Introduction
Lateral medullary syndrome (LMS), also known as Wallenberg syndrome, is a neurological disorder affecting the dorsolateral medulla oblongata, most commonly caused by ischemic infarction of the posterior inferior cerebellar artery (PICA) or the vertebral artery (VA) (see Image. Lateral Medullary Syndrome). This condition was named after Adolf Wallenberg (1862–1949), a German neurologist and neuroanatomist who reported the first case of LMS. The LMS and resulting ischemic injury affect multiple neural structures in the posterolateral medulla, located posterior to the inferior olivary nucleus.
Affected structures include the inferior cerebellar peduncle, spinocerebellar tract, spinothalamic tract, descending sympathetic fibers, spinal trigeminal nucleus and tract, vestibular nuclei, and the nuclei and fibers of cranial nerves IX and X. Involvement of these structures results in a classic constellation of symptoms, including ipsilateral facial sensory loss, contralateral loss of pain and temperature sensation in the body, vertigo, ataxia, dysphagia, hoarseness, and Horner syndrome.[1][2][3][4] Recent systematic reviews continue to underscore the clinical heterogeneity of LMS and emphasize the importance of early multimodal neuroimaging for diagnosis.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
LMS is most commonly caused by an acute ischemic stroke affecting the dorsolateral medulla, due to a thrombus or embolism of the VA or PICA. Less common causes include injury to the VA in the neck, dissection, arteriovenous malformation, or arteritis. The most important risk factors for LMS are hypertension, diabetes, and cigarette smoking. The common etiologies of LMS are as follows:
- Atherosclerotic disease: This is typically due to thromboembolism or, less commonly, hemodynamic insufficiency.
- Cardiogenic embolism: This is associated with atrial fibrillation,[6] mechanical heart valves, intracardiac thrombi, dilated cardiomyopathy, myocardial infarction, and infective endocarditis. Cocaine misuse, neck manipulation, medullary tumors, radiation necrosis, and hematomas may also lead to embolism.
- VA dissection: This is particularly seen in younger individuals presenting with headache and neurological symptoms consistent with LMS.[7]
- Hypoplastic VA: This is a rare cause, especially if additional risk factors are present in younger patients.[8]
- Surgical trauma: PICA is often exposed and injured during surgeries, especially telovelar approaches.[9]
- Arteriovenous malformation and aneurysms: This is a common cause of LMS, especially involving the PICA.
- Vertebrobasilar dolichoectasia and ectatic PICA: These malformations compress the medulla and cranial nerves, causing neurovascular compression syndromes.[9]
- Connective tissue diseases: Ehlers-Danlos syndrome, Marfan syndrome, and fibromuscular dysplasia, can weaken the walls of blood vessels.
Less common causes include subclavian steal syndrome, rotational vertebral artery compression syndrome, Fabry disease, mitochondrial encephalopathy, lactic acidosis, and migraines.[10][11]
Epidemiology
Posterior circulation strokes represent about 20% of all ischemic strokes. In a 2024 registry of more than 2900 strokes, posterior circulation strokes accounted for 28.8%, with LMS representing 12%.[12] Notably, lateral medullary infarcts occur more frequently in those who consume alcohol.[13] Angiography showing VA involvement is more common (67%) than isolated PICA involvement (10%). PICA-related infarcts are often due to cardiogenic embolism.[8] Between 51% and 94% of patients with lateral medullary syndrome experience some degree of dysphagia.[1][3]
Pathophysiology
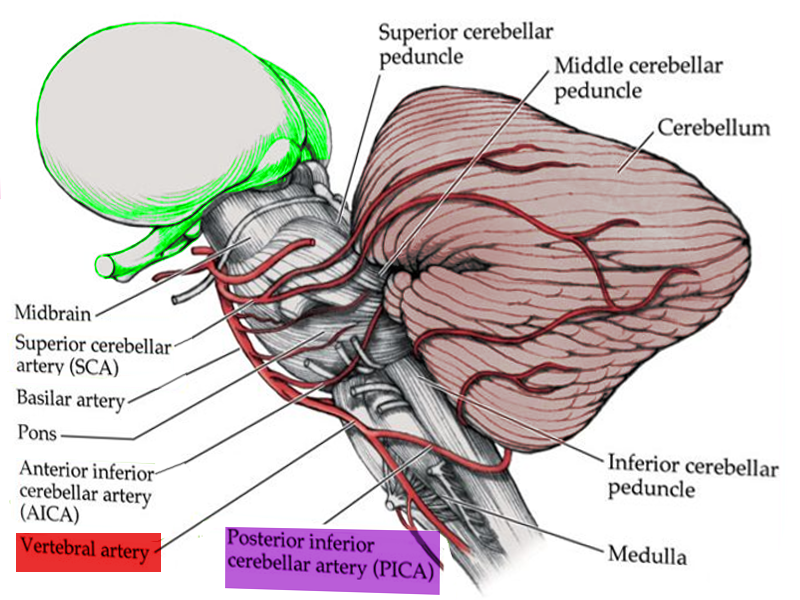
