Introduction
Lyme disease, or Lyme borreliosis, is the most commonly transmitted tick-borne infection in the United States (US) and among the most frequently diagnosed tick-borne infections worldwide. Lyme disease is divided into 3 stages: early localized, early disseminated, and late. Early localized disease is distinguished by the red ring-like expanding rash of erythema migrans at the site of a recent tick bite. Other symptoms experienced at this stage may be malaise, headache, fever, myalgia, and arthralgia. Most patients only experience the symptoms of early localized disease. About 20% of patients develop early disseminated disease, with the most common symptoms being multiple erythema migrans lesions.
Other symptoms of the disseminated stage are flu-like symptoms, lymphadenopathy, arthralgia, myalgia, palsies of the cranial nerves, especially of cranial nerve VII, ophthalmic conditions, and lymphocytic meningitis. Additionally, cardiac manifestations, including conduction abnormalities, myocarditis, or pericarditis, may occur. The most common late-stage manifestation is arthritis, which is usually pauciarticular and affects large joints, especially the knees.[1][2] Lyme disease is a curable condition if identified and treated appropriately.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
In the US, Lyme disease is caused by the bacterial spirochete Borrelia burgdorferi (and rarely Borrelia mayonii) and is transmitted by the bite of an Ixodes genus black-legged tick. In Europe and Asia, the predominant causes of Lyme disease are B burgdorferi, Borrelia afzelii, and Borrelia garinii.[3][4] In Asia, B garinii is the most common cause of Lyme disease. B burgdorferi has a particular affinity for joints, while B garinii has a predilection for nervous system tissue. B afzelii has an affinity for the skin. In the US, Borrelia spp are transmitted by ticks, with Ixodes scapularis being the main vector in the Northeastern and upper Midwest regions and Ixodes pacificus in the Western region. Ixodes ricinus and Ixodes persulcatus are the main species in Europe and Asia, respectively.
The tick lifecycle is significant to understanding when they are infectious. They have a 2- to 3-year life span and cycle through 4 life stages: egg, larva, nymph, and adult. The larva and nymph must take a blood meal to advance to the next life stage, and the female ticks require blood to reproduce. During these meals, ticks become infected with the bacteria. Ticks attach to an animal host reservoir, typically birds or other small mammals such as mice and voles, and feed anywhere from 3 to 7 days. While ticks can feed on any mammal, deer are not a natural reservoir of the spirochete. Those infected nymphs and adult ticks spread the bacteria each time they subsequently feed.
Epidemiology
Lyme disease is most commonly reported in the Northeastern and upper Midwestern US regions. The primary states with endemic Lyme disease are Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont, Virginia, and Wisconsin. Sporadic cases have been reported in northern California, Oregon, and Washington.[5][6]
The infection tends to occur during late spring, summer, and early fall, coinciding with activity of the nymph feeding and more interaction of people in spaces that increase the risk for a tick bite. In 2022, 62,551 cases of Lyme disease in the US were reported to the US Centers for Disease Control and Prevention (CDC).cdc.gov/lyme Other estimates place the actual number of cases much higher. More men than women tend to be affected. The age distribution is bimodal, with more children younger than 15 and adults 45 and older affected. Lyme disease can affect people of all ethnic backgrounds, but most cases reported are in White individuals.
Pathophysiology
The Borrelia spirochete lives in the midgut of the tick. Once the tick feeds, blood from the affected host triggers the proliferation of the spirochetes and induces the spirochete to express a protein called outer surface protein C, which allows the spirochete to invade the tick salivary glands by moving from the midgut closer to the tick mouthpiece. This process takes time and varies by species of tick and spirochete, but it explains why transmission to human hosts requires a tick to be attached to the host for more than 15 hours in most cases. During transmission, the spirochetes are deposited in the skin and then disseminated from the site of inoculation to other areas of the body.
The infected host's innate and adaptive immune system triggers macrophage- and antibody-mediated killing of the spirochetes. In some cases, the immune system can clear the infection without evidence of developing disease. However, due to sophisticated mechanisms by the spirochete to evade the host immune response, the infection may persist and develop clinically evident disease.[7] The host inflammatory response is responsible for causing tissue damage in organs, including the heart, nervous tissue, and joints. The spirochete itself is not known to be directly toxic.
