Introduction
Mallory-Denk bodies (MDBs), also called "Mallory bodies," are cytoplasmic hyaline inclusions found in hepatocytes. Initially considered specific to alcohol-related steatohepatitis (ASH), MDBs also appear in metabolic dysfunction-associated steatohepatitis (MASH, formerly nonalcoholic steatohepatitis or NASH), cholestatic liver diseases, primary biliary cirrhosis (PBC), and hepatocellular carcinoma (HCC).[1][2]
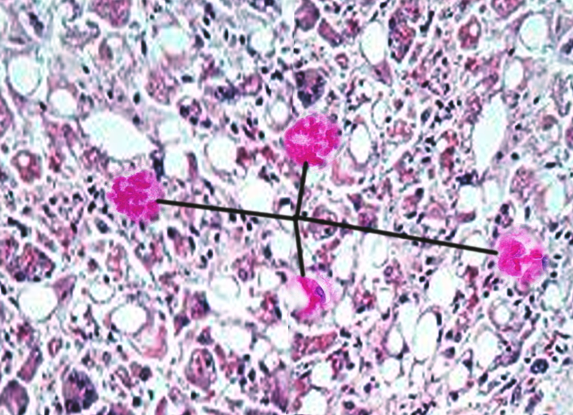
In 1911, Frank Burr Mallory identified these histological structures in the hepatocytes of patients with alcoholic hepatitis. In 1975, Helmut Denk established the first animal model by administering griseofulvin to mice, prompting the shift from the term "Mallory bodies" to "Mallory-Denk bodies" (see Image. Mallory-Denk Bodies).[3] Human studies subsequently revealed that MDBs primarily consist of keratins, molecular chaperones, cotransporters, and additional aggregated proteins. Histologically, clinicians classify MDBs into subtypes—classic, nonkeratinous, rounded, and variants—to guide grading and staging during liver biopsy evaluation.
MDB formation reflects cellular stress and dysregulated cell cycle control, which impairs hepatocyte regeneration and affects other proliferating cell types. Since cell cycle dysregulation is central to the liver pathologies listed above, MDBs serve as a histological marker with clinical relevance. In the early stages of inflammation, MDB formation may offer protective effects by limiting damage and delaying the progression of fibrosis. However, persistent or compounded stressors can worsen disease severity.
The learning activity below explores the molecular pathways underlying MDB formation, the triggers that induce their development, and evidence-based associations with clinical outcomes. Interprofessional teams, including primary care, gastroenterology, and pathology, contribute to disease staging and management. Students in these fields become familiar with MDB morphology as part of their histopathological training.
Causes
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Causes
Hepatocytes
MDB formation occurs in various liver diseases. Documented conditions include hepatitis B and C, alcoholic liver disease, metabolic function-associated steatotic liver disease (MASLD, formerly nonalcoholic fatty liver disease or NAFLD), HCC, focal nodular hyperplasia, Wilson disease, and copper toxicosis.[4] Gallbladder-related conditions with MDB involvement include PBC, chronic cholestasis, and cholangiocarcinoma.
Additional associations include glucocorticoid therapy, intestinal bypass surgery for obesity, Weber-Christian disease, von Gierke disease, radiation pneumonitis, asbestosis, amiodarone toxicity, β-lipoproteinemia, porphyria cutanea tarda, α1-antitrypsin deficiency, Indian childhood cirrhosis, perhexiline maleate–induced hepatitis, cirrhosis, 2′3′-dideoxyinosine diethylaminoetheoxyhex-estriol–induced hepatitis, hepatic adenoma, sclerosing hyaline necrosis in Bloom syndrome, and congenital hepatic fibrosis.[5]
Nonhepatic Cells
MDBs rarely appear outside hepatocytes. However, documented examples include type 2 pneumocytes in asbestosis, renal cell carcinoma, and scattered reports in other tissues. The clinical relevance of MDBs in these contexts remains under investigation and is discussed further in the sections below.
Anatomical Pathology
MDBs primarily consist of p62 (a sequestosome), ubiquitin, and intermediate filament proteins, particularly keratins 8 (K8) and 18 (K18). Under normal physiological conditions, K8 and K18 are expressed in a 1:1 ratio.[6] Disruption of this balance, specifically when the K8-K18 ratio is increased, along with protein misfolding, proteasome overload, and transamidation of K8, contributes to the formation of MDB.[7] Since K8 is relatively insoluble and resistant to proteasomal degradation, its accumulation promotes the development of inclusion bodies. Overexpression of K18 suppresses MDB formation.