Large artery atherothrombosis accounts for nearly 75% of LMS cases, followed by cardioembolism in 17%, and VA dissection in 8%.[4] Pathophysiologically, the clinical features of LMS correlate with the affected structures in the dorsolateral medulla, an area supplied by the PICA, a branch of the VA (see Image. Anatomy of Brain Vascular Territories). Therefore, LMS is commonly caused by occlusion of the VA. Clinical features of LMS are correlated with dysfunction of neuroanatomical structures as outlined below:
- Vestibular nuclei: Dizziness, vertigo, nystagmus, skew deviation, nausea, and vomiting
- Spinocerebellar tracts and the inferior cerebellar peduncle: Ipsilateral ataxia and lateral pulsion [1][3][8]
- Caudal trigeminal nucleus and tract: Ipsilateral facial sensation abnormalities, primarily involving pain and temperature
- Spinothalamic tract: Contralateral pain and temperature loss affecting the arm, body, and leg
- Descending sympathetic fibers: Ipsilateral Horner syndrome
- Nucleus ambiguus: Ipsilateral dysphagia, dysarthria, and dysphonia [8][14]
- Cough reflex may also be impaired.[3]
- Nucleus and tractus solitarius: Ipsilateral taste impairment
A 2025 prospective flexible endoscopic evaluation of swallowing and high-resolution pharyngeal manometry study demonstrated severe pharyngeal sensory loss and delayed swallow initiation in medullary strokes compared with cerebellar strokes.[15] Hyponatremia after a brain injury occurs due to the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) and cerebral salt wasting syndrome. SIADH has been reported in patients with lateral medullary infarction. The proposed pathophysiology involves failure of nonosmotic stimuli to propagate from the carotid sinus via the vagus nerve due to a lesion in the nucleus tractus solitarius of the medulla. This lesion causes the disinhibition of antidiuretic hormone secretion by the pituitary gland, causing SIADH.[16]
Other clinical findings are rarer but have corresponding anatomical variations. Ipsilateral hemiparesis (Opalski variant) has been attributed to LMS affecting the lateral medulla, caudal to the corticospinal decussation, as discussed in a 2024 case report.[17] Life-threatening bradycardia precipitated by vertebral dissection-related LMS has also been reported.[18] Moreover, persistent hiccups are believed to result from the involvement of the nucleus ambiguus or adjacent respiratory regulatory centers.
History and Physical
Many patients with LMS are older adults with multiple vascular risk factors. Typical symptoms include acute onset of vertigo, dizziness, nystagmus, ataxia, nausea, vomiting, dysphagia, hiccups, dysphonia, facial pain, visual disturbances, and headaches. Sensory signs and symptoms are the most frequent manifestations (96%). Ipsilateral limb ataxia, Horner syndrome, and sensory impairments are also common. Less common clinical presentations include dysarthria, skew deviation, oscillopsia due to nystagmus, and ipsilateral lateral pulsion.
Gradual onset occurs in approximately 25% of cases, progressing over several hours to days, with symptoms of headache, dizziness, vertigo, or gait ataxia, followed by sensory deficits, hiccups, hoarseness, and dysphagia. Weakness is a relatively uncommon feature of LMS, often resulting in a missed diagnosis. Partial syndromes are also more common than complete syndromes [1][2][8][10][16]
Classic signs of LMS include:
- Impairment of pain and thermal sensation over the contralateral side of the trunk and limbs
- Impairment of pain and thermal sensation over the ipsilateral face
- Ipsilateral Horner syndrome (partial ptosis, miosis, and facial anhidrosis)
- Ipsilateral limb ataxia
- Ipsilateral taste impairment
- Dysphagia
- Nystagmus,
- This may be horizontal or horizontal–rotational, beating opposite to the side of the lesion, or directionally changing gaze-evoked, is often accompanied by skew deviation.
