Introduction
Opsoclonus is a rare oculomotor dyskinesia characterized by rapid, repetitive, conjugate eye movements that are involuntary, arrhythmic, chaotic, and multidirectional (horizontal, vertical, and torsional) without intersaccadic intervals.[1][2] The movements appear most pronounced when the individual is awake and attempting fixation but persist during convergence, with closed eyelids, in darkness, and during sleep.[3][4] Visual blur and oscillopsia commonly result from the large amplitude and high frequency of the oscillations.
Opsoclonus differs from opsochoria, which involves dysconjugate eye movements, as well as ocular flutter, which is restricted to the horizontal plane. Unlike nystagmus, the phase moving the eye away from the target is always a saccade. When opsoclonus occurs with myoclonus or ataxia, encephalopathy, generalized tremor, or impaired cognition and behavioral changes, the presentation is classified as opsoclonus-myoclonus syndrome (OMS), also known as dancing eye and dancing feet syndrome.[5]
Often called "saccadomania," opsoclonus serves as a hallmark of central nervous system (CNS) dysfunction and frequently accompanies paraneoplastic, autoimmune, postinfectious, or toxic-metabolic disorders. Unlike typical nystagmus, which follows a rhythmic and directional pattern, opsoclonus consists of random, uncontrolled bursts of eye movement in all planes, making it highly specific for a neurological abnormality. Given this symptom's frequent association with systemic or neurological illness, early recognition and targeted investigations are crucial for identifying an underlying cause and initiating appropriate treatment.[6]
OMS is a rare but significant neuroinflammatory disorder in pediatric and adult populations. In children, neuroblastoma-associated OMS represents the most well-known form. In adults, paraneoplastic syndromes, viral encephalitis, and autoimmune encephalitis are frequent contributors. Dysfunction of the brainstem and cerebellar circuits, particularly the omnipause neurons of the pontine reticular formation, underlies the disorder, as these neurons normally suppress inappropriate saccadic activity. Damage to these inhibitory systems leads to disinhibition of the fastigial nucleus and burst neurons, causing excessive saccadic eye movements. This mechanism explains why opsoclonus often coexists with truncal ataxia, myoclonus, and cognitive dysfunction, all characteristic of OMS.[7]
Opsoclonus is an extremely rare neurological finding in pediatric and adult populations. The estimated incidence of OMS in children is 1 in 5 million per year, with most cases occurring between 12 months and 4 years of age. In pediatric patients manifesting with opsoclonus, neuroblastoma is identified in approximately 50% of cases, making this manifestation a paraneoplastic red flag that warrants immediate tumor screening. The early identification of opsoclonus has improved neuroblastoma detection, contributing to better survival rates and neurological outcomes and emphasizing the importance of timely diagnosis.[8]
In adults, the epidemiology varies, with paraneoplastic opsoclonus often linked to small cell lung cancer (SCLC), breast cancer, ovarian teratoma, and Hodgkin lymphoma. However, nonparaneoplastic cases are more prevalent, often occurring in postviral or postvaccination autoimmune encephalitis, toxic-metabolic syndromes, or idiopathic immune-mediated diseases. Opsoclonus rarely presents in isolation and frequently accompanies diffuse neurological dysfunction, necessitating a thorough interprofessional evaluation involving neurologists, ophthalmologists, oncologists, and immunologists.[9]
Dysfunction within the brainstem-cerebellar circuits underlies opsoclonus, particularly involving the fastigial nucleus, vestibulocerebellar tracts, and omnipause neurons in the pontine reticular formation. The fastigial nucleus plays a critical role in integrating ocular movements and posture. Lesions or autoimmune-mediated dysfunction affecting this structure disrupt saccadic control, leading to the characteristic disorganized bursts.
Omnipause neurons in the nucleus raphe interpositus normally suppress saccades when the eyes are at rest. Damage to these inhibitory neurons removes this suppression, generating uncontrolled saccadic activity. Paraneoplastic opsoclonus is believed to result from autoantibodies targeting neuronal antigens, triggering widespread neuroinflammation in the brainstem and cerebellum. Anti-Hu and anti-Ri antibodies have been implicated in many cases, particularly those associated with SCLC and breast cancer.
Opsoclonus arises from various pathological processes affecting the CNS, including immune-mediated, toxic-metabolic, and infectious mechanisms. As mentioned, this neurological sign may also occur as a paraneoplastic phenomenon associated with neuroblastoma in children and SCLC, breast cancer, ovarian cancer, and lymphoma, particularly Hodgkin lymphoma, in adults. A systematic approach to the differential diagnosis is essential for guiding the appropriate workup and treatment strategy.
Postinfectious and autoimmune etiologies have also been implicated. Viral encephalitis caused by West Nile virus, Epstein-Barr virus (EBV), Coxsackievirus, or COVID-19 has been reported as a trigger. Postvaccination immune-mediated encephalopathy and autoimmune encephalitis, including anti-N-methyl-D-aspartate receptor (anti-NMDA) encephalitis, anti-glutamic acid decarboxylase (anti-GAD) antibody-associated syndromes, and opsoclonus related to Sjögren syndrome, have also been described.
Toxic and metabolic disturbances contribute to opsoclonus as well. Lithium toxicity, serotonin syndrome, and metabolic encephalopathies, including those due to hepatic or uremic dysfunction or severe vitamin B12 deficiency, have been linked to opsoclonus development. Certain medications, such as phenytoin, amiodarone, and metronidazole, have been associated with cerebellar toxicity leading to this manifestation.[10]
Structural and degenerative disorders associated with opsoclonus include brainstem infarcts, multiple sclerosis, and autoimmune demyelinating syndromes. Neurodegenerative conditions such as ataxia-telangiectasia, Creutzfeldt-Jakob disease (CJD), and progressive supranuclear palsy (PSP) have also been implicated.
A comprehensive diagnostic approach is essential due to the strong association between opsoclonus and paraneoplastic or autoimmune syndromes. Brain and spine magnetic resonance imaging (MRI) is necessary to evaluate brainstem or cerebellar pathology and potential paraneoplastic syndromes. Paraneoplastic antibody testing, including anti-Hu, anti-Ri, and anti-Yo antibodies, aids in identifying autoantibody-mediated neurological conditions.
