Introduction
Human herpesvirus 6 (HHV-6) was first identified in the blood lymphocytes of adults with lymphoproliferative diseases or AIDS and was initially called human B-lymphotropic virus. The virus was later found in CD4+ lymphocytes and classified as a member of the herpesvirus family. As the sixth herpesvirus to be isolated, the organism was renamed human herpesvirus 6. Like other herpesviruses, HHV-6 establishes acute, persistent, and lifelong infections.
HHV-6 encompasses 2 genetically distinct double-stranded DNA viruses—HHV-6A and HHV-6B. These viruses are now considered separate species within the herpesvirus family. HHV-6A remains less understood but is more commonly detected in immunocompromised patients. In contrast, HHV-6B is the known cause of exanthema subitum (roseola infantum), a common childhood illness.[1] HHV-6B infection is highly prevalent, with over 90% of individuals acquiring it by age 3, making it a frequent encounter in pediatric and emergency care settings.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
HHV-6A, HHV-6B, and HHV-7 share partial homology with human cytomegalovirus, the only other human β-herpesvirus. Both HHV-6A and HHV-6B replicate in T cells but differ in their mechanisms of cellular entry. HHV-6A uses CD46, whereas HHV-6B primarily binds to CD134, a receptor expressed only on activated T cells.[2] Like Epstein–Barr virus and HHV-8, also known as Kaposi sarcoma-associated herpesvirus, HHV-6 exhibits oncogenic potential and causes damage to immune cells. Once bound to its receptor, HHV-6 establishes latency in lymphocytes and exerts strong immunomodulatory effects that may lead to immunosuppression or chronic inflammation.[3]
By adulthood, more than 95% of individuals test seropositive for HHV-6A, HHV-6B, or both. Current serologic techniques do not reliably differentiate between the 2 organisms.[4] HHV-6 is now recognized as the primary cause of roseola infantum in children, with HHV-6B predominantly infecting infants and accounting for most cases of viral reactivation in both immunocompetent and immunocompromised hosts.
Most adults with chronic HHV-6 infection remain asymptomatic. Ongoing research continues to explore the role of HHV-6 in the central nervous system (CNS). In one study, over 70% of children with primary HHV-6 infection had detectable viral DNA in the cerebrospinal fluid (CSF) and presented with neurologic manifestations, such as frequent febrile seizures. In contrast, other studies report a 0% to 4% detection rate of HHV-6 DNA in the CSF of children with febrile seizures and adults with AIDS-related neurologic symptoms.[5] HHV-6 has been implicated in several illnesses among immunocompromised patients and may contribute to the development of Hodgkin lymphoma and other malignancies. Documented cases of opportunistic HHV-6A and HHV-6B infections include encephalitis, hepatitis, colitis, and pneumonitis.[6]
Epidemiology
Researchers have identified HHV-6B as the primary pathogen responsible for most symptomatic HHV infections. Although most individuals acquire HHV during early childhood, primary infection typically occurs within the first 2 years of life. During this period, the virus often presents as an undifferentiated febrile illness, though some children exhibit the characteristic features of roseola infantum. Seropositivity appears to decline with age, indicating a gradual decline in antibody levels over time. Although primary HHV infection is rare in adults, viral reactivation can occur throughout life. HHV affects individuals regardless of sex or race and is distributed globally.
In a large prospective study of North American children, HHV acquisition peaked between 6 and 9 months of age. Similar trends have been observed in the United Kingdom and Japan, where 97% to 100% of primary infections are attributed to HHV-6B. Although less is known about HHV-6A, studies suggest it is acquired later in life and often remains asymptomatic. However, symptomatic HHV-6A infections have been reported in children from Africa and the United States. In an endemic region of sub-Saharan Africa, HHV-6A emerged as the predominant variant among HIV-positive infants.[7] Seroprevalence varies widely by population, with reports ranging from 20% in pregnant Moroccan women to 100% in asymptomatic Chinese adults. Among ethnically diverse adult populations in Tanzania, Malaysia, Thailand, and Brazil, reported seroprevalence ranged from 39% to 80%.
