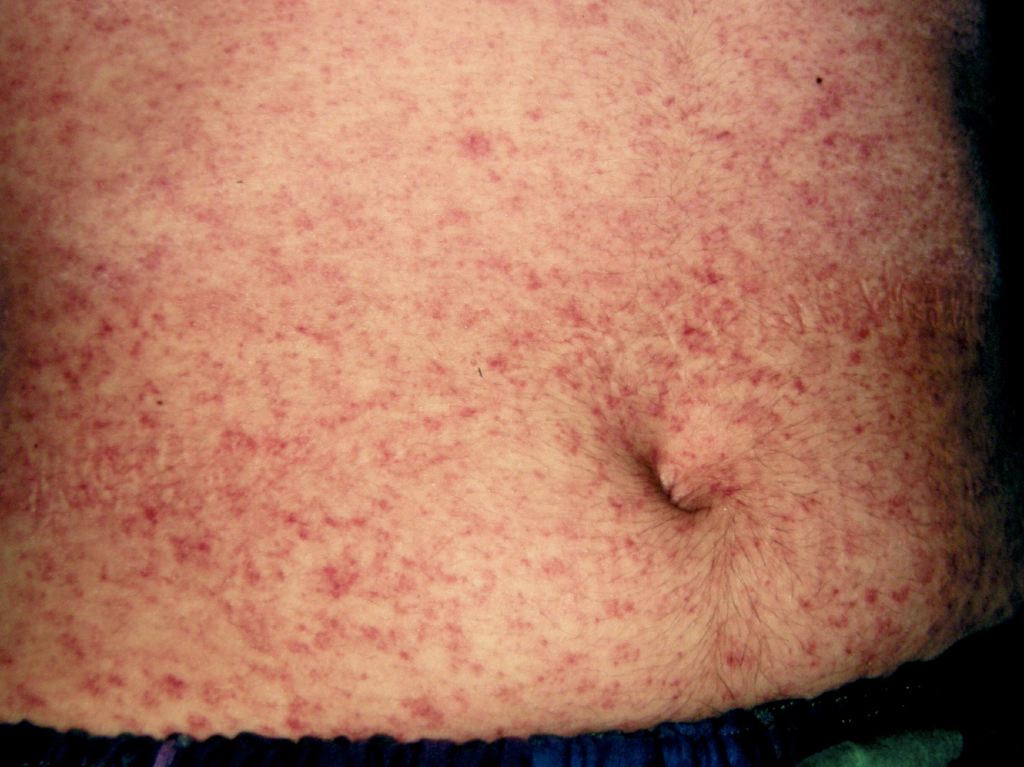
Introduction
Measles, or rubeola, is a preventable, highly contagious, acute febrile viral illness; this condition remains an important cause of global mortality and morbidity, particularly in Africa and Southeast Asia, and accounts for about 100,000 deaths annually despite the availability of an effective vaccine.[1][2] Public health officials declared the elimination of measles from the United States (US) in 2000, marking the absence of continuous disease transmission for 1 year, and from the region of the Americas in 2016. However, outbreaks continue to occur through imported disease and transmission among unvaccinated groups of children in the community (see Image. Measles Infection). Since May 2025, 935 confirmed cases have been reported across multiple states (US Centers for Disease Control and Prevention [CDC]-Measles cases and outbreaks, 2025). Measles is a reportable disease in most nations, including the US.[3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The causative organism of measles is the measles virus, an enveloped, single-stranded, negative-sense ribonucleic acid (RNA) virus belonging to the genus Morbillivirus in the family Paramyxoviridae (see Image. Paramyxovirus Virion, Transmission Electron Micrograph). The genome encodes 6 structural proteins and 2 non-structural proteins, V and C. The structural proteins are nucleoprotein, phosphoprotein, matrix, fusion, haemagglutinin (HA), and large protein. The HA protein is responsible for virus attachment to the host cell.[2]
Epidemiology
The epidemiology of measles is variable globally and relates to immunization levels achieved in a particular region. Before implementing widespread vaccination programs, measles accounted for an estimated 2.6 million deaths. Despite vaccination in the present era, the World Health Organization (WHO) reported that in 2023, there were an estimated 107,500 measles deaths globally, mostly among unvaccinated or under-vaccinated children younger than 5 (WHO-Measles, 2025). Measles is resurging in the US due to vaccine hesitancy and lower vaccination rates.[4] As of May 1, 2025, the CDC has reported 935 confirmed measles cases, mostly in unvaccinated or vaccination status unknown children.[5] Texas reported most of these cases (683), with 89 hospitalizations and 2 deaths.[6]
The measles virus has no animal reservoir and occurs only in humans. The virus is highly contagious, with each case capable of causing 14 to 18 secondary cases among susceptible populations, contrasting with the SARS-CoV-2 virus, which contributes from 1 to 6 cases per infected person.[7] Respiratory droplets, small particle aerosols, and close contact transmit measles from person to person. The incubation period ranges from 6 to 21 days, with a median of 13 days; longer periods have also been documented.
