Introduction
A labyrinthine, or perilymphatic, fistula is an abnormal communication between the inner ear and a neighboring space, most often the middle ear or mastoid cavity. Connections with the intracranial space are less common, with fistulas into the carotid and facial nerve canals being rarer still. Most cases of perilymphatic fistula (PLF) are associated with some traumatic event, but 20% to 40% remain idiopathic even after a thorough evaluation.[1][2]
Idiopathic PLF may be especially challenging to recognize, as the symptoms, particularly mild or intermittent manifestations, are otologically nonspecific, including hearing loss, disequilibrium, aural fullness, autophony, and tinnitus. Of note, some authors differentiate between the terms "labyrinthine fistula" and "perilymphatic fistula," defining the former as a defect in the dense otic capsule bone that surrounds the inner ear, thus exposing the membranous labyrinth, while the latter is a subset of the former that also involves disruption of the membranous labyrinth and leakage of perilymph. In this activity, however, the 2 terms will be used interchangeably.
Clinical interest in the PLF phenomenon arose initially in the 1960s as the stapedectomy procedure for otosclerosis gained popularity. Escape of perilymph through the oval window during manipulation of the stapes footplate, poor sealing of the oval window membrane around the stapes piston prosthesis, and malposition or postoperative migration of the prosthesis have all been implicated as potential causes of PLF.[3] During the subsequent decade, however, reports of similar clinical presentations to those of postoperative stapedectomy patients began to appear, but occurring in patients without prior otologic surgery.[4] Further research resulted in Goodhill's early hypothesis of "implosive" and "explosive" routes of PLF development, with the former including increased intracranial pressure and barotrauma, eg, Valsalva maneuvers, pinched-nose sneezing, and straining.[5][6]
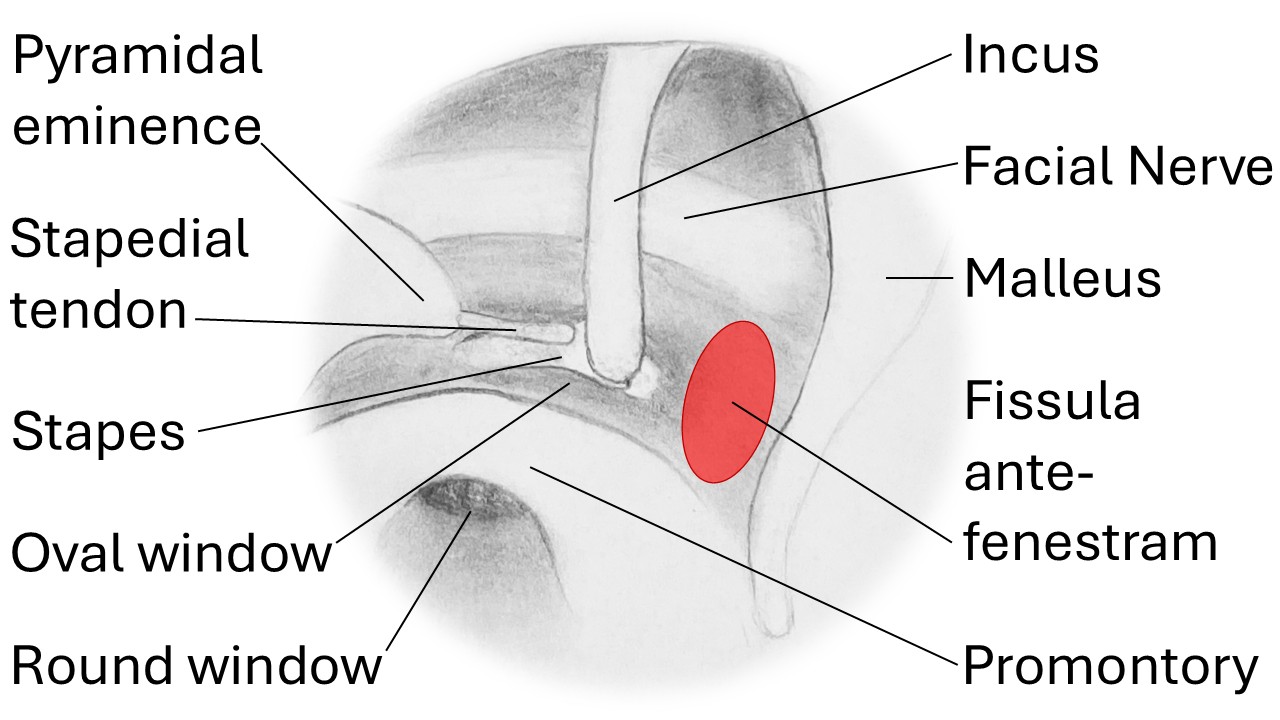
While the oval and round windows (or vestibular and cochlear fenestrae, respectively) may be the most common locations for PLFs to occur, several other sites have been reported as well, eg, the fissula ante fenestram and the horizontal semicircular canal, depending upon the patient's medical history and anatomy (see Image. Internal Ear). The inner ear consists of both hearing and balance elements, with membrane-bound endolymphatic and perilymphatic compartments spanning the 2 sections (see Images. Cochlea, Cross Section and Internal Ear or Labyrinth, Transverse Section).