Pathophysiology
Primary HHV-6 infection ranks among the most common causes of acute febrile illness in young children and accounts for a significant number of emergency visits, hospitalizations, and febrile seizures. HHV-6 is a member of the Betaherpesvirinae subfamily and the Roseolovirus genus. The virion has the typical herpesvirus structure, consisting of a central DNA core, an icosahedral capsid, a protein-rich tegument, and an outer lipid envelope.
The virus primarily targets mature CD4+ T cells and demonstrates broad immunomodulatory effects, including interference with natural killer cell activity. In vivo, HHV-6 infects CD4+ lymphocytes through the CD46 receptor and enters cells through receptor-mediated endocytosis. After entry, the virus replicates within the host cell, and its DNA persists in peripheral blood mononuclear cells following primary infection.
Although the exact mechanisms of HHV-6 transmission remain under investigation, saliva has been identified as the predominant route, particularly in maternal-infant transmission. An infectious virus has been detected in the saliva of nearly all individuals tested. One study found HHV-6 DNA in 90% of salivary samples, whereas another reported a lower detection rate through polymerase chain reaction (PCR) despite 63% of salivary gland biopsies testing positive. HHV-6 DNA has also been found in the CSF of children during both primary and latent infections and in the brain tissue of healthy adults on autopsy, supporting the CNS and salivary glands as potential reservoirs for latency and persistent infection.
History and Physical
Although the full spectrum of clinical features and disease associations linked to HHV-6 is still under investigation, substantial evidence supports its role in CNS disorders and disease in immunocompromised individuals, including transplant recipients.[8] In most cases, HHV-6 infection is benign and self-limiting. Symptomatic disease typically occurs in infants or immunocompromised adults experiencing viral reactivation.
HHV-6B is the established cause of exanthema subitum (roseola infantum), a common childhood illness marked by high fever and a transient rash. This variant accounts for 10% to 17% of acute febrile visits to the emergency department in children younger than 36 months.[9] Among children aged 12 to 15 months, primary HHV-6 infection causes over 36% of acute febrile episodes, with nearly all cases attributed to HHV-6B rather than HHV-6A.[10]
The diagnosis of roseola infantum is clinical. The condition typically begins with the abrupt onset of high fever, often reaching 40 °C (104 °F), lasting for 3 to 5 days. During this febrile phase, some children may exhibit periorbital edema, conjunctivitis, or tympanic membrane inflammation, although many remain active and appear well. Other pediatric findings can include lymphadenopathy, gastrointestinal symptoms, liver dysfunction, hepatitis, and bulging fontanelles.[11] Hepatitis and encephalitis-like symptoms are more common in adults. In transplant recipients, clinical manifestations may include fever, graft-versus-host disease, signs of graft rejection, interstitial pneumonitis, myelitis, and rash.[12]
Physical examination generally mirrors the clinical symptoms. In children, resolution of fever is often followed by a blanching, rose-pink maculopapular rash, measuring approximately 2 to 5 mm, typically surrounded by a pale halo. The rash spreads centrifugally and resolves within 1 to 2 days. However, some cases present with fever without rash.[13] Additional findings in children may include inflamed tympanic membranes and upper or lower respiratory tract signs, whereas adults may present with fever, hepatosplenomegaly, and lymphadenopathy.
Evaluation
HHV-6 can be diagnosed through PCR, serologic testing, or viral culture, with PCR being the most commonly used diagnostic method. However, in immunocompetent individuals, laboratory testing is rarely pursued due to the disease's typically self-limiting course. Saliva serves as the primary reservoir for viral transmission, with HHV-6 frequently isolated from salivary glands. No reports confirm transmission through blood transfusion or breastfeeding, but HHV-6 has been documented following organ transplantation. Most evidence indicates that HHV-6B is typically acquired in early childhood, while HHV-6A is more often acquired later in life. Both variants of the virus have been detected simultaneously in adults, suggesting that chronic infection affects a large portion of the population.