The pathophysiology of posttreatment Lyme disease syndrome (PTLDS) is unclear. Several theories have been suggested, though no evidence has been reported to support the existence of chronic B burgdorferi infection. Some active pathology may be present after the initial clearance of the microbes, the persistence of inappropriate immune activation and inflammation, or some combination of both. No one leading proposal has been established, and more research is needed in this area.[8]
For patients who develop postinfectious Lyme arthritis, a complex interplay is proposed between bacterial and host factors. No active infection can be identified in these cases, yet there is objective evidence of ongoing inflammation of the joints. The persistence of bacterial debris may continue to trigger immune activation. Additionally, immune system overactivation by protein-specific bacteria is possible.[9]
Histopathology
Erythema Migrans
A biopsy of erythema migrans is not needed to establish a diagnosis. Erythema migrans histologic findings are nonspecific, usually showing a perivascular cellular infiltrate consisting of histiocytes, lymphocytes, and plasma cells. Rarely are mast cells and neutrophils identified. A biopsy may show eosinophilic infiltrates, representing a local reaction to the bite. Spirochetes may be identified using antibody-labeled or silver stains but are typically rarely identified in skin specimens and should not be used as a basis for diagnosis.
Acrodermatitis Chronica Atrophicans
Histopathology is essential for the diagnosis of acrodermatitis chronica atrophicans, which may overlap and be confused clinically with morphea, mycosis fungicides, or lichen sclerosis.[10] Interstitial granulomatous infiltrates, which are CD68-positive, are common, and CD3-positive lymphocytes are predominant. Histiocytes are also part of the infiltrates in all cases. Plasma cells may be seen rarely but can be visualized by staining for CD138. Thickened collagen bundles and band-like infiltrates of CD4 T cells are other common features. Interstitial fibroblasts are also reduced and can be seen by staining for CD34.[10]
Borrelial Lymphocytoma
Histologic examination is performed in patients with suspected Borrelia lymphocytoma when the history is unclear enough to support a diagnosis, but the histopathology is generally nonspecific. Evaluating for other causes of nodules and ruling out neoplasm can help. The biopsy tends to show a dense dermal polyclonal lymphocytic infiltrate with lymphoid follicles and pseudoterminal centers or a pseudolymphoma.[11]
History and Physical
Lyme disease is classified into 3 stages: early localized, early disseminated, and late. Approximately 1.6% to 7% of individuals may have an asymptomatic Lyme infection.[7] In most cases, however, patients develop symptoms of Lyme disease, with erythema migrans being the most common initial manifestation. Erythema migrans is characteristic of early localized disease, occurring 1 to 2 weeks after a tick bite. Patients may develop early disseminated or late disease if the infection persists or goes untreated.
Early disseminated disease is associated with neurologic and cardiac manifestations. Neurologic Lyme disease manifestations include facial nerve (cranial nerve VII) palsy, lymphocytic meningitis, or radiculopathy. Cardiac involvement may consist of myopericarditis and heart block. Lyme arthritis is typically a late disease manifestation and may be monoarticular or pauciarticular, generally involving large joints, most commonly the knee, and occurring months after the initial tick bite.[12] Because the symptoms are not specific, clinicians should consider other conditions and infections transmitted by ticks (eg, coinfection with Babesia microti and Ehrlichia). Coinfection has been reported in approximately 10% of patients.
Early Localized Disease
The early localized phase of Lyme disease may present with an erythema migrans rash and low-grade fever. This stage usually occurs within 1 to 28 days following the tick bite. The erythema migrans rash is seen in 70% of patients and may develop between 7 to 14 days following the tick bite. The uniform rash usually occurs at the tick bite site and may burn, itch, or be asymptomatic. The rash tends to expand for a few days, and concentric rings may be visible (see Image. Lyme Disease "Bulls-Eye" Rash).
If left untreated, the rash persists for 2 to 3 weeks and may expand to 20 cm in diameter. About 20% of patients may have recurrent episodes of the rash, and multiple lesions are not uncommon. At the same time, flu-like symptoms may be present. A low-grade fever may be associated with myalgia, neck stiffness, and headache. Visual problems include eye redness and tearing.
Early Disseminated Disease
Stage 2 is the early disseminated phase of the disease process. This stage usually develops 3 to 12 weeks after the initial infection. Features may include general malaise, fever, neurological symptoms (dizziness and headache), muscle pain, or cardiac symptoms (chest pain, palpitations, and dyspnea). About 20% of patients have centrl nervous system involvement, including meningitis, cranial neuropathies, radiculopathy, peripheral neuropathy, cerebellar symptoms, or rarely encephalomyelitis. Bell palsy affecting the facial nerve is seen in approximately 5% of patients. The classic triad includes meningitis, cranial neuropathy, and motor or sensory radiculoneuropathy, but these features may occur alone.
