 Metabolic Dysfunction-Associated Steatotic Liver Disease (MΑSLD)
Metabolic Dysfunction-Associated Steatotic Liver Disease (MΑSLD)
Introduction
Metabolic dysfunction-associated steatotic liver disease (MΑSLD) is a broad term used to cover a spectrum of conditions characterized by evidence of hepatic steatosis on imaging or histology.[1] MASLD is a broad term used to encompass a spectrum of conditions characterized by evidence of hepatic steatosis on imaging or histology in patients with at least 1 metabolic risk factor (eg, obesity, diabetes mellitus, dyslipidemia, hypertension).[2]
MASLD represents a significant evolution in the terminology and conceptual framework surrounding hepatic steatosis. Historically, liver fat accumulation without significant alcohol intake was categorised under nonalcoholic fatty liver disease (NAFLD).[3] However, recent shifts in nomenclature arose primarily to address 2 core issues. Firstly, the term "fatty" was replaced with "steatotic" to mitigate the stigma associated with the former term. Secondly, defining a disease by exclusion, eg, "nonalcoholic," was widely criticised for its inherent ambiguity and lack of specificity, akin to labelling conditions like myocardial infarction as "non-cocaine myocardial infarction," which is considered conceptually inadequate.
The pivotal shift in terminology was initiated by the landmark paper from Eslam et al, introducing the term Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD), thereby directly associating hepatic steatosis with underlying metabolic dysfunction rather than excluding alcohol consumption alone. This redefinition marked a significant departure from NAFLD, emphasizing pathophysiological mechanisms linked to components of metabolic syndrome. Subsequently, following broader international consensus, MAFLD was refined further to MASLD, establishing clearer diagnostic criteria and positioning it within a broader spectrum of conditions collectively termed Steatotic Liver Disease (SLD). This evolution reflects a more precise and mechanistically informed approach to understanding hepatic steatosis, enhancing clinical and research clarity.
Types of Steatotic Liver Disease
Steatotic liver disease is categorized into the following types:
- Metabolic dysfunction-associated steatotic liver disease (MASLD): MASLD is defined by ≥5% hepatic steatosis and the presence of at least 1 cardiometabolic risk factor (eg, dyslipidemia or obesity), with no other underlying causes and minimal or no alcohol intake (ie, <20 g/day for females and <30 g/day for males). Formerly known as NAFLD, MASLD is the hepatic manifestation of metabolic syndrome.
- Metabolic dysfunction-associated steatohepatitis (MASH): MASH, previously called nonalcoholic steatohepatitis (NASH), is a progressive form of MASLD marked by liver inflammation and hepatocellular injury, with or without fibrosis.
- Metabolic dysfunction and alcohol-associated liver disease (MetALD): MetALD refers to patients with hepatic steatosis, at least 1 metabolic risk factor, and moderate alcohol consumption, defined as 20 to 50 g/day for females and 30 to 60 g/day for males. This condition may result from a combination of metabolic dysfunction and moderate alcohol consumption, representing a spectrum between MASLD-predominant and alcohol-predominant disease.
- Alcohol-associated liver disease (ALD): Patients with steatosis and heavy alcohol use (>50 g/day for females, >60 g/day for males), with a standard drink containing approximately 14 g of alcohol, are classified with alcohol-associated liver disease (ALD).[4]
Although MASLD can coexist with other causes of steatosis, eg, celiac disease, medications, or genetic conditions, this discussion focuses on MASLD in the absence of those factors. A diagnostic approach for steatotic liver diseases (SLD) other than MASLD, MetALD, and ALD has been established, highlighting critical clinical features and recommended investigations (see Image. Steatotic Liver Disease Diagnosis). Understanding the etiology and pathophysiology of MASLD and its subtypes is critical for clinicians, emphasizing the need for interprofessional collaboration in diagnosis and management.
The cardiometabolic risk factors defining metabolic dysfunction include:
- Excess adiposity
- Body-mass index (BMI): A BMI of ≥25 kg/m² in the general population or ≥23 kg/m² in individuals of Asian ancestry.
- Central (visceral) obesity (waist circumference thresholds): • Waist circumference of ≥94 cm in men and ≥80 cm in women of European ancestry • Waist circumference of ≥90 cm in men and ≥80 cm in women of South-Asian or Chinese ancestry • Waist circumference of ≥85 cm in men and ≥90 cm in women of Japanese ancestry
- Disorders of glucose metabolism
- Prediabetes: Glycated haemoglobin (HbA1c) 5.7% to 6.4 %; fasting plasma glucose ranging from 100 to 125 mg/dL
- Type 2 diabetes mellitus: HbA1c of ≥6.5%; fasting plasma glucose of ≥126 mg/dL
- Hypertriglyceridemia: Plasma triglycerides ≥150 mg/dL or treatment with lipid-lowering agents
- High-density lipoprotein (HDL) cholesterol: <40 mg/dL in men or ≤50 mg/dL in women
- Arterial hypertension: Resting blood pressure of 130/85 mm Hg or greater or ongoing antihypertensive treatment.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Obesity, diabetes, dyslipidemias, insulin resistance, and metabolic syndrome are known to be associated with the development of MASLD.[6] Due to its close association with metabolic syndrome, MASLD correlates with cardiovascular risk factors, which also contribute to mortality in these patients, in addition to end-stage liver cirrhosis and hepatocellular carcinoma.[7]
Epidemiology
MASLD incidence is rapidly increasing worldwide. The prevalence has been reported to be the highest in Latin America at 44.4% and lowest in Western Europe at 25.1%; however, the global prevalence of MASLD is anticipated to rise to 55.4% by 2040.[8] MASLD is considered to be the liver manifestation of metabolic syndrome. Between 50% and 70% of people with diabetes are found to have MASLD. Rising obesity levels, increasing incidence of childhood obesity, sedentary lifestyles, consumption of unhealthy quick eats, and a longer lifespan are some of the likely contributors.