K8 exhibits a greater tendency to convert its α-helical structure into cross-β sheet conformations, a critical step in MDB aggregation. MDBs do not form in the absence of K8, supporting the requirement of cross-β sheet architecture in their pathogenesis.[8][9][10] Similar cross-linking occurs in other protein aggregation disorders, including prion disease and Alzheimer disease.
Recent studies correlate MDBs with markers of cellular senescence, such as p16 and p21, suggesting that MDBs may reflect broader stress-induced cell cycle dysregulation beyond hepatic injury.[11] Macrophages recruited during MDB-associated inflammation demonstrate transcriptional heterogeneity, further implicating MDBs in complex inflammatory signaling.[12] Aggregates such as MDBs may activate nuclear factor-κB (NF-κB), although the precise downstream effects on inflammation remain unclear.[13] The mechanism by which inflammation is amplified likely involves multiple checkpoints in hepatocyte regeneration and senescence.
Clinical Pathology
MDBs commonly appear in ASH, MASH, PBC, HCC, and other gastrointestinal disorders. Hepatotoxic medications also contribute to MDB formation. Most recently, amiodarone has been directly implicated.[14] Pathologists may consider the presence or absence of MDBs when establishing a final diagnosis.
Recent findings have identified MDBs in patients with COVID-19, suggesting a potential link to pneumocyte injury and pathological transformation.[15] MDB-like inclusions have also been reported in ovarian fibromas following torsion.[16] Although these findings suggest broader systemic associations, MDBs in extrahepatic conditions remain nondiagnostic. MDBs indicate underlying pathology but generally do not predict prognosis or influence mortality.
In HCC, cytoplasmic inclusion bodies, including MDBs and intracytoplasmic hyaline bodies (IHBs), may be visualized histologically.[17] MDBs are specifically associated with the steatohepatitic variant of HCC, whereas IHBs are not. Notably, IHBs correlate with shorter survival compared to MDBs.[18] Despite some histological overlap, MDBs and IHBs are distinguishable: MDBs contain ubiquitin, p62, and keratins, whereas IHBs consist only of ubiquitin and p62. IHBs may represent a morphological precursor to MDBs.[19]
Morphology
MDBs are predominantly filamentous cytoplasmic inclusions measuring 3 to 24 nm in diameter, compared to the 10-nm diameter of typical intermediate filaments. These structures are classified into the following subtypes:
- Type I: Parallel filaments
- Type II: Randomly oriented filaments, typically found at the periphery
- Type III: Granular and amorphous structures, primarily located around the center
MDBs form within ballooned hepatocytes, although not all ballooned hepatocytes contain MDBs. Both features reflect active tissue inflammation and cellular stress. MDB formation progresses in distinct stages, beginning with the accumulation of misfolded proteins that would normally be targeted for degradation by the proteasome pathway. These aggregates initially appear as young MDBs within ballooned hepatocytes and gradually mature into more organized inclusions.
Although hematoxylin-eosin staining can reveal MDBs, immunohistochemical staining for cytokeratin or ubiquitin offers greater sensitivity and diagnostic specificity. Hepatocytes containing MDBs are often surrounded by pericellular fibrosis and infiltrating neutrophils, a phenomenon known as satellitosis.[20] The intracellular distribution of MDBs reflects their stage of development. Early-stage MDBs appear as small cytoplasmic globules. Mature forms present as large paranuclear inclusions. Older forms are typically found at the cell periphery.
The zonal distribution of MDBs varies by disease. In PBC and Wilson disease, MDBs localize to zone 1 of the hepatic lobule, whereas in ASH and MASLD, they are predominantly found in zone 3.
Mechanisms
Ballooning of hepatocytes typically precedes the formation of MDBs and reflects a response to oxidative stress. This stress may result from the abnormal accumulation of proteins (eg, heat shock proteins) or lipid overload, both of which disrupt cellular homeostasis. The ensuing cytoplasmic swelling is due to water accumulation within the hepatocyte. Elevated expression of heat shock proteins signals impaired protein handling and cellular dysfunction.
MDB formation arises from 3 interconnected cellular stress mechanisms, each targeting different points in the cell cycle—replication, transcription, or translation. These pathways may occur independently, sequentially, or simultaneously, depending on the inciting factor.