- Intractable hiccups (may lead to diagnostic delay)
The clinical presentation varies depending on the size and location of the infarct.[4]
Evaluation
Evaluating patients with LMS should include a detailed clinical history, physical examination, and appropriate testing, including:
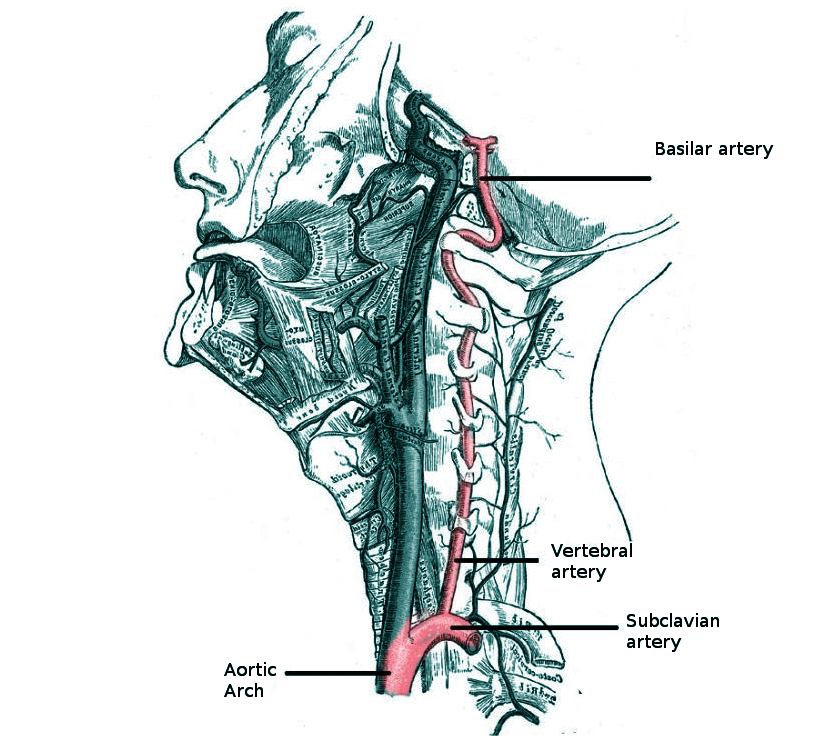
- Screening of stroke risk factors such as hypertension, diabetes mellitus, smoking history, and heart disease. A history of neck pain, trauma, or headache, particularly in younger patients, may suggest vertebral artery dissection (see Image. Vertebral Anatomy in Neck Region).[8][11]
- A complete neurological examination is critical because LMS is a clinical diagnosis based on a characteristic history and a constellation of physical findings.
- Routine blood testing, including blood glucose and serum electrolytes, is appropriate.
- An electrocardiogram is necessary to screen for atrial fibrillation.
- Transthoracic echocardiography with Doppler and neck imaging, such as carotid ultrasound or computed tomography (CT)/magnetic resonance (MR) angiography.
- Clinical and instrumental swallowing evaluations can assess swallowing, which are carried out by videofluoroscopy and/or fiberendoscopic examination.[3]
Diagnostic Imaging Tests
A CT scan of the brain is typically the initial imaging test for patients with lateral medullary syndrome, as with any emergent evaluation for a patient with acute ischemic stroke symptoms. However, CT gives suboptimal visualization of the posterior fossa structures due to obscuration by bony structures. Early ischemic changes are often not visible.[11] Magnetic resonance imaging (MRI) of the brain provides better visualization of the soft tissue structures. For example, an imaging protocol that includes fluid attenuation inversion recovery sequences improves the visualization of medullary infarction.[8][10] Additionally, diffusion-weighted MRI can detect an infarct earlier.
Neurovascular Studies
CT angiography and MR angiography examinations are advanced imaging studies that may be clinically indicated, often in consultation with a neurologist.
Treatment / Management
Similar to the management of any acute ischemic stroke, the principle of "time is brain" underscores the need for rapid evaluation. An algorithmic approach should be developed within each hospital.[19] Management in certified stroke centers has been shown to improve overall patient outcomes.[20] Treatment aims to reduce the size of the infarction and prevent any medical complications to improve patient outcomes and prognosis.[4]
Standard acute ischemic stroke algorithms apply, including intravenous thrombolysis, blood pressure control, and antiplatelet/anticoagulation (see Image. Blood Supply to Medulla). Intravenous fibrinolytic therapy with alteplase or tenecteplase is indicated for LMS due to acute ischemic infarction within 4.5 hours of onset.[19] Intravenous fibrinolytic treatment is also indicated for an acute stroke of unknown onset if the MRI shows an infarct in the diffusion-weighted image but a negative fluid-attenuation inversion recovery sequence image.[21]
Thrombectomy for large vessel occlusion in the anterior circulation can be efficacious up to 24 hours from the onset (see Image. Diagram of Brain Circulation). The outcomes with thrombectomy for posterior circulation large vessel occlusion, including the VA and basilar artery, are mostly uncertain.[21] These patients should be treated at certified stroke centers with interventional neuroradiologists and receive individualized treatment plans to achieve the best outcomes.[22]
Acute management of LMS also includes the following:
- Intensive care monitoring should be used for respiratory failure and increased intracranial pressure.[13] Signs of increased intracranial pressure may include disorientation, lethargy, headache, and vomiting. Late signs include bradycardia, hypertension with widened pulse pressure, or an irregular respiratory pattern (Cushing triad). Treatment includes head elevation to 30°, blood pressure management to maintain cerebral perfusion, targeted hyperventilation, neurosurgical consultation, and occasionally osmotic agents (such as 3% saline or mannitol).[23]
- Extracranial VA stenting may be beneficial during endovascular therapy for tandem posterior circulation lesions. A 2025 registry analysis demonstrated improved 1-year functional outcomes with this intervention.[24]
- Secondary stroke prevention can be achieved with antiplatelets, antihypertensives, and statins.[11]
- An enteral nutrition evaluation with nasogastric or gastrostomy feeding is useful if severe dysphagia persists.