The incidence and prevalence of MASLD are underestimated, as ultrasonography is commonly used to screen for fatty liver disease. The prevalence of MASLD is 80% to 90% in obese adults, 30% to 50% in patients with diabetes mellitus, 90% or more in patients with hyperlipidemia, and as high as 40% to 70% among children with obesity.[9] An estimated 7% to 14% of children and adolescents in the general population have MASLD.[8]
Pathophysiology
Both environmental and genetic factors contribute to the development and progression of MASLD. First-degree relatives of patients with MASLD are at higher risk than the general population.
Day and James proposed a 2-hit model of MASLD pathogenesis in 1998. The first hit is caused by insulin resistance, which leads to the accumulation of fat droplets, specifically triglycerides, in the cytoplasm of hepatocytes, resulting in the development of steatosis. Insulin resistance causes an excess delivery of free fatty acids and triglycerides to the liver, resulting in decreased excretion and accumulation. Additionally, excess carbohydrates serve as a stimulus for de novo fatty acid synthesis in the liver.
The second hit, causing hepatocellular injury and the development of MASH, is multifactorial. Excessive fatty acids in the liver make the liver more vulnerable to injury. Peroxisomal fatty acid oxidation, reactive oxygen species (ROS) production from the mitochondrial respiratory chain, cytochrome P450 metabolism of fatty acids, and hepatic metabolism of gut-derived alcohol are hypothesized to cause the injury. Obesity also contributes to the second hit, as adipose tissue releases inflammatory mediators, eg, leptin, tumor necrosis factor-alpha (TNF-alpha), and interleukin (IL)-6, causing hepatocyte damage. The hepatocytes undergo ballooning, cytoskeletal aggregation, apoptosis, and necrosis.[10]
Insulin resistance is also a part of the second hit. The sinusoidal collagen deposition, caused by the activation of hepatic stellate cells, and the portal fibrosis resulting from ductular proliferation lead to the development and progression of MASH. These changes have correlated with insulin resistance, which is now believed to cause the progression of steatosis to MASH and progressive fibrosis.[11]
Histopathology
MASLD is characterized by more than 5% of hepatocytes with fat droplets on liver biopsy. Functionally, the liver is subdivided into 3 zones based on the oxygen supply. Zone 1 has the highest oxygenation (oxygenated blood from hepatic arteries) and encircles the portal tracts, and zone 3 encircles the central veins, where the oxygenation is poor.
The American Association for the Study of Liver Diseases (AASLD) has defined the histopathological abnormalities required for the diagnosis of MASH, which include steatosis, lobular inflammation, and hepatocellular ballooning, most clearly seen in zone 3 steatotic liver cells. Fibrosis, although not necessary for the diagnosis, is usually present. Other findings observed include Mallory-Denk bodies (MDB, eosinophilic intracytoplasmic inclusions), megamitochondria, glycogenated nuclei, and iron deposition.[12]
Steatosis manifests as hepatocytes containing large lipid vacuoles, which displace the nuclei peripherally. Ballooning degeneration presents as enlarged hepatocytes with granular cytoplasm, reflecting cytoskeletal disruption. Lobular inflammatory infiltrates are dispersed throughout liver tissue, while mild portal inflammation may also be present.
MASH must be differentiated histologically from alcoholic steatohepatitis (ASH), which commonly exhibits prominent hepatocyte ballooning surrounded by neutrophils, abundant Mallory-Denk bodies, and centrilobular sclerosing hyaline necrosis. Nonetheless, substantial histological overlap exists between MASH and ASH, necessitating correlation with the clinical context for accurate diagnosis.[13][14][15]
Several scoring systems exist for evaluating MASLD severity on liver biopsy, notably the Brunt system, the NASH Clinical Research Network (NASH-CRN) scoring system, and the Steatosis, Activity, and Fibrosis (SAF) system. The Brunt system grades steatosis severity, ballooning, and inflammation, and stages fibrosis severity, but does not explicitly differentiate between MASH and MASLD. The NASH-CRN system provides a numerical NAFLD activity score (0-8), integrating steatosis, lobular inflammation, and hepatocellular ballooning, and further subclassifies fibrosis severity. Developed primarily for clinical trials, it similarly does not independently establish a MASH diagnosis. In contrast, the SAF scoring system directly diagnoses MASH based on inflammation and ballooning, requiring an activity score of 2 or more, with the presence of steatosis essential but its severity not influencing the activity score. This reflects the pathophysiological role of fatty acids and metabolites rather than triglycerides in liver injury, although it complicates scoring in advanced or "burnt-out" MASH cases.
Fibrosis begins in zone 3 of the acinar zone and exhibits a chicken-wire appearance due to the deposition of collagen and other extracellular matrices along the sinusoids. MASH-related cirrhosis is macronodular or mixed. When cirrhosis develops, the other histological features may not be evident.[11]
History and Physical
Clinical Features of Metabolic Dysfunction-Associated Steatotic Liver Disease
Patients with MASLD can present with several nonspecific symptoms long before the diagnosis is made, although most patients are asymptomatic. Fatigue is one of the most common presenting symptoms. Sharp or dull aching upper abdominal pain, thirst, bloating, and sleep disturbances.[16] Patients who develop NASH-associated cirrhosis, end-stage liver disease, or hepatocellular carcinoma (HCC) present with various symptoms, including:
- Nausea
- Vomiting
- Jaundice
- Pruritis
- Ascites
- Memory impairment
- Easy bleeding
- Loss of appetite
The most common clinical sign is mild to moderate hepatomegaly. Advanced stages of the spectrum can demonstrate signs of end-stage liver disease, including:
- Jaundice
- Spider angiomas
- Palmar erythema
- Caput medusae
- Gynecomastia
- Dupuytren contracture
- Ascites
- Petechiae
Evaluation
Metabolic Dysfunction-Associated Steatotic Liver Disease Diagnostic Evaluation
Mildly elevated serum aminotransferases are the primary abnormality in MASLD, although the liver enzymes are normal in the majority of patients. The ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT) is less than 1. Gamma-glutamyl transferase (GGT), when elevated in MASLD, can serve as a marker of increased mortality, with the progression of the disease often accompanied by hypoalbuminemia, hyperbilirubinemia, thrombocytopenia, and a prolonged prothrombin time, all of which are indicative of hepatic synthetic dysfunction.
