Introduction
The heart's electrical conduction system operates through structures composed of specialized pacemaker cells. The primary pacemaker is the sinoatrial node, located subepicardially in the right atrium wall near the junction with the superior vena cava. The atrioventricular node lies subendocardially within the triangle of Koch, which is bordered posteriorly by the coronary sinus ostium, superiorly by the tendon of Todaro, and anteriorly by the septal leaflet of the tricuspid valve. The atrioventricular node tapers into the bundle of His, which bifurcates into right and left bundle branches that course along the interventricular septum and give rise to Purkinje fibers in the subendocardial regions of the ventricles.
The sinoatrial node receives blood supply from the sinoatrial nodal artery, a branch of the right coronary artery in approximately 60% of individuals and of the left circumflex coronary artery in the remaining 40%. The atrioventricular node is supplied by the atrioventricular nodal branch, most commonly from the right coronary artery (90%) and less frequently from the left circumflex artery (10%), depending on whether the coronary circulation is right- or left-dominant. Under normal conditions, electrical impulses originate in the sinoatrial node, initiating atrial contraction. The impulse then travels to the atrioventricular node, where conduction slows briefly before continuing through the bundle of His and Purkinje fibers to trigger ventricular contraction.
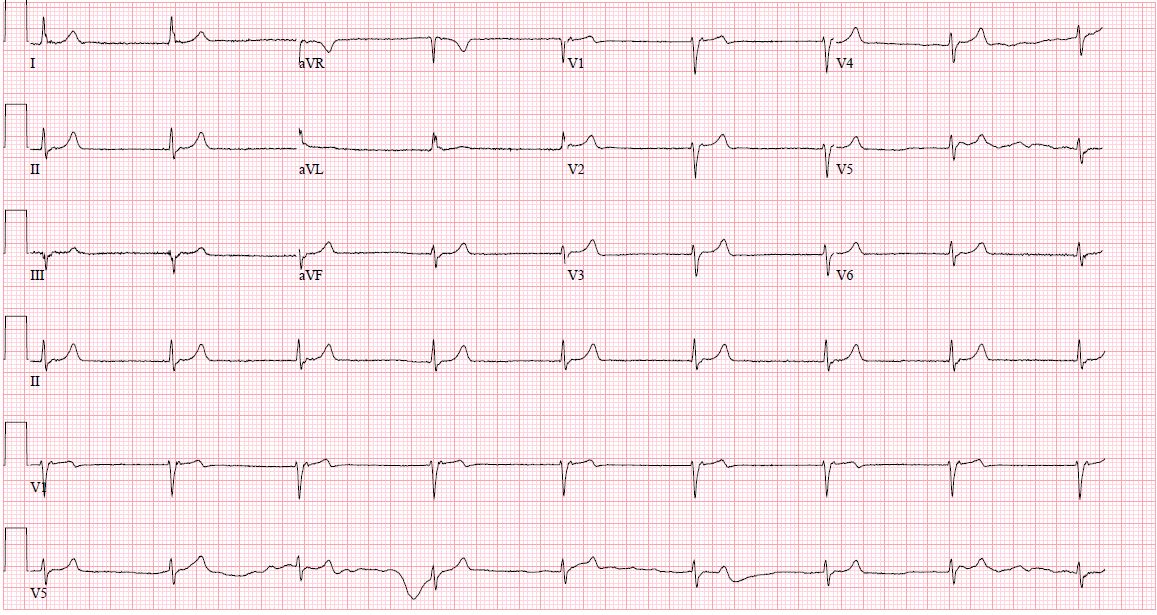
Each region containing specialized pacemaker cells can independently sustain the heart rate. Under physiologic conditions, secondary pacemaker sites remain suppressed due to overdrive inhibition by the faster depolarization rate of the sinoatrial node, maintaining normal sinus rhythm. If the sinoatrial node slows to a rate below that of another pacemaking structure, the faster region assumes control as the dominant pacemaker. Since each region has a distinct intrinsic depolarization rate, the resulting heart rate and rhythm depend on which site becomes predominant. This mechanism underlies the various forms of junctional rhythm (see Image. Junctional Rhythm).[1][2][3][4][5][6]
The terminology used to classify junctional rhythms is based on the ventricular rate and includes the following categories:
- Junctional bradycardia: Heart rate below 40 bpm
- Junctional escape rhythm: Heart rate between 40 and 60 bpm
- Accelerated junctional rhythm: Heart rate between 60 and 100 bpm
- Junctional tachycardia: Heart rate exceeding 100 bpm
Classifying junctional rhythms by rate enables precise identification of underlying electrophysiologic disturbances. Such distinctions are critical for optimizing patient care.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
A junctional rhythm develops when electrical impulses from the sinoatrial node are blocked or fall below the intrinsic automaticity of the atrioventricular node or bundle of His. Multiple conditions and medications can impair sinoatrial node function and precipitate a junctional rhythm.[7][8][9][10][11][12][13][14]
The most commonly reported triggers fall into the following categories:
- Structural and inflammatory causes: Chest trauma, myocarditis, pericarditis, radiation therapy, repair of congenital heart disease, rheumatic fever, ischemic heart disease, acute myocardial infarction, acute and chronic coronary artery disease, amyloidosis, Lyme disease
- Conduction system disorders: Sick sinus syndrome, familial conduction disorders, inherited channelopathy, high-grade second-degree heart block, third-degree heart block
- Neuromuscular conditions: Neuromuscular disorders, X-linked muscular dystrophy
- Systemic conditions: collagen vascular disease, hypothyroidism, sleep apnea, hypoxia
- Neurologic conditions: Intracranial hypertension, anorexia nervosa
- Autonomic and reflex causes: Vasovagal stimulation (eg, endotracheal suctioning), carotid sinus hypersensitivity