Laboratory findings in HHV-6 may include leukocytosis, leukopenia, or anemia. In patients with renal transplants or liver dysfunction, renal and hepatic function tests are recommended to assess for electrolyte abnormalities or hepatitis.
Radiologic imaging is guided by clinical presentation and is more often warranted in immunocompromised individuals. Chest radiographs may help evaluate for pneumonia or pneumonitis in adults, whereas imaging is less commonly necessary in children. Computed tomography of the head, with or without contrast, can help exclude other treatable conditions when CNS involvement is suspected. Lumbar puncture is appropriate in cases of suspected neurologic involvement. CSF typically reveals mild pleocytosis and elevated protein in HHV-6 infection. HHV-6 PCR testing of CSF may confirm the diagnosis (see Image. Human Herpesvirus 6 Cellular Infection).[14]
Treatment / Management
Currently, no approved compound exists exclusively for the treatment of HHV-6, and no vaccine is available. Antiviral prophylaxis for HHV-6 infection is generally not recommended. Early antiviral treatment is advised, particularly in cases of HHV-6 encephalitis. First-line therapy with intravenous ganciclovir and foscarnet is recommended for 3 to 4 weeks. In stem-cell transplant recipients, ganciclovir has demonstrated benefits and is the antiviral of choice.[15](B3)
HHV-6 infections in immunocompetent children are self-limiting and do not require treatment. Treatment for infants with roseola infantum is generally supportive, with antipyretics such as acetaminophen or ibuprofen recommended for high-grade fever and febrile seizure risk. Antiepileptics are unnecessary if a febrile seizure occurs. Patients with CNS involvement, including febrile seizures, should be admitted to the hospital.
Differential Diagnosis
The differential diagnosis of HHV-6 infection includes the following conditions:
- Infectious mononucleosis
- Cytomegalovirus infection
- Viral hepatitis
- Herpes simplex virus infection
- Meningitis
- Rubella
- Viral pneumonia
- Drug rash with eosinophilia and systemic symptoms (DRESS syndrome)
Several diseases may present with fever and rash, but HHV-6 is the primary etiologic agent in children with classic roseola infantum. Accurate diagnosis requires distinguishing HHV-6 from more severe conditions that demand prompt intervention.
In immunocompetent adults, HHV-6 infection can mimic mononucleosis, necessitating the exclusion of EBV and cytomegalovirus. In immunocompromised individuals, especially transplant recipients and those with AIDS, symptomatic HHV-6 infection is common and often occurs alongside cytomegalovirus, warranting immediate antiviral treatment. HHV-6 has also been associated with hepatitis, herpes simplex virus, meningitis, rubella, viral pneumonia, and DRESS syndrome.[16] Additional reports have documented HHV-6 in cases of multiple sclerosis encephalitis and interstitial pneumonitis.[17] Notably, only HHV-6A has been linked to Hashimoto thyroiditis.
Prognosis
Generally, primary HHV-6 infection is self-limiting, and immunocompetent individuals recover without sequelae. In contrast, immunosuppressed individuals may experience more severe illness. Reports have documented pneumonitis, hepatitis, and organ rejection in transplant recipients, with fatalities in patients who developed encephalitis or meningoencephalitis.[18] A survey from 2003 to 2004 in Japan found an unusually poor prognosis among patients with exanthema subitum–associated encephalitis, including 2 fatalities in children with HHV-6 encephalopathy and underlying mitochondrial disorders.[19][20] More recently, HHV-6 has also been linked to CNS infections in children in Sudan.[21]
Complications
HHV-6 primary infection is typically benign, with spontaneous resolution within 5 to 7 days. The most common complication of roseola infantum is febrile seizures. Additional complications often result from the virus's neurotrophic properties, particularly when the CNS is involved, as in meningoencephalitis or encephalopathy. In such cases, the virus can invade the brain during primary infection and remain dormant in brain tissue.[22] Instances of acute or subacute encephalitis occasionally accompanied by diffuse or multifocal demyelination have been documented. HHV-6 infrequently causes opportunistic infections in immunocompromised individuals. Reactivation of HHV-6A and HHV-6B has been detected following renal, liver, and bone marrow transplants.