In pediatric cases, neuroblastoma screening should include abdominal ultrasound, urine catecholamines such as vanillylmandelic acid (VMA) and homovanillic acid (HVA), and imaging with MRI or metaiodobenzylguanidine (MIBG) scanning. Cerebrospinal fluid (CSF) analysis and autoimmune testing, including oligoclonal bands, anti-GAD antibodies, and NMDA receptor antibodies, help identify immune-mediated etiologies.[11]
Opsoclonus represents a rare but clinically significant neurological disorder that frequently signals an underlying malignancy or immune-mediated process. Chaotic, multidirectional saccadic eye movements serve as an important neurological red flag, prompting urgent evaluation and targeted management. Early recognition remains critical, as timely tumor detection, immunotherapy, and supportive care significantly improve outcomes. Ongoing research into paraneoplastic and autoimmune mechanisms continues to advance treatment strategies, with targeted immunomodulatory therapies offering hope for better disease control and improved long-term prognosis.[12]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Opsoclonus and OMS occur in various disease states, most commonly in paraneoplastic syndromes, systemic infections, or toxic, metabolic, and drug-induced conditions. In adults, the most frequently described paraneoplastic causes include SCLC, breast carcinoma, and ovarian teratoma, with less common associations such as non-Hodgkin lymphoma, melanoma, and renal adenocarcinoma. A study reviewing a large cohort of individuals with opsoclonus found a low probability of an underlying neoplasm, aside from ovarian teratoma, in those younger than 40. In children, paraneoplastic neuroblastic tumors, including neuroblastoma, ganglioneuroblastoma, and ganglioneuroma, represent the most common cause of opsoclonus and OMS, with over half of pediatric cases linked to neuroblastoma.
Opsoclonus in neuroblastoma correlates with a better prognosis. Other rare paraneoplastic causes in children include ovarian teratoma and hepatoblastoma. Parainfectious triggers include HIV or immune reconstitution following antiretroviral therapy initiation, Mycoplasma pneumoniae, Salmonella enterica, Rickettsia conorii, Streptococcus, Lyme disease, rotavirus, cytomegalovirus, human herpesvirus 6, and hepatitis C, West Nile, and varicella-zoster viruses.[13] Systemic diseases linked to opsoclonus include celiac disease, sarcoidosis, and multiple sclerosis.
Toxins such as chlordecone, organophosphates, strychnine, thallium, and toluene have been implicated. Medications, including amitriptyline, cocaine, lithium, and phenytoin with diazepam, have also been associated with opsoclonus. Despite extensive investigations, many cases remain without an identified cause, leading to a classification of idiopathic opsoclonus.
Opsoclonus results from dysfunction within brainstem and cerebellar circuits, specifically involving omnipause neurons in the pons and the fastigial nucleus of the cerebellum. Identifying the underlying cause remains essential, as early intervention in secondary opsoclonus can significantly improve neurological and functional outcomes.[14]
Paraneoplastic Opsoclonus (Neuroblastoma- and Cancer-Associated Syndromes)
Paraneoplastic opsoclonus results from an immune-mediated attack on neural tissues triggered by tumor-associated antigens. In pediatric cases, neuroblastoma represents the most common malignancy associated with opsoclonus. Approximately 50% to 60% of pediatric cases of OMS are linked to neuroblastoma. Opsoclonus often precedes neuroblastoma diagnosis. The pathogenesis involves autoimmune-mediated dysfunction of the cerebellum and brainstem due to tumor-directed anti-neuronal antibodies, including anti-neuronal nuclear antibody type 1 (anti-Hu) and anti-neuronal nuclear antibody type 2 (anti-Ri).
In adults, paraneoplastic opsoclonus occurs more frequently in older individuals, particularly those with SCLC, breast cancer, ovarian teratoma, or lymphoma, including Hodgkin and non-Hodgkin lymphoma.[15] The autoimmune mechanism involves anti-Hu and anti-Ri antibodies targeting neuronal structures in the brainstem and cerebellum, leading to opsoclonus.[16]
Any individual with unexplained opsoclonus should undergo malignancy screening, including computed tomography (CT) of the chest, abdomen, and pelvis, along with tumor marker evaluation. In children, neuroblastoma screening involves abdominal ultrasound, urine VMA and HVA testing, and MIBG scintigraphy.[17]
Autoimmune and Postinfectious Opsoclonus
Opsoclonus can result from an immune response triggered by viral infections, autoimmune diseases, or postvaccination reactions. These cases involve immune-mediated inflammation of the cerebellum and brainstem without direct neuronal invasion.
Viral encephalitis represents the most common infectious cause of postinfectious opsoclonus. Cases have been associated with Coxsackievirus, EBV, HIV, and West Nile, influenza A, and Zika viruses. Opsoclonus may emerge weeks after infection as a postviral autoimmune response.
Postvaccination opsoclonus is rare but has been reported following immunization with influenza, human papillomavirus, measles, and tetanus toxoid vaccines. Molecular mimicry is thought to underlie this phenomenon by triggering an immune-mediated attack on neuronal structures.[18]
Autoimmune Encephalitis and Central Nervous System Disorders
Opsoclonus can occur in autoimmune disorders, often alongside other neurological or systemic symptoms. Anti-NMDA receptor encephalitis may present with opsoclonus in addition to psychiatric symptoms, seizures, and dysautonomia. Anti-GAD autoimmune cerebellitis has been reported in both paraneoplastic and nonparaneoplastic cases. Opsoclonus has also been observed in demyelinating disorders such as multiple sclerosis and neuromyelitis optica when lesions affect the brainstem. In Sjögren syndrome and systemic lupus erythematosus (SLE), autoimmune vasculitis or neuroinflammation may involve the brainstem, leading to opsoclonus.[19]
CSF analysis, including oligoclonal bands and autoantibody panels, along with contrast-enhanced MRI, is essential in evaluating autoimmune-associated opsoclonus. These cases often respond well to corticosteroids, intravenous immunoglobulin (IVIG), or plasma exchange (PLEX).
Toxic and Metabolic Causes of Opsoclonus
Opsoclonus may also arise from toxic exposure, adverse drug reactions, or metabolic disturbances affecting cerebellar function. Drug-induced opsoclonus has been associated with lithium toxicity, commonly observed in individuals undergoing treatment for bipolar disorder. Serotonin syndrome, triggered by selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), or serotonin agonists, may also cause opsoclonus. Metronidazole is known for its effects on cerebellar function and has been implicated in some cases. Other medications linked to cerebellar toxicity and opsoclonus include amiodarone, phenytoin, and various anticonvulsants. Drug-induced opsoclonus typically resolves after discontinuation of the offending agent.
Metabolic and nutritional deficiencies can also contribute to opsoclonus. Severe vitamin B12 deficiency may result in opsoclonus due to subacute combined degeneration (SCD) affecting the spinal cord and brainstem. Uremic or hepatic encephalopathy can disrupt cerebellar function, leading to abnormal ocular movements. Thiamine (vitamin B1) deficiency, as seen in Wernicke encephalopathy, may present with opsoclonus alongside nystagmus and ataxia.[20]
Structural and Degenerative Causes
Opsoclonus may also result from structural brainstem or cerebellar lesions, as well as neurodegenerative conditions that disrupt brainstem circuits. Identifying these causes requires a brain MRI with contrast to assess for underlying pathology.