Cardiac involvement may present with arrhythmias or transient heart block. Conduction abnormalities are not uncommon, but most cases are isolated and rarely last more than a few days. Seldom does a patient require permanent pacing. Sporadic cases of sudden cardiac death have occurred due to fulminant carditis.
Ocular manifestations have also been described, with conjunctivitis being the most common feature and keratitis, iridocyclitis, retinal vasculitis, optic neuropathy, and uveitis being rare. Borrelial lymphocytoma is a rare presentation of early Lyme disease reported mainly in Europe; this condition tends to occur weeks to several months after the initial infection. Borrelial lymphocytoma presents as a slow-growing, painless plaque or nodule, which is red-bluish in coloration and usually occurs on the ear lobe in children or the breast in adults.[11] The lesion may resolve spontaneously.
Late Disease
Late-stage Lyme disease may occur months after the initial infection. The typical features include neurological and musculoskeletal involvement. Many patients may not have a history of erythema migrans. However, these individuals may present with sensory axonal polyneuropathy, encephalomyelitis, or mononeuropathy. Cognitive deficits are common. Encephalopathy presents with deficits in concentration, cognition, memory loss, and personality changes. Extreme irritability and depression are also common. Encephalomyelitis is rare and can present with ataxia, seizures, hemiparesis, autonomic dysfunction, and hearing loss.
Lyme arthritis tends to affect large joints, especially the knee. Symptoms manifest as joint pain with swelling and stiffness. Smaller joints, such as the elbow, may be involved, and episodes of bursitis or tendinitis may occur. Over time, in most cases, symptoms tend to subside even without intervention. In a small proportion of patients with Lyme arthritis, chronic inflammatory arthritis similar to rheumatoid arthritis may develop. This complication of Lyme disease is termed postinfectious Lyme arthritis.
Acrodermatitis chronica atrophicans is typically seen in older women and tends to occur on the dorsum of the hands and feet. This rash presents as bluish-red discoloration with some swelling that progresses very slowly as an expansive lesion, leading the skin to become atrophic over time; this may occur years following the primary infection, has been described chiefly in European cohorts, and may be confused with morphea.
Evaluation
A challenge in evaluating Lyme disease is that many patients diagnosed with the disease often have no recollection of a tick bite or a rash. Symptoms may be vague in most patients, and testing is needed in those cases where Lyme disease is suspected as an underlying etiology. Therefore, the CDC recommends a 2-step serologic testing process using US Food and Drug Administration (FDA)-cleared assays.
Serologic testing is unreliable during the first few weeks of infection, given the time needed for seroconversion. Patients presenting with erythema migrans rash and a history of residing in or traveling to an endemic region of Lyme disease may be treated based on clinical findings. In later stages of the disease, the 2-step approach is recommended for the serologic diagnosis of Lyme disease. The first step is to perform a quantitative screening test for serum antibodies to B burgdorferi using a sensitive enzyme immunoassay (EIA) or immunofluorescent antibody assay.
If this step is negative, no further testing is recommended. A Western blot should follow specimens with positive or equivocal results. Standard 2-tier testing uses EIA as the first step and Western blot as the 2nd step. According to the CDC, an immunoblot is positive if at least 2 of 3 bands are present on the immunoglobulin (Ig) IgM immunoblot within 30 days of symptom onset or 5 to 10 bands are present on the IgG immunoblot at any time.[13] Serologic diagnosis is greater than 80% sensitive for patients with neurologic or cardiac manifestations.[14][15][16]
Sequential serological testing is not recommended in the acute state because antibody titers often remain elevated for a long time. Testing the tick is also not recommended. False positive results may occur in patients with other conditions, including syphilis, rheumatoid arthritis, relapsing fever, and Epstein-Barr infection.
Suspected Cardiac Manifestations
Serologic testing is recommended for patients presenting with acute myocarditis or pericarditis of unknown cause in an appropriate epidemiologic setting. Specific cardiac testing with a screening electrocardiogram is recommended for patients presenting with dyspnea, edema, palpitations, lightheadedness, chest pain, and syncope and living in a Lyme endemic area. For those presenting with more severe potential manifestations, including exercise intolerance, palpitations, presyncope, syncope, pericardial pain, evidence of pericardial effusion, elevated biomarkers, edema, shortness of breath, or evidence of PR interval prolongation of more than 300 milliseconds, admission to the hospital for continuous electrocardiogram monitoring is recommended.