The 1st mechanism involves epigenetic modifications, such as acetylation, methylation, and ubiquitination of histones. These changes alter gene expression, contributing to the hepatocyte’s response to injury. Cells exposed to repeated toxic insults, such as alcohol- or drug-induced liver injury, can retain epigenetic “memory,” resulting in gene silencing and persistent vulnerability to MDB formation.
The 2nd mechanism centers on a shift in protein degradation pathways. Under normal conditions, the 26S proteasome clears intracellular proteins, including cytosolic, nuclear, and membrane proteins. However, stressors like 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) or alcohol disrupt this system, prompting a shift from the 26S proteasome to the immunoproteasome. This change impairs protein clearance, leading to intracellular protein accumulation. When molecular chaperones, such as heat shock protein 70, fail to manage misfolded proteins, these aggregates are tagged with ubiquitin and directed toward degradation via the p62 pathway. If this system is overwhelmed or dysfunctional, inclusion bodies such as MDBs begin to form.
The 3rd mechanism involves chronic activation of toll-like receptors (TLRs), which stimulate proinflammatory and cell growth pathways. Inflammatory cytokines, such as tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ), activate TLR signaling, promoting the upregulation of the immunoproteasome and downregulation of the 26S proteasome. This imbalance further hinders protein degradation and contributes to the accumulation of MDB. Agents such as DDC not only disrupt proteasome function but also enhance TLR signaling by increasing tumor necrosis factor α and interferon γ expression, creating a feedback loop that amplifies cellular stress and MDB formation.[21][22][23]
Clinicopathologic Correlations
Liver Diseases
The diagnostic relevance of MDBs in MASLD depends on the histological scoring system. While the Matteoni system highlights MDB formation as a key feature, the more widely accepted Kleiner system focuses on fibrosis, steatosis, lobular inflammation, and hepatocyte ballooning, excluding MDBs as a diagnostic criterion. Some studies suggest that the K8-K18 ratio may serve as a potential biomarker for HCC.
Among individuals who consume alcohol, ubiquitin immunostaining offers a sensitive and specific method to detect MDBs. These inclusions appear not only in the liver but also in muscle and neural tissues, supporting the theory of a shared intracellular pathway for protein synthesis, degradation, and stress response. MDBs may act as a transient protective response to cellular injury or as a step toward progressive hepatic damage. In animal models, MDBs reappear within 2 to 3 days after alcohol cessation, indicating a rapid recurrence linked to prior exposure.
Metabolic Dysfunction-Associated Steatohepatitis
In MASH, clinical features such as jaundice, leukocytosis, and fever may correlate with classic histologic signs of steatosis, but MDBs are often absent or poorly developed. Compared to ASH, MASH typically exhibits less severe hepatic injury, which may explain the relative scarcity of MDBs. Notably, pediatric cases of MASH rarely show MDB formation, suggesting that age-related factors contribute to their development.
Clinical Significance
Areas for Further Research
Abnormal protein folding contributes to several diseases, including Alzheimer disease. A recent literature review identified a mechanistic overlap between Alzheimer pathology and MDB formation. Both processes involve the accumulation of β-sheet-rich protein aggregates. Given this similarity, future studies should explore whether betaine or S-adenosyl-L-methionine (SAMe)—agents shown to prevent MDB formation—might also have therapeutic potential in Alzheimer disease.
While 70% to 75% of patients with alcoholic liver disease exhibit MDBs, the frequency in MASLD is highly variable, with estimates spanning 7% to 90%. This variability likely stems from inconsistent classification of alcohol consumption levels. For instance, patients who exceed a certain threshold may be reclassified as having alcohol-related disease rather than MASLD. In cases of severe alcoholic hepatitis, nonresponders to corticosteroid therapy demonstrate higher histologic features of hepatocyte ballooning and MDBs, suggesting that tissue findings may help predict treatment response.[24]
Autophagy plays a role in the degradation of MDBs, alongside other intracellular clearance mechanisms.[25][26] Fenofibrate, a lipid-lowering fibric acid derivative, has been shown to prevent MDB formation by preserving intermediate filament integrity and limiting cytoskeletal disruption.[27]
Methyl donors such as betaine and SAMe inhibit MDB formation through distinct mechanisms. SAMe prevents histone demethylation, a process induced by DDC exposure, thereby preserving epigenetic regulation. Betaine interrupts MDB formation by maintaining methionine metabolism, specifically by supporting the conversion of homocysteine to methionine via betaine-homocysteine methyltransferase (BHMT).[28] Both agents also downregulate the TLR and p62 pathways implicated in MDB pathogenesis.