Ultrasound of the abdomen is routinely used to evaluate fatty liver, but a liver biopsy is considered the gold standard for the diagnosis of MASLD.[17] The primary objective of staging patients with MASLD is to assess the severity of fibrosis. Noninvasive imaging methods are generally used, while liver biopsy is reserved for cases that remain inconclusive.
Noninvasive assessment of hepatic fibrosis in metabolic dysfunction-associated steatotic liver disease
Current noninvasive tests, encompassing serum biomarker panels and imaging-based elastography, evaluate either circulating markers or liver stiffness. Their diagnostic accuracy declines outside specialist cohorts because performance is prevalence-dependent; therefore, tools validated in referral centres require separate validation before use in primary care.
Serum-based indices
Combining routine biochemistry with anthropometric data outperforms isolated transaminase measurement. Frequently used scores include the Fibrosis-4 index (FIB-4 = age × AST ÷ [platelet count × √ALT]), the AST-to-Platelet Ratio Index (APRI = AST ÷ upper-limit-of-normal × 100 ÷ platelets) and the NAFLD Fibrosis Score (NFS = composite of age, body-mass index, impaired fasting glucose, AST/ALT ratio, platelets and albumin). FIB-4 is the most accessible, but it loses accuracy in its intermediate zone (1.3–2.67), particularly in older adults, individuals with type 2 diabetes, and those younger than 35 years.
Panels reflecting active collagen turnover provide complementary information: the Enhanced Liver Fibrosis (ELF™) score combines serum hyaluronic acid and amino-terminal propeptide of type III collagen.III procollagen (PIIINP) and tissue inhibitor of metalloproteinase-1 (TIMP-1); the ADAPT algorithm (Age, Diabetes, PRO-C3, and Platelets) is effective both in tertiary care and in low-risk cohorts.[18][19][20][21][19]
Imaging-based elastography
Fibrosis-related stiffening is measured by vibration-controlled transient elastography (VCTE), point or 2-dimensional shear-wave elastography, and magnetic resonance elastography (MRE). Ultrasound-based techniques are inexpensive but less reliable in class II obesity, whereas MRE, although costlier and less available, provides equal or superior staging accuracy and is weight-independent. Quantitative MRI techniques such as proton-density fat fraction (PDFF) and corrected T1 mapping (LiverMultiScan®) further characterize steatosis and extracellular expansion, although cost and availability restrict routine use. The controlled attenuation parameter (CAP) derived from VCTE and the liver attenuation index from an unenhanced computed tomography scan offers only approximate estimates of steatosis.[22]
Hybrid algorithms
Composite models leverage complementary modalities. The Magnetic Resonance Elastography, AST, and Steatosis (MAST) score integrates MRE, PDFF, and AST. The FibroScan–AST (FAST) score combines VCTE stiffness, CAP, and AST. MEFIB (MRE + FIB-4) merges MRE with FIB-4. Comparative studies have yet to establish a universal superiority, and performance varies with case mix.[23]
Adults with diabetes, abdominal obesity, plus 1 or more additional metabolic risk factors, or persistent transaminase elevation are advised to undergo initial screening with FIB-4. Values less than 1.3 indicate low risk and merit reevaluation every 1 to 3 years. Scores 1.3 to 2.67 (or >2.0 in individuals older than 65 years) prompt either immediate elastography or a 12-month program of lifestyle modification and intensified cardiometabolic management, followed by repeat FIB-4 testing. Persistent elevation should lead to elastography or collagen biomarker testing. This sequential approach effectively triages high-risk individuals into hepatology care pathways and predicts liver-related events. Serum biomarker panels and elastography are suitable for excluding advanced fibrosis; elastography offers the highest positive predictive value for fibrosis stage F3 or more. None of the current noninvasive tests detect steatohepatitis-defining lesions (ballooning or lobular inflammation). Liver biopsy remains indispensable for the definitive diagnosis of MASH.
Assessment of comorbidities
Comprehensive comorbidity assessment requires systematic evaluation across multiple domains, with specific reference parameters and supplementary investigations tailored to individual clinical presentations. For obesity assessment, primary parameters include body mass index, waist circumference, and waist-to-height ratio, with additional body composition analysis and thyroid function evaluation (TSH and free thyroxine) when hypothyroidism is suspected. Type 2 diabetes and insulin resistance evaluation encompasses fasting plasma glucose, HbA1c, oral glucose tolerance testing with a 2-hour postload glucose measurement, fasting plasma insulin and C-peptide levels, and HOMA-IR calculation.
More sophisticated insulin resistance indices, derived from oral glucose tolerance tests or mixed meal tests, are also available for comprehensive metabolic assessment. Dyslipidemia screening requires fasting plasma triglycerides, total cholesterol, LDL and HDL cholesterol measurements, with a one-time lipoprotein-a assessment, and may be supplemented by nonesterified fatty acids and apolipoprotein B quantification. Kidney disease assessment involves measuring plasma and urine creatinine, as well as serum and urine albumin levels, and calculating the estimated glomerular filtration rate.
Cardiovascular disease evaluation incorporates fasting plasma uric acid, serum high-sensitivity C-reactive protein, serum ferritin, and blood pressure measurements, with optional 24-hour ambulatory blood pressure monitoring, echocardiography for heart failure assessment, serum NT-ProBNP, and transferrin saturation analysis. Atherosclerosis assessment requires complete blood count with platelet evaluation, recognizing that elevated lipoprotein-a represents an independent causal risk factor for atherosclerotic cardiovascular disease. Supplementary investigations include fibrinogen, homocysteine, von Willebrand factor antigen, carotid artery intima-media thickness, echo-Doppler plaque instability assessment, and coronary artery calcification scoring.