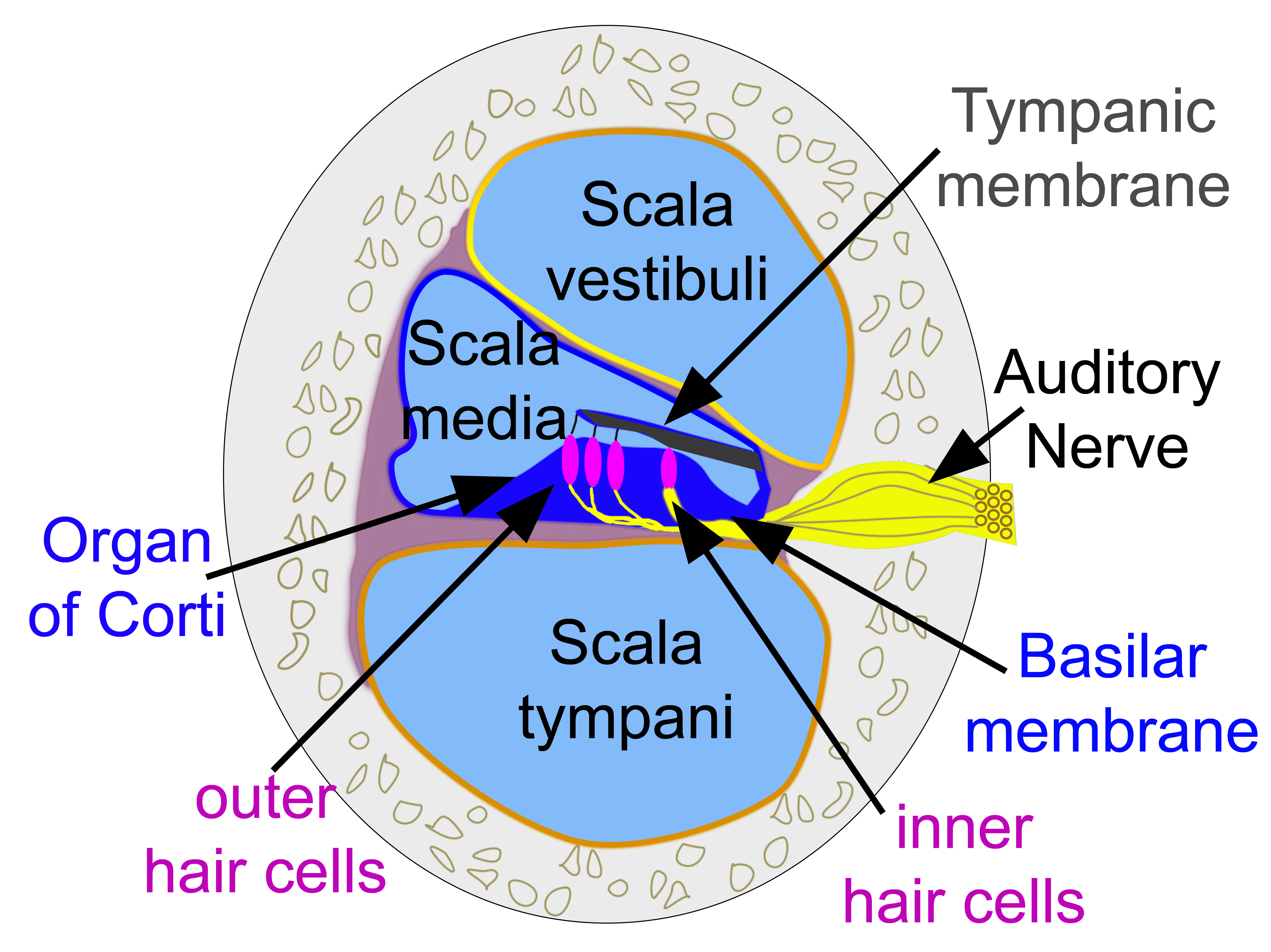
For this reason, a decrease in perilymph volume relative to that of endolymph may result in auditory and/or vestibular symptoms. Within the cochlea, the endolymph is contained in the scala media, while the perilymph resides inside the scala tympani and the scala vestibuli, which are contiguous via the helicotrema at the cochlear apex (see Image. Cochlea and Vestibule). The scala tympani ends at the round window and the scala vestibuli at the oval window, beneath the stapes footplate. Anterior to the oval window lies the fissula ante fenestram, a small bony cleft filled with connective tissue that, if incompletely closed during fetal development or traumatically disrupted, may provide a connection between the middle ear space and the vestibule of the inner ear (see Image. View of the right middle ear.).
Perilymph efflux may also occur via bony microfissures that have been observed extending between the ampulla of the posterior semicircular canal and the round window. Beyond these normal anatomical structures, however, a broad range of variations may predispose patients to PLF formation, including congenital enlargement of the vestibular aqueduct, Mondini malformation, and erosion of the horizontal semicircular canal by cholesteatoma.[7]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
PLFs typically result from mechanical injuries, although some patients may be more predisposed to develop PLFs than others, making predicting what may or may not cause a fistula nearly impossible. The most common etiologies are traumatic and iatrogenic, with roughly 1% of stapes procedures resulting in PLF.[8] Traumatic insults may be pressure-related, eg, a blast injury or even a stifled sneeze, penetrating, as from a foreign body that passes though the tympanic membrane or a cholesteatoma that erodes into the inner ear, or blunt, which may be associated with a temporal bone fracture.[9]
Temporal bone fractures have historically been classified as either longitudinal or transverse, describing the course of the fracture relative to the long axis of the temporal bone's petrous pyramid; longitudinal fractures are more likely to result in conductive hearing loss due to hemotympanum and disruption of the ossicular chain (particularly the incudomalleolar joint), while transverse fractures are more likely to disrupt the otic capsule, causing vertigo and sensorineural hearing loss from the resulting PLF as well as potential facial paralysis.[10][11] PLFs caused by physical exertion like weightlifting, coughing, or sneezing are thought to result from increased cerebrospinal fluid (CSF) pressure that is transferred to the inner ear via the cochlear aqueduct or the internal auditory canal.[12] More unusual causes of PLF reported in the literature include whiplash injuries, lightning strikes, airbag injuries, straining during childbirth, and acoustic trauma from the sirens of emergency vehicles.[3]
PLFs occur in 4% to 15% of cholesteatomas arising from chronic otitis media.[13] The horizontal semicircular canal is the most common location, although other sites within the otic capsule may also be affected.[14] When erosion of the horizontal canal occurs due to cholesteatoma, clinicians should have a high index of suspicion for damage to the facial nerve canal or the tegmen tympani/floor of the middle cranial fossa as well, which occurs in 60% and 39% of cholesteatoma-related PLF.[15]
Beyond characterizing barotraumatic PLFs as either implosive or explosive, Matsuda and colleagues developed the following classification system for PLFs based on presumed etiology.
- Category 1: PLF etiologies include fistulas resulting from trauma, otologic disease, and otologic surgery
- Category 2: PLFs caused by external barotrauma, either high pressure (eg, diving, blast injury, and boxing the ear) or low pressure (eg, air travel)
- Category 3: Barotrauma from internal sources, eg, coughing, sneezing, and Valsalva maneuvers
- Category 4: PLFs of idiopathic origin [16]
However, the clinical relevance of this schema is debatable and has not gained wide acceptance.
Epidemiology
Labyrinthine fistulas are rare; their incidence and prevalence are not well characterized, although Fiedler and colleagues estimate a prevalence of 1.5 per 100,000 adults.[17] In cases of chronic otitis media with cholesteatoma, the prevalence of PLFs is reported to range between 4% and 15%.[13] Children with idiopathic hearing loss, on the other hand, may have congenital PLFs in ≥6% of cases.[18] A 2024 study published by Sasaki et al used a biomarker specific for perilymph, cochlin-tomoprotein, to identify PLFs in 22% of sudden sensorineural hearing loss patients, with a higher proportion of elderly patients testing positive.[19] The incidence of PLF is higher in women than in men, potentially as a consequence of the higher incidence of otosclerosis in women, which makes women more likely to undergo stapes surgery.
Pathophysiology
The symptoms PLFs cause can be divided into vestibular and auditory, with disequilibrium occurring approximately twice as frequently as hearing loss.[20] The means by which these symptoms are produced is unclear and may depend on multiple mechanisms. One potential cause is the "third window" effect, in which a dehiscence in the otic capsule bone allows the endosteal membrane that contains the perilymph to vibrate in the same way that the oval and round windows do (see Image. Computed Tomography of Temporal Bones).
While the mobility of the round window is critical to allow vibrations from the stapes footplate and oval window to propagate along the basilar membrane of the cochlea and stimulate the hair cells' stereocilia during normal sound conduction, the addition of a third window, with or without actual leakage of perilymph, may cause loss of vibratory energy in what should otherwise be a closed system, thus resulting in conductive hearing loss. The presence of a third window through which vibratory energy may escape can also alter how fluid waves propagate through the labyrinth, causing sound pressure waves to deflect the cupula of a semicircular canal and produce a sensation of motion or vertigo when no movement is occurring.