Suspected Neurologic Manifestations
Testing is recommended for patients presenting with meningitis, painful radiculoneuritis, mononeuropathy multiplex including confluent mononeuropathy multiplex, acute cranial neuropathies, or in patients with evidence of spinal cord or brain inflammation, and with epidemiologically plausible exposure to ticks infected with B burgdorferi.[17]
Suspected Arthritic Manifestations
Joint aspiration is only recommended if a clinician suspects inflammatory arthritis. When assessing possible Lyme arthritis, serum antibody testing is recommended over polymerase chain reaction or culture of blood, synovial fluid, or tissue. However, a polymerase chain reaction of the synovial fluid or tissue can be performed if the diagnosis remains under question despite positive Lyme serologies.
Treatment / Management
Lyme Disease Management
Lyme disease treatment is divided into the following categories:
Asymptomatic patients with potential exposure: Asymptomatic children and adults with a known tick bite should be treated with prophylactic antibiotics of a single dose of oral doxycycline 200 mg for adults and 4.4 mg/kg (up to a maximum dose of 200 mg) for children if the tick bite was from any of the following:
- An identified Ixodes spp vector species
- Occurred in a highly endemic area
- The tick was attached for ≥36 hours [17] (A1)
Erythema migrans rash: Patients with 1 or more classic appearing erythema migrans rash in an endemic area for Lyme disease should be treated without further testing. Oral antibiotic therapy should be used with a 10-day course of doxycycline or a 14-day course of amoxicillin or cefuroxime axetil. Second-line therapy is 5 to 10 days of azithromycin for those patients intolerant of or allergic to the former antibiotics.[17] To establish a diagnosis before antibiotic treatment, serologic testing is recommended for patients with an atypical rash but suggestive of erythema migrans. (A1)
Neurologic manifestations: Intravenous (IV) ceftriaxone, cefotaxime, penicillin G, or oral doxycycline is recommended over other antimicrobials for 14 to 21 days. IV is preferred, but choosing oral or IV antibiotics should be based on patient-specific factors such as tolerance, access, or risk for adverse effects.[17](A1)
Cardiac manifestations: For patients with symptomatic heart block, a temporary pacemaker may be placed over a permanent pacemaker, as in most cases with antibiotic treatment, heart block is reversible. Oral doxycycline as an outpatient is sufficient for patients with Lyme carditis and mild symptoms. For patients with Lyme carditis and more severe manifestations requiring hospitalization and monitoring, IV ceftriaxone is preferred with a switch to an oral antibiotic as the patient clinically improves. A total of 14 to 21 days of antibiotics is recommended.[17](A1)
Arthritis: Lyme arthritis should be treated with oral doxycycline for 28 days. A second course of oral antibiotics may be administered to those with a partial response. For those who have had minimal or no response, IV ceftriaxone for 2 to 4 weeks can be administered.[17] For patients who have developed postantibiotic Lyme arthritis refractory to a course of oral and a course of IV antibiotics, referral to a rheumatologist for consideration of intra-articular corticosteroid injections, use of disease-modifying agents or other advanced therapies should be considered. (A1)
Borrelial lymphocytoma: Oral antibiotic therapy for 14 days is recommended.[17](A1)
Acrodermatitis chronica atrophicans: Oral antibiotic therapy for 21 to 28 days is recommended.[17] (A1)
Posttreatment Lyme Disease Syndrome
Ten to 20% of patients with Lyme disease will not respond to treatment and can develop PTLDS after using antibiotics.[18] For patients with no objective manifestations of Lyme disease, such as active rash, cardiac or neurologic findings, or arthritis, but with persistent or recurring nonspecific symptoms such as fatigue, pain, or cognitive impairment following recommended treatment for Lyme disease, there is no evidence that additional antibiotic therapy confers any benefit. Patients are treated for symptom management. For arthralgia and myalgia, nonsteroidal anti-inflammatory drugs, acetaminophen, heating pads, and physical therapy may be used. For neurocognitive symptoms, cessation of any medications that may contribute to neurocognitive symptoms should be considered. These medications may include benzodiazepines, anticholinergics, antihistamines, and opioids.[8] Referrals to rheumatology, neurology, physical rehabilitation, and psychiatry specialists may be helpful. (B3)
Antibiotic Refractory Lyme Arthritis
About 10% of patients with Lyme arthritis will experience persistent inflammatory arthritis that does not respond to 1 or more round of antibiotic treatments. If no evidence of persistent infection is identified, this manifestation may be called postinfectious Lyme arthritis. A referral to a rheumatologist is indicated for further evaluation and management.