- Dysphagia management can be achieved with postural/dietary modifications, swallowing exercises, or botulinum toxin for trismus.[1][3] Persistent dysphagia mandates early swallow assessment, enteral support, and goal-directed therapy with long-term rehabilitation.[25]
- Low-molecular-weight heparin prophylaxis is useful for deep vein thrombosis.
- Speech-language therapy can be effective.
- Gabapentin is useful for persistent hiccups or chronic facial pain.[2]
- Managing autonomic complications is essential; cardiac pacing can help with acute heart failure due to bradycardia.[26]
- An ophthalmology evaluation can be useful for neurotrophic keratopathy.[14]
- Regular evaluation of speech and motor function are essential.[10] (B2)
Differential Diagnosis
Depending on the predominant symptoms, the differential diagnosis of LMS will vary. The most common presentation is dizziness and vertigo with nausea and vomiting. The most important differential diagnosis is acute peripheral vestibulopathy, or acute vestibular neuritis. A careful evaluation should differentiate the 2 using the head impulse-nystagmus-skew deviation (HINTS) test. The head impulse test is negative in LMS but positive in cases of peripheral vestibulopathy. Nystagmus is unilateral, away from the side of the lesion with vestibulopathy, but bilateral and/or vertical for LMS. Skew deviation is present in LMS but absent in peripheral vestibulopathy. The HINTS test may identify patients at risk for stroke who require MRI and hospitalization.[27] Hemorrhagic stroke is much less common, and headache is a prominent symptom. Acute demyelination, as in multiple sclerosis, may present with medullary symptoms, but the patients are generally younger women with a history of a demyelinating disease.[4]
Other differential diagnoses include:
- Headache (migraine or cluster)
- Intracerebral hemorrhage
- Brainstem or cerebellar tumor, including primary and secondary malignancies
- Psychiatric conversion disorders
- Reversible cerebral vasoconstriction syndrome [11]
- Vasculitis of the large and medium-sized vessels, such as giant cell arteritis
- Vertebral artery dissection
Prognosis
LMS generally has a favorable prognosis. Significant functional recovery is common, including improvement of dysphagia.[1][8]
Complications
Complications of LMS can include the following:
- Respiratory complications may include aspiration pneumonia, respiratory failure, and central hypoventilation syndrome (eg, Ondine curse). Respiratory failure due to autonomic dysfunction can be observed rarely and causes apnea and death.
- Syndrome of inappropriate antidiuretic hormone can be a complication, although rare in LMS compared to other types of stroke.
- Neurotrophic keratopathy (corneal damage and infection) is a potential complication.[14][28]
- Severe dysphagia may occur in lateral medullary syndrome due to involvement of the nucleus ambiguus, which innervates muscles responsible for swallowing.
- Trigeminal neuralgia can result from disruption of the spinal trigeminal nucleus and tract, leading to facial pain and sensory disturbances.
- Cardiac complications are rare and may occur when the nucleus tractus solitarius, which is involved in cardiovascular regulation, is affected.[26]
- Obstructive hydrocephalus may develop as a rare complication of lateral medullary syndrome due to brainstem edema or infarct-related compression of cerebrospinal fluid pathways, such as the fourth ventricle.
Deterrence and Patient Education
Patients with lateral medullary syndrome require early initiation of physical and occupational therapy to support recovery of strength, mobility, and functional independence. Secondary stroke prevention strategies, including risk factor modification and antithrombotic therapy, should be discussed to optimize long-term outcomes. Patients with dysphagia should undergo formal swallowing rehabilitation. In cases of severe dysphagia, placement of a gastrostomy tube may be necessary to maintain adequate nutrition. Regular follow-up with a speech-language pathologist is recommended to monitor progress and adjust therapeutic interventions accordingly.
Pearls and Other Issues
A physical examination, including the HINTS test, is the most crucial clinical examination used to differentiate between vestibular neuritis and stroke in patients with an acute onset of vertigo. Loss of pain and temperature sensation on the ipsilateral face and the contralateral side of the body, arm, and leg is most diagnostic of an acute LMS. Weakness is uncommon in LMS. The prognosis for patients with LMS depends on the size of the infarct; however, most patients generally have a better outcome than other ischemic stroke syndromes, except for some lacunar syndromes. Gait instability or ataxia is the most typical sequela. Intractable hiccups may occur.[4]
Enhancing Healthcare Team Outcomes
Optimal outcomes in managing LMS require a collaborative, interprofessional approach. Emergency clinicians and hospitalists play a crucial role in the early recognition and stabilization of patients presenting with symptoms suggestive of brainstem infarction. Neurologists confirm diagnoses, guide acute management, and coordinate appropriate imaging and interventions. Nurses provide continuous monitoring and patient education, while physical and occupational therapists help address balance, coordination, and activities of daily living to maximize functional recovery.