Besides the third window effect, imaging of temporal bones with PLFs often shows pneumatosis of the labyrinth, which also impedes fluid wave propagation through the cochlea.[9] Symptom reduction after exploratory tympanotomy and PLF repair has been reported to correspond to radiographically apparent reduction in the volume of air within the labyrinth.[21] The amount of air visible on a patient's imaging may also fluctuate, which is consistent with the often fluctuating nature of PLF patients' symptoms.
According to Black and colleagues, approximately half of PLFs occur at the oval window (52%), with 20% at the round window, and 22% occurring at both sites.[20] Muntarbhorn and Webber further associated the site of the fistula with the reported mechanism, noting that blunt head trauma tends to produce a fistula at the oval window, while barotraumatic and idiopathic fistulas are most likely to develop at the round window.[22]
History and Physical
Clinical History
A thorough history and careful physical examination are critical, as a PLF can easily be overlooked or misdiagnosed. Patients with labyrinthine fistulas complain of hearing loss or disequilibrium, often induced by loud sounds. Aural fullness and autophony, in which patients describe hearing their own voices more loudly than usual, may occur as well.[23] Tinnitus is not commonly encountered with PLFs, although roaring tinnitus has been reported at the onset of symptoms, similar to that described with Ménière disease. While many patients are unable to identify or recall an inciting event that preceded the onset of symptoms, 50% to 80% of patients will.[16]
A labyrinthine fistula should be suspected in patients who sustain temporal bone fracture, penetrating ear injury, or potential barotrauma situations, including the Valsalva maneuver, nose blowing, blast injuries, air travel, and SCUBA diving, before symptom onset.[3] The primary symptoms of a traumatic PLF are sensorineural hearing loss (15%) and dizziness (64%).[14] Symptoms may appear immediately after the inciting event or arise progressively over several days. Additionally, the anatomic defects required to produce PLFs are often so small that they may close spontaneously and recur with minimal additional trauma, leading to fluctuations in symptoms that further hinder diagnosis.
Posttraumatic vestibular dysfunction often presents as disequilibrium, severe vertigo, or positional dizziness.[12] A PLF should also be suspected if a patient has vestibular disturbances or hearing loss after undergoing a stapedectomy. The stapes prosthesis may become dislodged postoperatively, leading to perilymph leakage. Dizziness may also result after stapes surgery; however, it may also result from the inadvertent use of an excessively long prosthesis that contacts and stimulates the vestibular saccule when it vibrates, causing sound-induced vertigo. For this reason, stapes surgery is often performed with the patient conscious during prosthesis placement, thereby permitting the surgeon to ensure the selected prosthesis is of the appropriate length. Finally, a PLF should be suspected in patients with suggestive symptomatology in the setting of chronic otitis media. Development of cholesteatoma may lead to erosion of the otic capsule bone, causing a third window or even a fistula proper, most often where the horizontal semicircular canal protrudes into the mastoid antrum.
Physical Examination
Several physical examination techniques are employed to screen for PLFs. Otoscopy may reveal a cholesteatoma by identifying tympanic membrane retraction or perforation and the presence of squamous debris or a keratin pearl, most often in the posterosuperior portion of the ear drum (see Image. Cholesteatoma). Alternatively, a hemotympanum in the setting of head trauma may indicate a temporal bone fracture, associated clear rhinorrhea likely representing a CSF leak.
The so-called "fistula test" is also easy to employ and will typically elicit a Hennebert sign (evoked nystagmus) if a PLF is present. This maneuver is performed by applying pulsatile pressure to the tragus to induce pressure changes in the external auditory canal, which are then transmitted to the middle and inner ear via tympanic membrane vibrations. Alternatively, air insufflation during pneumatic otoscopy may be more effective. A positive test results in nystagmus, typically accompanied by a sensation of dizziness.
A similar finding is the Tullio phenomenon, in which dizziness and nystagmus occur with exposure to loud sounds. A tuning fork examination should also be performed, which may help identify conductive or sensorineural hearing loss, although audiometry is a more conclusive test.[9] The direction of the nystagmus may help to localize the labyrinthine defect, with horizontal nystagmus alone indicating horizontal semicircular canal involvement and torsional nystagmus with an upward slow phase indicating superior canal dehiscence.[24][25] Horizontal nystagmus with a torsional component whose slow phase beats downward most likely results from posterior semicircular canal dehiscence.[26]
Office-based vestibular testing
Office-based vestibular examination should also include evaluation of the vestibulo-ocular and vestibulo-spinal reflexes. Head shake and head thrust testing will help to identify unilateral vestibular hypofunction by eliciting corrective ocular saccades. The head shake test requires that the patient rhythmically shake their head to the left and the right for several seconds to stimulate the vestibular system bilaterally. Then the examiner tells the patient to stop shaking and quickly bring their head back to midline, looking straight forward at the examiner's nose. Corrective saccades indicate insufficiency of the vestibulo-ocular reflex.
A similar maneuver is the head thrust test, in which the examiner shakes the patient's head back and forth for several seconds before suddenly snapping the head back into midline and asking the patient to look at the examiner's nose; resulting corrective saccades likewise indicate vestibular hypofunction. The Dix-Hallpike test is also typically performed, although this maneuver identifies benign paroxysmal positional vertigo and is an integral part of any complete vestibular assessment. Additionally, the Fukuda/Unterberger test can identify vestibular hypofunction by producing postural instability.[3] The patient is asked to walk forward with eyes closed and then, on command, turn with 1 foot and stop. The test is positive if the patient becomes unsteady after stopping.
Evaluation
Definitively identifying a labyrinthine fistula can be challenging, as no international consensus regarding diagnosis has been established. However, diagnostic criteria has been suggested by the Intractable Hearing Loss Research Committee of the Japanese Ministry of Health and Welfare, which are based on the presence of hearing impairment, aural fullness, tinnitus and disequilibrium in the context of middle/inner ear pathology or barotrauma, with or without visualization of a fistula via tympanotomy or the presence of cochlin-tomoprotein in the tympanic fluid.[16] Patients with suggestive symptoms and history only are considered to have a probable PLF, while additional confirmation via tympanotomy or laboratory studies constitutes a definite PLF.
Audiological Studies
Numerous audiological studies may be employed to provide additional diagnostic information before undertaking surgical confirmation and repair. Audiometry is needed to determine the type and degree of hearing loss, if any is present, and video or electro-nystagmography will quantify any vestibular hypofunction.[27] Electrocochleography often demonstrates an elevated summating potential (SP) to action potential (AP) ratio if a PLF is present, similar to results in cases of Ménière disease, although the SP increases in Ménière disease, while the AP may decrease with a labyrinthine fistula.
Electrocochleography is also useful for postoperative follow-up, as the SP/AP ratio typically returns to normal after successful surgical repair of the fistula.[28] Vestibular-evoked myogenic potential testing will likely demonstrate decreased thresholds for sternocleidomastoid contraction from 90 dB down to 70 dB when impulse sounds are presented on the affected side. However, this may be seen in cases of third window phenomena, eg, superior semicircular canal dehiscence as well as PLF.[29] Dynamic platform posturography can be added to standard fistula testing to quantify any imbalance that insufflation of the external auditory canal produces.[30] Swaying as a result of pressure changes in the ear strongly correlates with the presence of a PLF.