Identified markers associated with MDBs represent potential targets for therapeutic intervention across all stages of protein structure, from primary through quaternary, and in patients receiving treatment for related conditions.[29][30] Although findings from animal models do not fully translate to clinical practice, particularly in cases where MDBs revert or recur, these insights may offer value to clinicians managing gastrointestinal diseases.
Interprofessional collaboration
The identification and characterization of MDBs continues to inform diagnostic approaches across specialties. Standardized inclusion and exclusion criteria based on histologic presence or absence of MDBs could enhance diagnostic precision. Although key mechanisms—including epigenetic modification, proteasome shift, TLR signaling, and immunoregulation—are increasingly understood, downstream effects remain unclear. Given their similarity to aggregates in other protein-folding disorders, MDBs may also play underrecognized roles in pulmonary and reproductive pathology. Further investigation into these associations may expand the clinical relevance of MDBs beyond hepatology.
Media
(Click Image to Enlarge)
References
Rinella ME. Examining the Nomenclature Change From NAFLD and NASH to MASLD and MASH. Gastroenterology & hepatology. 2023 Nov:19(11):697-699 [PubMed PMID: 38405223]
Aishima S, Fujita N, Mano Y, Iguchi T, Taketomi A, Maehara Y, Oda Y, Tsuneyoshi M. p62+ Hyaline inclusions in intrahepatic cholangiocarcinoma associated with viral hepatitis or alcoholic liver disease. American journal of clinical pathology. 2010 Sep:134(3):457-65. doi: 10.1309/AJCP53YVVJCNDZIR. Epub [PubMed PMID: 20716803]
Level 2 (mid-level) evidenceZatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, Omary MB. From Mallory to Mallory-Denk bodies: what, how and why? Experimental cell research. 2007 Jun 10:313(10):2033-49 [PubMed PMID: 17531973]
Level 3 (low-level) evidenceBasaranoglu M, Turhan N, Sonsuz A, Basaranoglu G. Mallory-Denk Bodies in chronic hepatitis. World journal of gastroenterology. 2011 May 7:17(17):2172-7. doi: 10.3748/wjg.v17.i17.2172. Epub [PubMed PMID: 21633525]
French SW, Mendoza AS, Peng Y. The mechanisms of Mallory-Denk body formation are similar to the formation of aggresomes in Alzheimer's disease and other neurodegenerative disorders. Experimental and molecular pathology. 2016 Jun:100(3):426-33. doi: 10.1016/j.yexmp.2016.03.010. Epub 2016 Apr 9 [PubMed PMID: 27068270]
Golob-Schwarzl N, Bettermann K, Mehta AK, Kessler SM, Unterluggauer J, Krassnig S, Kojima K, Chen X, Hoshida Y, Bardeesy NM, Müller H, Svendova V, Schimek MG, Diwoky C, Lipfert A, Mahajan V, Stumptner C, Thüringer A, Fröhlich LF, Stojakovic T, Nilsson KPR, Kolbe T, Rülicke T, Magin TM, Strnad P, Kiemer AK, Moriggl R, Haybaeck J. High Keratin 8/18 Ratio Predicts Aggressive Hepatocellular Cancer Phenotype. Translational oncology. 2019 Feb:12(2):256-268. doi: 10.1016/j.tranon.2018.10.010. Epub 2018 Nov 12 [PubMed PMID: 30439626]
French SW, Bardag-Gorce F, Li J, French BA, Oliva J. Mallory-Denk body pathogenesis revisited. World journal of hepatology. 2010 Aug 27:2(8):295-301. doi: 10.4254/wjh.v2.i8.295. Epub [PubMed PMID: 21161012]
Mahajan V, Klingstedt T, Simon R, Nilsson KP, Thueringer A, Kashofer K, Haybaeck J, Denk H, Abuja PM, Zatloukal K. Cross β-sheet conformation of keratin 8 is a specific feature of Mallory-Denk bodies compared with other hepatocyte inclusions. Gastroenterology. 2011 Sep:141(3):1080-1090.e1-7. doi: 10.1053/j.gastro.2011.05.039. Epub 2011 May 27 [PubMed PMID: 21699779]