Speech language pathologists play an integral role in assessing and treating dysphagia and communication difficulties, which are common in patients with lateral medullary syndrome. Registered dietitians ensure nutritional needs are safely met, especially when swallowing is impaired. Social workers support patients and families by addressing psychosocial needs, arranging resources, and facilitating discharge planning. Stroke prevention, supportive care, and caregiver education are crucial in reducing the risk of recurrent events and optimizing long-term outcomes. Ongoing follow-up and rehabilitation are vital to promote patient safety, independence, and quality of life.
Media
(Click Image to Enlarge)
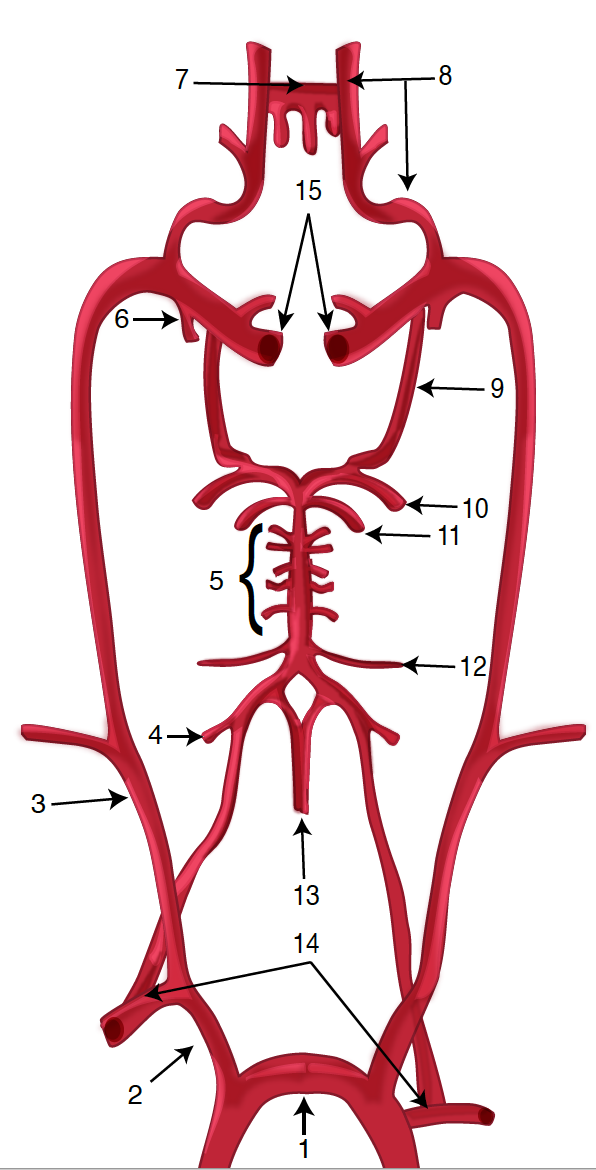
Diagram of the Brain Blood Circulation. Each number corresponds to the following neuroanatomy: 1) aortic arch; 2) brachiocephalic artery; 3) common carotid artery; 4) posterior inferior cerebellar artery; 5) pontine arteries; 6) anterior choroidal artery; 7) anterior communicating artery; 8) anterior cerebral artery; 9) posterior communicating artery; 10) posterior cerebral artery; 11) superior cerebellar artery; 12) anterior inferior cerebellar artery; 13) anterior spinal artery; 14) arches of vertebral arteries; and 15) internal carotid arteries.
Contributed by O Kuybu, MD
(Click Image to Enlarge)
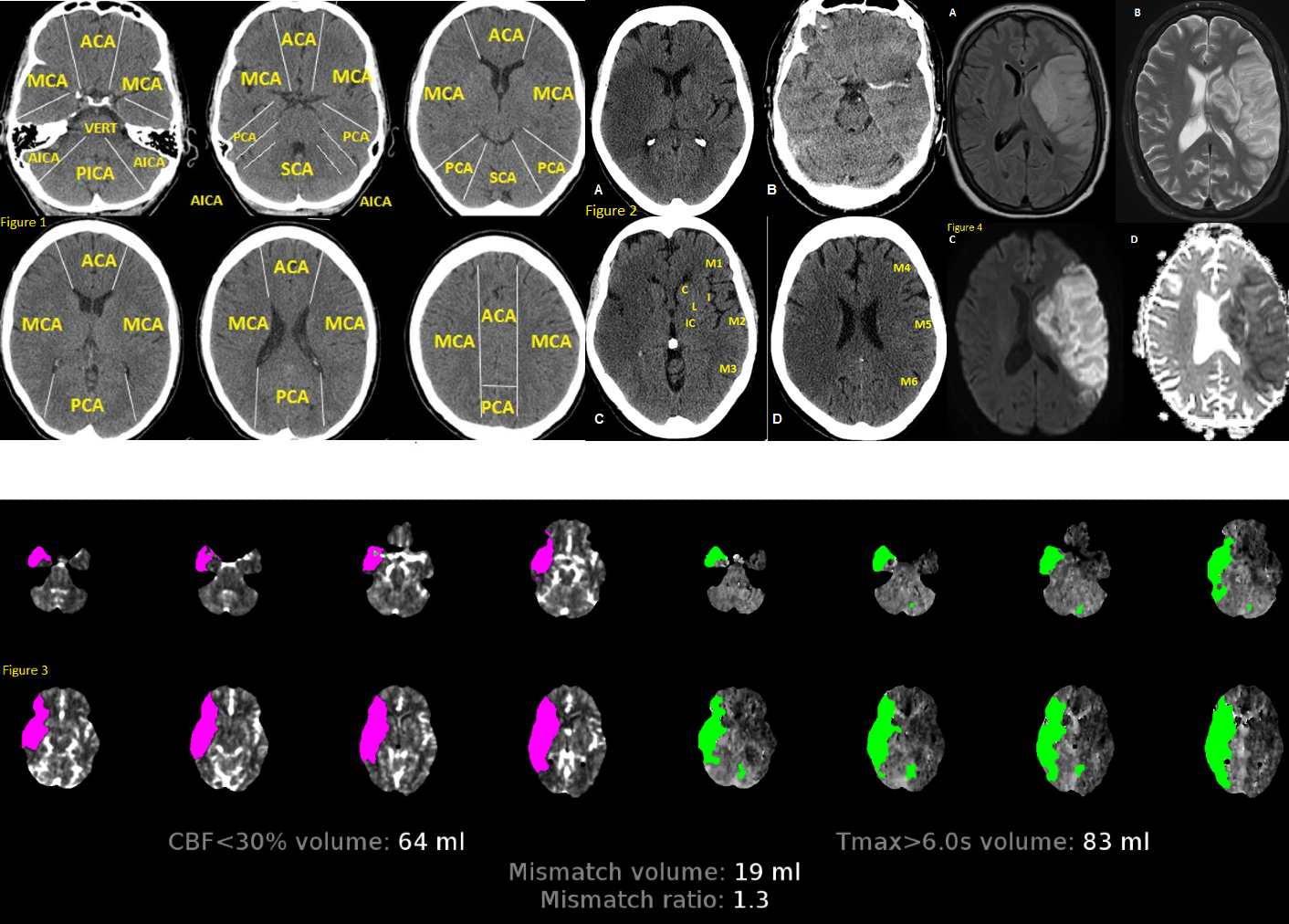