Imaging Studies
With respect to imaging, computed tomography (CT) is the most reliable method for identifying labyrinthine fistula preoperatively, as pneumolabyrinth seen on a CT scan confirms the diagnosis and indicates the need for surgical intervention (see Image. Pneumolabyrinth).[9] If the pneumolabyrinth is very small, however, visualization on CT may be improved with coronal views of the temporal bone because the air tends to rise to the top of the semicircular canals, which are well visualized from this perspective.[9]
Other advantages of CT scanning are that it is particularly adept at characterizing the extent of cholesteatoma erosion of the otic capsule bone and revealing congenital anomalies of the inner ear that can increase the risk of PLF, including enlarged vestibular (>1 mm) and cochlear aqueducts, and Mondini deformities (see Images. Cholesteatoma CT, Enlarged Vestibular Aqueduct, and Mondini Deformity).[31] The sensitivity of CT scanning alone for identification of a PLF is >80%, while that of CT and magnetic resonance imaging combined approaches 100%.[14][32]
Invasive Diagnostic Evaluation
Invasive evaluation for labyrinthine fistula may be performed via transtympanic endoscopy, either as the first step of exploratory tympanotomy or as an office-based procedure. A radial myringotomy is made, with topical phenol anesthesia if the patient is awake, and a small (1.9 mm), rigid endoscope is introduced to visualize the oval and round windows. Because PLFs may be intermittent, a finding of clear fluid at the oval or round window may not be present at the time of evaluation.
For this reason, some surgeons advocate using the Valsalva maneuver to provoke perilymph leakage, although this may increase the risk of hearing loss. Despite the high-quality view of the middle ear provided by endoscopy, findings often differ from those encountered with formal exploratory tympanotomy, even when surgery is performed immediately after the endoscopy.[33][34] Endoscopy does, nevertheless, retain one advantage: its ability to obtain fluid samples for preoperative analysis, permitting identification of cochlin-tomoprotein and potentially β-2 transferrin as well.
Use of β-2 transferrin to confirm the presence of perilymph remains controversial though, as assays are unlikely to detect it in the extremely small sample volumes obtained from the middle ear unless the assays are highly sensitive; the highly sensitive assays, however, are liable to detect the protein even in serum, which may contaminate middle ear fluid samples.[35][36] More recently, flexible endoscopy via the Eustachian tube has been used to visualize the middle ear cleft without requiring a myringotomy, although the views of the oval and round windows this technique provides are generally inferior to those provided by transtympanic endoscopy.[37]
Treatment / Management
Nonsurgical Management
Because of a lack of definitive diagnostic criteria, conservative management is preferred initially with head elevation, bed rest, and avoidance of straining, lifting heavy objects, and other activities that increase intracranial pressure. If symptoms persist or are recurrent, an intratympanic blood patch or exploratory tympanotomy would be the next step. Surgery has been shown to decrease vestibular symptoms reliably but rarely improves hearing, sometimes even resulting in iatrogenic sensorineural hearing loss.[13][12]
On the other hand, the blood patch procedure is performed in the office and requires only 0.5 mL of the patient's blood injected into the middle ear, followed by maintaining a supine position for half an hour.[38] As with an epidural blood patch, the intratympanic procedure likely creates a mechanical and inflammatory seal at the round and oval windows. Blood patching has a high success rate, although symptomatic relief is only temporary for some patients. If the blood patch improves the symptoms, but they relapse, the patient is likely a good candidate for tympanotomy; however, if the blood patch produces no symptomatic improvement, the diagnosis of labyrinthine fistula may be called into question.[21]
Surgical Management
Currently, no consensus exists on the optimal surgical management of labyrinthine fistula.[13] Goto et al concluded that urgent tympanotomy was unnecessary due to poor hearing outcomes.[39] Seltzer and McCabe, however, found that hearing improvement was still possible with repair even after many years of symptoms.[40] While the role of operative intervention in cases of idiopathic PLF remains unclear, surgery is more widely accepted as appropriate for PLFs with known causes, with earlier intervention preferred to prevent the progression of hearing loss.[41] (B3)
Repair of labyrinthine fistula in the middle ear is classically accomplished via a tympanotomy, using a postauricular approach. This involves elevating a tympanomeatal flap with incisions in the posterior aspect of the external auditory canal and reflection of the skin-tympanic membrane flap anteriorly to expose the posterior aspect of the middle ear, including the ossicles, the chorda tympani nerve, and the round window.
Curettage of the posterosuperior bony scutum permits translocation of the chorda tympani and wider visualization of the stapes footplate. Inspection of the footplate and round window niche may reveal clear fluid, which can be indicative of a PLF; however, these concavities may also collect mucous transudate and local anesthetic solution, making it difficult to identify perilymph without laboratory confirmation of β-2 transferrin or cochlin-tomoprotein, which cannot be performed with a rapid assay suitable for intraoperative use.[33]
While increasing intracranial pressure via the Valsalva maneuver may make perilymph leakage more visually apparent, it should be avoided due to the danger of causing additional hearing loss.[20] Regardless of whether or not a fistula is visualized, the majority of surgeons will use temporalis fascia, parotid fascia, or tragal perichondrium to reinforce the round and oval windows during every exploratory tympanotomy for PLF (see Image. Standard Surgical Techniques of Perilymph Fistulas Repair).[42] Before the placement of the grafts, the mucosa should be denuded to improve adhesion.(B3)
Any fascial grafts placed onto the oval window should be very thin to avoid causing conductive hearing loss by dampening stapes footplate vibrations. Fat grafting is avoided entirely because it is less likely to prevent the recurrence of a fistula in the long term.[21] Some surgeons advocate the use of endoscopic tympanotomy over an open approach with a microscope due to its less invasive nature, higher magnification, and wider field of view. However, using an operating microscope provides better depth perception and the ability to operate with 2 hands simultaneously, as one hand is not occupied while the endoscope is being operated.[23](B2)