Structural brain lesions linked to opsoclonus include brainstem infarcts and strokes, which can impair neural pathways controlling eye movements. Cerebellar tumors, particularly medulloblastomas and metastatic lesions, may also contribute. Brainstem demyelination, commonly seen in multiple sclerosis, can affect the pathways involved in ocular coordination. Congenital or acquired structural abnormalities, such as Arnold-Chiari malformation and posterior fossa compression syndromes, have also been associated with opsoclonus.[21]
Neurodegenerative disorders affecting the brainstem and cerebellum can lead to opsoclonus as part of their disease course. PSP, characterized by midbrain atrophy and eye movement abnormalities, may present with opsoclonus in some cases. Ataxia-telangiectasia, a genetic disorder involving progressive cerebellar degeneration, rarely manifests with opsoclonus. Opsoclonus has been observed in rare cases of CJD, a prion disorder causing rapid neurodegeneration.[22]
Opsoclonus is a complex neuroophthalmic movement disorder with diverse underlying causes. Paraneoplastic opsoclonus, particularly in children with neuroblastoma, and postinfectious autoimmune opsoclonus represent the most common etiologies. However, toxic, metabolic, structural, and degenerative disorders must also be considered in the differential diagnosis. A systematic diagnostic approach, incorporating brain MRI, paraneoplastic antibody panels, autoimmune testing, neuroblastoma screening, and toxicology evaluation, is essential for identifying the underlying cause. Early recognition and targeted intervention—whether immunotherapy for autoimmune cases, tumor-directed therapy for paraneoplastic cases, or withdrawal of neurotoxic medications—can significantly improve outcomes.[23]
Epidemiology
Opsoclonus has an annual incidence of 1 in 5 million. Although rare, this neuroophthalmic movement disorder is clinically significant, affecting both pediatric and adult populations. The symptom's strong association with paraneoplastic syndromes, postviral encephalitis, and autoimmune diseases underscores its diagnostic importance. Epidemiology varies by age group, underlying cause, and geographical distribution, highlighting the need for detailed understanding to facilitate early recognition and appropriate management.[24]
Incidence and Prevalence
Opsoclonus is a rare neurological disorder, with prevalence and incidence estimates varying based on the underlying etiology. In pediatric cases, the incidence is approximately 1 in 5 million children per year. The condition most commonly affects those between 12 months and 4 years of age and is rarely observed after 6 years. Neuroblastoma accounts for 50% to 60% of pediatric cases, while 40% to 50% remain idiopathic or follow viral infections.
Opsoclonus is even less common in adults, though precise incidence rates are difficult to determine due to underreporting and misdiagnosis. The mean age of onset ranges from 40 to 60 years, with a slight female predominance. Paraneoplastic opsoclonus constitutes 20% to 50% of adult cases, whereas nonparaneoplastic causes, such as postviral and autoimmune encephalitis, are more frequently identified in this group.[25]
Age Distribution
Opsoclonus follows a distinct bimodal age distribution, with peak incidence occurring in young children and older adults. In children, the highest risk period is between 1 and 4 years of age, with neuroblastoma being the most common underlying cause. Paraneoplastic opsoclonus due to neuroblastoma is most frequently observed before 5 years of age, while postinfectious and autoimmune forms occur less frequently.
In adults, the condition typically presents between 40 and 60 years of age and arises from a broader range of etiologies, including paraneoplastic, autoimmune, toxic-metabolic, and neurodegenerative disorders. Paraneoplastic syndromes, particularly those associated with SCLC, are a significant concern. Autoimmune opsoclonus is more common in middle-aged adults, whereas neurodegenerative causes, such as multiple system atrophy (MSA), are more frequently observed in older individuals.
Sex Distribution
A mild female predominance is observed in adult opsoclonus cases, particularly in autoimmune-related and idiopathic forms. In contrast, pediatric cases show a slight male predominance, likely due to the higher incidence of neuroblastoma in boys.[26]
Geographic Distribution
Opsoclonus has been reported worldwide, with no significant racial or geographic predilection. However, variations arise due to differences in the prevalence of underlying causes. Neuroblastoma, the leading cause of pediatric opsoclonus, occurs more frequently in developed countries such as the United States and Europe, with incidence rates of 10 to 12 per million children annually, while lower rates are observed in sub-Saharan Africa and South Asia. In adults, paraneoplastic opsoclonus, particularly associated with SCLC, is more prevalent in regions with higher smoking rates, including North America, Europe, and East Asia.[27]
Opsoclonus and Cancer Associations
Paraneoplastic opsoclonus accounts for a significant proportion of cases, with distinct cancer associations in children and adults. In pediatric cases, neuroblastoma is responsible for 50% to 60% of occurrences, making it the most common malignancy linked to opsoclonus. These tumors are often small and remain undetected until opsoclonus manifests, serving as a critical paraneoplastic red flag. In adults, SCLC is the most frequent paraneoplastic cause, accounting for 20% to 40% of cases. Other malignancies associated with opsoclonus include breast cancer, ovarian teratomas, and lymphoma, including both Hodgkin and non-Hodgkin subtypes. Tumor-associated antineuronal antibodies, such as anti-Hu and anti-Ri, contribute to opsoclonus pathogenesis.
Postinfectious and Autoimmune Opsoclonus
Postviral opsoclonus accounts for 20% to 30% of pediatric cases and can occur in both children and adults. Common viral triggers include EBV, Coxsackievirus, HIV, COVID-19, and West Nile and influenza A viruses. Opsoclonus often emerges weeks after the initial infection, likely due to an immune-mediated neuroinflammatory response. Autoimmune opsoclonus has been linked to anti-NNMDA receptor encephalitis, Sjögren syndrome, SLE, and anti-GAD antibody syndromes. This neurologic manifestation is more frequently observed in middle-aged adults and typically responds well to immunotherapy, including IVIG, corticosteroids, and PLEX.
Prognosis Based on Etiology
The long-term outcome of opsoclonus depends on the underlying cause, age of onset, and treatment response. Paraneoplastic opsoclonus associated with neuroblastoma has a good prognosis if the tumor is treated early, although some individuals may experience residual cognitive impairment. In contrast, paraneoplastic opsoclonus linked to SCLC, breast cancer, or ovarian cancer carries a guarded prognosis due to the aggressive nature of these malignancies.