Anatomy of Brain Vascular Territories. Figure 1: ACA (anterior cerebral artery), MCA (middle cerebral artery), PCA (posterior cerebral artery), AICA (anterior inferior cerebellar artery), PICA (posterior inferior cerebellar artery), and SCA (superior cerebellar artery). Figure 2: Noncontrast CT shows loss of gray-white matter differentiation in the right MCA territory, consistent with acute large right MCA infarction. Figure 3: CT perfusion imaging reveals stroke; areas with increased mean transit time and time-to-peak/time-to-maximum, along with decreased cerebral blood volume and cerebral blood flow, indicate infarct core. Figure 4: MRI shows large left MCA infarction. It appears hyperintense on FLAIR (A) and T2 (B), with mass effect suggesting subacute infarction. Diffusion restriction is confirmed by hyperintensity on DWI (C) and hypointensity on ADC map (D).
Contributed by O Shafaat, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
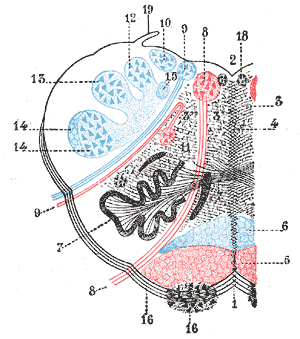
Lateral Medullary Syndrome. This syndrome, also known as Wallenberg syndrome, is a neurological disorder affecting the dorsolateral medulla oblongata, most commonly caused by ischemic infarction of the posterior inferior cerebellar artery or the vertebral artery.
1. Anterior median fissure. 2. Fourth ventricle. 3. Formatio reticularis. 4. Raphe. 5. Pyramid. 6. Lemniscus. 7. Inferior olivary nucleus with the two accessory olivary nuclei. 8. Hypoglossal nerve. 10. Lateral dorsal acoustic nucleus. 11. Nucleus ambiguus. 12. Gracile nucleus. 13. Cuneate nucleus. 14. Head of posterior column. 15. Fasciculus solitarius. 16. Anterior external arcuate fibers. 17. Nucleus lateralis 18. Nucleus of fasciculus teres. 19. Ligula.