Corticosteroids administered intravenously during surgery have been associated with better hearing outcomes, and they may also be injected intratympanically in the office during episodes of acute vertigo. Obliteration of the mastoid has also been reported to improve postoperative dizziness in some patients, particularly those with cholesteatoma-induced erosion of the horizontal semicircular canal.[13]
Management of labyrinthine fistula due to penetrating trauma or cholesteatoma requires not only repair of the leak itself but also removal of the offending pathology. In the case of cholesteatoma, this requires complete extirpation of the matrix and resurfacing of the bony defect with fascia, cartilage, bone pâté, or bone chips, typically at the horizontal semicircular canal but potentially at the oval window or the cochlear promontory as well.[13]
Differential Diagnosis
Differential diagnoses for concomitant vertigo and hearing loss include:
- Ménière disease/primary endolymphatic hydrops (more common than PLF and can present with similar symptoms)
- Secondary endolymphatic hydrops (due to head trauma, allergy, or autoimmune disorders such as Cogan disease) [43][13]
- Medication ototoxicity (cochleotoxicity or vestibulotoxicity, most commonly from aminoglycoside antibiotics, antineoplastic agents, loop diuretics, and aspirin)
- Acoustic neuroma (vestibular schwannoma)
- Labyrinthitis
- Vestibular neuronitis
- Vestibular migraine
- Semicircular canal dehiscence
- Enlarged vestibular aqueduct
- Enlarged cochlear aqueduct
Prognosis
Surgical intervention has been shown to improve vestibular symptoms more than hearing loss, the latter of which may or may not improve with surgical intervention.[44] However, some evidence has demonstrated that the prognosis for improvement of symptoms correlates inversely with the magnitude of the inciting event and the severity of symptoms at onset.[12]
The more severe the symptoms or the more traumatic the inciting events, the less likely a spontaneous resolution will occur or surgical intervention will succeed. Improvement in hearing after exploratory tympanotomy and fistula repair was reported to only be 9% to 17% from the data collected by Goto's and Black's groups. However, with early repair, resolution of vestibular symptoms is very likely, and hearing improvement may occur in up to half of patients.[39][20]
Complications
The most common complications of PLFs themselves are hearing loss and vertigo. However, when a fistula occurs due to a congenital inner ear anomaly, recurrent meningitis may be the presenting complaint due to an abnormal connection between the middle ear and the CSF-containing spaces.[45][46] Complications may also occur with fistula repair. These include tympanic membrane perforation due to traumatic tympanomeatal flap elevation, postoperative conductive or sensorineural hearing loss, and chorda tympani nerve injury with resulting taste loss or dysgeusia.[47] Fortunately, postoperative hearing loss and chorda tympani injury are temporary in many patients, lasting only a few months.[16]
Deterrence and Patient Education
Patients diagnosed with a PLF should avoid loud noise exposure and activities that elicit symptoms. These include straining and heavy lifting, pressure changes from SCUBA diving, air travel, the Valsalva maneuver, and the forceful nose blowing. Should these activities prove unavoidable, the patient should seek the assistance of a colleague, friend, or family member to provide support if imbalance or dizziness occur and to take over operation of heavy machinery or driving, if necessary. Additionally, the simple act of wearing hearing protection may substantially limit sound-induced vestibular symptoms in noisy environments.
Enhancing Healthcare Team Outcomes
Effective management of PLF requires a skilled interprofessional team working together to enhance patient-centered care and safety. Physicians, particularly otolaryngologists and neurotologists, play a crucial role in diagnosing and determining the appropriate treatment strategy, whether conservative management or surgical intervention. Advanced practitioners and nurses contribute by obtaining thorough patient histories, recognizing symptoms such as vertigo and hearing loss, and facilitating referrals to specialists. Audiologists are instrumental in quantifying auditory deficits through specialized testing, helping guide the clinical decision-making process. Pharmacists ensure safe medication use, particularly when prescribing vestibular suppressants or post-surgical treatments, minimizing adverse effects and optimizing patient outcomes.
Interprofessional communication and care coordination are essential for improving patient outcomes and ensuring team efficiency. Surgeons and anesthesia teams must collaborate closely, particularly during stapes surgery, where patient cooperation is critical for proper prosthesis placement. Nurses play a key role in postoperative monitoring, educating patients on symptom management, and coordinating follow-up care to prevent complications such as persistent dizziness or worsening hearing loss. By fostering open communication and clearly defining team responsibilities, healthcare professionals can improve diagnostic accuracy, optimize treatment approaches, and enhance overall team performance in managing PLF, ultimately leading to better patient safety and satisfaction.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
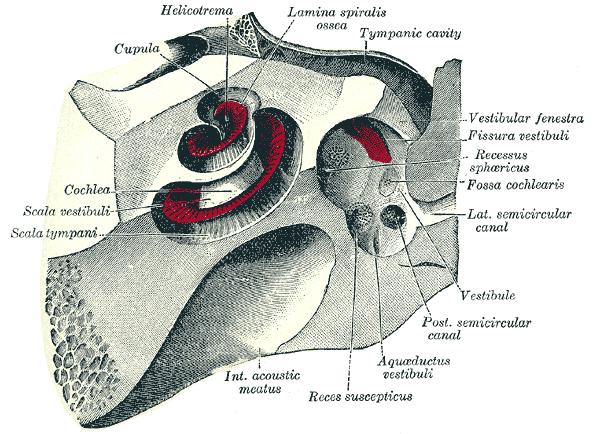
Cochlea and Vestibule. The cochlea and vestibule, viewed from above, including the helicotrema, cupula, cochlea, scala vestibuli, scala tympani, and recess suscepticus.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
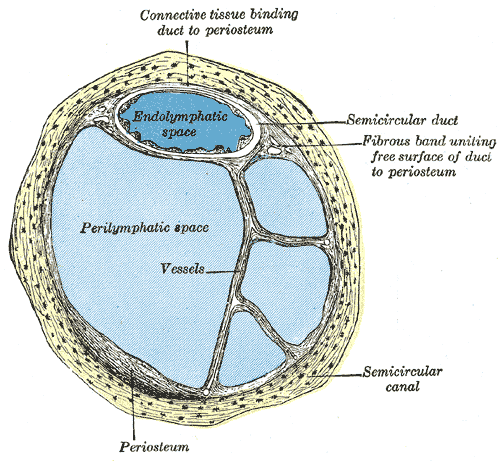
Internal Ear or Labyrinth, Transverse Section. The image is a transverse section of a human semicircular canal and duct, endolymphatic space, perilymphatic space, and a fibrous band uniting the free surface of the duct to the periosteum.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
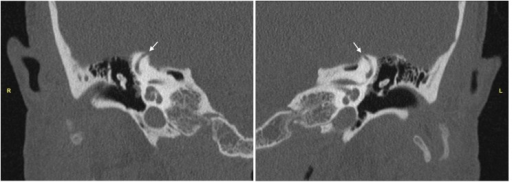
Computed Tomography of Temporal Bones. High-resolution computed tomography scans of the temporal bones demonstrating the area of dehiscence (arrow) at the apex of the (R) right superior semicircular canal and (L) left superior semicircular canal.
Chung LK, Lagman C, Nagasawa DT, et al. Superior semicircular canal dehiscence in a patient with Ehlers-Danlos Syndrome: a case report. Cureus. 2017;9(4):e1141.
(Click Image to Enlarge)
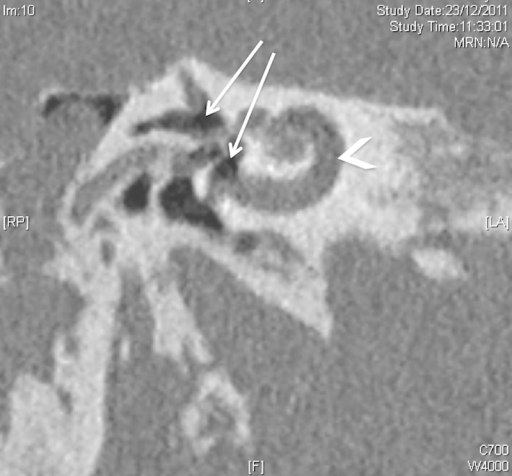
Pneumolabyrinth. The pneumolabyrinth extended into the vestibule and the superior and lateral canals (arrows) but did not extend into the cochlea (arrowhead). This may explain the conservation of the auditory function up to the 2 weeks after injury and the initial recuperation of hearing function after the surgery.