Postviral and autoimmune cases show variable outcomes. Many respond well to immunotherapy but remain at risk for relapse. Toxic-metabolic causes, such as drug reactions, vitamin B12 deficiency, uremia, or hepatic encephalopathy, generally have an excellent prognosis when the inciting condition is corrected. However, neurodegenerative disorders, including multiple sclerosis, PSP, CJD, and ataxia-telangiectasia, are associated with a poor prognosis due to the progressive nature of the underlying disease. Early diagnosis and intervention improve prognosis, particularly in paraneoplastic and autoimmune cases.
Key Epidemiological Insights
Opsoclonus is a rare but clinically significant neurological disorder with a bimodal age distribution. In children, neuroblastoma is the leading cause, accounting for 50% to 60% of cases and serving as a critical paraneoplastic red flag. In adults, SCLC is the most common paraneoplastic association, while autoimmune and postinfectious forms are more frequently observed than in pediatric cases. Geographic variations in incidence reflect differences in tumor prevalence and infectious exposures. Prognosis varies widely, with the most favorable outcomes seen in postviral and metabolic cases, whereas neurodegenerative causes carry the poorest prognosis.
Recognizing the epidemiology of opsoclonus is essential for early diagnosis and intervention. Advances in immunotherapy and neurooncology have improved outcomes, particularly in paraneoplastic and autoimmune cases. However, further research is needed to refine treatment strategies and better understand the long-term neurological impact of this disorder.
Pathophysiology
While the exact pathophysiology of opsoclonus remains unclear, 3 primary theories have been proposed. Given that the abnormal eye movements are saccadic in nature, the 1st theory suggests that damage to omnipause neurons in the pontine nucleus raphe interpositus may lead to saccadic intrusions. Omnipause neurons prevent unwanted saccades by inhibiting burst cell activity in the paramedian pontine reticular formation and the rostral interstitial nucleus of Cajal, which generate saccadic commands. Disruption of omnipause neuron function may result in uninhibited burst cell activity and subsequent involuntary ocular dyskinesia. However, no neuropathological evidence supports this hypothesis, and documented lesions in the pontine nucleus raphe interpositus have been associated with slow saccades rather than oscillatory saccades.
The 2nd theory, known as the brainstem theory, proposes that opsoclonus arises from altered synaptic membrane properties of burst cells, making them either excessively responsive to postinhibitory rebound excitation following omnipause neuron inhibition or unresponsive to effective omnipause neuron inhibition. Clinical evidence for this theory remains lacking, and such membrane changes would likely produce smaller amplitude saccadic oscillations than those observed in opsoclonus.
The 3rd theory, known as the cerebellar theory, proposes that dysfunction of cerebellar Purkinje cells leads to failure in inhibiting the fastigial nucleus, reinforcing omnipause neuron inhibition and causing unopposed burst cell oscillation, ultimately resulting in opsoclonus. Functional MRI findings of increased fastigial nucleus activation and single-photon emission computed tomography (SPECT) evidence of dysfunctional Purkinje cells in individuals with opsoclonus support this theory. Additionally, a heterozygous missense mutation with a large deletion in the potassium channel domain has been identified in cases of OMS with myoclonic epilepsy, further implicating the cerebellum in the pathophysiology of oscillatory saccades. Histopathological evidence of damage to afferent projections to the fastigial nucleus in one patient with opsoclonus provides additional support for this hypothesis.
Recent findings suggest that both humoral and cell-mediated immune mechanisms contribute to paraneoplastic and idiopathic opsoclonus. The clinical response to immunosuppressive therapy further supports an immunological pathogenesis. Humoral involvement in paraneoplastic opsoclonus is suggested by the presence of various antineuronal antibodies, including anti-Ri (strongly associated with breast cancer), anti-Yo, anti-Hu, anti-Ma1, anti-Ma2, anti-amphiphysin, anti-CRMP-5/anti-CV2, anti-Zic2, anti-neurofilament (NF210K antibody), anti-neuroleukin, anti-gliadin (immunoglobulin A and G subtypes), anti-endomysial antibodies, and anti-Purkinje cell antibodies.
The variability in the presence of these autoantibodies and the absence of identifiable antibodies in many cases make it unclear whether they play a direct role in opsoclonus pathogenesis or are merely an epiphenomenon associated with tumors. No definitive link has been established between these autoantibodies and the neurological abnormalities observed in OMS. The involvement of cell-mediated immunity has also been proposed due to the presence of lymphocytic pleocytosis in serum or CSF samples in some individuals with opsoclonus. Further support for this hypothesis comes from the clinical response to treatment with anti-CD20 monoclonal antibodies such as rituximab.
Histopathology
Opsoclonus primarily involves neuroinflammatory processes affecting the brainstem and cerebellum. Histopathological findings vary based on the underlying etiology, including paraneoplastic syndromes, autoimmune encephalitis, and viral infections. Examination of affected brain regions provides critical insights into disease mechanisms, particularly in cases linked to paraneoplastic OMS, viral encephalitis, and autoimmune-mediated cerebellitis.[28]
Key Histopathological Features of Opsoclonus
Opsoclonus predominantly affects the brainstem, cerebellum, and cortical regions, leading to inflammation, neuronal loss, and gliosis. Histopathological studies of postmortem and biopsy specimens reveal perivascular lymphocytic infiltration, particularly in autoimmune and paraneoplastic cases. The presence of T lymphocytes and plasma cells within the cerebellum, brainstem, and spinal cord suggests an immune-mediated pathogenesis. CD4+ and CD8+ T-cell infiltration is most pronounced in the fastigial nucleus, pontine tegmentum, and deep cerebellar white matter.[29]
Neuronal degeneration is another hallmark feature, with Purkinje cell loss in the cerebellar cortex contributing to ataxia and abnormal eye movements. Damage to omnipause neurons in the nucleus raphe interpositus disrupts saccadic inhibition, leading to uncontrolled ocular oscillations. Additionally, selective neuronal apoptosis in the fastigial nucleus, a structure critical for motor coordination, further implicates cerebellar involvement in opsoclonus.[30]
Demyelination and gliosis are commonly observed, particularly in autoimmune opsoclonus. Focal demyelination within the cerebellar white matter and brainstem reflects inflammatory-mediated neuronal injury. The presence of astrocytic gliosis and microglial activation in paraneoplastic cases suggests a chronic neuroinflammatory response, reinforcing the role of immune dysfunction in the pathogenesis of opsoclonus.[31]
Paraneoplastic Opsoclonus: Anti-Neuronal Autoantibody-Mediated Damage