Level 3 (low-level) evidenceMurray KA, Hughes MP, Hu CJ, Sawaya MR, Salwinski L, Pan H, French SW, Seidler PM, Eisenberg DS. Identifying amyloid-related diseases by mapping mutations in low-complexity protein domains to pathologies. Nature structural & molecular biology. 2022 Jun:29(6):529-536. doi: 10.1038/s41594-022-00774-y. Epub 2022 May 30 [PubMed PMID: 35637421]
Somlapura M, Gottschalk B, Lahiri P, Kufferath I, Pabst D, Rülicke T, Graier WF, Denk H, Zatloukal K. Different Roles of p62 (SQSTM1) Isoforms in Keratin-Related Protein Aggregation. International journal of molecular sciences. 2021 Jun 9:22(12):. doi: 10.3390/ijms22126227. Epub 2021 Jun 9 [PubMed PMID: 34207662]
Denk H, Abuja PM, Zatloukal K. Mallory-Denk bodies and hepatocellular senescence: a causal relationship? Virchows Archiv : an international journal of pathology. 2024 Apr:484(4):637-644. doi: 10.1007/s00428-024-03748-1. Epub 2024 Jan 30 [PubMed PMID: 38289501]
Zhang R, Zhong B, He J, Yang X, He M, Zeng W, Pan J, Fang Z, Jia J, Liu H. Single-cell transcriptomes identifies characteristic features of mouse macrophages in liver Mallory-Denk bodies formation. Experimental and molecular pathology. 2022 Aug:127():104811. doi: 10.1016/j.yexmp.2022.104811. Epub 2022 Jul 16 [PubMed PMID: 35850229]
Liu Y, Trnka MJ, Guan S, Kwon D, Kim DH, Chen JJ, Greer PA, Burlingame AL, Correia MA. A Novel Mechanism for NF-κB-activation via IκB-aggregation: Implications for Hepatic Mallory-Denk-Body Induced Inflammation. Molecular & cellular proteomics : MCP. 2020 Dec:19(12):1968-1986. doi: 10.1074/mcp.RA120.002316. Epub 2020 Sep 10 [PubMed PMID: 32912968]
Pop A, Halegoua-DeMarzio D, Barnhart H, Kleiner D, Avigan M, Gu J, Chalasani N, Ahmad J, Fontana RJ, Lee W, Barritt AS, Durazo F, Hayashi PH, Navarro VJ. Amiodarone and Dronedarone Causes Liver Injury with Distinctly Different Clinical Presentations. Digestive diseases and sciences. 2024 Apr:69(4):1479-1487. doi: 10.1007/s10620-023-08251-2. Epub 2024 Feb 28 [PubMed PMID: 38416280]
Zubieta-Calleja GR, Zubieta-DeUrioste N, de Jesús Montelongo F, Sanchez MGR, Campoverdi AF, Rocco PRM, Battaglini D, Ball L, Pelosi P. Morphological and functional findings in COVID-19 lung disease as compared to Pneumonia, ARDS, and High-Altitude Pulmonary Edema. Respiratory physiology & neurobiology. 2023 Mar:309():104000. doi: 10.1016/j.resp.2022.104000. Epub 2022 Nov 29 [PubMed PMID: 36460252]
Krishnamurthy K, Stillman IE, Hecht JL, Vyas M. Defining the Nature and Clinicopathologic Significance of Mallory-Denk-like Inclusions in Ovarian Fibromas: A Potential Degenerative Phenomenon Associated With Torsion. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2024 May 1:43(3):290-295. doi: 10.1097/PGP.0000000000000974. Epub 2023 Jul 18 [PubMed PMID: 37562060]
Level 2 (mid-level) evidenceKawasaki M, Shioya A, Takata M, Tsubata Y, Okanemasa Y, Takenaka M, Terauchi T, Yamashita M, Kumagai M, Yamada S. A case of bone metastasis of hepatocellular carcinoma: Mallory hyaline bodies can lead to the correct cytological diagnosis. Diagnostic cytopathology. 2023 Feb:51(2):E70-E74. doi: 10.1002/dc.25072. Epub 2022 Nov 8 [PubMed PMID: 36345980]
Level 3 (low-level) evidenceAigelsreiter A, Neumann J, Pichler M, Halasz J, Zatloukal K, Berghold A, Douschan P, Rainer F, Stauber R, Haybaeck J, Denk H, Lackner C. Hepatocellular carcinomas with intracellular hyaline bodies have a poor prognosis. Liver international : official journal of the International Association for the Study of the Liver. 2017 Apr:37(4):600-610. doi: 10.1111/liv.13325. Epub 2017 Jan 12 [PubMed PMID: 27885796]