Roaelina TR, Elziere M, Michel J, Devèze A. Intralabyrinthine penetrating ventilation tube with preservation of hearing: an unusual clinical situation. Int Arch Otorhinolaryngol. 2015;19(2):183-186.
(Click Image to Enlarge)
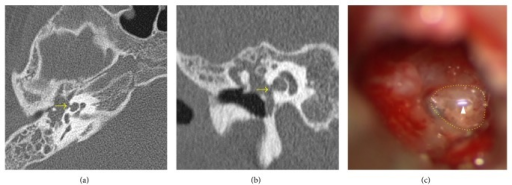
Cholesteatoma CT. Axial (a) and coronal (b) CT scans of the right temporal bone showing cholesteatoma with a cochlear fistula (arrow). Intraoperative findings (c) confirmed the fistula with pulsatile fluid. The arrowhead indicates light reflex in the fluid.
Kuo CL, Shiao AS, Yung M, et al. Updates and knowledge gaps in cholesteatoma research. Biomed Res Int. 2015;2015:854024.
(Click Image to Enlarge)
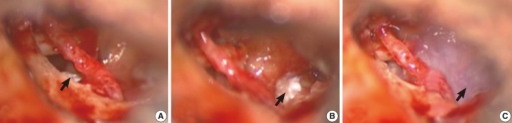
Standard Surgical Techniques of Perilymph Fistulas Repair. (A) Soft tissue was covered over the oval window and stapes (arrow). (B) Soft tissue was covered over the round window (arrow). (C) Fibrin glue was applied in the middle ear cavity (arrow).
Park GY, Byun HY, Moon IJ, et al. Effects of early surgical exploration in suspected barotraumatic perilymph fistulas. Clin Exp Otorhinolaryngol. 2012;5(2):74-80.
(Click Image to Enlarge)
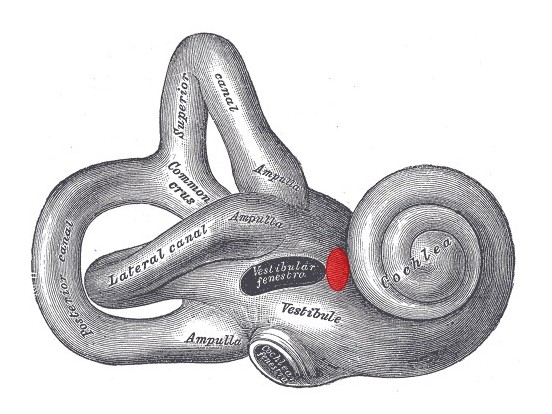
Internal Ear. Lateral view of the right osseous labyrinth with labeled components, including the lateral canal, common crus, ampulla, vestibule, and cochlea. The region of the fissula antefenestram is highlighted in red. Vestibular fenestra = oval window. Cochlear fenestra = round window.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)

Enlarged Vestibular Aqueduct. Computed tomography scan of the temporal bone for a patient with a bilateral enlarged vestibular aqueduct.
Yazdanpanahi N, Tabatabaiefar MA, Farrokhi E, et al. Compound heterozygosity for two novel SLC26A4 mutations in a large Iranian pedigree with Pendred Syndrome. Clin Exp Otorhinolaryngol. 2013;6(4):201-208.
(Click Image to Enlarge)
(Click Image to Enlarge)
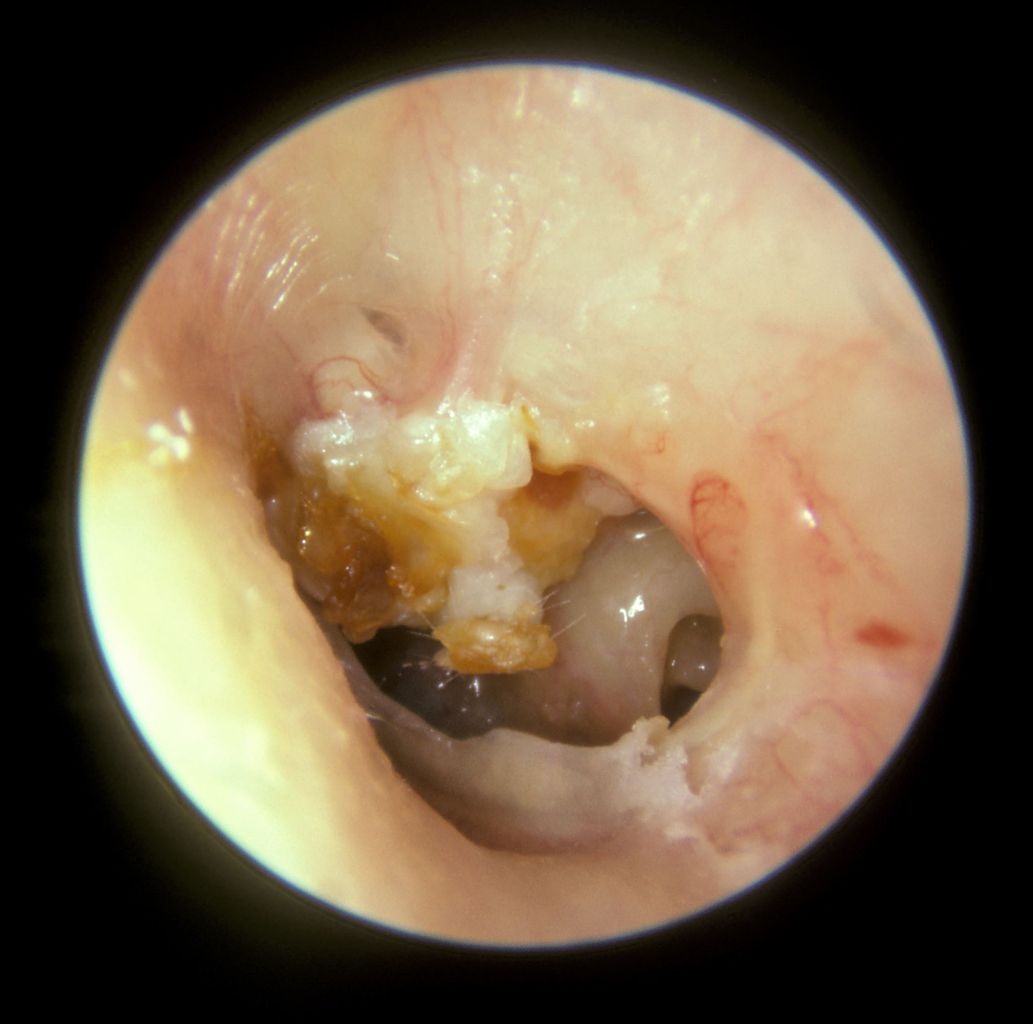
Cholesteatoma. A large mass of white keratin debris is visible in the upper left quadrant of the left tympanic membrane, indicating a cholesteatoma. The majority of the tympanic membrane is missing due to perforation. In the lower right quadrant, the round window niche on the medial wall of the middle ear is visible.