Henry Van Dyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Kim H, Lee HJ, Park JW. Clinical course and outcome in patients with severe dysphagia after lateral medullary syndrome. Therapeutic advances in neurological disorders. 2018:11():1756286418759864. doi: 10.1177/1756286418759864. Epub 2018 Feb 28 [PubMed PMID: 29511384]
Level 3 (low-level) evidenceSampath V, Gowda MR, Vinay HR, Preethi S. Persistent hiccups (singultus) as the presenting symptom of lateral medullary syndrome. Indian journal of psychological medicine. 2014 Jul:36(3):341-3. doi: 10.4103/0253-7176.135397. Epub [PubMed PMID: 25035568]
Level 3 (low-level) evidenceBattel I, Koch I, Biddau F, Carollo C, Piccione F, Meneghello F, Merico A, Palmer K, Marchese Ragona R. Efficacy of botulinum toxin type-A and swallowing treatment for oropharyngeal dysphagia recovery in a patient with lateral medullary syndrome. European journal of physical and rehabilitation medicine. 2017 Oct:53(5):798-801. doi: 10.23736/S1973-9087.17.04499-9. Epub 2017 Mar 6 [PubMed PMID: 28264544]
Lui F, Tadi P, Anilkumar AC. Wallenberg Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 29262144]
Thapliyal K, Garg A, Singh VP. Lateral medullary syndrome: Case report and review of literature. Journal of family medicine and primary care. 2022 Nov:11(11):7438-7441. doi: 10.4103/jfmpc.jfmpc_667_22. Epub 2022 Dec 16 [PubMed PMID: 36993088]
Level 3 (low-level) evidenceShrestha S, Maharjan S, Ghimire B, Mainali N, Gurung K, Yadav HR, Bhandari K, Shrestha S, Halder A, Rajak K, Jaiswal V. Lateral medullary syndrome resulting from atrial fibrillation due to rheumatic heart disease: A case report and literature review. Clinical case reports. 2024 Jul:12(7):e9124. doi: 10.1002/ccr3.9124. Epub 2024 Jun 28 [PubMed PMID: 38947544]
Level 3 (low-level) evidenceGiannopoulos S, Markoula S, Kosmidou M, Pelidou SH, Kyritsis AP. Lateral medullary ischaemic events in young adults with hypoplastic vertebral artery. Journal of neurology, neurosurgery, and psychiatry. 2007 Sep:78(9):987-9 [PubMed PMID: 17702781]
Level 3 (low-level) evidenceKim JS. Pure lateral medullary infarction: clinical-radiological correlation of 130 acute, consecutive patients. Brain : a journal of neurology. 2003 Aug:126(Pt 8):1864-72 [PubMed PMID: 12805095]
Level 2 (mid-level) evidenceMiao HL, Zhang DY, Wang T, Jiao XT, Jiao LQ. Clinical Importance of the Posterior Inferior Cerebellar Artery: A Review of the Literature. International journal of medical sciences. 2020:17(18):3005-3019. doi: 10.7150/ijms.49137. Epub 2020 Oct 18 [PubMed PMID: 33173421]
Shetty SR, Anusha R, Thomas PS, Babu SG. Wallenberg's syndrome. Journal of neurosciences in rural practice. 2012 Jan:3(1):100-2. doi: 10.4103/0976-3147.91980. Epub [PubMed PMID: 22346215]
Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Frontiers in neurology. 2014:5():30. doi: 10.3389/fneur.2014.00030. Epub 2014 Apr 7 [PubMed PMID: 24778625]
Imam YZ, Chandra P, Singh R, Hakeem I, Al Sirhan S, Kotob M, Akhtar N, Kamran S, Al Jerdi S, Muhammad A, Haroon KH, Hussain S, Perkins JD, Elalamy O, Alhatou M, Ali L, Abdelmoneim MS, Joseph S, Morgan D, Uy RT, Bhutta Z, Azad A, Ayyad A, Elsotouhy A, Own A, Deleu D. Incidence, clinical features, and outcomes of posterior circulation ischemic stroke: insights from a large multiethnic stroke database. Frontiers in neurology. 2024:15():1302298. doi: 10.3389/fneur.2024.1302298. Epub 2024 Feb 7 [PubMed PMID: 38385041]
Aynaci O, Gok F, Yosunkaya A. Management of a patient with Opalski's syndrome in intensive care unit. Clinical case reports. 2017 Sep:5(9):1518-1522. doi: 10.1002/ccr3.1111. Epub 2017 Jul 30 [PubMed PMID: 28878917]
Level 3 (low-level) evidenceCidad P, Boto A, Del Hierro A, Capote M, Noval S, Garcia A, Santiago S. Unilateral punctate keratitis secondary to Wallenberg Syndrome. Korean journal of ophthalmology : KJO. 2014 Jun:28(3):278-83. doi: 10.3341/kjo.2014.28.3.278. Epub 2014 May 19 [PubMed PMID: 24882965]