Unvaccinated young children and pregnant women are at elevated risk of contracting measles; the virus is contagious from 5 days before the rash's appearance to 4 days after its appearance. To prevent widespread transmission, 95% of the population should be immune, but in 2023, only 83% of children globally were immune.[4][8] There were 16 outbreaks in the US in 2024; 40% of those were hospitalized (CDC Measles cases and outbreaks, March 21, 2025). Infants born to mothers with acquired immunity receive passive antibody protection against measles, but become susceptible as these antibodies decrease. Recent data suggest this may not be true in the current era, as vaccine-mediated passive immunity in early infancy may not be as protective.[9] Infectiousness is greatest from 4 days before to 4 days after rash onset, coinciding with peak viremia a cough, conjunctivitis, and coryza.[3]
Pathophysiology
The inhaled virus from the exposed droplets initially infects the respiratory tract’s lymphocytes, dendritic cells, and alveolar macrophages. Then the virus spreads to the adjacent lymphoid tissue and disseminates throughout the bloodstream, resulting in viremia and spreading to distant organs. The virus residing in the dendritic cells and lymphocytes transfers to the epithelial cells of the respiratory tract, which are shed and expelled as respiratory droplets during coughing and sneezing, infecting others and perpetuating the cycle. The initial inflammation leads to symptoms of coryza, conjunctivitis, and cough. The appearance of fever coincides with the development of viremia. The skin rash occurs after dissemination due to perivascular and lymphocytic infiltrates.
During the prodromal phase, the measles virus depresses host immunity by suppressing interferon production through its nonstructural proteins, V and C. The increasing viral replication then triggers both humoral and cellular immunological responses. The initial humoral response consists of immunoglobulin (Ig)M antibody production, which is detectable three to four days after the rash appears and can persist for 6 to 8 weeks. Subsequently, IgG antibodies are produced, primarily against viral nucleoprotein. Cellular immune responses are essential for recovery, as demonstrated by elevated T-helper (Th)1-dependent plasma interferon-gamma levels during the acute phase and subsequent elevation of Th2-dependent interleukin 4, interleukin 10, and interleukin 13 levels.[10] Lymph node biopsy will show the characteristic (fused lymphocytes) in a background of paracortical hyperplasia.[11]
The measles virus can induce immunosuppression that can last for weeks to years. This causes increased susceptibility to secondary bacterial and other infections. While the mechanisms causing this phenomenon are unclear, it is hypothesized that measles infection induces the proliferation of measles-specific lymphocytes that replace the previously established memory cells, causing "immune amnesia." This results in enhanced susceptibility of the host to secondary infections, leading to most of the morbidity and mortality associated with measles. Neutralizing IgG antibodies against hemagglutinin is responsible for lifelong immunity as it blocks host cell receptors from binding to the virus.