Michael Hawke, MD, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Weider DJ, Johnson GD. Perilymphatic fistula: a New Hampshire experience. The American journal of otology. 1988 May:9(3):184-96 [PubMed PMID: 3177601]
McCabe BF. Perilymph fistula: the Iowa experience to date. The American journal of otology. 1989 Jul:10(4):262 [PubMed PMID: 2801889]
Hornibrook J. Perilymph fistula: fifty years of controversy. ISRN otolaryngology. 2012:2012():281248. doi: 10.5402/2012/281248. Epub 2012 Jul 31 [PubMed PMID: 23724269]
Level 3 (low-level) evidenceComacchio F, Mion M. Sneezing and Perilymphatic Fistula of the Round Window: Case Report and Systematic Review of the Literature. The journal of international advanced otology. 2018 Apr:14(1):106-111. doi: 10.5152/iao.2018.4336. Epub [PubMed PMID: 29764784]
Level 3 (low-level) evidenceGoodhill V. Labyrinthine membrane ruptures in sudden sensorineural hearing loss. Proceedings of the Royal Society of Medicine. 1976 Aug:69(8):565-72 [PubMed PMID: 981244]
Goodhill V. Ben H. Senturia lecture. Leaking labyrinth lesions, deafness, tinnitus and dizziness. The Annals of otology, rhinology, and laryngology. 1981 Mar-Apr:90(2 Pt 1):99-106 [PubMed PMID: 7224522]
Rosito LPS, Canali I, Teixeira A, Silva MN, Selaimen F, Costa SSD. Cholesteatoma labyrinthine fistula: prevalence and impact. Brazilian journal of otorhinolaryngology. 2019 Mar-Apr:85(2):222-227. doi: 10.1016/j.bjorl.2018.01.005. Epub 2018 Mar 9 [PubMed PMID: 29599061]
Hall AC, Mandavia R, Selvadurai D. Total endoscopic stapes surgery: Systematic review and pooled analysis of audiological outcomes. The Laryngoscope. 2020 May:130(5):1282-1286. doi: 10.1002/lary.28294. Epub 2019 Sep 30 [PubMed PMID: 31566754]
Level 1 (high-level) evidenceKita AE, Kim I, Ishiyama G, Ishiyama A. Perilymphatic Fistula After Penetrating Ear Trauma. Clinical practice and cases in emergency medicine. 2019 May:3(2):115-118. doi: 10.5811/cpcem.2019.1.37404. Epub 2019 Mar 4 [PubMed PMID: 31061965]
Level 3 (low-level) evidenceGhorayeb BY, Yeakley JW. Temporal bone fractures: longitudinal or oblique? The case for oblique temporal bone fractures. The Laryngoscope. 1992 Feb:102(2):129-34 [PubMed PMID: 1738283]
Level 3 (low-level) evidenceDahiya R, Keller JD, Litofsky NS, Bankey PE, Bonassar LJ, Megerian CA. Temporal bone fractures: otic capsule sparing versus otic capsule violating clinical and radiographic considerations. The Journal of trauma. 1999 Dec:47(6):1079-83 [PubMed PMID: 10608536]
Level 2 (mid-level) evidencePark GY, Byun H, Moon IJ, Hong SH, Cho YS, Chung WH. Effects of early surgical exploration in suspected barotraumatic perilymph fistulas. Clinical and experimental otorhinolaryngology. 2012 Jun:5(2):74-80. doi: 10.3342/ceo.2012.5.2.74. Epub 2012 Jun 12 [PubMed PMID: 22737287]
Geerse S, de Wolf MJF, Ebbens FA, van Spronsen E. Management of labyrinthine fistula: hearing preservation versus prevention of residual disease. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2017 Oct:274(10):3605-3612. doi: 10.1007/s00405-017-4697-2. Epub 2017 Aug 10 [PubMed PMID: 28799140]
Sagar P, Devaraja K, Kumar R, Bolu S, Sharma SC. Cholesteatoma Induced Labyrinthine Fistula: Is Aggressiveness in Removing Disease Justified? Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2017 Jun:69(2):204-209. doi: 10.1007/s12070-017-1072-y. Epub 2017 Jan 19 [PubMed PMID: 28607891]
Manolidis S. Complications associated with labyrinthine fistula in surgery for chronic otitis media. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2000 Dec:123(6):733-7 [PubMed PMID: 11112967]
Matsuda H, Sakamoto K, Matsumura T, Saito S, Shindo S, Fukushima K, Nishio SY, Kitoh R, Shibasaki O, Ito A, Araki R, Usami SI, Suzuki M, Ogawa K, Hasegawa T, Hagiwara Y, Kase Y, Ikezono T. A nationwide multicenter study of the Cochlin tomo-protein detection test: clinical characteristics of perilymphatic fistula cases. Acta oto-laryngologica. 2017:137(sup565):S53-S59. doi: 10.1080/00016489.2017.1300940. Epub 2017 Apr 3 [PubMed PMID: 28368720]
Level 2 (mid-level) evidenceFiedler T, Boeger D, Buentzel J, Esser D, Hoffmann K, Jecker P, Mueller A, Radtke G, Häfke D, Bitter T, Guntinas-Lichius O. Middle ear surgery in Thuringia, Germany: a population-based regional study on epidemiology and outcome. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2013 Jul:34(5):890-7. doi: 10.1097/MAO.0b013e318280dc55. Epub [PubMed PMID: 23370571]
Reilly JS. Congenital perilymphatic fistula: a prospective study in infants and children. The Laryngoscope. 1989 Apr:99(4):393-7 [PubMed PMID: 2784526]
Sasaki A, Ikezono T, Matsuda H, Araki R, Matsumura T, Saitoh S, Wasano K, Matsubara A. Prevalence of perilymphatic fistula in patients with sudden-onset sensorineural hearing loss as diagnosed by Cochlin-tomoprotein (CTP) biomarker detection: its association with age, hearing severity, and treatment outcomes. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2024 May:281(5):2373-2381. doi: 10.1007/s00405-023-08368-0. Epub 2023 Dec 21 [PubMed PMID: 38123733]
Black FO, Pesznecker S, Norton T, Fowler L, Lilly DJ, Shupert C, Hemenway WG, Peterka RJ, Jacobson ES. Surgical management of perilymphatic fistulas: a Portland experience. The American journal of otology. 1992 May:13(3):254-62 [PubMed PMID: 1609855]
Sarna B, Abouzari M, Merna C, Jamshidi S, Saber T, Djalilian HR. Perilymphatic Fistula: A Review of Classification, Etiology, Diagnosis, and Treatment. Frontiers in neurology. 2020:11():1046. doi: 10.3389/fneur.2020.01046. Epub 2020 Sep 15 [PubMed PMID: 33041986]
Muntarbhorn K, Webber PA. Labyrinthine window rupture with round window predominance: a long-term review of 32 cases. Clinical otolaryngology and allied sciences. 1987 Apr:12(2):103-8 [PubMed PMID: 3581487]
