926357. Epub 2020 Nov 13 [PubMed PMID: 33184253]
Level 3 (low-level) evidenceBhagat H, Singhal V, Dash HH, Mahajan S, Mishra N, Pandia MP. Comparative evaluation of intraoperative use of normal saline, Ringer's lactate, and combination of normal saline and Ringer's lactate in neurosurgical patients - A preliminary randomized clinical trial. Neurology India. 2019 Mar-Apr:67(2):452-458. doi: 10.4103/0028-3886.258047. Epub [PubMed PMID: 31085860]
Level 1 (high-level) evidenceNakamura M, Ikeda K, Uezono S. Metabolic acidemia due to saline absorption during transurethral and transcervical surgery: a report of 2 cases. BMC anesthesiology. 2024 Feb 10:24(1):62. doi: 10.1186/s12871-024-02437-5. Epub 2024 Feb 10 [PubMed PMID: 38341531]
Level 3 (low-level) evidenceFlorez ID, Sierra J, Pérez-Gaxiola G. Balanced crystalloid solutions versus 0.9% saline for treating acute diarrhoea and severe dehydration in children. The Cochrane database of systematic reviews. 2023 May 17:5(5):CD013640. doi: 10.1002/14651858.CD013640.pub2. Epub 2023 May 17 [PubMed PMID: 37196992]
Level 1 (high-level) evidenceHayes W. Ab-normal saline in abnormal kidney function: risks and alternatives. Pediatric nephrology (Berlin, Germany). 2019 Jul:34(7):1191-1199. doi: 10.1007/s00467-018-4008-1. Epub 2018 Jul 9 [PubMed PMID: 29987459]
Pfortmueller C, Funk GC, Potura E, Reiterer C, Luf F, Kabon B, Druml W, Fleischmann E, Lindner G. Acetate-buffered crystalloid infusate versus infusion of 0.9% saline and hemodynamic stability in patients undergoing renal transplantation : Prospective, randomized, controlled trial. Wiener klinische Wochenschrift. 2017 Sep:129(17-18):598-604. doi: 10.1007/s00508-017-1180-4. Epub 2017 Mar 2 [PubMed PMID: 28255797]
Level 1 (high-level) evidenceLobo DN, Awad S. Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent 'pre-renal' acute kidney injury?: con. Kidney international. 2014 Dec:86(6):1096-105. doi: 10.1038/ki.2014.105. Epub 2014 Apr 9 [PubMed PMID: 24717302]
Kamar FB, McQuillan RF. Hyperchloremic Metabolic Acidosis due to Cholestyramine: A Case Report and Literature Review. Case reports in nephrology. 2015:2015():309791. doi: 10.1155/2015/309791. Epub 2015 Sep 3 [PubMed PMID: 26425378]
Level 3 (low-level) evidenceWang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl(-)/HCO3(-) exchanger in the small intestine. American journal of physiology. Gastrointestinal and liver physiology. 2002 Mar:282(3):G573-9 [PubMed PMID: 11842009]
Ruiz-Sánchez JG, Calle-Pascual AL, Rubio-Herrera MÁ, De Miguel Novoa MP, Gómez-Hoyos E, Runkle I. Clinical manifestations and associated factors in acquired hypoaldosteronism in endocrinological practice. Frontiers in endocrinology. 2022:13():990148. doi: 10.3389/fendo.2022.990148. Epub 2022 Oct 11 [PubMed PMID: 36303866]
Abdalla M, Dave JA, Ross IL. Addison's disease associated with hypokalemia: a case report. Journal of medical case reports. 2021 Mar 25:15(1):131. doi: 10.1186/s13256-021-02724-6. Epub 2021 Mar 25 [PubMed PMID: 33761983]
Level 3 (low-level) evidenceZemlin AE, Sigwadhi LN, Wiese OJ, Jalavu TP, Chapanduka ZC, Allwood BW, Tamuzi JL, Koegelenberg CF, Irusen EM, Lalla U, Ngah VD, Yalew A, Erasmus RT, Matsha TE, Zumla A, Nyasulu PS, COVID-19 Research Response Collaboration. The association between acid-base status and clinical outcome in critically ill COVID-19 patients admitted to intensive care unit with an emphasis on high anion gap metabolic acidosis. Annals of clinical biochemistry. 2023 Mar:60(2):86-91. doi: 10.1177/00045632221134687. Epub 2022 Nov 7 [PubMed PMID: 36220779]
Level 2 (mid-level) evidenceChen J, Dai C, Yang Y, Wang Y, Zeng R, Li B, Liu Q. The association between anion gap and in-hospital mortality of post-cardiac arrest patients: a retrospective study. Scientific reports. 2022 May 6:12(1):7405. doi: 10.1038/s41598-022-11081-3. Epub 2022 May 6 [PubMed PMID: 35524151]
Level 2 (mid-level) evidenceKraut JA, Madias NE. Metabolic acidosis: pathophysiology, diagnosis and management. Nature reviews. Nephrology. 2010 May:6(5):274-85. doi: 10.1038/nrneph.2010.33. Epub 2010 Mar 23 [PubMed PMID: 20308999]
Aydın SŞ, Aksakal E. Relationship Between Albumin-Corrected Anion Gap and Mortality in Hospitalized Heart Failure Patients. Cureus. 2023 Sep:15(9):e45967. doi: 10.7759/cureus.45967. Epub 2023 Sep 25 [PubMed PMID: 37900402]