Byrnes K, Bailey NT, Baral K, Mercer A, Joshi S, Wahby N, Rorison T, Liu G, Yin XM, Khambu B. Impaired hepatic autophagy exacerbates hepatotoxin induced liver injury. Cell death discovery. 2023 Feb 21:9(1):71. doi: 10.1038/s41420-023-01368-3. Epub 2023 Feb 21 [PubMed PMID: 36810855]
Denk H, Stumptner C, Zatloukal K. Mallory bodies revisited. Journal of hepatology. 2000 Apr:32(4):689-702 [PubMed PMID: 10782920]
Level 3 (low-level) evidenceBardag-Gorce F, Oliva J, Villegas J, Fraley S, Amidi F, Li J, Dedes J, French B, French SW. Epigenetic mechanisms regulate Mallory Denk body formation in the livers of drug-primed mice. Experimental and molecular pathology. 2008 Apr:84(2):113-21. doi: 10.1016/j.yexmp.2007.12.004. Epub 2008 Jan 11 [PubMed PMID: 18281034]
Level 3 (low-level) evidenceLivneh I, Cohen-Kaplan V, Cohen-Rosenzweig C, Avni N, Ciechanover A. The life cycle of the 26S proteasome: from birth, through regulation and function, and onto its death. Cell research. 2016 Aug:26(8):869-85. doi: 10.1038/cr.2016.86. Epub 2016 Jul 22 [PubMed PMID: 27444871]
Qian H, Ding WX. SQSTM1/p62 and Hepatic Mallory-Denk Body Formation in Alcohol-Associated Liver Disease. The American journal of pathology. 2023 Oct:193(10):1415-1426. doi: 10.1016/j.ajpath.2023.02.015. Epub 2023 Mar 9 [PubMed PMID: 36906265]
Level 2 (mid-level) evidenceShasthry SM, Rastogi A, Bihari C, Vijayaraghavan R, Arora V, Sharma MK, Sarin SK. Histological activity score on baseline liver biopsy can predict non-response to steroids in patients with severe alcoholic hepatitis. Virchows Archiv : an international journal of pathology. 2018 Apr:472(4):667-675. doi: 10.1007/s00428-018-2330-4. Epub 2018 Mar 7 [PubMed PMID: 29516163]
Harada M. Autophagy is involved in the elimination of intracellular inclusions, Mallory-Denk bodies, in hepatocytes. Medical molecular morphology. 2010 Mar:43(1):13-8. doi: 10.1007/s00795-009-0476-5. Epub 2010 Mar 26 [PubMed PMID: 20340001]
Level 3 (low-level) evidenceKe PY. Diverse Functions of Autophagy in Liver Physiology and Liver Diseases. International journal of molecular sciences. 2019 Jan 13:20(2):. doi: 10.3390/ijms20020300. Epub 2019 Jan 13 [PubMed PMID: 30642133]
Nikam A, Patankar JV, Somlapura M, Lahiri P, Sachdev V, Kratky D, Denk H, Zatloukal K, Abuja PM. The PPARα Agonist Fenofibrate Prevents Formation of Protein Aggregates (Mallory-Denk bodies) in a Murine Model of Steatohepatitis-like Hepatotoxicity. Scientific reports. 2018 Aug 28:8(1):12964. doi: 10.1038/s41598-018-31389-3. Epub 2018 Aug 28 [PubMed PMID: 30154499]
Oliva J, Bardag-Gorce F, Li J, French BA, Nguyen SK, Lu SC, French SW. Betaine prevents Mallory-Denk body formation in drug-primed mice by epigenetic mechanisms. Experimental and molecular pathology. 2009 Apr:86(2):77-86. doi: 10.1016/j.yexmp.2008.11.002. Epub 2008 Nov 24 [PubMed PMID: 19073172]
Level 3 (low-level) evidenceNeuman MG, Mueller J, Mueller S. Non-invasive Biomarkers of Liver Inflammation and Cell Death in Response to Alcohol Detoxification. Frontiers in physiology. 2021:12():678118. doi: 10.3389/fphys.2021.678118. Epub 2021 Jul 7 [PubMed PMID: 34305638]
Zhong B, Dong J, Zhang R, He M, Zeng W, Pan J, He J, Tao A, Yang R, Fu B, French SW, Liu H. Altered regulation of LncRNA analysis of human alcoholic hepatitis with Mallory-Denk Bodies (MDBs) is revealed by RNA sequencing. Experimental and molecular pathology. 2020 Dec:117():104559. doi: 10.1016/j.yexmp.2020.104559. Epub 2020 Oct 27 [PubMed PMID: 33121977]