Introduction
The interpleural analgesic technique was first described by Reiestad et al. for the treatment of acute postoperative pain.[1]. This technique is believed to enable the spread of injected medication to reach multiple intercostal nerves using a single interpleural injection site. Stromskag and colleagues suggest that after the medication is injected into the interpleural space, it spreads to the intercostal nerves in a retrograde fashion.[2] Therefore, the technique of interpleural analgesia is best suited for thoracic and abdominal pain. When used appropriately, this analgesic modality improves pain control, reduces opioid consumption, and in specific situations may even improve pulmonary function.[3]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
When providing interpleural analgesia, medication is injected into the pleural cavity. Before accessing the pleural cavity, the provider must first pass a needle through the intercostal space of the posterior or lateral thoracic wall. The intercostal spaces contain 3 muscle layers. The external intercostal muscles form the most superficial muscular layer of the intercostal space. The internal intercostal muscles form the next muscular layer. The innermost intercostal muscles form the deepest muscle layer of the intercostal space. A neurovascular bundle, containing an intercostal vein, artery, and nerve, is also located in the intercostal space.
The intercostal nerve is derived from the anterior rami of the thoracic spinal nerve. These rami of the thoracic spinal nerves exit the intervertebral foramina and are typically initially located in the endothoracic fascia, just superficial to the parietal pleura. However, as these nerves course more distally from the neuraxis, they begin to pass superficially at the angle of the ribs. At this point, the nerves will continue their distal course between the internal and innermost intercostal muscles. As these nerves pass through the intercostal space, they tend to be located just under the rib along with the other components of the neurovascular bundles. Two serous membranes form the pleural cavity.[4] These 2 membranes are referred to as the parietal pleura and the visceral pleura. The parietal pleura covers the internal side of the chest wall, while the visceral pleura covers the lungs and other intrathoracic structures, for example, blood vessels, and bronchi, among others. The parietal pleura is adherent to the thoracic wall via the endothoracic fascia.
Indications
Interpleural analgesia may be utilized to treat acute postoperative pain that results from thoracic and upper abdominal surgeries.[1] Multiple studies have found interpleural analgesia to be especially useful in treating the pain that results from cholecystectomies.[3],[5] VadeBoncouer et al. found that interpleural local anesthetic administration not only resulted in decreased opioid consumption but also improved pulmonary function. Furthermore, rib fractures, acute herpes zoster, and pain secondary to upper extremity ischemia are other acute pain syndromes that may be treated with interpleural analgesia. Interpleural analgesia is not specific to acute pain, as chronic pain conditions such as cancer pain, post-herpetic neuralgia, chronic pancreatitis, and complex regional pain syndrome find relief with this intervention.[1]
Contraindications
Absolute Contraindications
These include patient refusal, true local anesthetic allergy, and local infection at the insertion site.[6][7]
Relative Contraindications
Relative contraindications include coagulopathy, phrenic nerve palsy on the contralateral side, and pulmonary pathology that may include:
Equipment
The following equipment is needed for the performance of the interpleural analgesic injection and catheter placement (see Image. Interpleural Catheter Components):
- Ultrasound machine (if the procedure is ultrasound-guided)
- Topical antiseptic
- Sterile gloves, gauze, and towels or drape
- 16- to 18-gauge Tuohy needle
- Multi-orifice catheter
- 25-gauge needle (for anesthetizing procedure site on the awake patient)
- Glass or plastic loss-of-resistance syringe
- Pulse oximetry, a non-invasive blood pressure monitoring device, continuous electrocardiogram
- Bag of crystalloid solution
- Intravenous tubing with a three-way connector
- Local anesthetic (if bupivacaine is used, a concentration of 0.25% to 0.5% and a volume of 10 to 30 mL are recommended)
Preparation
The patient should be placed in either the supine or lateral position. Typically, the procedure site is selected within the region of the thorax spanning from the 4 to 7 intercostal space. The chosen insertion site needs to be immediately cephalad of the rib to avoid direct trauma to the neurovascular bundle, which is located inferior to each rib. If placed in the lateral position, the patient should be lying with the operative site in the dependent position (down). In this position, the posterior axillary line, or a site that is 8 to 10 cm lateral from the midline is identified as the insertion site. If the procedure is to be performed on the patient in the supine position, the anterior or mid-axillary line is used to identify the needle insertion site. The operative area is cleaned with antiseptic, and a sterile drape or towels are placed to prevent contamination.