Obstructive sleep apnea screening involves measuring neck circumference and assessing the Epworth score, with confirmatory sleep studies, including overnight pulse oximetry, polysomnography, and CPAP trial evaluation. Finally, polycystic ovary syndrome assessment requires comprehensive sex hormone evaluation, including LH, FSH, testosterone, and SHBG measurements, accompanied by ovarian ultrasonography.[24][25][26][27]
Assessment of genetic risk factors
Clinicians in specialized centers may consider genetic risk profiling, including assessment of the PNPLA3 p.I148M variant and polygenic risk scores, to enhance personalized risk stratification and facilitate disease sub-phenotyping in patients with MASLD. Genetic risk variants serve as valuable tools for clinical studies examining disease progression patterns. Individuals presenting with strong family histories of severe hepatic disease in first-degree relatives or early-onset severe phenotypes, particularly in the absence of conventional metabolic triggers or in patients with normal body weight, warrant consideration for comprehensive genetic evaluation through next-generation sequencing approaches to identify coexisting treatable genetic causes of liver disease.[28]
Hepatocellular carcinoma surveillance in MASLD
Given the limited sensitivity of ultrasound-based surveillance for early-stage hepatocellular carcinoma detection, particularly in adults with MASLD-related cirrhosis and obesity, alpha-fetoprotein measurement may be integrated with ultrasound screening in high-risk individuals, while cross-sectional MRI imaging represents a valuable option for selected high-risk adults with persistent poor ultrasound visualization, especially those presenting with dysplastic or regenerative nodules. Factors associated with elevated hepatocellular carcinoma risk in non-cirrhotic MASLD include the presence and duration of type 2 diabetes and obesity, advanced age, concurrent alcohol intake and smoking, FIB-4 scores exceeding 3.25, and liver stiffness measurements above 10 kPa with progressive increases over time, necessitating tailored surveillance approaches based on individual risk profiles.[29][2]
Treatment / Management
Lifestyle changes are recommended for all patients with MASLD, even without MASH, as these patients have metabolic derangements and are at risk for the development of cardiovascular diseases. The recommended weight loss is 3% to 5% in simple steatosis and 7% to 10% in NASH. Adequate control of risk factors, eg, hyperlipidemia with statins (which also protects against cardiovascular risks) and hypertension, as well as adequate glycemic control, is required. Please see StatPearls' companion resource, "Liver Transplantation," for more information on hepatitis and nonalcoholic steatohepatitis.
Metabolic Dysfunction-Associated Steatotic Liver Disease Management
Management of MASLD involves weight management, alcohol avoidance, medical therapy, and regular monitoring for fibrosis.
Weight management
Weight loss is recommended for patients with obesity, as it can lead to histologic improvement. Patients are advised to lose 5% to 7% of body weight at a rate of 0.5 to 1.0 kg/week (1–2 lb/week) through lifestyle changes, including a balanced diet and regular exercise.
Alcohol avoidance
Abstinence from alcohol is recommended for all patients with MASLD.
Pharmacologic therapy
Medications that are utilized in the treatment of MASLD include:
- Resmetirom (thyroid hormone receptor-beta agonist): Resmetirom is used for patients with MASH and fibrosis stage F2 or F3 without significant weight loss. Patients with cirrhosis were excluded from prior clinical trials, but ongoing studies are assessing its safety in this group. The recommended dosage for patients weighing less than 100 kg is 80 mg orally once daily; for those weighing 100 kg or more, 100 mg orally once daily should be administered. Before prescribing resmetirom, clinicians should evaluate potential drug interactions. Resmetirom should not be used with OATP 1B1/1B3 inhibitors (eg, cyclosporine) or strong CYP2C8 inhibitors (eg, gemfibrozil).[30]
- Vitamin E: Vitamin E is suggested for patients with MASH (stage 2 fibrosis or above) who do not have diabetes at a dosage of 800 IU daily. The risks and benefits should be discussed with the patient, as high-dose vitamin E may slightly increase all-cause mortality based on observational studies.
Other pharmacological interventions for MASLD include bile acid derivatives, eg, ursodeoxycholic acid, which demonstrates biochemical efficacy without histological improvement, and obeticholic acid, which shows improvement in fibrosis (although discontinued for this indication due to safety concerns). Glucose-lowering agents may also be utilized, including GLP1 receptor agonists (eg, liraglutide and semaglutide), which achieve steatohepatitis resolution without fibrosis improvement, SGLT2 inhibitors (eg, empagliflozin and dapagliflozin) that have shown modest liver lipid reduction, pioglitazone, which improves steatohepatitis without clear fibrosis regression, and metformin, which lacks histological efficacy despite biochemical benefits.
Adjunctive therapies, eg, omega-3 fatty acids, which have shown no histological benefit, statins that have demonstrated cardiovascular protection and potential hepatoprotective effects, and silymarin, which improves liver enzymes without histological improvement, may also be considered for treating MASLD. While dual and triple incretin co-agonists (tirzepatide, cotadutide, survodutide, retatrutide) and pan-PPAR agonists (lanifibranor) show promising preliminary results with dose-dependent histological improvements, none of these agents are currently recommended as targeted MASH therapies due to insufficient evidence from large phase III trials, though they remain safe and indicated for their respective metabolic conditions. Sarolitazar is a PPAR agonist that is approved in India for use in MASLD without cirrhosis.[31]
Surgical and endoscopic therapies
Bariatric surgery procedures, including Roux-en-Y gastric bypass, sleeve gastrectomy, and gastric banding, demonstrate significant efficacy in adults with noncirrhotic MASLD who meet approved indications, inducing long-term beneficial hepatic effects alongside remission of type 2 diabetes and improvement of cardiometabolic risk factors. In patients with MASLD-related compensated advanced chronic liver disease or compensated cirrhosis, bariatric surgery may be considered following careful interprofessional evaluation, with Roux-en-Y gastric bypass showing superior outcomes in achieving steatohepatitis improvement [32]
Endoscopic bariatric procedures, including intragastric balloon, endoscopic sleeve gastroplasty, and various other minimally invasive techniques, show promise for improving liver steatosis and fibrosis, with lower complication rates and affordability compared to surgical interventions. However, these approaches require further validation as targeted MASH therapy, given the limited histological data and predominantly retrospective study designs.[33]
Diabetes Management in Metabolic Dysfunction-Associated Steatohepatitis