Special Populations
Doxycycline is used in most patients except in young children and those who are pregnant. In children, amoxicillin remains the drug of choice. For patients older than 8 with early, localized disease, doxycycline is recommended for 10 days. Patients younger than 8 should receive amoxicillin or cefuroxime for 14 days to avoid the potential for tooth staining caused by tetracycline use in young children.[19][20][21]
Clinicians should monitor patients for the Jarisch-Herxheimer reaction when starting therapy, which involves a transient worsening of symptoms during the first 24 hours of treatment. The Jarisch-Herxheimer reaction is a cytokine-driven reaction to the antibiotic-mediated destruction of spirochetes. This reaction is seen in 5% to 15% of patients and usually resolves within 1 to 2 days. Treatment should not be interrupted if this reaction occurs, but symptom therapy with acetaminophen and oral nonsteroidal anti-inflammatory medications can be helpful.
Differential Diagnosis
In patients with erythema migrans, a careful history and physical examination are primarily what is required to diagnose Lyme disease. However, many patients with Lyme disease present without erythema migrans, and the diagnosis becomes a challenge. In those cases, erythema migrans may never have occurred, may not have been recognized, or may not have been correctly diagnosed by the clinician. The differential diagnoses for Lyme disease considered are guided by the clinical manifestation observed, including:
- Erythema migrans: Aarthropod hypersensitivity reaction, Southern tick-associated rash illness (STARI), cellulitis, erysipelas, erythema multiforme, tinea, nummular eczema, granuloma annular, contact dermatitis, urticaria, fixed drug eruption, pityriasis rosea, parvovirus B19 infection, dermatomyositis, systemic lupus erythematosus
- Neuroborreliosis: Other causes of facial palsy, viral meningitis, mechanical radiculopathy, multiple sclerosis, and other autoimmune-mediated causes of encephalomyelitis
- Carditis: Other infectious and noninfectious causes of conduction disturbances or myopericarditis
- Arthritis: Gout, calcium pyrophosphate deposition disease, septic arthritis, viral arthritis, psoriatic arthritis, reactive arthritis, early rheumatoid arthritis, seronegative spondyloarthritis, and sarcoid arthritis
- Acrodermatitis chronica atrophicans: Aging skin, chilblains, venous insufficiency, superficial thrombophlebitis, eczema, morphea, scleroderma
- Borrelial lymphocytoma: Centrofollicular B cell lymphoma, follicular B cell lymphoma, Paget disease, inflammatory breast cancer, sarcoidosis, chronic infection, insect bite
- Posttreatment Lyme disease syndrome: Long COVID, depression, hypothyroidism, fibromyalgia, myalgic-encephalomyelitis, micronutrient deficiencies (eg, vitamin B12)
Prognosis
Treatment is usually curative for early cases. However, treatment may be complicated due to late diagnosis, antibiotic treatment failure, and concurrent infection with other tick-borne diseases. For patients with early Lyme disease, more than 80% of patients have complete resolution of symptoms following recommended treatment.[7]
Approximately 10% to 20% of patients have lingering symptoms of fatigue, pain, or joint and muscle aches after treatment. These symptoms, known as posttreatment Lyme disease syndrome, can last for 6 or more months. Currently, a novel vaccine directed against the outer surface protein A target of Borrelia has been developed and is in a 3-year clinical trial (beginning in 2022). If successful, this vaccine may provide another means of prevention of Lyme disease and related complications in at-risk populations.
Complications
The complications that can manifest with Lyme disease include:
- Arthritis
- Carditis
- Neurological deficits
- Ocular manifestations
- Acrodermatitis chronica atrophicans
- Lymphocytoma
Consultations
Consultations that are typically requested for patients with this condition include the following:
- Infectious diseases
- Dermatology
- Neurology
- Rheumatology
- Cardiology
Deterrence and Patient Education
Lyme disease is a treatable and possibly preventable condition in the right circumstances. While many patients are unaware of a tick bite, those at risk of exposure are advised to implement personal protective measures to prevent the risk of exposure and infection by tick-borne pathogens.[17] Chemical repellants containing N,N-Diethylmeta-toluamide (DEET), picaridin, ethyl-3-(N-n-butyl-Nacetyl) aminopropionate (IR3535), oil of lemon eucalyptus (OLE), p-methane-3,8-diol (PMD), 2-undecanone, or permethrin are recommended.[17]
Additional prevention measures include wearing shoes, long-sleeved shirts, and pants when outside, showering after coming in from outside and checking the clothes and body for ticks after being outdoors. Children should be examined for ticks, particularly on the scalp, around the ears, and in the groin and buttock regions. Found ticks should be removed mechanically (eg, a clean, fine-tipped tweezer). Observing the tick for its shape, size, and any identifying features can be helpful in reporting to the healthcare clinician.