A potential association between HHV-6 and tumor necrosis factor-α promoter polymorphisms has been recently investigated in relation to severe depressive disorders.[23] Although HHV-6 has also been suspected in the pathogenesis of Hashimoto thyroiditis, this association has not been supported by research evidence.[24]
Deterrence and Patient Education
In otherwise healthy infants with roseola infantum, parent education plays a key role in reducing anxiety related to fever and the risk of febrile seizures. Caregivers should be counseled on the importance of supportive care, including the use of antipyretics, and the lack of benefit from antibiotics in viral infections. In immunocompromised individuals, the potential for complex symptomatology and concurrent viral illnesses warrants careful counseling, with emphasis on the need for prompt medical evaluation when symptoms develop.
Pearls and Other Issues
HHV-6B is widely recognized as the primary variant responsible for initial infections, whereas HHV-6A is typically acquired later through asymptomatic transmission.[25] Differentiating primary HHV-6 infection, particularly when presenting with the characteristic roseola infantum rash, is essential to exclude more serious conditions. Roseola infantum remains the most common complication of HHV-6 infection, accounting for approximately 10% to 20% of febrile illnesses in children aged 6 months to 3 years. Febrile seizures and gastrointestinal or respiratory symptoms are less frequent and generally resolve without intervention. Supportive care, including rest and hydration, is the recommended treatment approach in this population.
Like other herpesviruses, HHV-6 replicates in salivary glands and establishes latency in peripheral blood mononuclear cells. In immunocompetent individuals, laboratory confirmation is rarely necessary. However, in immunocompromised patients, PCR, serologic testing, or viral cultures may be required to confirm HHV-6 infection.
More severe disease associations include mononucleosis, colitis, myocarditis, hepatitis, and CNS complications such as encephalitis and meningoencephalitis. Early identification and intervention are critical in mitigating neurologic sequelae. Individuals with cancer, those undergoing allogeneic transplantation, and patients with immunosuppressive conditions face a higher risk of HHV-6 reactivation. Presenting symptoms in this group often include fever, rash, and cytopenias affecting granulocytes and erythroid cells. Although no therapies are approved exclusively for HHV-6, antiviral agents such as ganciclovir and foscarnet have demonstrated efficacy in managing HHV-6–related neurologic complications.
Enhancing Healthcare Team Outcomes
HHV-6 often manifests as a rash in children, which is commonly observed by emergency physicians and pediatricians. Although rashes can be diagnostically challenging, identifying coexisting symptoms is crucial for distinguishing benign illnesses from more serious conditions. Most HHV-6 infections are asymptomatic, self-limited, and do not require antiviral therapy. Pediatric consultation is recommended for infants with roseola infantum who experience febrile seizures. Complications from HHV-6 do occur, and individuals with concerning signs such as altered mental status or organ dysfunction benefit from an interprofessional care model. In such cases, early involvement of neurology and infectious disease specialists is essential. When HHV-6–associated myocarditis or heart failure is suspected in immunocompetent adults, prompt cardiology consultation is critical.[26]
Skilled nursing care is essential for monitoring clinical status, including early detection of deterioration and changes in vital signs. Laboratory teams contribute by expediting preferred diagnostic tests, including PCR for blood or CSF or immunohistochemical staining of tissue samples for viral antigen detection. Pharmacists play a crucial role in guiding and dispensing antiviral therapies, such as ganciclovir or foscarnet, particularly in the treatment of HHV-6–associated encephalitis.[27] A coordinated, patient-centered approach among clinicians, nurses, laboratory personnel, and pharmacists is recommended to improve outcomes in complex presentations of HHV-6 infection.