In paraneoplastic opsoclonus, histopathological findings are associated with anti-neuronal antibodies such as anti-Hu, anti-Ri, anti-Yo, and anti-Ma2, which target neural structures.[32] These antibodies contribute to immune-mediated neuronal damage, particularly in the cerebellum and brainstem. In neuroblastoma-associated opsoclonus, Purkinje cells often appear shrunken or are completely lost, with T-cell infiltration further supporting an autoimmune mechanism. Paraneoplastic encephalitis extends beyond the cerebellum, affecting brainstem neurons and contributing to the disorder's complex neurological manifestations.[33]
Brainstem involvement is evident, particularly in the pons, where the loss of omnipause neurons leads to disinhibited burst neuron activity and chaotic saccades. Chronic lymphocytic infiltration of the inferior olivary nucleus and vestibulocerebellar pathways further correlates with the myoclonus component of OMS.[34]
Autoimmune and Postinfectious Opsoclonus: Neuroinflammation and Demyelination
Autoimmune-mediated opsoclonus presents with diffuse inflammatory infiltration, gliosis, and axonal damage in the cerebellum and brainstem.[35] Postviral cases, such as those linked to West Nile virus and EBV, exhibit perivascular cuffing with CD8+ T cells and microglial activation. Glial nodules and perivascular demyelination suggest secondary immune-mediated neurotoxicity. In some autoimmune cases, cerebellar white matter demyelination resembles multiple sclerosis-like plaques, with astrocytosis and perivascular gliosis indicating chronic neuroinflammation.[36]
Opsoclonus in Toxic and Metabolic Encephalopathy
Opsoclonus caused by toxic-metabolic conditions, such as lithium toxicity, hepatic encephalopathy, and vitamin B12 deficiency, presents distinct histopathological changes. In toxic opsoclonus, metronidazole-induced cerebellar dysfunction is associated with spongiform degeneration of Purkinje cells, axonal swelling, and myelin vacuolation, often leading to reversible deficits. Metabolic opsoclonus, as seen in vitamin B12 deficiency or hepatic encephalopathy, involves diffuse microvascular injury, edema, and hypoxic damage in the deep cerebellar nuclei, along with cerebellar granule cell loss due to chronic metabolic stress.[37]
Opsoclonus in Neurodegenerative Disorders
Opsoclonus can also occur in progressive neurodegenerative diseases, including PSP, CJD, and MSA. In PSP, tauopathy with neurofibrillary tangles is occasionally observed in the superior colliculus and pretectal nucleus. CJD may present with rapidly progressive opsoclonus, characterized by spongiform vacuolation in cerebellar Purkinje cells. In MSA, widespread α-synuclein deposition, gliosis, and neuronal loss in the brainstem and cerebellum can contribute to opsoclonus.[38]
Table. Summary of Key Histopathological Findings
A summary of the previously detailed histopathological findings across various causes of opsoclonus is provided in the table below.
|
Etiology |
Histopathological Features |
|
Paraneoplastic opsoclonus (neuroblastoma, SCLC, breast cancer, lymphoma) |
Perivascular lymphocytic infiltration, Purkinje cell degeneration, anti-neuronal antibodies targeting the cerebellum and brainstem. |
|
Autoimmune opsoclonus (anti-NMDA, anti-GAD, Sjögren’s, SLE) |
T-cell-mediated cerebellar inflammation, perivascular demyelination, astrocytosis. |
|
Postviral encephalitis (West Nile, EBV, influenza, COVID-19) |
Microglial activation, meningoencephalitis, CD8+ T-cell infiltration in the cerebellum. |
|
Toxic-metabolic (lithium, metronidazole, vitamin B12 deficiency, hepatic encephalopathy) |
Spongiform degeneration of Purkinje cells, axonal swelling, myelin vacuolation. |
|
Neurodegenerative (PSP, CJD, MSA) |
Tauopathy (PSP), prion-related spongiform vacuolation (CJD), α-synucleinopathy (MSA). |
Paraneoplastic and autoimmune opsoclonus are marked by prominent perivascular inflammation, while toxic-metabolic causes primarily involve Purkinje cell swelling and axonal degeneration. Neurodegenerative forms may feature tauopathies, synucleinopathies, or prion-related changes. These histopathological findings provide critical insights into opsoclonus pathogenesis, reinforcing its immune-mediated, paraneoplastic, and degenerative origins. Recognizing these microscopic patterns aids early diagnosis and guides targeted treatment, particularly in paraneoplastic and autoimmune cases where immunotherapy may alter disease progression.[39]
Toxicokinetics
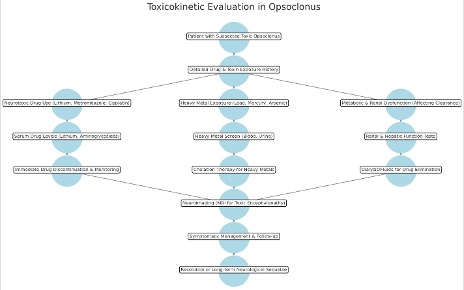
Toxicokinetics describes the absorption, distribution, metabolism, and excretion (ADME) of toxins or drugs implicated in opsoclonus. Understanding these properties is essential for diagnosing and managing drug-induced opsoclonus or toxic-metabolic encephalopathy while preventing recurrent toxicity (see Image. Toxicokinetics in Opsoclonus).
Absorption
Opsoclonus-inducing toxins enter the body through oral, intravenous, or inhalational routes. Systemic absorption of drugs like lithium, metronidazole, and neurotoxic chemotherapy agents can precipitate opsoclonus. Environmental toxins, including heavy metals (lead, mercury, arsenic) and organic solvents, may also induce neurotoxicity. Absorption varies based on chemical properties. Metronidazole and lithium exhibit high oral bioavailability (~90%–100%), while heavy metals and solvents penetrate mucosal and dermal barriers, leading to accumulation and delayed clearance.[40]
Distribution
Neurotoxic agents implicated in opsoclonus must cross the blood-brain barrier to affect the cerebellum and brainstem. Lipophilic drugs such as metronidazole, lithium, and benzodiazepines readily penetrate the blood-brain barrier, leading to direct neurotoxicity. In contrast, hydrophilic compounds like aminoglycosides and platinum-based chemotherapeutics accumulate more slowly in neural tissues, resulting in a delayed onset of symptoms. Lithium preferentially distributes in the cerebellum and brainstem, while metronidazole accumulates in the deep cerebellar and dentate nuclei, leading to reversible neurotoxicity. Heavy metals such as lead and mercury deposit in neurons, oligodendrocytes, and astrocytes, disrupting neurotransmission and contributing to persistent neurological dysfunction.[41]
Metabolism
Hepatic metabolism plays a critical role in determining the risk of drug-induced opsoclonus. Metronidazole undergoes cytochrome enzyme-mediated metabolism and its neurotoxic potential increases in individuals with hepatic dysfunction. Lithium, in contrast, is not metabolized but is excreted unchanged by the kidneys, making renal impairment a key factor in its toxic accumulation within the CNS. Platinum-based chemotherapeutics such as cisplatin and carboplatin contribute to opsoclonus through oxidative stress and mitochondrial dysfunction in Purkinje cells. Genetic variations further influence susceptibility, as cytochrome enzyme polymorphisms can alter drug metabolism, increasing the risk of neurotoxicity in predisposed individuals. Additionally, reduced glutathione conjugation capacity heightens vulnerability to heavy metal-induced opsoclonus.[42]
Excretion and Elimination
Renal excretion plays a crucial role in the clearance of opsoclonus-inducing toxins. Lithium is eliminated unchanged by the kidneys, with a half-life ranging from 18 to 36 hours. Nephrotoxic agents such as aminoglycosides and cisplatin can prolong lithium excretion, increasing the risk of chronic toxicity. Heavy metals are excreted primarily via the kidneys (lead, cadmium) or the biliary system (mercury, arsenic), but their slow clearance leads to cumulative neurotoxicity.
