Introduction
The female reproductive system, unlike the male, undergoes regular cyclic changes known as the menstrual cycle, which serves as the body’s periodic preparation for ovulation and potential pregnancy. The most noticeable aspect of the female reproductive system is menstruation, or cyclic vaginal bleeding, which occurs alongside a series of coordinated hormonal shifts. Menstruation, also known as menarche when it first begins, typically starts around puberty with a median age of 12.4.[1] Menstrual cycles cease at menopause, which has an average onset around age 51. Please see StatPearls' companion resource, "Physiology, Female Reproduction," for more information.[2][3]
Definitions
When discussing timing within the menstrual cycle, the first day of heavy menstrual flow is considered day 1. According to the International Federation of Gynecology and Obstetrics (FIGO), normal menstrual cycles should have consistent frequency, regularity, duration, and volume of flow.[4] Normal menstrual frequency is defined as cycles occurring every 24 to 38 days. Infrequent menstruation is defined as cycle lengths longer than 38 days, while frequent menstruation refers to cycle lengths shorter than 24 days.[4] Amenorrhea describes the complete absence of menstrual bleeding. Normal menstrual duration is defined as bleeding lasting 8 days or less, while bleeding beyond 8 days is considered prolonged menses.[4]
The volume of menstrual flow is classified as light, normal, or heavy. No defined objective thresholds separate these classifications, as they are often impractical in clinical settings. For research purposes, heavy menstrual bleeding is defined as blood loss exceeding 80 mL per cycle, based on weighed menstrual products. Heavy menstrual bleeding is a subjective symptom rather than a formal diagnosis. The National Institute for Health and Care Excellence (NICE) defines it as excessive menstrual bleeding that interferes with a person's physical, social, emotional, and/or material quality of life. Notably, 2 patients with the same objective volume of blood loss may have significantly different perceptions of their flow volume.
Light menstrual bleeding is rarely associated with underlying pathology, although it can occur in patients with intrauterine adhesions or cervical stenosis. For research purposes, light menstrual bleeding is typically defined as less than 5 mL of blood loss per cycle. Several factors can influence the volume of blood loss during menstruation, including medications, endometrial thickness, and bleeding or clotting disorders.
Menstrual regularity is defined by the variation in cycle lengths from one cycle to the next. Although slight variations in cycle lengths are normal, cycles are considered regular if the difference between the shortest and longest cycle lengths is 7 days or less for individuals aged 26 to 41 and 9 days or less for those aged 18 to 25 or 42 to 45. FIGO notes that for practical purposes, normal variation in cycle length can also be expressed as an average cycle length of ±4 days.[4]
The menstrual cycle is considered irregular when cycle lengths vary by 8 days or more for individuals aged between 26 and 41 or by 10 days or more for those aged between 18 and 25 or between 42 and 45. For example, a patient aged 43 with cycle lengths of 25, 28, and 34 days has a 9-day difference between her shortest and longest cycles, indicating regular cycles for her age. In contrast, the same cycle history in a patient aged 26 would suggest an irregular cycle. Intermenstrual bleeding is defined as bleeding that occurs between cyclically regular menstrual periods. This type of bleeding can be random, meaning it is unpredictable or cyclic, indicating that it occurs consistently at the same point in each cycle.[4]
The Ovarian and Endometrial Cycles
The menstrual cycle comprises 2 distinct cycles—one within the ovary and another within the endometrium. The phases of the ovarian cycle include the follicular phase, ovulation, and the luteal phase. The endometrial cycle consists of the proliferative phase, the secretory phase, and the menstrual phase. Generally, the ovarian follicular phase corresponds to the menstrual and proliferative phases of the endometrium, while the luteal phase of the ovarian cycle corresponds to the secretory phase of the endometrial cycle. These phases are discussed in more detail below.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
The complex nature of the menstrual cycle means that abnormal menstrual patterns often indicate underlying health issues. Clinicians must be familiar with what constitutes normal menstruation to effectively identify any abnormal patterns. For example, for menstruation to occur, the hypothalamus-pituitary-ovarian (HPO) axis must function properly, the endometrium must be healthy, and the uterine outflow tract must be patent. Numerous conditions can impact the HPO axis, including endocrine, metabolic, inflammatory, infectious, autoimmune, infiltrative, genetic, and neoplastic processes. Please see StatPearls' companion resource, "Amenorrhea," for more information.