Patients who develop Lyme disease symptoms after a tick bite (eg, flu-like symptoms, fevers, rash or nerve, joint, and heart problems), should seek medical attention immediately. For patients who develop no symptoms after a tick bite, no antibiotic therapy is recommended unless it is from a high-risk tick, in which case prophylactic antibiotic therapy may be considered.[17] Patients should be advised to monitor for the development of rash or systemic symptoms and return to a healthcare clinician to seek additional medical attention.
Pearls and Other Issues
Based on the geographic distribution of the shared vector Ixodes scapularis, coinfections with Lyme disease and human granulocytic anaplasmosis or babesiosis can occur. Co-infected patients may be more severely ill at presentation, have a persistent fever longer than 48 hours after initiating antibiotic therapy for Lyme disease, or present with anemia, leukopenia, and thrombocytopenia. When coinfection is suspected or confirmed, treatment with an appropriate antimicrobial regimen for each infection is necessary to resolve the illness.
Enhancing Healthcare Team Outcomes
The key to Lyme disease is prevention, recognition of complications, and prompt initiation of treatment once confirmed. Management spans the interprofessional continuum from the primary care setting to the specialist. Primary care clinicians, including family medicine, internal medicine, and pediatrics, can play a role in educating patients on preventing tick bites while hiking or working outdoors. These front-line clinicians may also help parents by showing them how to inspect their children for ticks at the end of an outdoor event in an endemic area and manage a tick bite if it occurs.
Primary care, urgent care, and emergency medicine professionals play a critical role in recognizing the symptoms and signs of Lyme disease, ranging from appropriate identification of a bull's eye rash and prompt initiation of treatment to recognition and evaluation for later-stage findings of the disease. These individuals must know how to appropriately order and interpret serological tests for Lyme disease and when to refer patients who develop cardiac, neurological, musculoskeletal, or dermatologic manifestations.
Primary care clinicians can also work with infectious diseases to determine the accuracy of diagnosis or in more complex cases where management decisions require further input. Other specialists can help interpret advanced testing such as cardiac imaging, electrocardiography findings, radiologic imaging, lab results, and additional advanced testing. Having a coordinated approach with the necessary specialist's expertise is critical to making a diagnosis and initiating prompt treatment. Effective communication between interprofessional healthcare team members, including nurses, advanced clinicians, physicians, and pharmacists, is essential to enhancing patient outcomes with Lyme disease.
Media
(Click Image to Enlarge)
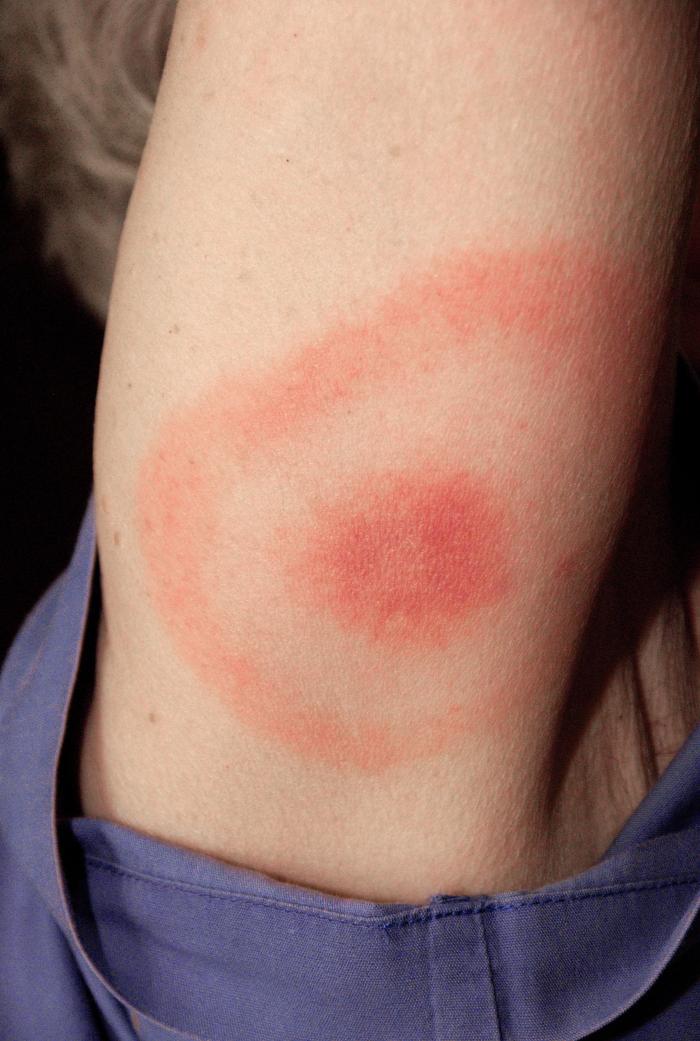
Lyme Disease "Bulls-Eye" Rash. This image illustrates the characteristic rash present in some cases of Lyme disease.