- Electrolyte and metabolic disturbances: Hyperkalemia
- Medications: Clonidine, reserpine, adenosine, cimetidine, antiarrhythmics (class I to IV), lithium, amitriptyline, β-blockers, calcium channel blockers, digoxin, ivabradine, opioids, cannabinoids, isoproterenol infusion
A wide range of pathologies and pharmacologic agents can precipitate junctional rhythms by disrupting sinoatrial node automaticity. Identifying these causes enables precise treatment strategies.
Epidemiology
A junctional rhythm may occur in any patient population and affects females and males equally. Morbidity and mortality associated with this rhythm depend directly on the underlying cause. Consequently, junctional rhythm can represent a benign, intermittent finding in healthy children, athletes, or individuals with increased vagal tone during sleep. This rhythm is also frequently reported as a complication following cardiac procedures, including transcatheter aortic valve replacement and surgeries for congenital cardiac disorders.[15][16]
Pathophysiology
The intrinsic depolarization rate decreases at each successive pacemaker site as the electrical impulse travels through the heart’s conduction system. Junctional rhythms commonly result from sinoatrial node dysfunction, causing its intrinsic rate to slow or cease. In rare cases, increased automaticity of the atrioventricular node may allow it to override a normally functioning sinoatrial node. Consequently, junctional rhythms may present with rates slower than, equal to, or faster than the usual heart rate.
Intrinsic pacemaker sites and their depolarization rates are as follows:
- Sinoatrial node: 60 to 100 bpm
- Atria: less than 60 bpm
- Atrioventricular node: 40 to 60 bpm
- Ventricles: 20 to 40 bpm
Junctional rhythms reflect shifts in the dominant pacemaker driven by changes in intrinsic depolarization rates. Recognizing these rate patterns aids in diagnosing and tailoring treatment for this type of arrhythmia.
Histopathology
Histopathological findings vary according to the underlying cause of the junctional rhythm. Conditions contributing to sinoatrial node remodeling include myocardial fibrosis, atrophy, hypoplasia, and amyloid deposition. However, histological abnormalities may be absent despite sinoatrial node dysfunction.[17][18]
History and Physical
Patients with junctional rhythm may present with various symptoms or be asymptomatic. Key history elements include underlying heart disease, recent cardiac procedures, medications, drug use, and a thorough review of systems to identify potential triggers. A history of congenital heart disorder or lack of standard newborn screening warrants particular concern in pediatric patients.
Symptoms largely depend on the underlying cause of the junctional rhythm. For example, a patient with heart failure exacerbation may experience shortness of breath and lower extremity edema. Individuals with rheumatic fever may present with a heart murmur caused by valvular damage, fever, joint pain, and rash. Other patients might report nonspecific symptoms such as dizziness, fatigue, syncope or near-syncope, and palpitations. Physical examination may reveal nonspecific findings, including pulsating veins and a regular heart rate ranging from 20 to over 100 bpm.[19][20][21]
Evaluation
History and physical examination are critical components in assessing a patient presenting with signs and symptoms that may be attributed to a junctional rhythm. Hemodynamic stability should be evaluated, and the examination must include vital signs such as respiratory rate, blood pressure, temperature, and heart rate. The history should encompass a review of the patient’s medication list. Diagnostic workup must include an electrocardiogram (ECG). The patient should be evaluated for underlying ischemic heart disease or heart failure. Consideration should be given to thyroid function testing, routine blood tests, and echocardiography as part of the initial assessment.