For patients with MASH and diabetes, glucose-lowering therapy should be selected with liver health in mind. While metformin is the first-line therapy for type 2 diabetes, pioglitazone and GLP-1 receptor agonists (eg, liraglutide, semaglutide) are reasonable alternatives for those needing additional glucose control or unable to take metformin, as they may improve liver histology. Metformin demonstrates efficacy in adults with compensated cirrhosis and preserved renal function, improving ALT levels and potentially providing protective effects against HCC. Metformin is contraindicated in decompensated cirrhosis due to lactic acidosis risk, while sulfonylureas should be avoided in hepatic decompensation given the increased risk of hypoglycemia. GLP1 receptor agonists show promise in Child-Pugh class A cirrhosis with demonstrated improvements in steatosis, liver enzymes, body weight, and glycemic control, while SGLT2 inhibitors can be utilized in Child-Pugh class A and B cirrhosis, providing benefits in glucose control, weight reduction, and cardiovascular outcomes despite increased drug exposure in advanced disease.[34]
Patients with biopsy-proven MASH undergo regular noninvasive assessments for advanced fibrosis, with the frequency determined by their clinical progress. Patients with MASH are to be followed by hepatologists or gastroenterologists. NASH with cirrhosis requires hepatocellular carcinoma surveillance with an ultrasound every 6 months.[35]
Statins represent crucial therapeutic interventions for cardiovascular risk reduction in chronic liver disease, including compensated cirrhosis, with observational evidence suggesting hepatocellular carcinoma prevention and reduced cirrhotic complications, though ALT elevation may occur in up to 3% of patients without significant liver injury. High-dose statin therapy in decompensated cirrhosis requires careful consideration due to increased risk of severe adverse events, including liver toxicity and rhabdomyolysis, particularly in Child-Pugh class B or C patients, necessitating individualized risk-benefit assessment.[36]
Differential Diagnosis
The differential diagnoses of MASLD, conditions that can also cause hepatic steatosis, include:
- Alcoholic liver disease
- Hepatitis C, particularly genotype 3
- Wilson disease
- Medications, eg, amiodarone, methotrexate, tamoxifen, glucocorticoids, valproate, anti-retroviral agents for HIV
- Reye syndrome
- Mitochondrial hepatopathies
- Kwashiorkor/anorexia nervosa
- Mitochondrial disorders
Staging
The following criteria are used for grading and staging MASLD:
- Grades
- Grade 1 (mild): Steatosis up to 66%, occasional ballooning in zone 3, scattered polymorphs with or without lymphocytes, mild or no portal inflammation
- Grade 2 (moderate): Any degree of steatosis, obvious ballooning predominantly in zone 3, intralobular inflammation with polymorphs and chronic inflammation, and mild to moderate portal inflammation
- Grade 3 (severe): Panacinar steatosis, ballooning, and apparent disarray predominantly in zone 3, intralobular inflammation with scattered polymorphs with or without mild chronic and mild to moderate portal inflammation [37][38][12]
- Stages
Prognosis
Patients with MASLD exhibit increased mortality rates when compared to the general population. These patients have a high risk of mortality from cardiovascular causes, as they have metabolic derangements. Cardiovascular causes of mortality are higher in these patients than liver causes.[40] MASLD can be a slowly progressive disease; simple steatosis is reversible and nonprogressive, whereas MASH can progress to cirrhosis. In a 13-year follow-up study by Ekstedt et al, the progression of cirrhosis was observed in 41% of cases.[41] A meta-analysis conducted by White et al showed that in cohorts of MASLD or MASH with few or no cases of cirrhosis, the risk of developing HCC was minimal at 0% to 3% over 20 years. In cohorts with MASH with cirrhosis, the risk was high at 2.4 % over 7 years.[42]
Complications
The most important complications of MASLD include:
- Cardiovascular disease
- Hepatocellular carcinoma
- End-stage liver disease
The severity of these complications is proportional to the severity of the histological stage and grade of liver disease.
Deterrence and Patient Education
Patient education on dietary decisions and portions is essential. Nutrition and lifestyle education are the mainstays of therapy. During every healthcare practitioner encounter, the issues of food choices, portion sizes, and exercise, including weight-bearing exercises, should be emphasized and reviewed. The American Diabetes Association and other organizations offer excellent dietary and lifestyle advice.
Enhancing Healthcare Team Outcomes
MASLD is a prevalent liver condition linked to metabolic risk factors such as obesity, diabetes, and dyslipidemia. Formerly known as NAFLD, MASLD can progress to steatohepatitis (MASH), fibrosis, cirrhosis, or hepatocellular carcinoma. Early detection, risk stratification, and coordinated care are essential to prevent complications and improve long-term outcomes. The growing prevalence of MASLD, primarily driven by rising obesity rates and sedentary lifestyles, calls for a coordinated, interprofessional approach to patient care.
Managing MASLD effectively requires collaboration among primary care physicians, advanced practitioners, hepatologists or gastroenterologists, nutritionists, endocrinologists, bariatricians, specialty-trained nurses, and pharmacists. Each team member brings essential expertise to address the multifactorial nature of MASLD, including metabolic syndrome, cardiovascular risks, and liver-related complications. Physicians and advanced practitioners lead in diagnosis, staging, and ongoing management, while nurses provide patient education, symptom monitoring, and care coordination. Nutritionists and bariatricians support lifestyle modification, which is foundational to improving outcomes through sustainable weight loss, smoking cessation, and interventions involving diet and exercise. Endocrinologists manage diabetes and dyslipidemia, both of which are critical components of MASLD care.
Interprofessional communication and collaboration are essential for enhancing patient-centered outcomes and ensuring patient safety. Open dialogue among team members ensures that care plans are cohesive, timely, and aligned with patients' goals. Pharmacists contribute by reviewing medication regimens for interactions and supporting adherence, especially when agents like GLP-1 receptor agonists or vitamin E are introduced. Nurses and allied health professionals often serve as the frontline in patient engagement, reinforcing education and helping patients navigate complex treatment plans. Together, these roles create a shared responsibility model that enhances team performance, minimizes gaps in care, and fosters a continuous feedback loop that keeps the patient at the center of every decision. Effective care coordination improves health outcomes, reduces complications, and supports long-term disease management in MASLD.