Technique or Treatment
Before starting the procedure, ensure proper patient and site. Hang an isotonic crystalloid solution with tubing to connect it to a 3-way connector. Connect an empty glass syringe and the 16-gauge Tuohy needle to the remaining 2 ports on the 3-way connector. Ensure that the tubing, needle, 3-way connector, and glass syringe have all been filled with the crystalloid solution before moving on to the next step. Advance the Tuohy needle (A) over the rib and into the intended intercostal space after the procedure site has been anesthetized. Advancement of the needle is performed during expiration to reduce the risk of visceral pleura puncture. Ensure that the 3-way connector is closed to the bag of crystalloid solution (B), but open to allow for continuity between the Tuohy needle and the glass syringe. Using continuous pressure on the loss-of-resistance syringe (C), slowly advance the needle until the parietal pleura has been punctured, which can be confirmed by a loss of resistance on the glass syringe. Once the intrapleural space has been entered, the provider may proceed in 1 of 2 ways: placement of a continuous catheter or administration of a single injection. A continuous catheter is often selected to provide prolonged pain relief through intermittent dosing. Whereas the single injection technique offers short-term pain relief that is limited by the uptake of the medication after injection.
If proceeding with the placement of a continuous catheter, after the loss of resistance has been confirmed, the 3-way connector should be open to three ports to allow the crystalloid solution to flow freely while the syringe is completely depressed. Observing the flow of the crystalloid in the tubing chamber ensures that fluid is flowing through the Tuohy needle. This provides further confirmation that the Tuohy needle tip is in the pleural cavity.[6][7] At this stage, the syringe (C) may be disconnected, and the crystalloid solution should flow out of the port on the 3-way connector that is on the side of the provider (between C and D). This flow of liquid will prevent the entrainment of air into the pleural cavity.
At this point, a multi-orifice catheter may be threaded so that the tip of the catheter is inserted 5 to 10 cm into the pleural cavity. Once the catheter is in place and held firmly by the provider, the Tuohy needle and 3-way connector should be removed from the catheter. Single bolus, intermittent dosing, or continuous infusion may be used for the interpleural catheter. The catheter site should be regularly checked for signs of complications, such as bleeding and infection. For catheter removal, simply ensure a closed system and pull on the catheter with gradual pressure until it is completely removed. Ensure that the catheter tip is intact. Alternatively, if only a single injection is intended, after confirming a loss of resistance and closing the 3-way connector to the provider, the saline syringe may be replaced by a syringe containing injectate (usually local anesthetic). Once the medication is injected, the entire system may be removed. Again, it is important to maintain a closed system once the Tuohy needle has entered the intrapleural space to avoid the entrainment of air.
Complications
The most common complication with the interpleural block is pneumothorax. A retrospective review of 703 procedures found that the incidence of pneumothorax may be as high as 2%.[6][7] However, techniques that avoid the entrainment of air into the intrapleural space may reduce the incidence of pneumothorax when performing the interpleural regional anesthetic. As with any procedure involving the use of local anesthetic, the interpleural block carries the risk of local anesthetic toxicity, especially when large volumes or doses are required for effective analgesia. Additionally, given the proximity of the phrenic nerve and upper thoracic sympathetic ganglia, interpleural analgesia may result in hemidiaphragm paralysis or Horner syndrome. The following is a list of other potential complications:
- Bleeding or hemothorax
- Local infection
- Catheter migration, misplacement, or failed block
- Pleural Effusion
- Bronchial trauma or formation of a bronchopleural fistula
Clinical Significance
Interpleural analgesia provides a safe and effective regional anesthetic modality to improve pain control. How interpleural analgesia works is likely by taking advantage of the retrograde spread of injectate to the intercostal nerves from the pleural cavity. It is particularly effective for thoracic and upper abdominal pain. The procedure may be used in the treatment of acute pain, but also for the treatment of chronic pain. Given the ease of performance, safety, and efficacy, interpleural analgesia may be deemed a reasonable alternative to epidural analgesia.