History and Physical
The WHO clinical case definition of measles is "any person with fever, generalized maculopapular rash, cough, coryza, or conjunctivitis." Measles is an acute febrile exanthema characterized by the 3 “Cs”: cough, coryza, and conjunctivitis (see Image. Koplik Spots, Measles). Small white papules on the buccal mucosa are pathognomonic for measles and appear 1 to 2 days before the rash, although they are not always seen; the fever precedes the rash. The morbilliform rash appears around the hairline and face about 2 weeks after exposure, then spreads caudally. Uncomplicated measles typically resolves a week after the rash onset in the same order it initially appeared, followed frequently by desquamation.[5][12]
Breakthrough measles, or modified measles, is rare due to waning vaccine immunity. This condition tends to be a mild illness and can be challenging to diagnose as the rash may not follow the typical progression and can start on the trunk or extremities. The combination of cough, coryza, and conjunctivitis may appear singly or not and can present as a mild flu-like illness. Transmission of infection from cases of modified measles can occur, but is rare.[13]
Evaluation
The diagnosis of measles hinges on a high clinical suspicion, especially when evaluating children with febrile illness and a maculopapular rash. A complete blood count may show leukopenia, particularly lymphopenia, and thrombocytopenia. Electrolyte abnormalities may occur in children with poor intake or diarrhea. Reverse transcriptase-polymerase chain reaction (RT-PCR) may detect the viral RNA in throat, nasal, nasopharyngeal secretions, peripheral blood mononuclear cells, and urine samples up to 10 days after rash appearance, but is most sensitive in the first 3 days.[5] RT-PCR is the most commonly used laboratory method to diagnose measles.[14]
Identifying measles-virus-specific IgM antibodies in serum or plasma confirms the diagnosis. However, it may be a false negative in up to 25% of cases when performed early (less than 3 days of rash onset). These antibodies usually peak within one to three weeks after the onset of a rash and become undetectable by 4 to 8 weeks. False-positive IgM test reactions can be due to the presence of rheumatoid factor or during infection with rubella, parvovirus, or human herpes virus 6.[14] See Images. Rubella Virus, Transmission Electron Micrograph and German Measles, Rubella. The gold standard test is the highly sensitive plaque reduction neutralization assay. The measles virus can be cultured from nasopharyngeal secretions, but this is labor-intensive and impractical.[14]. Both measles infection and vaccination confer lifelong immunity in most populations.
Treatment / Management
There is no specific antiviral therapy for measles; treatment is primarily supportive. The mainstay of therapy is the control of fever, prevention and correction of dehydration, and infection control measures, including appropriate isolation.[15] The WHO and American Academy of Pediatrics recommend administering daily doses of vitamin A for 2 days (or longer for malnourished children).[16][17] While vitamin A supplementation has been shown to reduce measles complications in malnourished children, it is neither a measles prevention strategy nor an antiviral agent. Vitamin A toxicity from excessive doses can cause dangerous neurologic and hepatic complications.[18] No established scientific data support using antibacterials or inhaled steroids in treating measles.(A1)
Measles Vaccine
The single best way to treat measles is to prevent it. To that end, the measles-mumps-rubella (MMR) vaccine has a well-deserved, celebrated history of being one of the safest and most effective vaccines ever produced. See StatPearls companion article, MMR Vaccine, for additional details. Before the vaccine's widespread use, measles was responsible for 7 to 8 million childhood deaths globally per year.[19] Approximately 1 in 1000 measles-infected children will develop life-threatening encephalitis as an early complication. An additional 5 to 10 in 100,000 children will develop a late-appearing measles sequela, subacute sclerosing panencephalitis. Pneumonia is the leading cause of death due to measles. Keratoconjunctivitis and otitis media due to measles can result in blindness and hearing loss, respectively.[20][21]
The measles vaccine provides 97% protection after 2 doses; it has been administered to several million recipients globally over decades, and has proven safe and effective by dramatically reducing the number of measles cases. Secondary vaccine failure rates due to waning immunity are approximately 5% at 10 to 15 years after vaccination. Breakthrough infection from waning immunity is rare and typically occurs only after intense and prolonged exposure to an active measles case.[13] Given the absence of an animal reservoir and the availability of a safe and highly effective vaccine, it is theoretically possible to eliminate measles as a cause of disease.[19] For those individuals who have not been fully vaccinated, catch-up vaccination is highly recommended.