Media
(Click Image to Enlarge)
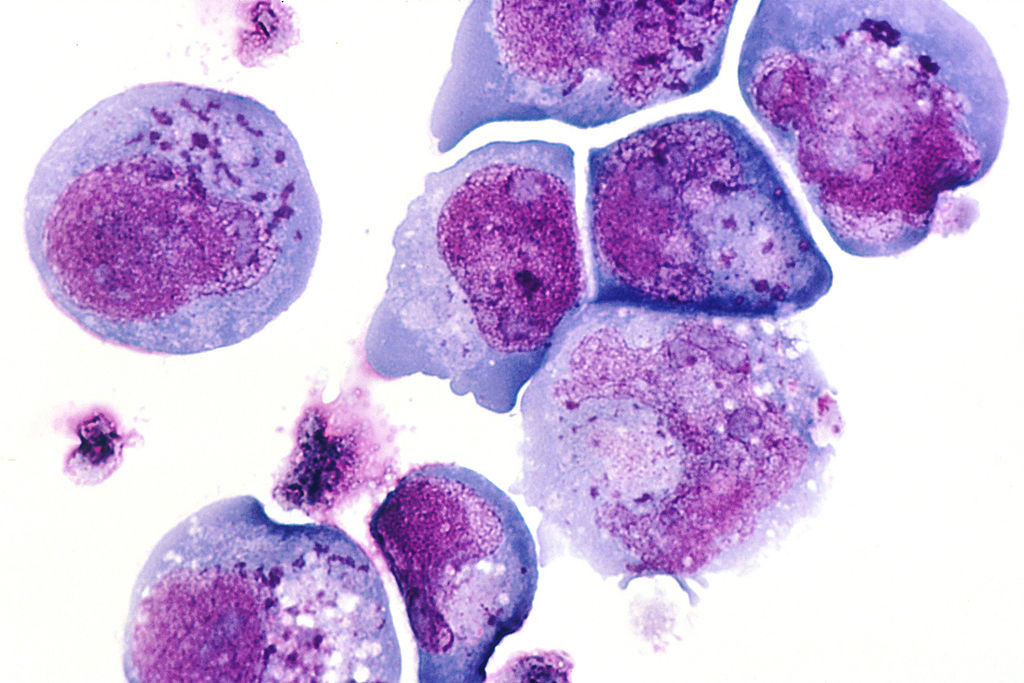
Human Herpes Virus 6 Cellular Infection. This histological slide shows cells infected with human herpesvirus 6 (HHV-6), previously known as human B-lymphotropic virus (HBLV), a herpesvirus identified in October 1986. The photomicrograph highlights infected cells with inclusion bodies present in both the nucleus and cytoplasm. The slide is stained with hematoxylin and eosin. This virus is the causative agent of roseola infantum.