Enhancing Healthcare Team Outcomes
Acute pain in the chest and thoracic region that often results from local trauma or surgical procedures may result in significant morbidity and mortality.[8],[9] Poor outcomes in these patients are often associated with increased opioid consumption and diminished pulmonary function.[8][9] The use of regional anesthetic techniques improves both pain control and pulmonary function. These techniques include thoracic epidural, thoracic paravertebral, erector spinae, serratus plane, and intercostal blocks. Interpleural analgesia is another regional anesthetic modality that is beneficial in the treatment of thoracic and upper abdominal pain. Although concerns for pneumothorax and local anesthetic toxicity have limited the use of interpleural analgesia, it remains a safe and effective modality that has few complications and contraindications.[6],[7]
Anesthesiologists are often consulted to manage interpleural analgesia for acute pain. Coordination between the consultant and the consulting physician is important in the management of these patients. Cardiothoracic or trauma surgeons may need to be involved in the management of these patients if complications from the procedure have developed. Additionally, the radiologist may be able to provide further insight into the pathology that is causing the pain and determine if complications occur, for example, pneumothorax, and hemothorax, among others. Nurses also play a crucial role in routinely monitoring these patients for acute changes in clinical condition, assessing the adequacy of pain control, and observing the development of complications from interpleural analgesia. Nonetheless, the management of interpleural analgesia requires impeccable communication between all involved clinical personnel, including physicians, nurses, and pharmacists.
After a comprehensive review of current literature, the American Society of Regional Anesthesia and Pain Medicine, the American Society of Anesthesiologists, and the American Pain Society established a clinical practice guideline for the management of postoperative pain. The guideline did not recommend the use of interpleural analgesia for the management of pain after thoracic surgery.[10] The current guideline advises against interpleural analgesia in this setting due to the inconsistent data showing benefits and the potential for systemic local anesthetic toxicity. In contrast, a review of pain management in thoracic trauma found interpleural analgesia to be a reasonable option for pain control, especially when other modalities such as the thoracic epidural or paravertebral are contraindicated.[8]
Media
(Click Image to Enlarge)
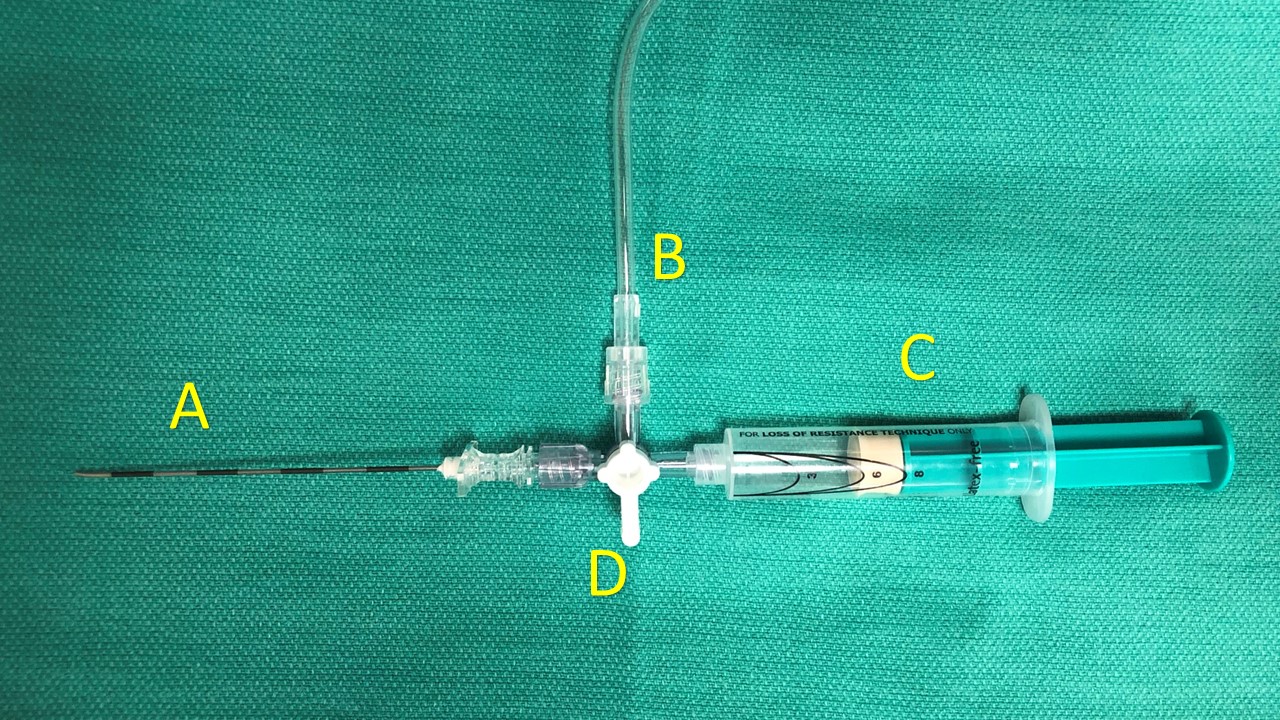
Interpleural Catheter Components. This image portrays an arrangement of components that may be used to place an interpleural catheter. The individual components include an 18 g Tuohy needle (A), intravenous tubing (B), a plastic loss-of-resistance syringe (C), and a three-way connector (D). Each port of the three-way connector (D) may be closed by moving the white handle in line with the port.