Normal P waves measure less than 0.12 seconds (3 small boxes) and appear upright in leads I and II, and inverted in aVR. Inverted P waves in the inferior leads (II, III, aVF) indicate a non-sinoatrial origin. In junctional rhythms, the electrical impulse often arises at or near the atrioventricular node, frequently rendering the P wave invisible. When visible, the P wave may occur just before or after the QRS complex or be embedded within it. The morphology of visible P waves is often inverted or retrograde rather than upright. Other specific ECG findings depend on the precise classification of the junctional rhythm. (Source: Bhattad and Jain, 2020)
Treatment / Management
Treatment of a junctional rhythm primarily depends on its underlying cause. Termination should be avoided when the rhythm results from sinus node dysfunction that causes asystole or bradycardia, as the junctional rhythm maintains the heart rate. Therefore, identifying the underlying etiology is essential before establishing a management plan. Otherwise healthy individuals with a junctional rhythm who are asymptomatic typically require no medical intervention. This rhythm usually reflects increased vagal tone, which suppresses the sinoatrial node’s intrinsic automaticity.
In cases of digoxin toxicity, treatment includes atropine and digoxin-specific antibodies. If pharmacologic therapy fails and junctional tachycardia develops, intravenous phenytoin may be administered in a monitored setting due to the risk of hypotension. In the pediatric population, persistent symptomatic junctional tachycardia warrants consideration of percutaneous radiofrequency ablation. A permanent pacemaker implantation is indicated in patients with sick sinus syndrome or complete or high-grade atrioventricular block.[22][23][24][25][26](B2)
Differential Diagnosis
The differential diagnosis includes the following:
- Digoxin toxicity
- Atrioventricular nodal reentrant tachycardia
- Atrioventricular reentrant tachycardia
- Sinus node dysfunction
- High-grade second-degree heart block
- Third-degree heart block
Given the overlap in presentation, a detailed assessment is required to differentiate junctional rhythm from other supraventricular arrhythmias and conduction blocks. Proper identification ensures timely and targeted intervention.
Prognosis
As with management, outcomes depend largely on the underlying etiology and the specific type of junctional rhythm. For instance, congenital junctional ectopic tachycardia in pediatric patients carries higher morbidity and mortality compared to the postoperative form of the same arrhythmia. Prompt identification and treatment of the rhythm and its underlying cause are essential for optimizing outcomes. For additional information, see StatPearls’ companion reference about junctional ectopic tachycardia.[27]
Complications
Symptoms such as syncope, fatigue, or dizziness may arise when junctional rhythms are not promptly identified. Some forms can result in cardiac dilation, leading to congestive heart failure or progression to more serious arrhythmias such as ventricular fibrillation or complete heart block. Others may cause severe hypotension, particularly in patients with underlying autonomic dysfunction.[28]
Deterrence and Patient Education
Patient education should be provided using accessible and familiar resources, such as pamphlets or trusted online platforms. Patients should be encouraged to track symptoms like palpitations or dizziness and report any changes promptly. Emphasizing medication adherence and regular follow-up visits can also support early detection and management of recurrent or worsening junctional rhythms.
Pearls and Other Issues
Integrating clinical history with focused diagnostic testing can expedite management and improve outcomes. For example, in patients with a junctional rhythm taking digoxin, a serum digoxin level should always be obtained to evaluate for possible toxicity. In patients with a history of tick exposure, empirical antibiotic therapy should be initiated while awaiting Lyme disease serologic results, as early treatment may prevent progression of underlying infectious causes.
Enhancing Healthcare Team Outcomes
Junctional rhythms encompass multiple subtypes with varied presentations, prognoses, and potential complications. They can arise from a wide range of conditions, diseases, and pharmacologic agents. When a junctional rhythm is identified, awareness and role-specific competence among all members of the healthcare team are essential.