Differential Diagnosis
The following are differential diagnoses for measles:
- Drug eruptions
- Erythema Infectiosum
- Kawasaki disease
- Meningococcemia
- Rocky Mountain Spotted Fever
- Infectious Mononucleosis
- Parvovirus B19 infection
- Pediatric enteroviral infections
- Pediatric rubella
- Pediatric sepsis
- Pediatric toxic shock syndrome
- Scarlet fever
- Multisystem inflammatory syndrome in children
- Dengue
- Zika
- Syphilis
Prognosis
Most patients will recover from measles without complications, but there is still a risk of a poor prognosis. Some common complications due to infection with measles include otitis media, keratoconjunctivitis, diarrhea, and secondary bacterial infections. Blindness and hearing loss represent potential lifelong sequelae. Those more likely to have severe complications include infants and children younger than 5, adults older than 20, pregnant women, and those who are immunocompromised. Neurological sequelae are associated with significant functional impairment and potential mortality. Measles continues to cause 100,000 to 200,000 deaths annually in the unvaccinated.[21]
Complications
Complications of measles occur most commonly in young infants, pregnant women, and malnourished or immunocompromised children. The most common complication is pneumonia, which can be due to the measles virus itself (eg, Hecht giant cell pneumonia) or a secondary bacterial infection with organisms such as Streptococcus pneumoniae, Haemophilus influenzae, group A streptococcus, and Staphylococcus aureus. Other complications include croup, otitis media, diarrhea, and, less commonly, myocarditis, pericarditis, appendicitis, and thrombocytopenic purpura.[15]
Pregnant women with measles are at increased risk for maternal death, spontaneous abortion, intrauterine fetal death, and low birth-weight infants. Measles keratoconjunctivitis occurs mostly in children with vitamin A deficiency and can lead to blindness. Central nervous system complications include acute disseminated encephalomyelitis (ADEM), measles inclusion body encephalitis (MIBE), and subacute sclerosing panencephalitis (SSPE).[22][23] ADEM is an autoimmune demyelinating disease that occurs within days to weeks.[24][25] MIBE is thought to be a progressive brain infection in patients with impaired cellular immunity occurring within months of the initial infection. SSPE is a progressive neurological disease that presents 5 to 10 years after the acute illness and is thought to be caused by an abnormal host response to the production of mutated virions.[23][26] This condition usually occurs among children who developed measles before 2 years of age and manifests with seizures and progressive loss of cognitive and motor function.
Consultations
Institutional infection control should be consulted promptly to initiate appropriate airborne isolation measures and identify potential healthcare exposures if measles infection is suspected. Clinicians should notify the proper public health authorities if a measles infection is suspected to assist with contact tracing and outbreak containment. Specialist consultations from infectious disease, neurology, pulmonology, and critical care may be necessary in severe cases or those with measles complications.
Deterrence and Patient Education
The recent resurgence of measles is a vexing public health issue since prevention strategies in the form of safe and effective vaccines have existed for decades. Past measles epidemics have a corresponding epidemic of measles vaccine misinformation that currently circulates to the detriment of public health. At all levels of healthcare professions, in resource-rich and resource-poor areas of the world, there is a need to combat vaccine misinformation campaigns. This will require clinicians to become familiar with existing sound science and disseminate accurate information to the general public.
Pearls and Other Issues
Measles is preventable due to the availability of a safe, inexpensive, and effective vaccine. The vaccine is a live attenuated measles strain used either as a single component or a combination vaccine (MMR and measles-mumps-rubella-varicella). The WHO recommends 2 doses of measles vaccination beginning at 9 to 12 months and 15 to 18 months in countries where incidence and mortality are still high in the first year of life. In the US and other developed countries, the first vaccine is given at 12 to 15 months and the second at 4 to 5 years. In the setting of an outbreak or travel to endemic areas, the CDC recommends earlier vaccination between the ages of 6 and 11 months.[5] The Pan American Health Organization (PAHO) reported that the Americas were verified as measles-free in 2024. Still, this designation is at risk due to the recent outbreaks in the US (PAHO-Measles in Americas, April 2025). Simulation modeling by Kiang et al suggests that recent declines in childhood vaccination rates could increase the number and size of outbreaks of previously eliminated, vaccine-preventable diseases such as measles.[27] To eliminate measles, vaccination rates of the population must be in the 93% to 95% range.