Level 3 (low-level) evidenceRawal R, Zhao X, Lipson S, R Brodsky J. Endoscopic Repair of Traumatic Perilymphatic Fistula in Children: A Case Series. The journal of international advanced otology. 2021 Mar:17(2):182-185. doi: 10.5152/JIAO.2021.8390. Epub [PubMed PMID: 33893790]
Level 2 (mid-level) evidenceMusat GC, Tataru CP, Musat O, Preda MA, Radu M, Musat AAM, Mitroi MR. Ocular Movement Examination in Peripheral Vestibular Disorders as a Tool to Improve Diagnosis: A Literature Review. Medicina (Kaunas, Lithuania). 2024 Oct 11:60(10):. doi: 10.3390/medicina60101665. Epub 2024 Oct 11 [PubMed PMID: 39459452]
Cremer PD, Minor LB, Carey JP, Della Santina CC. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology. 2000 Dec 26:55(12):1833-41 [PubMed PMID: 11134382]
Castellucci A, Dumas G, Abuzaid SM, Armato E, Martellucci S, Malara P, Alfarghal M, Ruberto RR, Brizzi P, Ghidini A, Comacchio F, Schmerber S. Posterior Semicircular Canal Dehiscence with Vestibulo-Ocular Reflex Reduction for the Affected Canal at the Video-Head Impulse Test: Considerations to Pathomechanisms. Audiology research. 2024 Mar 24:14(2):317-332. doi: 10.3390/audiolres14020028. Epub 2024 Mar 24 [PubMed PMID: 38666899]
Korver AM, Smith RJ, Van Camp G, Schleiss MR, Bitner-Glindzicz MA, Lustig LR, Usami SI, Boudewyns AN. Congenital hearing loss. Nature reviews. Disease primers. 2017 Jan 12:3():16094. doi: 10.1038/nrdp.2016.94. Epub 2017 Jan 12 [PubMed PMID: 28079113]
Meyerhoff WL, Yellin MW. Summating potential/action potential ratio in perilymph fistula. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1990 Jun:102(6):678-82 [PubMed PMID: 2115654]
Modugno GC, Magnani G, Brandolini C, Savastio G, Pirodda A. Could vestibular evoked myogenic potentials (VEMPs) also be useful in the diagnosis of perilymphatic fistula? European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2006 Jun:263(6):552-5 [PubMed PMID: 16482456]
Black FO, Lilly DJ, Peterka RJ, Shupert C, Hemenway WG, Pesznecker SC. The dynamic posturographic pressure test for the presumptive diagnosis of perilymph fistulas. Neurologic clinics. 1990 May:8(2):361-74 [PubMed PMID: 2359383]
Weissman JL, Weber PC, Bluestone CD. Congenital perilymphatic fistula: computed tomography appearance of middle ear and inner ear anomalies. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1994 Sep:111(3 Pt 1):243-9 [PubMed PMID: 8084632]
Venkatasamy A, Al Ohraini Z, Karol A, Karch-Georges A, Riehm S, Rohmer D, Charpiot A, Veillon F. CT and MRI for the diagnosis of perilymphatic fistula: a study of 17 surgically confirmed patients. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2020 Apr:277(4):1045-1051. doi: 10.1007/s00405-020-05820-3. Epub 2020 Feb 10 [PubMed PMID: 32040717]
Poe DS, Bottrill ID. Comparison of endoscopic and surgical explorations for perilymphatic fistulas. The American journal of otology. 1994 Nov:15(6):735-8 [PubMed PMID: 8572084]
Selmani Z, Pyykkö I, Ishizaki H, Marttila TI. Role of transtympanic endoscopy of the middle ear in the diagnosis of perilymphatic fistula in patients with sensorineural hearing loss or vertigo. ORL; journal for oto-rhino-laryngology and its related specialties. 2002 Sep-Oct:64(5):301-6 [PubMed PMID: 12417768]
Levenson MJ, Desloge RB, Parisier SC. Beta-2 transferrin: limitations of use as a clinical marker for perilymph. The Laryngoscope. 1996 Feb:106(2 Pt 1):159-61 [PubMed PMID: 8583846]
Skedros DG, Cass SP, Hirsch BE, Kelly RH. Sources of error in use of beta-2 transferrin analysis for diagnosing perilymphatic and cerebral spinal fluid leaks. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1993 Nov:109(5):861-4 [PubMed PMID: 8247566]
Ogawa K, Kanzaki J, Ogawa S, Tsuchihashi N, Inoue Y, Yamamoto M. Endoscopic diagnosis of idiopathic perilymphatic fistula. Acta oto-laryngologica. Supplementum. 1994:514():63-5 [PubMed PMID: 8073889]
Garg R, Djalilian HR. Intratympanic injection of autologous blood for traumatic perilymphatic fistulas. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2009 Aug:141(2):294-5. doi: 10.1016/j.otohns.2009.05.024. Epub [PubMed PMID: 19643271]
Goto F, Ogawa K, Kunihiro T, Kurashima K, Kobayashi H, Kanzaki J. Perilymph fistula--45 case analysis. Auris, nasus, larynx. 2001 Jan:28(1):29-33 [PubMed PMID: 11137360]
Level 3 (low-level) evidenceSeltzer S, McCabe BF. Perilymph fistula: the Iowa experience. The Laryngoscope. 1986 Jan:96(1):37-49 [PubMed PMID: 3941579]
Komori M, Yamamoto Y, Yaguchi Y, Ikezono T, Kojima H. Cochlin-tomoprotein test and hearing outcomes in surgically treated true idiopathic perilymph fistula. Acta oto-laryngologica. 2016 Sep:136(9):901-4. doi: 10.3109/00016489.2016.1165861. Epub 2016 Apr 8 [PubMed PMID: 27055739]
Hughes GB, Sismanis A, House JW. Is there consensus in perilymph fistula management? Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1990 Feb:102(2):111-7 [PubMed PMID: 2113234]
Level 3 (low-level) evidenceFerster APO, Cureoglu S, Keskin N, Paparella MM, Isildak H. Secondary Endolymphatic Hydrops. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2017 Jun:38(5):774-779. doi: 10.1097/MAO.0000000000001377. Epub [PubMed PMID: 28306649]
Supance JS, Bluestone CD. Perilymph fistulas in infants and children. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1983 Dec:91(6):663-71 [PubMed PMID: 6420749]
Tom LW, Bilaniuk L, Roa RA, Potsic WP. Recurrent meningitis and a congenital perilymph fistula. Ear, nose, & throat journal. 1992 Jul:71(7):287-90 [PubMed PMID: 1505375]
Desjardins R, Guerguerian AJ, Dube J, Deschamps N, Lavertu P. Meningitis and congenital fistula of the internal ear. The Journal of otolaryngology. 1982 Apr:11(2):97-100 [PubMed PMID: 7077735]
Chang EH, Menezes M, Meyer NC, Cucci RA, Vervoort VS, Schwartz CE, Smith RJ. Branchio-oto-renal syndrome: the mutation spectrum in EYA1 and its phenotypic consequences. Human mutation. 2004 Jun:23(6):582-9 [PubMed PMID: 15146463]