Media
(Click Image to Enlarge)
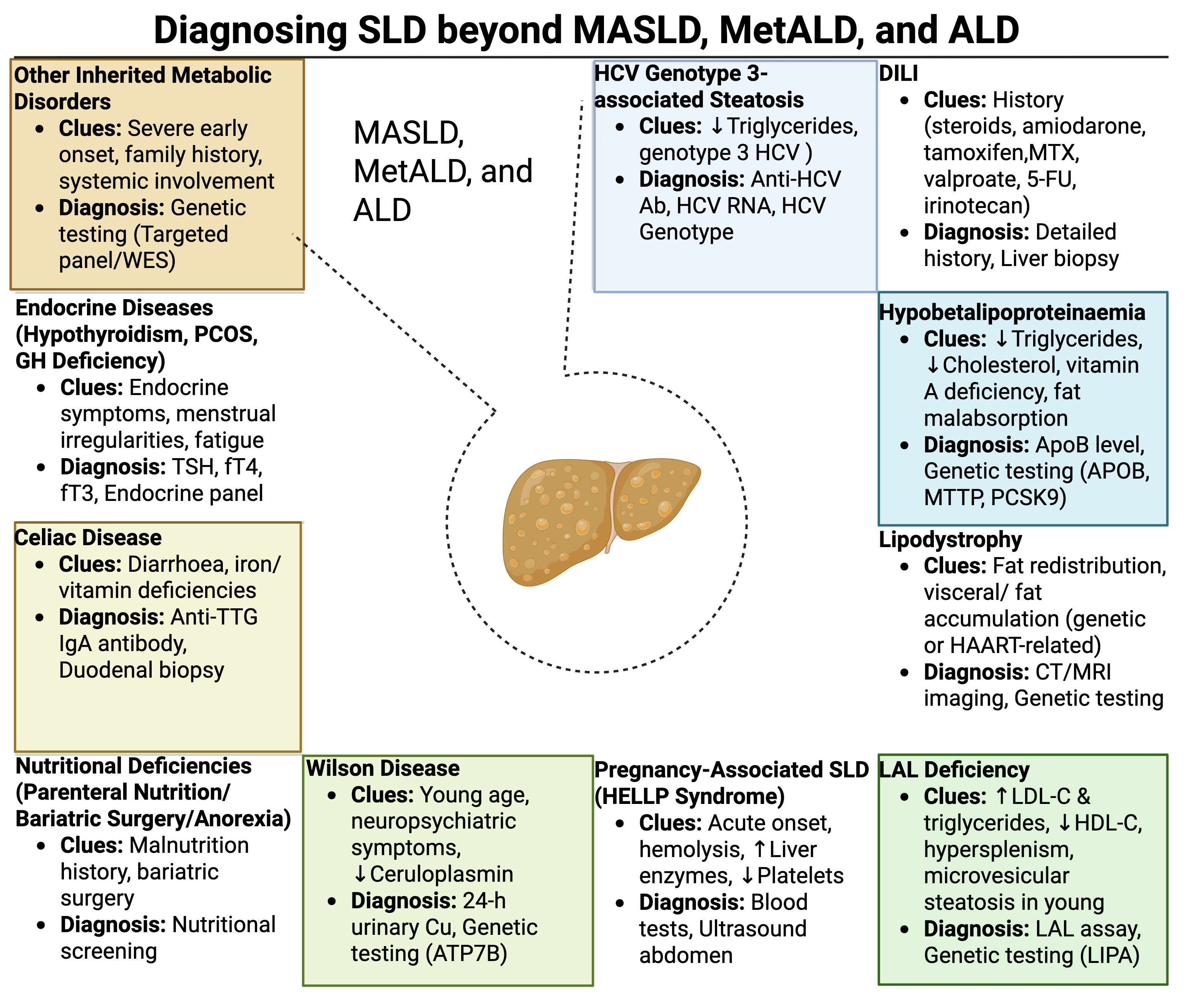
Steatotic Liver Disease Diagnosis.The infographic summarizes the diagnostic approach to steatotic liver disease (SLD) beyond MASLD, MetALD, and ALD using clinical clues and corresponding diagnostic tests for diverse etiologies of hepatic steatosis, emphasizing the importance of precise identification for optimal management.
Contributed by V Girish, MD
References
Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Narro GEC, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN, NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Annals of hepatology. 2024 Jan-Feb:29(1):101133. doi: 10.1016/j.aohep.2023.101133. Epub 2023 Jun 24 [PubMed PMID: 37364816]
Level 3 (low-level) evidenceEuropean Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD): Executive Summary. Diabetologia. 2024 Nov:67(11):2375-2392. doi: 10.1007/s00125-024-06196-3. Epub [PubMed PMID: 38869512]
Level 1 (high-level) evidenceHuang DQ, Wong VWS, Rinella ME, Boursier J, Lazarus JV, Yki-Järvinen H, Loomba R. Metabolic dysfunction-associated steatotic liver disease in adults. Nature reviews. Disease primers. 2025 Mar 6:11(1):14. doi: 10.1038/s41572-025-00599-1. Epub 2025 Mar 6 [PubMed PMID: 40050362]
Lee YH, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, Rhee EJ. Nonalcoholic Fatty Liver Disease in Diabetes. Part I: Epidemiology and Diagnosis. Diabetes & metabolism journal. 2019 Feb:43(1):31-45. doi: 10.4093/dmj.2019.0011. Epub [PubMed PMID: 30793550]
Diaz LA, Ajmera V, Arab JP, Huang DQ, Hsu C, Lee BP, Louvet A, Thiele M, Tavaglione F, Tincopa M, Pose E, Adams LA, Alazawi W, Arrese M, Bataller R, Duseja A, Liangpunsakul S, Lucey MR, Mathurin P, Mellinger J, Nakajima A, Ratziu V, Reau N, Rinella ME, Thursz M, Wong VW, Kamath PS, Loomba R. An Expert Consensus Delphi Panel in Metabolic Dysfunction- and Alcohol-associated Liver Disease: Opportunities and Challenges in Clinical Practice. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2025 Apr 30:():. pii: S1542-3565(25)00325-8. doi: 10.1016/j.cgh.2025.02.017. Epub 2025 Apr 30 [PubMed PMID: 40315973]
Level 3 (low-level) evidenceAguilera-Méndez A. [Nonalcoholic hepatic steatosis: a silent disease]. Revista medica del Instituto Mexicano del Seguro Social. 2019 Mar 15:56(6):544-549 [PubMed PMID: 30889343]
Hutchison AL, Tavaglione F, Romeo S, Charlton M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): Beyond insulin resistance. Journal of hepatology. 2023 Dec:79(6):1524-1541. doi: 10.1016/j.jhep.2023.08.030. Epub 2023 Sep 18 [PubMed PMID: 37730124]
Younossi ZM, Kalligeros M, Henry L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clinical and molecular hepatology. 2025 Feb:31(Suppl):S32-S50. doi: 10.3350/cmh.2024.0431. Epub 2024 Aug 19 [PubMed PMID: 39159948]
Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Digestive diseases (Basel, Switzerland). 2010:28(1):155-61. doi: 10.1159/000282080. Epub 2010 May 7 [PubMed PMID: 20460905]
Basaranoglu M, Neuschwander-Tetri BA. Nonalcoholic Fatty Liver Disease: Clinical Features and Pathogenesis. Gastroenterology & hepatology. 2006 Apr:2(4):282-291 [PubMed PMID: 28286458]
Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World journal of gastroenterology. 2010 Nov 14:16(42):5286-96 [PubMed PMID: 21072891]
Takahashi Y, Fukusato T. Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World journal of gastroenterology. 2014 Nov 14:20(42):15539-48. doi: 10.3748/wjg.v20.i42.15539. Epub [PubMed PMID: 25400438]
Level 3 (low-level) evidenceBrunt EM. Nonalcoholic Fatty Liver Disease: Pros and Cons of Histologic Systems of Evaluation. International journal of molecular sciences. 2016 Jan 13:17(1):. doi: 10.3390/ijms17010097. Epub 2016 Jan 13 [PubMed PMID: 26771611]
Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annual review of pathology. 2010:5():145-71. doi: 10.1146/annurev-pathol-121808-102132. Epub [PubMed PMID: 20078219]
Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology (Baltimore, Md.). 2012 Nov:56(5):1751-9. doi: 10.1002/hep.25889. Epub [PubMed PMID: 22707395]