Metronidazole has a half-life of approximately 8 hours, yet its neurotoxic effects can persist for weeks after discontinuation. Lithium's half-life extends significantly in renal dysfunction, amplifying its potential for neurotoxicity.
Chelation therapy accelerates the removal of heavy metals, with agents such as ethylenediaminetetraacetic acid (EDTA), dimercaprol, and penicillamine facilitating excretion. Hemodialysis may be required in cases of severe lithium toxicity, while intravenous fluids and diuretics can aid in the renal clearance of neurotoxic metabolites.[43]
Mechanisms of Toxicity Leading to Opsoclonus
Opsoclonus caused by toxins and drugs arises from various neurotoxic mechanisms that disrupt cerebellar and brainstem function. Lithium alters calcium signaling and inhibits Purkinje cell activity, leading to impaired motor control. Metronidazole induces axonal swelling in cerebellar pathways and disrupts γ-aminobutyric acid (GABA) transmission, contributing to abnormal eye movements. Platinum-based chemotherapeutic agents such as cisplatin and carboplatin trigger oxidative stress, mitochondrial dysfunction, and Purkinje cell apoptosis. Aminoglycosides, including gentamicin and tobramycin, damage vestibulocerebellar pathways, resulting in opsoclonus and ataxia. Heavy metals such as lead, mercury, and arsenic interfere with neurotransmission, promote oxidative injury, and impair cerebellar function, further compounding neurotoxicity.
Understanding toxicokinetics is essential in drug- and toxin-induced opsoclonus, as it influences symptom onset, severity, and reversibility. The absorption, distribution, metabolism, and excretion properties of implicated agents guide targeted interventions, enhance elimination strategies, and help prevent recurrent toxicity. Close monitoring of drug levels, renal function, and neurotoxic symptoms is particularly important in patients receiving lithium, metronidazole, aminoglycosides, or chemotherapy. Early identification, withdrawal of neurotoxic agents, and supportive management are crucial for preventing permanent neurological damage.
History and Physical
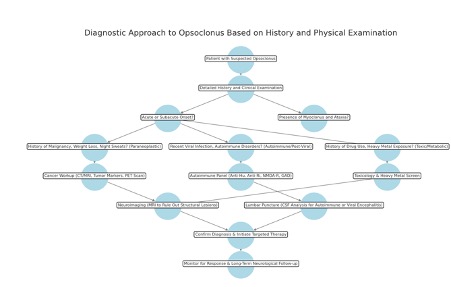
A thorough clinical history and physical examination are essential for identifying the underlying cause of opsoclonus (see Image. Diagnostic Approach in Opsoclonus). Opsoclonus often presents as part of OMS, which also encompasses ataxia, myoclonus, and cognitive or behavioral disturbances. A detailed history should evaluate patients for recent infections, toxin exposures, or malignancies. Reviewing eye movement recordings can aid in diagnosis by allowing for precise analysis of ocular motility. A comprehensive neuroophthalmological examination is necessary to evaluate for associated abnormalities and rule out alternative diagnoses.[44]
History-Taking
A detailed patient history is essential for identifying the underlying cause of opsoclonus, with a focus on neurological symptoms, systemic conditions, drug exposures, and environmental factors. The hallmark symptom, opsoclonus, presents as sudden-onset, chaotic, multidirectional saccadic eye movements that persist in all gaze positions and worsen with fixation attempts. Patients may also experience blurred vision and oscillopsia (visual instability). Additional motor symptoms include irregular myoclonic jerks of the limbs and trunk, gait instability, a broad-based ataxic stance, and dysmetria. Cognitive and behavioral changes such as irritability, sleep disturbances, altered consciousness, memory deficits, and speech difficulties may also occur, particularly in severe cases.[45]
Historical clues help narrow the differential diagnosis. In children younger than 3 years, opsoclonus is strongly associated with neuroblastoma, while in adults, it is linked to SCLC, breast cancer, or ovarian teratoma. A history of weight loss, night sweats, or a recent cancer diagnosis should raise suspicion for a paraneoplastic cause.
Autoimmune and postinfectious etiologies include autoimmune encephalitis associated with anti-Hu, anti-Ri, anti-Ma2, or anti-GAD antibodies, as well as postviral encephalitis following influenza, EBV, or COVID-19. Toxic and metabolic factors must also be considered, including medication-related neurotoxicity from lithium, metronidazole, aminoglycosides, or chemotherapy agents such as cisplatin and paclitaxel. Heavy metal exposure to lead, mercury, or arsenic can result in neurotoxicity, while metabolic disorders such as hepatic encephalopathy, vitamin B12 deficiency, and hypothyroidism may also contribute to the development of opsoclonus.[46]
Physical Examination
A comprehensive neurological examination is essential for assessing eye movements, cerebellar function, and systemic involvement in opsoclonus. The ocular assessment focuses on opsoclonus, characterized by rapid, random, multidirectional saccades without pauses. Unlike nystagmus, these movements persist with eye closure and do not suppress with fixation or pursuit. Pupillary responses remain normal unless brainstem pathology is present, and while convergence impairment may occur, the vestibuloocular reflex remains intact.[47]
Cerebellar and motor evaluations assess myoclonus and ataxia. Myoclonus manifests as irregular limb, trunk, or facial jerks that persist in wakefulness, often exaggerated by sudden stimuli. Ataxia features dysmetria on finger-nose and heel-knee-shin testing, truncal instability with a broad-based gait, and difficulty with rapid alternating movements. Hyperreflexia may be observed in autoimmune-related cases.[48]
A systemic evaluation helps identify underlying etiologies. Paraneoplastic syndromes may present with a palpable abdominal mass in children with neuroblastoma or Horner syndrome and lymphadenopathy in adults with SCLC or breast cancer. Infectious or autoimmune causes may be suggested by fever, meningeal signs, or skin rashes seen in conditions such as SLE or autoimmune encephalitis. Toxic and metabolic disorders should be suspected in the presence of jaundice and hepatomegaly, indicating hepatic encephalopathy, or tremors and peripheral neuropathy, suggestive of heavy metal toxicity.[49]
The physical examination provides critical clues for differentiating the underlying causes of opsoclonus. Distinguishing between paraneoplastic, autoimmune, and toxic-metabolic etiologies relies on the constellation of associated findings.