Effective management is best achieved through an interprofessional approach. Nurses often serve as the first to recognize junctional rhythms on telemetry monitoring. Initial evaluation is typically performed by primary care or emergency medicine clinicians, with cardiology referral warranted for most new diagnoses. Cardiologists provide ongoing care in both inpatient and outpatient settings.
Cardiology and emergency nurses are critical in administering treatments, monitoring responses, and collaborating with physicians or advanced practice providers to detect complications. Pharmacists review medications, identify potential drug interactions, and counsel patients on appropriate use and side effects. Educating at-risk patients and ensuring closed-loop communication between them and their healthcare team further optimizes the management of junctional rhythms.
Media
(Click Image to Enlarge)
References
Spodick DH. Normal sinus heart rate: sinus tachycardia and sinus bradycardia redefined. American heart journal. 1992 Oct:124(4):1119-21 [PubMed PMID: 1529897]
Thery C, Gosselin B, Lekieffre J, Warembourg H. Pathology of sinoatrial node. Correlations with electrocardiographic findings in 111 patients. American heart journal. 1977 Jun:93(6):735-40 [PubMed PMID: 871100]
Dobrzynski H, Anderson RH, Atkinson A, Borbas Z, D'Souza A, Fraser JF, Inada S, Logantha SJ, Monfredi O, Morris GM, Moorman AF, Nikolaidou T, Schneider H, Szuts V, Temple IP, Yanni J, Boyett MR. Structure, function and clinical relevance of the cardiac conduction system, including the atrioventricular ring and outflow tract tissues. Pharmacology & therapeutics. 2013 Aug:139(2):260-88. doi: 10.1016/j.pharmthera.2013.04.010. Epub 2013 Apr 20 [PubMed PMID: 23612425]
Level 3 (low-level) evidenceVan der Hauwaert LG, Stroobandt R, Verhaeghe L. Arterial blood supply of the atrioventricular node and main bundle. British heart journal. 1972 Oct:34(10):1045-51 [PubMed PMID: 5086972]
Pejković B, Krajnc I, Anderhuber F, Kosutić D. Anatomical aspects of the arterial blood supply to the sinoatrial and atrioventricular nodes of the human heart. The Journal of international medical research. 2008 Jul-Aug:36(4):691-8 [PubMed PMID: 18652764]
Chua K, Upadhyay GA, Lee E, Aziz Z, Beaser AD, Ozcan C, Broman M, Nayak HM, Tung R. High-resolution mapping of the triangle of Koch: Spatial heterogeneity of fast pathway atrionodal connections. Heart rhythm. 2018 Mar:15(3):421-429. doi: 10.1016/j.hrthm.2017.10.030. Epub 2017 Nov 26 [PubMed PMID: 29081398]
Semelka M, Gera J, Usman S. Sick sinus syndrome: a review. American family physician. 2013 May 15:87(10):691-6 [PubMed PMID: 23939447]
Trappe HJ. Tachyarrhythmias, bradyarrhythmias and acute coronary syndromes. Journal of emergencies, trauma, and shock. 2010 Apr:3(2):137-42. doi: 10.4103/0974-2700.62112. Epub [PubMed PMID: 20606790]
Silvestri NJ, Ismail H, Zimetbaum P, Raynor EM. Cardiac involvement in the muscular dystrophies. Muscle & nerve. 2018 May:57(5):707-715. doi: 10.1002/mus.26014. Epub 2017 Nov 28 [PubMed PMID: 29130502]
Patel P, Kelschenbach K. Case of Junctional Rhythm in the Setting of Acute Adrenal Insufficiency. Cureus. 2022 Aug:14(8):e27605. doi: 10.7759/cureus.27605. Epub 2022 Aug 2 [PubMed PMID: 36059370]
Level 3 (low-level) evidenceEid MM. COVID-19 patient with symptomatic bradycardia. Visual journal of emergency medicine. 2021 Jan:22():100920. doi: 10.1016/j.visj.2020.100920. Epub 2020 Nov 11 [PubMed PMID: 33200102]