A widely discredited and withdrawn article from The Lancet in 1998 created considerable misinformation, purporting a link between the MMR vaccine and autism that led to declining immunization rates, particularly in the United Kingdom and the US.[28] Subsequent studies have also refuted this misconception. Patients suspected of having measles in the community should be quarantined at home, and if hospitalized, should be isolated in a negative-pressure room. Clinicians should ensure that they are immune, and if not immune, should get revaccinated with the MMR vaccine and wear N95 or similar masks to prevent airborne infection. Postexposure prophylaxis with the MMR vaccine within 72 hours of exposure is recommended. If a patient is not eligible for the vaccine due to an immunocompromised state, consider immune globulin therapy.[5][29]
Enhancing Healthcare Team Outcomes
Measles is a preventable infection, and hence all healthcare professionals, including nurses and pharmacists, should educate patients on the importance of vaccination. Patients should be informed that the adverse events of the measles vaccination are rare and minor, and without vaccination, there is a high risk of acquiring the disease and transmitting it to others.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
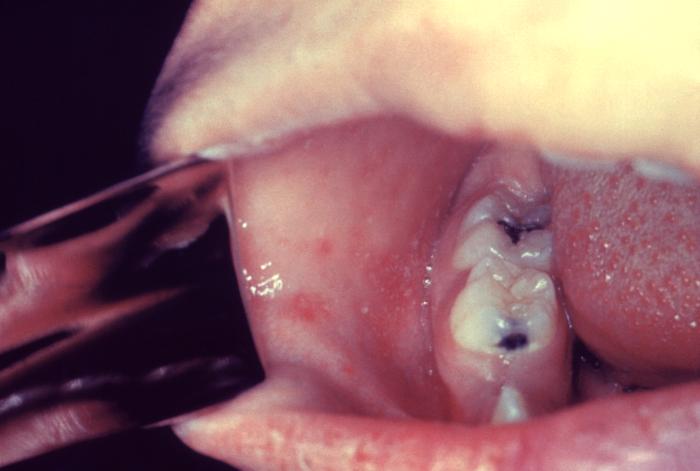
Koplik Spots, Measles. This patient presented on the third pre-eruptive day with “Koplik spots” indicative of the beginning onset of measles. In the prodromal or beginning stages, one of the signs of the onset of measles is the eruption of “Koplik spots” on the mucosa of the cheeks and tongue. These spots appear as irregularly shaped, bright red spots, often with a bluish-white central dot.
Centers for Disease Control and Prevention, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
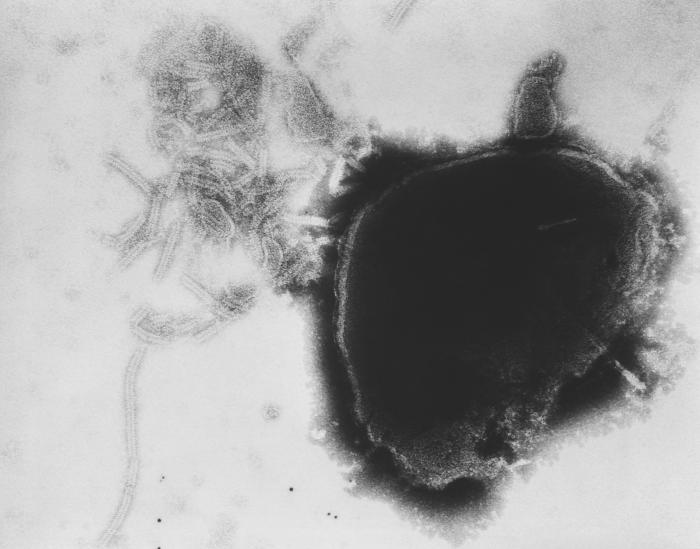
Paramyxovirus Virion, Transmission Electron Micrograph. The image displays the viral nucleocapsid of a paramyxovirus virion as visualized under a transmission electron microscope.