Opsoclonus is a persistent feature across all categories but may improve with immunotherapy in autoimmune cases or resolve following drug withdrawal in toxic-metabolic causes. Myoclonus tends to be severe with paraneoplastic syndromes, variable in autoimmune conditions, and milder but stimulus-sensitive in toxic-metabolic cases. Ataxia is prominent and progressive in paraneoplastic opsoclonus, moderate in autoimmune disorders, and typically mild and reversible in toxin-induced cases. Cognitive impairment ranges from mild to moderate in paraneoplastic opsoclonus, presents with severe encephalopathy in autoimmune conditions, and manifests as mild confusion that resolves with treatment in toxic-metabolic cases.
Additional systemic signs further aid differentiation. Paraneoplastic opsoclonus may present with an abdominal mass or lymphadenopathy, particularly in children with neuroblastoma or adults with SCLC. These findings are absent in autoimmune and toxic-metabolic cases. A history of drug or heavy metal exposure strongly suggests a toxic-metabolic cause. Opsoclonus persisting with eye closure and worsening with fixation is a key diagnostic clue distinguishing it from nystagmus or ocular flutter.
A thorough history should assess the onset of symptoms, cancer risk factors, recent infections, and potential exposure to neurotoxic drugs or environmental toxins. The physical examination confirms the presence of opsoclonus, myoclonus, and ataxia, while systemic findings help differentiate between paraneoplastic, autoimmune, and toxic etiologies. Early recognition of these features allows for targeted diagnostic investigations and timely intervention to address the underlying cause.[50]
Evaluation
Patients with opsoclonus or OMS require a comprehensive diagnostic evaluation. Neuroimaging of the entire neuroaxis with contrast and lumbar puncture should be performed first to exclude CNS disease. Once intracranial pathology is ruled out, further investigations should focus on identifying occult malignancy, as well as toxic, metabolic, and parainfectious causes. A CT scan of the chest, abdomen, and pelvis is recommended for all patients, with a positron emission tomography (PET) scan considered, particularly in those older than 40, if initial imaging is inconclusive. Although autoantibody testing has limited diagnostic utility, its detection supports a diagnosis of paraneoplastic opsoclonus or OMS. In women, mammography, a thorough gynecological evaluation, and anti-Ri antibody testing should be included.
In children, evaluation should prioritize the detection of neuroblastoma. Investigations should involve contrast-enhanced neuroaxis imaging and high-resolution CT of the chest, abdomen, and pelvis. Additional testing includes urine catecholamine analysis, specifically VMA and HVA levels, as well as an MIBG scan. Antibody screening should assess common markers such as anti-neurofilament (NF210K), anti-Purkinje cell, and immunoglobulin G autoantibodies. If initial investigations are negative, repeat evaluations should be conducted after a few months to detect occult tumors.
Clinical Assessment
A detailed history and neurological examination are essential to characterizing opsoclonus and identifying associated symptoms. The hallmark feature is rapid, chaotic, multidirectional saccadic eye movements that persist in all directions of gaze without an intersaccadic interval. Additional findings help refine the diagnosis: ataxia and myoclonus suggest OMS, while cognitive dysfunction and irritability are common in paraneoplastic and autoimmune cases. Systemic symptoms such as fever, weight loss, or night sweats may indicate an underlying malignancy or infection.
Differentiating opsoclonus from other ocular movement disorders is crucial. Unlike nystagmus, which is unidirectional and pauses between movements, opsoclonus is continuous and multidirectional. Ocular flutter, which also lacks pauses, is confined to horizontal movements. Another distinguishing feature is that opsoclonus persists in darkness and worsens with fixation, whereas nystagmus improves with fixation and disappears with eye closure. Recognizing these key differences facilitates accurate diagnosis and appropriate further investigation.[51]
Laboratory Investigations
Paraneoplastic and autoimmune screening involves testing serum and CSF for specific autoantibodies. Paraneoplastic opsoclonus is commonly associated with anti-Hu, anti-Ri, anti-Yo, and anti-Ma2 antibodies, while autoimmune forms may involve anti-NMDA, anti-GAD, and anti-amphiphysin antibodies. Anti-GQ1b antibodies suggest the Miller Fisher variant of Guillain-Barré syndrome. CSF analysis may reveal mild pleocytosis and oligoclonal bands in autoimmune encephalitis, while elevated protein levels point to an inflammatory or paraneoplastic process.[52]
Infectious causes should be explored through polymerase chain reaction (PCR) and serology testing for neurotropic viruses, including EBV, cytomegalovirus, COVID-19, and herpes simplex, West Nile, and influenza viruses. If bacterial infection is suspected, CSF Gram stain and culture are warranted.[53] Toxic and metabolic screening should include serum drug levels for lithium, metronidazole, and aminoglycosides, along with heavy metal testing for lead, mercury, and arsenic. Additionally, vitamin B12 and folate levels, as well as liver and kidney function tests, help assess for metabolic encephalopathy.[54]
Imaging Studies
Brain MRI, particularly T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences, can reveal cerebellar hyperintensities suggestive of autoimmune or paraneoplastic OMS. Brainstem involvement may indicate toxic-metabolic or paraneoplastic etiologies, while structural abnormalities such as demyelination, tumors, or infarcts must also be considered.[55]
When a paraneoplastic cause is suspected, whole-body PET-CT is recommended to detect occult malignancies. A CT scan of the chest, abdomen, and pelvis helps rule out neuroblastoma in children, as well as SCLC, breast cancer, or ovarian tumors in adults. In pediatric cases, imaging findings must be correlated with urinary catecholamine analysis, including VMA and HVA levels, to assess for neuroblastoma and assess the tumor burden.[56]
Electrophysiology
Electroencephalography (EEG) is typically normal in primary opsoclonus but may reveal epileptiform activity in cases associated with autoimmune encephalitis. Electromyography (EMG) and nerve conduction studies (NCS) can help evaluate peripheral nerve involvement in paraneoplastic myoclonus.[57]
Summary of Diagnostic Approach to Opsoclonus
Thus, the diagnostic approach to opsoclonus involves a thorough history and physical examination to assess for associated ataxia, myoclonus, and cognitive symptoms. Autoimmune testing includes serum and CSF analysis for antibodies such as anti-Hu, anti-Ri, anti-NMDA, and anti-GAD, along with CSF oligoclonal bands. Infectious workup requires PCR testing for neurotropic viruses, including EBV, COVID-19, and herpes simplex and West Nile viruses, as well as CSF analysis.