Contributed by Wikimedia Commons (Public Domain)
References
Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2001 Sep 15:33(6):829-33 [PubMed PMID: 11512088]
Level 3 (low-level) evidencePantry SN, Medveczky PG. Latency, Integration, and Reactivation of Human Herpesvirus-6. Viruses. 2017 Jul 24:9(7):. doi: 10.3390/v9070194. Epub 2017 Jul 24 [PubMed PMID: 28737715]
Eliassen E, Lum E, Pritchett J, Ongradi J, Krueger G, Crawford JR, Phan TL, Ablashi D, Hudnall SD. Human Herpesvirus 6 and Malignancy: A Review. Frontiers in oncology. 2018:8():512. doi: 10.3389/fonc.2018.00512. Epub 2018 Nov 13 [PubMed PMID: 30542640]
Braun DK, Dominguez G, Pellett PE. Human herpesvirus 6. Clinical microbiology reviews. 1997 Jul:10(3):521-67 [PubMed PMID: 9227865]
Level 3 (low-level) evidenceAnsari A, Li S, Abzug MJ, Weinberg A. Human herpesviruses 6 and 7 and central nervous system infection in children. Emerging infectious diseases. 2004 Aug:10(8):1450-4 [PubMed PMID: 15496247]
Agut H, Bonnafous P, Gautheret-Dejean A. Update on infections with human herpesviruses 6A, 6B, and 7. Medecine et maladies infectieuses. 2017 Mar:47(2):83-91. doi: 10.1016/j.medmal.2016.09.004. Epub 2016 Oct 20 [PubMed PMID: 27773488]
Ablashi D, Agut H, Alvarez-Lafuente R, Clark DA, Dewhurst S, DiLuca D, Flamand L, Frenkel N, Gallo R, Gompels UA, Höllsberg P, Jacobson S, Luppi M, Lusso P, Malnati M, Medveczky P, Mori Y, Pellett PE, Pritchett JC, Yamanishi K, Yoshikawa T. Classification of HHV-6A and HHV-6B as distinct viruses. Archives of virology. 2014 May:159(5):863-70. doi: 10.1007/s00705-013-1902-5. Epub 2013 Nov 6 [PubMed PMID: 24193951]
De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clinical microbiology reviews. 2005 Jan:18(1):217-45 [PubMed PMID: 15653828]
Tesini BL, Epstein LG, Caserta MT. Clinical impact of primary infection with roseoloviruses. Current opinion in virology. 2014 Dec:9():91-6. doi: 10.1016/j.coviro.2014.09.013. Epub 2014 Oct 14 [PubMed PMID: 25462439]
Level 3 (low-level) evidenceHall CB, Long CE, Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, Knott A, Dewhurst S, Insel RA, Epstein LG. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. The New England journal of medicine. 1994 Aug 18:331(7):432-8 [PubMed PMID: 8035839]
Level 3 (low-level) evidenceCristoforo T, Le NK, Rye-Buckingham S, Hudson WB, Carroll LF. The Not-So-Soft Spot: Pathophysiology of the Bulging Fontanelle in Association With Roseola. Pediatric emergency care. 2020 Oct:36(10):e576-e578. doi: 10.1097/PEC.0000000000001447. Epub [PubMed PMID: 29489601]
Agut H, Bonnafous P, Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clinical microbiology reviews. 2015 Apr:28(2):313-35. doi: 10.1128/CMR.00122-14. Epub [PubMed PMID: 25762531]
Arnež M, Avšič-Županc T, Uršič T, Petrovec M. Human Herpesvirus 6 Infection Presenting as an Acute Febrile Illness Associated with Thrombocytopenia and Leukopenia. Case reports in pediatrics. 2016:2016():2483183 [PubMed PMID: 27980872]
Level 3 (low-level) evidenceYavarian J, Gavvami N, Mamishi S. Detection of human herpesvirus 6 in cerebrospinal fluid of children with possible encephalitis. Jundishapur journal of microbiology. 2014 Sep:7(9):e11821. doi: 10.5812/jjm.11821. Epub 2013 Sep 1 [PubMed PMID: 25485059]
Prichard MN, Whitley RJ. The development of new therapies for human herpesvirus 6. Current opinion in virology. 2014 Dec:9():148-53. doi: 10.1016/j.coviro.2014.09.019. Epub 2014 Oct 22 [PubMed PMID: 25462447]