Khoonsari M, Mohammad Hosseini Azar M, Ghavam R, Hatami K, Asobar M, Gholami A, Rajabi A, Safarnezhad Tameshkel F, Amirkalali B, Sohrabi M. Clinical Manifestations and Diagnosis of Nonalcoholic Fatty Liver Disease. Iranian journal of pathology. 2017 Spring:12(2):99-105 [PubMed PMID: 29515630]
Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Experimental diabetes research. 2012:2012():145754. doi: 10.1155/2012/145754. Epub 2011 Oct 27 [PubMed PMID: 22110476]
Schreiner AD, Zhang J, Bolus V, Marsden J, Bays C, Mohamed A, Moran WP, Mauldin PD, Gebregziabher M, Rockey DC. Diagnosis of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) at Risk for Advanced Fibrosis in a Real-World Primary Care Setting: A Cross-Sectional Study. Journal of general internal medicine. 2025 Jul 7:():. doi: 10.1007/s11606-025-09659-4. Epub 2025 Jul 7 [PubMed PMID: 40624322]
Level 2 (mid-level) evidenceKöylü B, Sökmensüer C, Karçaaltıncaba M, Keskin O. Predicting Fibrosis Stage in MASH: The Role of Total Metabolic Syndrome Score and MMP-1. Medicina (Kaunas, Lithuania). 2025 Jun 17:61(6):. doi: 10.3390/medicina61061102. Epub 2025 Jun 17 [PubMed PMID: 40572790]
van Son KC, de Jong JCBC, Özsezen S, Caspers MPM, Augustijn QJJ, Snabel J, Galenkamp H, Hamer HM, van Dijk AM, van Koppen A, Mak AL, van den Born BJ, Nieuwdorp M, Drenth JPH, Gluud LL, Tushuizen ME, Hanemaaijer R, Verschuren L, Holleboom AG. Prognostic Value of the TLM3 Biomarker Panel for Early Fibrosis Development in MASLD Within the General Population. Liver international : official journal of the International Association for the Study of the Liver. 2025 Jul:45(7):e70169. doi: 10.1111/liv.70169. Epub [PubMed PMID: 40552595]
Thallapureddy K, Twitchell D, Ott K, Pedicone LD, Owo C, Kumar N, Gelfond J, Shankar N, Goros M, Kwok D, Liles A, Ozguc F, Kazi I, Nguyen H, Lawitz E, Tsai E, Rodas F, Landaverde CE, Poordad F. The accuracy of FibroScan, FIB-4, and nonalcoholic fatty liver disease fibrosis score in predicting biopsy-defined fibrosis and steatosis across all fibrosis stages in patients with metabolic dysfunction associated steatotic liver disease. Medicine. 2025 Apr 25:104(17):e42016. doi: 10.1097/MD.0000000000042016. Epub [PubMed PMID: 40295262]
Zhang YX, Feng YP, You CL, Zhang LY. The diagnostic value of MRI-PDFF in hepatic steatosis of patients with metabolic dysfunction-associated steatotic liver disease: a systematic review and meta-analysis. BMC gastroenterology. 2025 Jul 1:25(1):451. doi: 10.1186/s12876-025-04017-4. Epub 2025 Jul 1 [PubMed PMID: 40596891]
Level 1 (high-level) evidenceImajo K, Saigusa Y, Kobayashi T, Nagai K, Nishida S, Kawamura N, Doi H, Iwaki M, Nogami A, Honda Y, Kessoku T, Ogawa Y, Kirikoshi H, Kokubu S, Utsunomiya D, Takahashi H, Aishima S, Sumida Y, Saito S, Yoneda M, Dennis A, Kin S, Andersson A, Nakajima A. Head-to-head comparison among FAST, MAST, and multiparametric MRI-based new score in diagnosing at-risk MASH. European radiology. 2025 Jun:35(6):3599-3609. doi: 10.1007/s00330-024-11215-3. Epub 2024 Dec 5 [PubMed PMID: 39638942]
Xiao T, Liu HH, Tian N, Lian LY, Miao KW, Li YT, Chen LL, Yuan HY, Du M, Wu S, Sun F, Targher G, Byrne CD, Shapiro MD, Lip GYH, Zhou XD, Li JJ, Zheng MH, WMU MAFLD Clinical Research Working Group. Association of Plasma Lipoprotein(a) With Major Adverse Cardiovascular Events in MASLD With or Without Advanced Liver Fibrosis. Liver international : official journal of the International Association for the Study of the Liver. 2025 Aug:45(8):e70208. doi: 10.1111/liv.70208. Epub [PubMed PMID: 40607654]
Ochoa-Allemant P, Hubbard RA, Kaplan DE, Serper M. Adverse Liver Outcomes, Cardiovascular Events, and Mortality in Steatotic Liver Disease. JAMA internal medicine. 2025 Aug 1:185(8):986-995. doi: 10.1001/jamainternmed.2025.1809. Epub [PubMed PMID: 40522656]
Ebrahimi F, Ebrahimi R, Hagström H, Sundström J, Sun J, Bergman D, Forss A, Ludvigsson JF. Risk of Major Adverse Cardiovascular Outcomes in Families With MASLD: A Population-Based Multigenerational Cohort Study. Circulation. Cardiovascular quality and outcomes. 2024 Nov:17(11):e010912. doi: 10.1161/CIRCOUTCOMES.124.010912. Epub 2024 Nov 6 [PubMed PMID: 39503614]