Fred Murphy, MD, Public Health Image Library, Public Domain, Centers for Disease Control and Prevention
(Click Image to Enlarge)
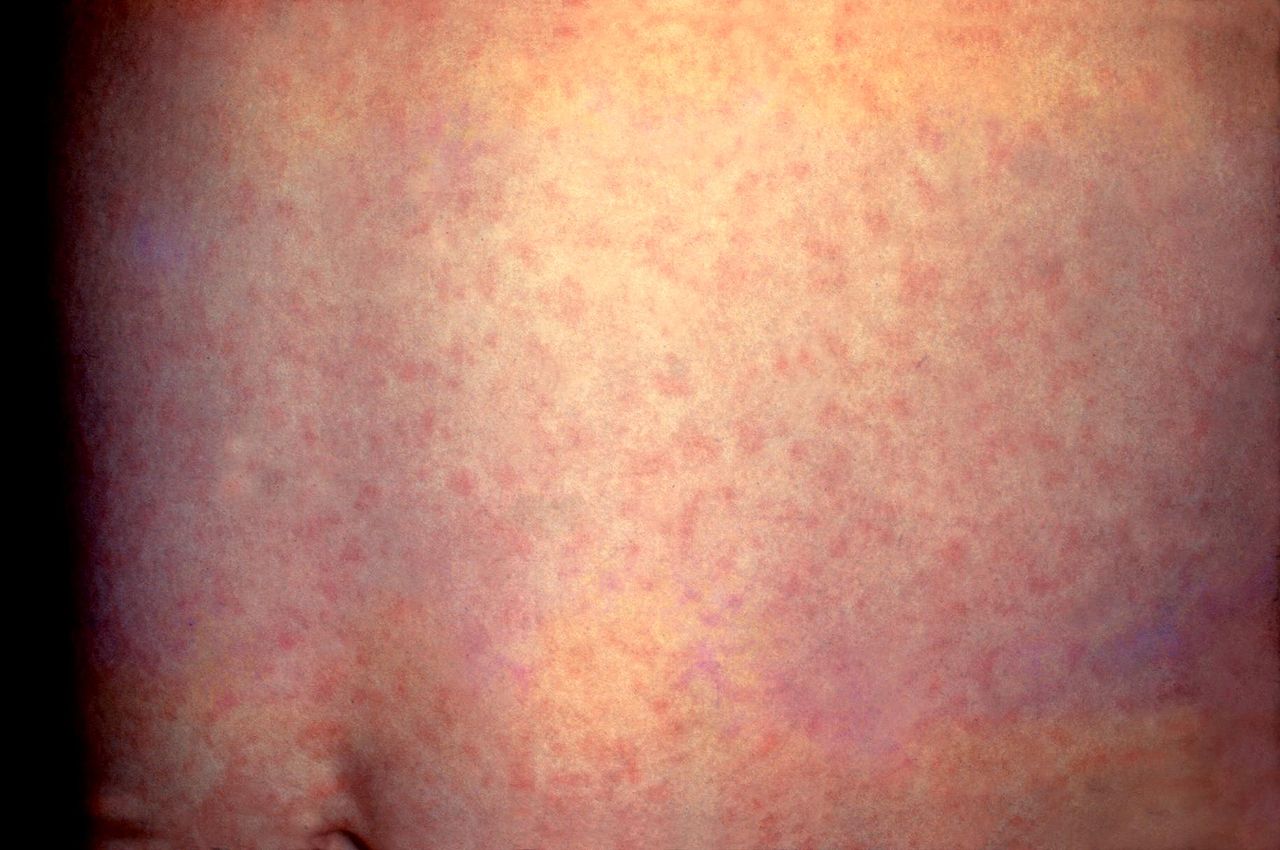
German Measles, Rubella. This patient presented with a generalized rash on the abdomen caused by German measles (rubella). The rash usually lasts about 3 days and may be accompanied by a low-grade fever.
Centers for Disease Control and Prevention, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
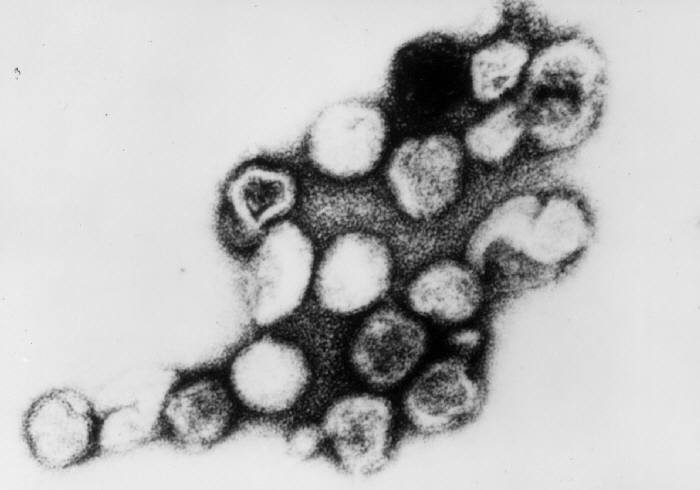
Rubella Virus, Transmission Electron Micrograph. Persons with measles can transmit the virus 4 days before and 4 days after the onset of the rash.
Erskine Palmer, MD, Public Domain, via Wikimedia Commons.