James Gathany, Public Domain, Public Health Image Library, Centers for Disease Control and Prevention
References
Cervantes J. [Lyme disease in Perú. A clinical and epidemiological review]. Revista peruana de medicina experimental y salud publica. 2018 Apr-Jun:35(2):292-296. doi: 10.17843/rpmesp.2018.352.3418. Epub [PubMed PMID: 30183906]
Level 2 (mid-level) evidenceBransfield RC. Neuropsychiatric Lyme Borreliosis: An Overview with a Focus on a Specialty Psychiatrist's Clinical Practice. Healthcare (Basel, Switzerland). 2018 Aug 25:6(3):. doi: 10.3390/healthcare6030104. Epub 2018 Aug 25 [PubMed PMID: 30149626]
Level 3 (low-level) evidenceBernard Q, Thakur M, Smith AA, Kitsou C, Yang X, Pal U. Borrelia burgdorferi protein interactions critical for microbial persistence in mammals. Cellular microbiology. 2019 Feb:21(2):e12885. doi: 10.1111/cmi.12885. Epub 2018 Jul 8 [PubMed PMID: 29934966]
Muhammad S, Simonelli RJ. Lyme Carditis: A Case Report and Review of Management. Hospital pharmacy. 2018 Jul:53(4):263-265. doi: 10.1177/0018578717749927. Epub 2018 Jan 2 [PubMed PMID: 30038446]
Level 3 (low-level) evidenceBerry K, Bayham J, Meyer SR, Fenichel EP. The allocation of time and risk of Lyme: A case of ecosystem service income and substitution effects. Environmental & resource economics. 2018 Jul:70(3):631-650. doi: 10.1007/s10640-017-0142-7. Epub 2017 Apr 13 [PubMed PMID: 30166775]
Level 3 (low-level) evidenceShapiro ED, Wormser GP. Lyme Disease in 2018: What Is New (and What Is Not). JAMA. 2018 Aug 21:320(7):635-636. doi: 10.1001/jama.2018.10974. Epub [PubMed PMID: 30073279]
Hatchette TF, Davis I, Johnston BL. Lyme disease: clinical diagnosis and treatment. Canada communicable disease report = Releve des maladies transmissibles au Canada. 2014 May 29:40(11):194-208 [PubMed PMID: 29769842]
Talbot NC, Spillers NJ, Luther P, Flanagan C, Soileau LG, Ahmadzadeh S, Viswanath O, Varrassi G, Shekoohi S, Cornett EM, Kaye AM, Kaye AD. Lyme Disease and Post-treatment Lyme Disease Syndrome: Current and Developing Treatment Options. Cureus. 2023 Aug:15(8):e43112. doi: 10.7759/cureus.43112. Epub 2023 Aug 8 [PubMed PMID: 37692614]
Bobe JR, Jutras BL, Horn EJ, Embers ME, Bailey A, Moritz RL, Zhang Y, Soloski MJ, Ostfeld RS, Marconi RT, Aucott J, Ma'ayan A, Keesing F, Lewis K, Ben Mamoun C, Rebman AW, McClune ME, Breitschwerdt EB, Reddy PJ, Maggi R, Yang F, Nemser B, Ozcan A, Garner O, Di Carlo D, Ballard Z, Joung HA, Garcia-Romeu A, Griffiths RR, Baumgarth N, Fallon BA. Recent Progress in Lyme Disease and Remaining Challenges. Frontiers in medicine. 2021:8():666554. doi: 10.3389/fmed.2021.666554. Epub 2021 Aug 18 [PubMed PMID: 34485323]
Brandt FC, Ertas B, Falk TM, Metze D, Böer-Auer A. Histopathology and immunophenotype of acrodermatitis chronica atrophicans correlated with ospA and ospC genotypes of Borrelia species. Journal of cutaneous pathology. 2015 Oct:42(10):674-92. doi: 10.1111/cup.12550. Epub 2015 Aug 10 [PubMed PMID: 26156537]