Figge J, Rossing TH, Fencl V. The role of serum proteins in acid-base equilibria. The Journal of laboratory and clinical medicine. 1991 Jun:117(6):453-67 [PubMed PMID: 2045713]
Seifter JL, Chang HY. Disorders of Acid-Base Balance: New Perspectives. Kidney diseases (Basel, Switzerland). 2017 Jan:2(4):170-186. doi: 10.1159/000453028. Epub 2016 Dec 10 [PubMed PMID: 28232934]
Level 3 (low-level) evidenceKraut JA, Kurtz I. Toxic alcohol ingestions: clinical features, diagnosis, and management. Clinical journal of the American Society of Nephrology : CJASN. 2008 Jan:3(1):208-25 [PubMed PMID: 18045860]
Hoffman RS, Smilkstein MJ, Howland MA, Goldfrank LR. Osmol gaps revisited: normal values and limitations. Journal of toxicology. Clinical toxicology. 1993:31(1):81-93 [PubMed PMID: 8433417]
Batlle D, Chin-Theodorou J, Tucker BM. Metabolic Acidosis or Respiratory Alkalosis? Evaluation of a Low Plasma Bicarbonate Using the Urine Anion Gap. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017 Sep:70(3):440-444. doi: 10.1053/j.ajkd.2017.04.017. Epub 2017 Jun 7 [PubMed PMID: 28599903]
Hanouneh M, Chen TK. Calcium Oxalate Crystals in Ethylene Glycol Toxicity. The New England journal of medicine. 2017 Oct 12:377(15):1467. doi: 10.1056/NEJMicm1704369. Epub [PubMed PMID: 29020580]
Kruse JA. Methanol and ethylene glycol intoxication. Critical care clinics. 2012 Oct:28(4):661-711. doi: 10.1016/j.ccc.2012.07.002. Epub [PubMed PMID: 22998995]
Himmelfarb J, Ikizler TA. Hemodialysis. The New England journal of medicine. 2010 Nov 4:363(19):1833-45. doi: 10.1056/NEJMra0902710. Epub [PubMed PMID: 21047227]
Kitabchi AE, Nyenwe EA. Hyperglycemic crises in diabetes mellitus: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinology and metabolism clinics of North America. 2006 Dec:35(4):725-51, viii [PubMed PMID: 17127143]
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive care medicine. 2017 Mar:43(3):304-377. doi: 10.1007/s00134-017-4683-6. Epub 2017 Jan 18 [PubMed PMID: 28101605]
Jaber S, Paugam C, Futier E, Lefrant JY, Lasocki S, Lescot T, Pottecher J, Demoule A, Ferrandière M, Asehnoune K, Dellamonica J, Velly L, Abback PS, de Jong A, Brunot V, Belafia F, Roquilly A, Chanques G, Muller L, Constantin JM, Bertet H, Klouche K, Molinari N, Jung B, BICAR-ICU Study Group. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet (London, England). 2018 Jul 7:392(10141):31-40. doi: 10.1016/S0140-6736(18)31080-8. Epub 2018 Jun 14 [PubMed PMID: 29910040]
Level 1 (high-level) evidenceBrent J. Fomepizole for ethylene glycol and methanol poisoning. The New England journal of medicine. 2009 May 21:360(21):2216-23. doi: 10.1056/NEJMct0806112. Epub [PubMed PMID: 19458366]
Duhon B, Attridge RL, Franco-Martinez AC, Maxwell PR, Hughes DW. Intravenous sodium bicarbonate therapy in severely acidotic diabetic ketoacidosis. The Annals of pharmacotherapy. 2013 Jul-Aug:47(7-8):970-5. doi: 10.1345/aph.1S014. Epub 2013 Jun 4 [PubMed PMID: 23737516]
Level 2 (mid-level) evidenceLee SW, Kim S, Na KY, Cha RH, Kang SW, Park CW, Cha DR, Kim SG, Yoon SA, Han SY, Park JH, Chang JH, Lim CS, Kim YS. Serum Anion Gap Predicts All-Cause Mortality in Patients with Advanced Chronic Kidney Disease: A Retrospective Analysis of a Randomized Controlled Study. PloS one. 2016:11(6):e0156381. doi: 10.1371/journal.pone.0156381. Epub 2016 Jun 1 [PubMed PMID: 27249416]
Level 1 (high-level) evidenceJung B, Martinez M, Claessens YE, Darmon M, Klouche K, Lautrette A, Levraut J, Maury E, Oberlin M, Terzi N, Viglino D, Yordanov Y, Claret PG, Bigé N, Société de Réanimation de Langue Française (SRLF), Société Française de Médecine d’Urgence (SFMU). Diagnosis and management of metabolic acidosis: guidelines from a French expert panel. Annals of intensive care. 2019 Aug 15:9(1):92. doi: 10.1186/s13613-019-0563-2. Epub 2019 Aug 15 [PubMed PMID: 31418093]
Jung B, Rimmele T, Le Goff C, Chanques G, Corne P, Jonquet O, Muller L, Lefrant JY, Guervilly C, Papazian L, Allaouchiche B, Jaber S, AzuRea Group. Severe metabolic or mixed acidemia on intensive care unit admission: incidence, prognosis and administration of buffer therapy. A prospective, multiple-center study. Critical care (London, England). 2011:15(5):R238. doi: 10.1186/cc10487. Epub 2011 Oct 13 [PubMed PMID: 21995879]