Frustaci A, Verardo R, Galea N, Lavalle C, Bagnato G, Scialla R, Chimenti C. Hypersensitivity Myocarditis after COVID-19 mRNA Vaccination. Journal of clinical medicine. 2022 Mar 16:11(6):. doi: 10.3390/jcm11061660. Epub 2022 Mar 16 [PubMed PMID: 35329986]
Vishnu VK, Jamshed N, Amrithanand VT, Thandar S. BRASH Syndrome: A Case Report. The Journal of emergency medicine. 2021 Jun:60(6):818-822. doi: 10.1016/j.jemermed.2021.01.033. Epub 2021 Feb 24 [PubMed PMID: 33640217]
Level 3 (low-level) evidenceDrumheller BC, Tuffy E, Gibney F, Stallard S, Siewers C, Korvek S. Severe bradycardia from severe hyperkalemia: Patient characteristics, outcomes and factors associated with hemodynamic support. The American journal of emergency medicine. 2022 May:55():117-125. doi: 10.1016/j.ajem.2022.03.007. Epub 2022 Mar 10 [PubMed PMID: 35306438]
Romhilt DW, Doyle M, Sagar KB, Hastillo A, Wolfgang TC, Lower RR, Hess ML. Prevalence and significance of arrhythmias in long-term survivors of cardiac transplantation. Circulation. 1982 Aug:66(2 Pt 2):I219-22 [PubMed PMID: 6177443]
Angsubhakorn N, Anderson M, Akdemir B, Bertog S, Garcia S, Sharma A, Tholakanahalli V, Adabag S. Prevalence and Implications of Junctional Rhythm During Transcatheter Aortic Valve Replacement. Cardiovascular revascularization medicine : including molecular interventions. 2021 May:26():61-62. doi: 10.1016/j.carrev.2020.11.014. Epub 2020 Nov 12 [PubMed PMID: 33203581]
Rodriguez RD, Schocken DD. Update on sick sinus syndrome, a cardiac disorder of aging. Geriatrics. 1990 Jan:45(1):26-30, 33-6 [PubMed PMID: 2403955]
Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004 Aug 24:110(8):897-903 [PubMed PMID: 15302799]
Kim D, Shinohara T, Joung B, Maruyama M, Choi EK, On YK, Han S, Fishbein MC, Lin SF, Chen PS. Calcium dynamics and the mechanisms of atrioventricular junctional rhythm. Journal of the American College of Cardiology. 2010 Aug 31:56(10):805-12. doi: 10.1016/j.jacc.2010.03.070. Epub [PubMed PMID: 20797495]
Level 3 (low-level) evidenceCools E, Missant C. Junctional ectopic tachycardia after congenital heart surgery. Acta anaesthesiologica Belgica. 2014:65(1):1-8 [PubMed PMID: 24988822]
Di Biase L, Gianni C, Bagliani G, Padeletti L. Arrhythmias Involving the Atrioventricular Junction. Cardiac electrophysiology clinics. 2017 Sep:9(3):435-452. doi: 10.1016/j.ccep.2017.05.004. Epub [PubMed PMID: 28838549]
Tuohy S, Saliba W, Pai M, Tchou P. Catheter ablation as a treatment of atrioventricular block. Heart rhythm. 2018 Jan:15(1):90-96. doi: 10.1016/j.hrthm.2017.08.015. Epub 2017 Aug 18 [PubMed PMID: 28823599]
Antman EM, Wenger TL, Butler VP Jr, Haber E, Smith TW. Treatment of 150 cases of life-threatening digitalis intoxication with digoxin-specific Fab antibody fragments. Final report of a multicenter study. Circulation. 1990 Jun:81(6):1744-52 [PubMed PMID: 2188752]
Level 2 (mid-level) evidenceHauptman PJ, Kelly RA. Digitalis. Circulation. 1999 Mar 9:99(9):1265-70 [PubMed PMID: 10069797]
Kelly RA, Smith TW. Recognition and management of digitalis toxicity. The American journal of cardiology. 1992 Jun 4:69(18):108G-118G; disc. 118G-119G [PubMed PMID: 1626485]
Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007 Apr 10:115(14):1921-32 [PubMed PMID: 17420362]
Level 3 (low-level) evidenceAshraf M, Collier SA. Junctional Ectopic Tachycardia. StatPearls. 2025 Jan:(): [PubMed PMID: 32809686]
Misumi I, Yamakawa M, Harada M, Sato K, Tabira A, Usuku H, Tsujita K. Severe hypotension during junctional rhythm in a patient with multiple cerebral infarcts. Journal of cardiology cases. 2023 Feb:27(2):84-87. doi: 10.1016/j.jccase.2022.10.010. Epub 2022 Oct 29 [PubMed PMID: 36788952]
Level 3 (low-level) evidence