Donval L, Thibault L, Langer A, Oufkir N, Rouzier R, Bonneau C. Borrelial lymphocytoma of the breast: A rare differential diagnosis of breast cancer. Journal of gynecology obstetrics and human reproduction. 2023 Jan:52(1):102502. doi: 10.1016/j.jogoh.2022.102502. Epub 2022 Nov 13 [PubMed PMID: 36379351]
Norris SJ. Catching up with Lyme Disease Antigenic Variation Computationally. Trends in microbiology. 2018 Aug:26(8):644-645. doi: 10.1016/j.tim.2018.05.017. Epub 2018 Jun 11 [PubMed PMID: 29903419]
Centers for Disease Control and Prevention (CDC). Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR. Morbidity and mortality weekly report. 1995 Aug 11:44(31):590-1 [PubMed PMID: 7623762]
Yeung C, Baranchuk A. Systematic Approach to the Diagnosis and Treatment of Lyme Carditis and High-Degree Atrioventricular Block. Healthcare (Basel, Switzerland). 2018 Sep 22:6(4):. doi: 10.3390/healthcare6040119. Epub 2018 Sep 22 [PubMed PMID: 30248981]
Level 1 (high-level) evidencePaparone P, Paparone PW. Variable clinical presentations of babesiosis. The Nurse practitioner. 2018 Oct:43(10):48-54. doi: 10.1097/01.NPR.0000545000.07640.11. Epub [PubMed PMID: 30234826]
Trayes KP, Savage K, Studdiford JS. Annular Lesions: Diagnosis and Treatment. American family physician. 2018 Sep 1:98(5):283-291 [PubMed PMID: 30216021]
Lantos PM, Rumbaugh J, Bockenstedt LK, Falck-Ytter YT, Aguero-Rosenfeld ME, Auwaerter PG, Baldwin K, Bannuru RR, Belani KK, Bowie WR, Branda JA, Clifford DB, DiMario FJ, Halperin JJ, Krause PJ, Lavergne V, Liang MH, Meissner HC, Nigrovic LE, Nocton JJJ, Osani MC, Pruitt AA, Rips J, Rosenfeld LE, Savoy ML, Sood SK, Steere AC, Strle F, Sundel R, Tsao J, Vaysbrot EE, Wormser GP, Zemel LS. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis and Treatment of Lyme Disease. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 Jan 23:72(1):1-8. doi: 10.1093/cid/ciab049. Epub [PubMed PMID: 33483734]
Level 1 (high-level) evidenceDeLong A, Hsu M, Kotsoris H. Estimation of cumulative number of post-treatment Lyme disease cases in the US, 2016 and 2020. BMC public health. 2019 Apr 24:19(1):352. doi: 10.1186/s12889-019-6681-9. Epub 2019 Apr 24 [PubMed PMID: 31014314]
Level 3 (low-level) evidenceAntony S. Mosquito and Tick-borne Illnesses in the United States. Guidelines for the Recognition and Empiric Treatment of Zoonotic Diseases in the Wilderness. Infectious disorders drug targets. 2019:19(3):238-257. doi: 10.2174/1871526518666180626123340. Epub [PubMed PMID: 29943705]
Centre for Food-borne, Environmental and Zoonotic Infectious Diseases. Synopsis: Lyme Disease in Canada - A Federal Framework. Canada communicable disease report = Releve des maladies transmissibles au Canada. 2017 Oct 5:43(10):212-214 [PubMed PMID: 29770048]
Patton SK, Phillips B. CE: Lyme Disease: Diagnosis, Treatment, and Prevention. The American journal of nursing. 2018 Apr:118(4):38-45. doi: 10.1097/01.NAJ.0000532071.32468.f7. Epub [PubMed PMID: 29543607]