References
Moss WJ. Measles. Lancet (London, England). 2017 Dec 2:390(10111):2490-2502. doi: 10.1016/S0140-6736(17)31463-0. Epub 2017 Jun 30 [PubMed PMID: 28673424]
Bester JC. Measles and Measles Vaccination: A Review. JAMA pediatrics. 2016 Dec 1:170(12):1209-1215. doi: 10.1001/jamapediatrics.2016.1787. Epub [PubMed PMID: 27695849]
Goodson JL, Seward JF. Measles 50 Years After Use of Measles Vaccine. Infectious disease clinics of North America. 2015 Dec:29(4):725-43. doi: 10.1016/j.idc.2015.08.001. Epub [PubMed PMID: 26610423]
Desai AN, Majumder MS. What Is Herd Immunity? JAMA. 2020 Nov 24:324(20):2113. doi: 10.1001/jama.2020.20895. Epub [PubMed PMID: 33074287]
Li CN, Kaplan SL, Edwards KM, Marshall GS, Parker S, Healy CM. What's Old Is New Again: Measles. Pediatrics. 2025 Apr 11:():. doi: 10.1542/peds.2025-071332. Epub 2025 Apr 11 [PubMed PMID: 40211105]
Tanne JH. Measles: Second child dies in Texas as RFK Jr finally recommends vaccination. BMJ (Clinical research ed.). 2025 Apr 9:389():r726. doi: 10.1136/bmj.r726. Epub 2025 Apr 9 [PubMed PMID: 40204340]
Guerra FM, Bolotin S, Lim G, Heffernan J, Deeks SL, Li Y, Crowcroft NS. The basic reproduction number (R(0)) of measles: a systematic review. The Lancet. Infectious diseases. 2017 Dec:17(12):e420-e428. doi: 10.1016/S1473-3099(17)30307-9. Epub 2017 Jul 27 [PubMed PMID: 28757186]
Level 1 (high-level) evidenceMinta AA, Ferrari M, Antoni S, Lambert B, Sayi TS, Hsu CH, Steulet C, Gacic-Dobo M, Rota PA, Mulders MN, Wimmer A, Bose AS, O'Connor P, Crowcroft NS. Progress Toward Measles Elimination - Worldwide, 2000-2023. MMWR. Morbidity and mortality weekly report. 2024 Nov 14:73(45):1036-1042. doi: 10.15585/mmwr.mm7345a4. Epub 2024 Nov 14 [PubMed PMID: 39541251]
Flannery DD, Zevallos Barboza A, Wade KC, Pfeifer MR, Gerber JS, Morris JS, Puopolo KM. Measles Serostatus Among Parturient Patients at 2 Philadelphia Hospitals in 2021. JAMA. 2023 Feb 28:329(8):682-684. doi: 10.1001/jama.2023.0166. Epub [PubMed PMID: 36735270]
Laksono BM, de Vries RD, McQuaid S, Duprex WP, de Swart RL. Measles Virus Host Invasion and Pathogenesis. Viruses. 2016 Jul 28:8(8):. doi: 10.3390/v8080210. Epub 2016 Jul 28 [PubMed PMID: 27483301]
Klassen-Fischer MK, Nelson AM, Neafie RC, Neafie FA, Auerbach A, Baker TP, Burke AP, Datta AA, Franks TJ, Horkayne-Szakaly I, Lack EE, Lewin-Smith MR, Luiña Contreras A, Mattu RH, Rush WL, Shick PC, Zhang Y, Rentas FJ, Moncur JT. The Reemergence of Measles. American journal of clinical pathology. 2023 Jan 4:159(1):81-88. doi: 10.1093/ajcp/aqac124. Epub [PubMed PMID: 36315019]
Cockbain BC, Bharucha T, Irish D, Jacobs M. Measles in older children and adults. BMJ (Clinical research ed.). 2017 Feb 16:356():j426. doi: 10.1136/bmj.j426. Epub 2017 Feb 16 [PubMed PMID: 28209781]
Fappani C, Gori M, Canuti M, Terraneo M, Colzani D, Tanzi E, Amendola A, Bianchi S. Breakthrough Infections: A Challenge towards Measles Elimination? Microorganisms. 2022 Aug 4:10(8):. doi: 10.3390/microorganisms10081567. Epub 2022 Aug 4 [PubMed PMID: 36013985]
Hübschen JM, Bork SM, Brown KE, Mankertz A, Santibanez S, Ben Mamou M, Mulders MN, Muller CP. Challenges of measles and rubella laboratory diagnostic in the era of elimination. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2017 Aug:23(8):511-515. doi: 10.1016/j.cmi.2017.04.009. Epub 2017 Apr 13 [PubMed PMID: 28412379]