Level 2 (mid-level) evidenceCalès P, Canivet CM, Costentin C, Lannes A, Oberti F, Fouchard I, Hunault G, de Lédinghen V, Boursier J. A new generation of non-invasive tests of liver fibrosis with improved accuracy in MASLD. Journal of hepatology. 2025 May:82(5):794-804. doi: 10.1016/j.jhep.2024.11.049. Epub 2024 Dec 13 [PubMed PMID: 39674323]
Kogiso T, Ogasawara Y, Taniai M, Tokushige K, Nakai Y. Extrahepatic Events in Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease and the Impact of Genetics and Alcohol Intake. Hepatology research : the official journal of the Japan Society of Hepatology. 2025 Jul 1:():. doi: 10.1111/hepr.14233. Epub 2025 Jul 1 [PubMed PMID: 40590763]
Sato-Espinoza K, Valdivia-Herrera M, Chotiprasidhi P, Diaz-Ferrer J. Hepatocellular carcinoma in patients without cirrhosis. World journal of gastroenterology. 2025 Jun 21:31(23):107100. doi: 10.3748/wjg.v31.i23.107100. Epub [PubMed PMID: 40575339]
Chen VL, Morgan TR, Rotman Y, Patton HM, Cusi K, Kanwal F, Kim WR. Resmetirom therapy for metabolic dysfunction-associated steatotic liver disease: October 2024 updates to AASLD Practice Guidance. Hepatology (Baltimore, Md.). 2025 Jan 1:81(1):312-320. doi: 10.1097/HEP.0000000000001112. Epub 2024 Oct 18 [PubMed PMID: 39422487]
Hu Y, Sun C, Chen Y, Liu YD, Fan JG. Pipeline of New Drug Treatment for Non-alcoholic Fatty Liver Disease/Metabolic Dysfunction-associated Steatotic Liver Disease. Journal of clinical and translational hepatology. 2024 Sep 28:12(9):802-814. doi: 10.14218/JCTH.2024.00123. Epub 2024 Jul 31 [PubMed PMID: 39280073]
Sahebkar A, Eid AH. Hope on the Horizon: Promising Therapies for Steatotic Liver Disease. Pharmacological reviews. 2024 Jun 14:76(4):561-563. doi: 10.1124/pharmrev.124.001269. Epub 2024 Jun 14 [PubMed PMID: 38876495]
Tasabehji D, Saleh S, Mokadem M. Impact of Bariatric Surgery and Endoscopic Therapies on Liver Health in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review. Journal of clinical medicine. 2025 Jun 6:14(12):. doi: 10.3390/jcm14124012. Epub 2025 Jun 6 [PubMed PMID: 40565758]
Horn P, Tacke F. Key takeaways from the updated multidisciplinary European MASLD guidelines. eGastroenterology. 2025:3(2):e100196. doi: 10.1136/egastro-2025-100196. Epub 2025 Jun 8 [PubMed PMID: 40510733]
Hosseini Shabanan S, Martins VF, Wolfson T, Weeks JT, Ceriani L, Behling C, Chernyak V, El Kaffas A, Borhani AA, Han A, Wang K, Fowler KJ, Sirlin CB. MASLD: What We Have Learned and Where We Need to Go-A Call to Action. Radiographics : a review publication of the Radiological Society of North America, Inc. 2024 Nov:44(11):e240048. doi: 10.1148/rg.240048. Epub [PubMed PMID: 39418184]
Knezović E, Hefer M, Blažanović S, Petrović A, Tomičić V, Srb N, Kirner D, Smolić R, Smolić M. Drug Pipeline for MASLD: What Can Be Learned from the Successful Story of Resmetirom. Current issues in molecular biology. 2025 Feb 27:47(3):. doi: 10.3390/cimb47030154. Epub 2025 Feb 27 [PubMed PMID: 40136408]
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. The American journal of gastroenterology. 1999 Sep:94(9):2467-74 [PubMed PMID: 10484010]
Level 2 (mid-level) evidenceBrown GT, Kleiner DE. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism: clinical and experimental. 2016 Aug:65(8):1080-6. doi: 10.1016/j.metabol.2015.11.008. Epub 2015 Dec 2 [PubMed PMID: 26775559]
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). Journal of hepatology. 2024 Sep:81(3):492-542. doi: 10.1016/j.jhep.2024.04.031. Epub 2024 Jun 7 [PubMed PMID: 38851997]
Level 1 (high-level) evidenceMachado MV, Cortez-Pinto H. Non-alcoholic fatty liver disease: what the clinician needs to know. World journal of gastroenterology. 2014 Sep 28:20(36):12956-80. doi: 10.3748/wjg.v20.i36.12956. Epub [PubMed PMID: 25278691]
Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology (Baltimore, Md.). 2006 Oct:44(4):865-73 [PubMed PMID: 17006923]
Level 2 (mid-level) evidenceWhite DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012 Dec:10(12):1342-1359.e2. doi: 10.1016/j.cgh.2012.10.001. Epub 2012 Oct 4 [PubMed PMID: 23041539]
Level 1 (high-level) evidence