Cellular Level
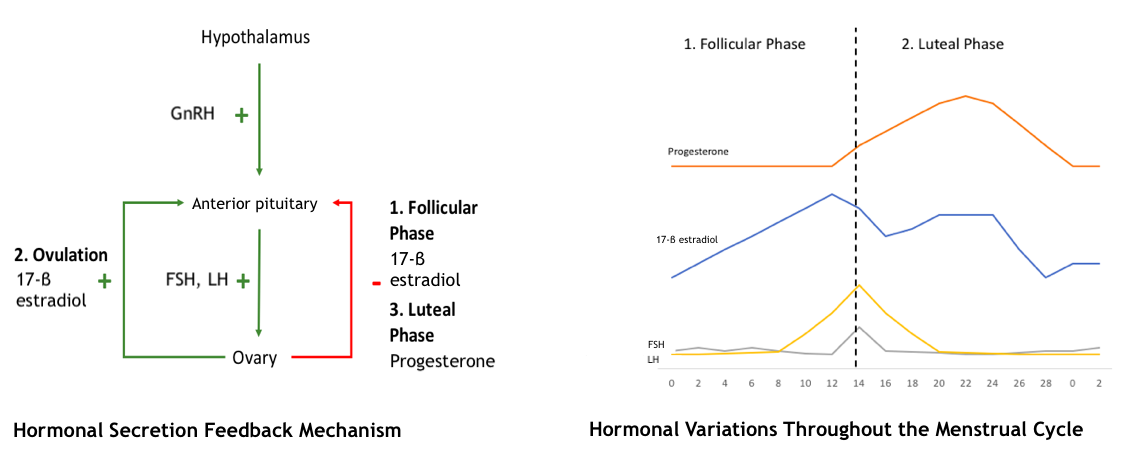
Hormones are secreted through both negative and positive feedback mechanisms to regulate the menstrual cycle (see Image. Hormonal Secretion and Feedback Mechanism During the Menstrual Cycle). Hormonal regulation begins in the hypothalamus, where gonadotropin-releasing hormone (GnRH) is secreted in an increased, pulsatile fashion starting at puberty. GnRH is transported to the anterior pituitary, where it activates its G protein–coupled receptor, signaling the pituitary gland to release follicle-stimulating hormone (FSH) and luteinizing hormone (LH). FSH and LH then travel through the bloodstream to the ovaries, stimulating the production of sex steroid hormones from follicular cells.
The ovarian follicle contains 2 cell types responsible for hormone production—theca cells and granulosa cells. LH stimulates theca cells to produce progesterone and androstenedione by activating the enzyme cholesterol desmolase. Androstenedione then diffuses into the adjacent granulosa cells, where FSH stimulates the enzyme aromatase within the granulosa cells to convert androstenedione to testosterone and then to 17-β estradiol. Both 17-β estradiol and progesterone are secreted into the bloodstream and affect various tissues, including the uterus and pituitary gland. In the uterus, these hormones promote the growth and maturation of the endometrium. At the anterior pituitary, these sex steroid hormones provide negative feedback, reducing the secretion of FSH and LH, which subsequently reduces the production of 17-β estradiol and progesterone by the ovaries.
An exception to this negative feedback loop occurs around the time of ovulation. When a critical level of 17-β estradiol is reached, it provides positive feedback to the anterior pituitary, leading to a surge in FSH and LH production. Granulosa cells within the feedback system also produce inhibin B and activin, which inhibit and stimulate FSH release from the anterior pituitary, respectively. This feedback mechanism is regulated by the upregulation or downregulation of GnRH receptors on the anterior pituitary.[5][6][7]
Organ Systems Involved
The menstrual cycle is regulated by the complex interaction of the hypothalamus, anterior pituitary gland, ovaries, and uterus. The uterine outflow tract, including the cervix and vagina, must remain patent to allow the outflow of menstrual fluid at the culmination of each cycle.
Function
The menstrual cycle prepares the female body for ovulation and potential pregnancy. Hormones secreted from the hypothalamus and pituitary gland promote the maturation of ovarian follicles and trigger ovulation. Ovarian hormones prepare the cervix to allow sperm entry into the endometrial cavity and stimulate endometrial growth and maturation for the potential implantation of a fertilized ovum. If pregnancy does not occur, the cycle "resets" the body for the next opportunity for conception.
Mechanism
Phase 1: The Follicular and Proliferative Phases
The initial stages of the menstrual cycle include the follicular and proliferative phases, which correspond to the maturation of ovarian follicles and the proliferation of the endometrium, respectively. The follicular phase, which varies in length, always begins on day 1 of the menstrual cycle—characterized by the first day of menstrual bleeding—and ends with ovulation. In a typical 28-day cycle, this phase lasts from days 1 to 14. Concurrently, the proliferative phase in the uterus starts after menstrual bleeding concludes and continues until ovulation.