Khan L. Measles in Children. Pediatric annals. 2018 Sep 1:47(9):e340-e344. doi: 10.3928/19382359-20180801-01. Epub [PubMed PMID: 30208191]
Bello S, Meremikwu MM, Ejemot-Nwadiaro RI, Oduwole O. Routine vitamin A supplementation for the prevention of blindness due to measles infection in children. The Cochrane database of systematic reviews. 2016 Aug 31:2016(8):CD007719. doi: 10.1002/14651858.CD007719.pub4. Epub 2016 Aug 31 [PubMed PMID: 27580345]
Level 1 (high-level) evidenceHuiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. The Cochrane database of systematic reviews. 2005 Oct 19:2005(4):CD001479 [PubMed PMID: 16235283]
Level 1 (high-level) evidenceChen G, Weiskirchen S, Weiskirchen R. Vitamin A: too good to be bad? Frontiers in pharmacology. 2023:14():1186336. doi: 10.3389/fphar.2023.1186336. Epub 2023 May 22 [PubMed PMID: 37284305]
Holzmann H, Hengel H, Tenbusch M, Doerr HW. Eradication of measles: remaining challenges. Medical microbiology and immunology. 2016 Jun:205(3):201-8. doi: 10.1007/s00430-016-0451-4. Epub 2016 Mar 2 [PubMed PMID: 26935826]
Paules CI, Marston HD, Fauci AS. Measles in 2019 - Going Backward. The New England journal of medicine. 2019 Jun 6:380(23):2185-2187. doi: 10.1056/NEJMp1905099. Epub 2019 Apr 17 [PubMed PMID: 30995368]
Moss WJ, Griffin DE. What's going on with measles? Journal of virology. 2024 Aug 20:98(8):e0075824. doi: 10.1128/jvi.00758-24. Epub 2024 Jul 23 [PubMed PMID: 39041786]
Fisher DL, Defres S, Solomon T. Measles-induced encephalitis. QJM : monthly journal of the Association of Physicians. 2015 Mar:108(3):177-82. doi: 10.1093/qjmed/hcu113. Epub 2014 May 26 [PubMed PMID: 24865261]
Baldolli A, Dargère S, Cardineau E, Vabret A, Dina J, de La Blanchardière A, Verdon R. Measles inclusion-body encephalitis (MIBE) in a immunocompromised patient. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2016 Aug:81():43-6. doi: 10.1016/j.jcv.2016.05.016. Epub 2016 Jun 2 [PubMed PMID: 27315036]
Chowdhary J, Ashraf SM, Khajuria K. Measles with acute disseminated encephalomyelitis (ADEM). Indian pediatrics. 2009 Jan:46(1):72-4 [PubMed PMID: 19179725]
Level 3 (low-level) evidenceAnilkumar AC, Foris LA, Tadi P. Acute Disseminated Encephalomyelitis. StatPearls. 2025 Jan:(): [PubMed PMID: 28613684]
Rocke Z, Belyayeva M. Subacute Sclerosing Panencephalitis. StatPearls. 2025 Jan:(): [PubMed PMID: 32809508]
Kiang MV, Bubar KM, Maldonado Y, Hotez PJ, Lo NC. Modeling Reemergence of Vaccine-Eliminated Infectious Diseases Under Declining Vaccination in the US. JAMA. 2025 Apr 24:():. doi: 10.1001/jama.2025.6495. Epub 2025 Apr 24 [PubMed PMID: 40272967]
Flaherty DK. The vaccine-autism connection: a public health crisis caused by unethical medical practices and fraudulent science. The Annals of pharmacotherapy. 2011 Oct:45(10):1302-4. doi: 10.1345/aph.1Q318. Epub 2011 Sep 13 [PubMed PMID: 21917556]
Kuppalli K, Omer SB. Measles: the urgent need for global immunisation and preparedness. Lancet (London, England). 2025 May 3:405(10489):1565-1567. doi: 10.1016/S0140-6736(25)00675-0. Epub 2025 Apr 8 [PubMed PMID: 40215988]