Contributed by S Dhanjal, MD
References
Murphy DF. Interpleural analgesia. British journal of anaesthesia. 1993 Sep:71(3):426-34 [PubMed PMID: 8398528]
Strømskag KE, Hauge O, Steen PA. Distribution of local anesthetics injected into the interpleural space, studied by computerized tomography. Acta anaesthesiologica Scandinavica. 1990 May:34(4):323-6 [PubMed PMID: 2343736]
Level 1 (high-level) evidenceVadeBoncouer TR, Riegler FX, Gautt RS, Weinberg GL. A randomized, double-blind comparison of the effects of interpleural bupivacaine and saline on morphine requirements and pulmonary function after cholecystectomy. Anesthesiology. 1989 Sep:71(3):339-43 [PubMed PMID: 2672898]
Level 1 (high-level) evidenceCharalampidis C,Youroukou A,Lazaridis G,Baka S,Mpoukovinas I,Karavasilis V,Kioumis I,Pitsiou G,Papaiwannou A,Karavergou A,Tsakiridis K,Katsikogiannis N,Sarika E,Kapanidis K,Sakkas L,Korantzis I,Lampaki S,Zarogoulidis K,Zarogoulidis P, Pleura space anatomy. Journal of thoracic disease. 2015 Feb [PubMed PMID: 25774304]
Strömskag KE, Reiestad F, Holmqvist EL, Ogenstad S. Intrapleural administration of 0.25%, 0.375%, and 0.5% bupivacaine with epinephrine after cholecystectomy. Anesthesia and analgesia. 1988 May:67(5):430-4 [PubMed PMID: 3364761]
Level 1 (high-level) evidenceDravid RM, Paul RE. Interpleural block - part 1. Anaesthesia. 2007 Oct:62(10):1039-49 [PubMed PMID: 17845657]
Dravid RM, Paul RE. Interpleural block - part 2. Anaesthesia. 2007 Nov:62(11):1143-53 [PubMed PMID: 17924896]
Karmakar MK, Ho AM. Acute pain management of patients with multiple fractured ribs. The Journal of trauma. 2003 Mar:54(3):615-25 [PubMed PMID: 12634549]
Kundra P, Varadharajan R, Yuvaraj K, Vinayagam S. Comparison of paravertebral and interpleural block in patients undergoing modified radical mastectomy. Journal of anaesthesiology, clinical pharmacology. 2013 Oct:29(4):459-64. doi: 10.4103/0970-9185.119133. Epub [PubMed PMID: 24249981]
Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, Carter T, Cassidy CL, Chittenden EH, Degenhardt E, Griffith S, Manworren R, McCarberg B, Montgomery R, Murphy J, Perkal MF, Suresh S, Sluka K, Strassels S, Thirlby R, Viscusi E, Walco GA, Warner L, Weisman SJ, Wu CL. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. The journal of pain. 2016 Feb:17(2):131-57. doi: 10.1016/j.jpain.2015.12.008. Epub [PubMed PMID: 26827847]
Level 1 (high-level) evidenceChen Y,Cai Y,Ye Y,Xia Y,Papadimos TJ,Liu L,Xu X,Wang Q,Shi K,Wu Y, Single and Repeated Intrapleural Ropivacaine Administration: A Plasma Concentration and Pharmacodynamics Study. Journal of pain research. 2021; [PubMed PMID: 33776475]