Follicular phase events: During the follicular phase, FSH stimulates a cohort of primordial follicles to mature into Graafian follicles and promotes the production of 17-β estradiol and inhibin B within the ovary. Studies indicate that most of the estrogen is produced by the dominant follicle.[8] Around cycle day 7, several 9 to 10 mm antral follicles are typically present in each ovary. As 17-β estradiol and inhibin B provide negative feedback to reduce FSH levels, the nondominant follicles begin to degenerate.
FSH stimulates the production of additional FSH receptors within the dominant Graafian follicle, increasing its sensitivity to FSH even as overall FSH levels decline. The dominant follicle continues to mature, growing approximately 2 mm/d until it reaches a diameter of 18 to 29 mm (average 23.6 mm).[9][10] FSH also induces the development of LH receptors within the dominant follicle, preparing it for the next step—ovulation.
Proliferative phase events: The 17-β estradiol produced by the growing follicles stimulates the development of the endometrial stroma and glands from the decidual basalis (the basal layer of the endometrium) and increases the depth of the spiral arteries supplying the endometrium. By the end of the proliferative phase, the endometrium reaches its maximal development, typically measuring between 8 and 12 mm, although this can vary.[11][12] These changes prepare the endometrium for a potential pregnancy following ovulation, which occurs at the end of the proliferative phase. Additionally, 17-β estradiol modifies the elasticity and protein content of the cervical mucus, creating channels that facilitate sperm entry into the uterine cavity.[13]
Ovulation
Ovulation typically occurs 14 days before the onset of menses; thus, in an average 28-day cycle, ovulation occurs on day 14. Throughout the follicular phase, estradiol levels rise, and at the end of this phase, 17-β estradiol shifts from providing negative feedback to positive feedback at the anterior pituitary.
This transition from negative to positive feedback is not fully understood and likely involves several factors; however, it typically occurs when a critical level of estradiol is reached. Elevated estradiol levels stimulate gonadotrophic cells in the pituitary to produce more GnRH receptors, enhancing the sensitivity of these cells to GnRH.[9] Estradiol may also prevent the breakdown of GnRH within pituitary cells and lower the threshold of GnRH needed to trigger LH release. Additionally, a nonsteroidal ovarian product, often referred to as gonadotropin surge-attenuating factor (GnSAF), is believed to counteract the sensitizing effects of estradiol during most of the follicular phase. As estradiol levels increase throughout the follicular phase, GnSAF is suppressed, allowing the sensitizing effects of estradiol to take effect at the end of the phase, leading up to ovulation.[9][14] These mechanisms result in a sudden surge of LH secretion, with LH levels increasing 10-fold during the LH surge, accompanied by smaller rises in FSH concentrations.
As a result of this hormonal milieu, the mature follicle secretes plasminogen activator and other cytokines, leading to the rupture of the follicle and the release of the oocyte. Ovulation typically occurs approximately 36 to 44 hours after the onset of the LH surge. The cervical changes that began during the follicular phase continue, resulting in increased, watery cervical mucus to facilitate sperm entry. At the end of ovulation, levels of 17-β estradiol decrease.
Phase 2: The Luteal and Secretory Phases
The next phase of the menstrual cycle includes the luteal and secretory phases, reflecting the role of the corpus luteum in the ovary and the secretory function of the mature endometrium in the uterus, respectively. This phase begins with ovulation and concludes when menstrual bleeding starts. Unlike the variable length of the follicular or proliferative phase, the luteal or secretory phase is relatively consistent within an individual, typically lasting 14 days.
The dominant hormone during this phase is progesterone, which is stimulated by LH. Progesterone stimulates the maturation of the endometrium in preparation for the potential implantation of a fertilized ovum. Progesterone promotes the development of complex glands, increases glycogen accumulation for energy, and increases the surface area of the spiral arteries. Additionally, progesterone thickens and decreases the elasticity of cervical mucus, making it more difficult for sperm to pass through. It also slightly raises basal body temperature.
A fertilized ovum releases human chorionic gonadotropin (hCG), which stimulates the corpus luteum to maintain progesterone production. However, without a fertilized ovum, the natural rise in progesterone slows LH release through negative feedback at the pituitary gland, resulting in a rapid decline in progesterone and estradiol levels at the end of the phase. As these hormone levels decline, hypothalamic GnRH is released from feedback inhibition, leading to an increase in secretion in preparation for the next cycle.
Normal Menstruation
The abrupt decline in progesterone and estradiol levels at the end of the luteal phase triggers the shedding of the endometrium, which can no longer be sustained without these hormones. This shedding is referred to as menses. The first day of menstrual bleeding is designated as day 1 of the menstrual cycle, meaning menstruation occurs during the initial days of the follicular phase.