Level 3 (low-level) evidenceHe Z, Xie M, Xie C, An D, Dai M, Wen H, Shan Y. Characteristics of Dysphagia in Medullary and Cerebellar Stroke: An Observational Study Based on HRPM and FEES. Archives of physical medicine and rehabilitation. 2025 May 20:():. pii: S0003-9993(25)00705-1. doi: 10.1016/j.apmr.2025.05.003. Epub 2025 May 20 [PubMed PMID: 40403865]
Level 2 (mid-level) evidenceKim JM, Park KY, Kim DH, Bae JH, Shin DW, Youn YC, Kwon OS. Symptomatic hyponatremia following lateral medullary infarction: a case report. BMC neurology. 2014 May 22:14():111. doi: 10.1186/1471-2377-14-111. Epub 2014 May 22 [PubMed PMID: 24886592]
Level 3 (low-level) evidenceAgarwal A, Agrawal P, Suri K, Theengh DP, Kaushal H. Ipsilateral Hemiparesis in Lateral Medullary Syndrome: A Case Report on Opalski's Syndrome. Cureus. 2024 Dec:16(12):e76105. doi: 10.7759/cureus.76105. Epub 2024 Dec 20 [PubMed PMID: 39834955]
Level 3 (low-level) evidenceSun B, Jin Y, Ye Z, Xu H, Luo W, Liu S. Vertebral artery dissection induced lateral medullary syndrome characterized with severe bradycardia: a case report and review of the literature. Annals of palliative medicine. 2022 Oct:11(10):3330-3336. doi: 10.21037/apm-22-1098. Epub [PubMed PMID: 36367000]
Level 3 (low-level) evidencePowers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec:50(12):e344-e418. doi: 10.1161/STR.0000000000000211. Epub 2019 Oct 30 [PubMed PMID: 31662037]
Boggs KM, Vogel BT, Zachrison KS, Espinola JA, Faridi MK, Cash RE, Sullivan AF, Camargo CA Jr. An inventory of stroke centers in the United States. Journal of the American College of Emergency Physicians open. 2022 Apr:3(2):e12673. doi: 10.1002/emp2.12673. Epub 2022 Feb 28 [PubMed PMID: 35252972]
Ahmed HK, Mathisen SM, Kurz K, Dalen I, Logallo N, Thomassen L, Kurz M. Thrombolysis in wake-up stroke based on MRI mismatch. Journal of the neurological sciences. 2024 Nov 15:466():123265. doi: 10.1016/j.jns.2024.123265. Epub 2024 Oct 4 [PubMed PMID: 39378794]
Baik SH, Kim JY, Jung C. A Review of Endovascular Treatment for Posterior Circulation Strokes. Neurointervention. 2023 Jun:18(2):90-106. doi: 10.5469/neuroint.2023.00213. Epub 2023 Jun 27 [PubMed PMID: 37365755]
Zhang Z, Guo Q, Wang E. Hyperventilation in neurological patients: from physiology to outcome evidence. Current opinion in anaesthesiology. 2019 Oct:32(5):568-573. doi: 10.1097/ACO.0000000000000764. Epub [PubMed PMID: 31211719]
Level 3 (low-level) evidenceLi W, Doheim MF, Qiu Z, Wang T, Chen Z, Zi W, Yang Q, Guan H, Qiao H, Liu W, Hu W, Liu X, Huang J, Han Z, Chen Z, Zhao Z, Sun W, Nogueira RG. Endovascular Treatment for Acute Posterior Circulation Tandem Lesions: Insights From the BASILAR and PERSIST Registries. Journal of stroke. 2025 Jan:27(1):75-84. doi: 10.5853/jos.2024.03055. Epub 2025 Jan 31 [PubMed PMID: 39916456]
Level 2 (mid-level) evidenceMagara J, Ita R, Tsutsui Y, Sakai H, Zhang M, Inoue M. A Case of Dysphagia Rehabilitation in a Patient in the Chronic Stage of Lateral Medullary Syndrome. Dysphagia. 2024 Jun:39(3):534-539. doi: 10.1007/s00455-024-10690-6. Epub 2024 Mar 23 [PubMed PMID: 38520512]
Level 3 (low-level) evidencevon Heinemann P, Grauer O, Schuierer G, Ritzka M, Bogdahn U, Kaiser B, Schlachetzki F. Recurrent cardiac arrest caused by lateral medulla oblongata infarction. BMJ case reports. 2009:2009():. pii: bcr02.2009.1625. doi: 10.1136/bcr.02.2009.1625. Epub 2009 Oct 12 [PubMed PMID: 21991295]
Level 3 (low-level) evidenceKrishnan K, Bassilious K, Eriksen E, Bath PM, Sprigg N, Brækken SK, Ihle-Hansen H, Horn MA, Sandset EC. Posterior circulation stroke diagnosis using HINTS in patients presenting with acute vestibular syndrome: A systematic review. European stroke journal. 2019 Sep:4(3):233-239. doi: 10.1177/2396987319843701. Epub 2019 Apr 10 [PubMed PMID: 31984230]
Level 1 (high-level) evidenceWu S, Li N, Xia F, Sidlauskas K, Lin X, Qian Y, Gao W, Zhang Q. Neurotrophic keratopathy due to dorsolateral medullary infarction (Wallenberg syndrome): case report and literature review. BMC neurology. 2014 Dec 4:14():231. doi: 10.1186/s12883-014-0231-y. Epub 2014 Dec 4 [PubMed PMID: 25472780]
Level 3 (low-level) evidence