Level 3 (low-level) evidenceShiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. The British journal of dermatology. 2007 May:156(5):1083-4 [PubMed PMID: 17381452]
Level 3 (low-level) evidenceMarks GL, Nolan PE, Erlich KS, Ellis MN. Mucocutaneous dissemination of acyclovir-resistant herpes simplex virus in a patient with AIDS. Reviews of infectious diseases. 1989 May-Jun:11(3):474-6 [PubMed PMID: 2546244]
Level 3 (low-level) evidenceBeović B, Pecaric-Meglic N, Marin J, Bedernjak J, Muzlovic I, Cizman M. Fatal human herpesvirus 6-associated multifocal meningoencephalitis in an adult female patient. Scandinavian journal of infectious diseases. 2001:33(12):942-4 [PubMed PMID: 11868775]
Level 3 (low-level) evidenceYoshikawa T, Ohashi M, Miyake F, Fujita A, Usui C, Sugata K, Suga S, Hashimoto S, Asano Y. Exanthem subitum-associated encephalitis: nationwide survey in Japan. Pediatric neurology. 2009 Nov:41(5):353-8. doi: 10.1016/j.pediatrneurol.2009.05.012. Epub [PubMed PMID: 19818937]
Level 3 (low-level) evidenceAl-Zubeidi D, Thangarajh M, Pathak S, Cai C, Schlaggar BL, Storch GA, Grange DK, Watson ME Jr. Fatal human herpesvirus 6-associated encephalitis in two boys with underlying POLG mitochondrial disorders. Pediatric neurology. 2014 Sep:51(3):448-52. doi: 10.1016/j.pediatrneurol.2014.04.006. Epub 2014 Apr 13 [PubMed PMID: 25160553]
Level 3 (low-level) evidenceAbdelrahim NA, Mohamed N, Evander M, Ahlm C, Fadl-Elmula IM. Human herpes virus type-6 is associated with central nervous system infections in children in Sudan. African journal of laboratory medicine. 2022:11(1):1718. doi: 10.4102/ajlm.v11i1.1718. Epub 2022 Sep 22 [PubMed PMID: 36263389]
Mason EE, Printen KJ. Metabolic considerations in reconstitution of the small intestine after jejunoileal bypass. Surgery, gynecology & obstetrics. 1976 Feb:142(2):177-83 [PubMed PMID: 813311]
Level 3 (low-level) evidenceSumala S, Ekalaksananan T, Pientong C, Buddhisa S, Passorn S, Duangjit S, Janyakhantikul S, Suktus A, Bumrungthai S. The Association of HHV-6 and the TNF-α (-308G/A) Promotor with Major Depressive Disorder Patients and Healthy Controls in Thailand. Viruses. 2023 Sep 8:15(9):. doi: 10.3390/v15091898. Epub 2023 Sep 8 [PubMed PMID: 37766304]
Darvish Molla Z, Kalbasi S, Kalantari S, Bidari Zerehpoosh F, Shayestehpour M, Yazdani S. Evaluation of the association between human herpes virus 6 (HHV-6) and Hashimoto's thyroiditis. Iranian journal of microbiology. 2022 Aug:14(4):563-567. doi: 10.18502/ijm.v14i4.10243. Epub [PubMed PMID: 36721502]
de Andrade RP. A multicenter clinical evaluation of a new monophasic combination: Minulet (gestodene and ethinyl estradiol). International journal of fertility. 1989 Sep:34 Suppl():22-30 [PubMed PMID: 2576253]
Ashrafpoor G, Andréoletti L, Bruneval P, Macron L, Azarine A, Lepillier A, Danchin N, Mousseaux E, Redheuil A. Fulminant human herpesvirus 6 myocarditis in an immunocompetent adult: role of cardiac magnetic resonance in a multidisciplinary approach. Circulation. 2013 Dec 3:128(23):e445-7. doi: 10.1161/CIRCULATIONAHA.113.001801. Epub [PubMed PMID: 24297820]
Level 3 (low-level) evidenceLe J, Gantt S, AST Infectious Diseases Community of Practice. Human herpesvirus 6, 7 and 8 in solid organ transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013 Mar:13 Suppl 4():128-37. doi: 10.1111/ajt.12106. Epub [PubMed PMID: 23465006]