The duration of menses is variable, but normal menses lasts 8 days or less.[4] Menstrual fluid contains blood, endometrial cells, vaginal secretions, and various biochemical molecules, including proteolytic enzymes, cytokines, and products of fibrinolysis.[15] Menstrual fluid typically does not contain clots unless the flow is very heavy.
Pathophysiology
Anovulatory Cycles
In some cases, ovulation may not occur at the end of the follicular phase, resulting in anovulatory cycles. These cycles are common during the first 12 to 18 months after menarche and again before menopause. Additionally, anovulatory cycles are frequently associated with endocrine and metabolic disorders that impact the hormones involved in the HPO axis, such as polycystic ovary syndrome (PCOS), thyroid disorders, and hyperprolactinemia.
When ovulation does not occur, no corpus luteum develops, and progesterone is not secreted in significant quantities.[16] Without progesterone secretion, the endometrium does not mature, and there is no withdrawal of progesterone to trigger synchronized shedding at the end of the cycle. Meanwhile, estrogen stimulates endometrial growth, causing the proliferative endometrium to thicken. Eventually, it reaches a point where it breaks down and sloughs off, typically at irregular intervals, with menstrual flow varying from scant to heavy.[17]
Abnormal Uterine Bleeding
Abnormal menstruation and abnormal uterine bleeding (AUB) in reproductive-aged females can arise from various causes. FIGO has developed a comprehensive classification system called the PALM-COEIN system, which categorizes AUB based on its underlying etiology.[4] The etiology of AUB can be identified through basic laboratory studies, pelvic ultrasound, and, in some cases, endometrial tissue sampling. The PALM-COEIN categories are divided into structural and nonstructural causes.
- Structural causes of AUB include Polyps, Adenomyosis, Leiomyomas, and Malignancy/hyperplasia.
- Nonstructural causes of AUB include:
- Coagulopathy.
- Ovulatory dysfunction, which includes endocrine and metabolic disorders affecting the HPO axis, such as PCOS, thyroid disorders, and elevated levels of prolactin, androgens, or cortisol.
- Endometrial dysfunction, which includes conditions such as Asherman syndrome (destruction of the basal layer of the endometrium), inflammation (endometritis), and other issues that disrupt typical endometrial hemostasis.
- Iatrogenic factors.
- Not otherwise classified.
Clinical Significance
The menstrual cycle is a crucial indicator of a female's reproductive health, and any abnormalities in menstruation necessitate thorough evaluation. A thorough understanding of menstrual physiology is important for clinicians, as it allows them to diagnose and manage various gynecological issues, including both hormonal and structural pathologies.
Moreover, understanding the menstrual cycle is crucial for guiding patients through fertility planning, pregnancy, and the menopausal transition. By comprehending the intricacies of hormonal fluctuations and their physiological effects, clinicians can deliver accurate assessments, tailor treatments, and provide informed advice, ultimately enhancing patient outcomes and quality of life.[18][19][20]
Media
(Click Image to Enlarge)
References
. ACOG Committee Opinion No. 651: Menstruation in Girls and Adolescents: Using the Menstrual Cycle as a Vital Sign. Obstetrics and gynecology. 2015 Dec:126(6):e143-e146. doi: 10.1097/AOG.0000000000001215. Epub [PubMed PMID: 26595586]
Level 3 (low-level) evidenceCoast E, Lattof SR, Strong J. Puberty and menstruation knowledge among young adolescents in low- and middle-income countries: a scoping review. International journal of public health. 2019 Mar:64(2):293-304. doi: 10.1007/s00038-019-01209-0. Epub 2019 Feb 10 [PubMed PMID: 30740629]
Level 2 (mid-level) evidencePan B, Li J. The art of oocyte meiotic arrest regulation. Reproductive biology and endocrinology : RB&E. 2019 Jan 5:17(1):8. doi: 10.1186/s12958-018-0445-8. Epub 2019 Jan 5 [PubMed PMID: 30611263]
Munro MG, Critchley HOD, Fraser IS, FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2018 Dec:143(3):393-408. doi: 10.1002/ijgo.12666. Epub 2018 Oct 10 [PubMed PMID: 30198563]
Harlow SD. Menstrual Cycle Changes as Women Approach the Final Menses: What Matters? Obstetrics and gynecology clinics of North America. 2018 Dec:45(4):599-611. doi: 10.1016/j.ogc.2018.07.003. Epub 2018 Oct 25 [PubMed PMID: 30401545]
Gibson DA, Simitsidellis I, Collins F, Saunders PTK. Endometrial Intracrinology: Oestrogens, Androgens and Endometrial Disorders. International journal of molecular sciences. 2018 Oct 22:19(10):. doi: 10.3390/ijms19103276. Epub 2018 Oct 22 [PubMed PMID: 30360364]
Pepe G, Locati M, Della Torre S, Mornata F, Cignarella A, Maggi A, Vegeto E. The estrogen-macrophage interplay in the homeostasis of the female reproductive tract. Human reproduction update. 2018 Nov 1:24(6):652-672. doi: 10.1093/humupd/dmy026. Epub [PubMed PMID: 30256960]
Pache TD, Wladimiroff JW, de Jong FH, Hop WC, Fauser BC. Growth patterns of nondominant ovarian follicles during the normal menstrual cycle. Fertility and sterility. 1990 Oct:54(4):638-42 [PubMed PMID: 2209884]
Messinis IE, Messini CI, Dafopoulos K. Novel aspects of the endocrinology of the menstrual cycle. Reproductive biomedicine online. 2014 Jun:28(6):714-22. doi: 10.1016/j.rbmo.2014.02.003. Epub 2014 Mar 4 [PubMed PMID: 24745832]
Kerin JF, Edmonds DK, Warnes GM, Cox LW, Seamark RF, Matthews CD, Young GB, Baird DT. Morphological and functional relations of Graafian follicle growth to ovulation in women using ultrasonic, laparoscopic and biochemical measurements. British journal of obstetrics and gynaecology. 1981 Feb:88(2):81-90 [PubMed PMID: 6450609]
Fleischer AC, Kalemeris GC, Machin JE, Entman SS, James AE Jr. Sonographic depiction of normal and abnormal endometrium with histopathologic correlation. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1986 Aug:5(8):445-52 [PubMed PMID: 3528523]
Raine-Fenning NJ, Campbell BK, Clewes JS, Kendall NR, Johnson IR. Defining endometrial growth during the menstrual cycle with three-dimensional ultrasound. BJOG : an international journal of obstetrics and gynaecology. 2004 Sep:111(9):944-9 [PubMed PMID: 15327609]
Herbison AE. A simple model of estrous cycle negative and positive feedback regulation of GnRH secretion. Frontiers in neuroendocrinology. 2020 Apr:57():100837. doi: 10.1016/j.yfrne.2020.100837. Epub 2020 Mar 30 [PubMed PMID: 32240664]
Fowler PA, Sorsa-Leslie T, Harris W, Mason HD. Ovarian gonadotrophin surge-attenuating factor (GnSAF): where are we after 20 years of research? Reproduction (Cambridge, England). 2003 Dec:126(6):689-99 [PubMed PMID: 14748688]
Yang H, Zhou B, Prinz M, Siegel D. Proteomic analysis of menstrual blood. Molecular & cellular proteomics : MCP. 2012 Oct:11(10):1024-35 [PubMed PMID: 22822186]
Thomas VG. The Link Between Human Menstruation and Placental Delivery: A Novel Evolutionary Interpretation: Menstruation and fetal placental detachment share common evolved physiological processes dependent on progesterone withdrawal. BioEssays : news and reviews in molecular, cellular and developmental biology. 2019 Jun:41(6):e1800232. doi: 10.1002/bies.201800232. Epub 2019 May 23 [PubMed PMID: 31119755]
Carlson LJ, Shaw ND. Development of Ovulatory Menstrual Cycles in Adolescent Girls. Journal of pediatric and adolescent gynecology. 2019 Jun:32(3):249-253. doi: 10.1016/j.jpag.2019.02.119. Epub 2019 Feb 14 [PubMed PMID: 30772499]
van Duursen MBM. Modulation of estrogen synthesis and metabolism by phytoestrogens in vitro and the implications for women's health. Toxicology research. 2017 Nov 1:6(6):772-794. doi: 10.1039/c7tx00184c. Epub 2017 Sep 8 [PubMed PMID: 30090542]
Gunn HM, Tsai MC, McRae A, Steinbeck KS. Menstrual Patterns in the First Gynecological Year: A Systematic Review. Journal of pediatric and adolescent gynecology. 2018 Dec:31(6):557-565.e6. doi: 10.1016/j.jpag.2018.07.009. Epub 2018 Jul 29 [PubMed PMID: 30064002]
Level 2 (mid-level) evidenceAlvergne A, Högqvist Tabor V. Is Female Health Cyclical? Evolutionary Perspectives on Menstruation. Trends in ecology & evolution. 2018 Jun:33(6):399-414. doi: 10.1016/j.tree.2018.03.006. Epub 2018 May 16 [PubMed PMID: 29778270]
Level 3 (low-level) evidence