Toxic and metabolic evaluations assess for lithium, metronidazole, heavy metal exposure, vitamin B12 deficiency, and liver and renal dysfunction. Neuroimaging with brain MRI can reveal cerebellar hyperintensities, while PET-CT is critical for identifying paraneoplastic causes. Cancer screening includes chest and abdominal CT scans, with urine catecholamine analysis in children to detect neuroblastoma. Whole-body PET-CT is recommended for adults with unexplained opsoclonus to rule out occult malignancies. A systematic, multimodal approach facilitates early diagnosis and targeted intervention, particularly in autoimmune and paraneoplastic cases where timely immunotherapy can improve outcomes.
Treatment / Management
Effective management of opsoclonus or OMS requires identifying the underlying etiology, particularly in paraneoplastic cases. In cancer-associated opsoclonus, treatment should prioritize targeted cancer therapy, including surgery, chemotherapy, and radiation, with immunotherapy as an adjunct when indicated. Given the immune-mediated pathogenesis, corticosteroids such as oral prednisone or intravenous dexamethasone pulses, along with adrenocorticotropic hormone (ACTH), have demonstrated efficacy. Recent evidence supports the addition of IVIG to steroid therapy for improved response rates.
For severe or refractory cases, cyclophosphamide or rituximab may be combined with corticosteroids, ACTH, and IVIG. PLEX and other immunomodulatory therapies may be considered in resistant cases. Pediatric treatment follows a similar approach, primarily using corticosteroids, ACTH, and IVIG, with low-dose cyclophosphamide or rituximab reserved for unresponsive cases. Plasmapheresis may provide benefits in refractory pediatric cases.[58]
Managing OMS requires a comprehensive approach that includes immunosuppression, treatment of underlying causes—especially malignancies—and rehabilitation. An interprofessional team is essential for optimizing long-term outcomes.
First-Line Immunotherapy
First-line immunotherapy for opsoclonus or OMS primarily involves corticosteroids, IVIG, and, in select cases, ACTH. Corticosteroids, such as oral prednisone or intravenous methylprednisolone pulse therapy, serve as the initial treatment due to their potent anti-inflammatory effects. Methylprednisolone is typically administered at 20 to 30 mg/kg/day (up to 1 g/day) for 3 to 5 days, while prednisone is given at 1 to 2 mg/kg/day, followed by a gradual taper over several months. Close monitoring is necessary for potential adverse effects, including hyperglycemia, weight gain, behavioral changes, and osteoporosis.[59]
IVIG is frequently used in conjunction with corticosteroids or as a steroid-sparing alternative. Administered at 2 g/kg over 2 to 5 days, IVIG modulates the immune response and helps reduce symptom recurrence. Common side effects include headaches, infusion reactions, and an increased risk of thrombosis.[60]
Although less commonly employed, ACTH remains a treatment option, particularly in pediatric cases. Depot ACTH (repository corticotropin injection) provides prolonged anti-inflammatory effects, with dosing regimens such as 75 IU/m² administered intramuscularly every other day. While its use has declined in favor of corticosteroids and IVIG, ACTH may still be beneficial in select patients.[61]
Second-Line and Adjunctive Immunotherapy
Rituximab, a monoclonal antibody targeting CD20-positive B cells, is used in refractory or relapsing OMS and cases with inadequate response to steroids and IVIG. This agent is administered at 375 mg/m² intravenously once a week for 4 weeks. Patients receiving rituximab require monitoring for infection risk, delayed neutropenia, and infusion-related reactions.[62]
Cyclophosphamide, an alkylating agent, is reserved for severe, chronic, or steroid-dependent cases. This drug is given as monthly intravenous pulse therapy at 500 to 750 mg/m². The use of this drug is typically limited due to its potential long-term toxic effects, including infertility, hemorrhagic cystitis, and malignancy.[63]
Mycophenolate mofetil (MMF) is an immunosuppressant that serves as a steroid-sparing alternative. Administered at 600 to 1200 mg/m²/day in divided doses, MMF has a slower onset of action but offers a safer long-term profile compared to cyclophosphamide.[64](A1)
Treatment of Underlying Etiology
In pediatric cases, neuroblastoma accounts for 50% to 80% of paraneoplastic OMS. Management involves surgical resection of the tumor, with chemotherapy or radiotherapy as needed based on tumor staging. Even after oncologic control, continued immunotherapy is necessary to manage persistent OMS symptoms.
In adults, paraneoplastic OMS is commonly associated with SCLC, breast cancer, or ovarian tumors. Treatment focuses on tumor-directed therapy, including chemotherapy, surgery, or radiation, while concurrent immunotherapy is essential for addressing neurological symptoms.[65]
Symptomatic and Supportive Therapy
Clonazepam or valproate can help manage disabling myoclonus. Levetiracetam may be considered in refractory cases, especially when seizures are present. Behavioral and psychiatric symptoms, including mood instability, irritability, and attention deficits, are common. SSRIs or mood stabilizers can address emotional lability, while stimulants like methylphenidate may benefit school-aged children with attention difficulties.[66]
Rehabilitation and Long-Term Management
Physical therapy helps improve gait, balance, and coordination, reducing long-term motor disability.[67] Occupational therapy focuses on enhancing fine motor skills and supporting daily functional activities. Speech and language therapy is essential for children with regression or speech delays, addressing articulation, comprehension, and expressive language development.[68] Cognitive rehabilitation targets memory, attention, and executive function, often incorporating school-based interventions for optimal support.(A1)
Monitoring and Follow-Up
Relapse prevention involves gradual tapering of immunotherapy, regular neurology evaluations, and repeat imaging if tumor recurrence is suspected.[69] Monitoring for side effects includes regular assessments of complete blood count (CBC) and liver and renal function tests during immunosuppressive therapy, as well as bone density (DEXA) scans for patients on long-term steroids. For tumor surveillance in paraneoplastic cases, periodic MIBG scans, CT, or MRI should be performed every 6 to 12 months, particularly for pediatric neuroblastoma.
Early and aggressive immunotherapy significantly improves outcomes in patients with OMS. While some children recover fully, others may experience persistent cognitive or behavioral deficits. Refractory or relapsing OMS carries the risk of long-term disability and requires prolonged immunosuppression. A personalized, stepwise treatment plan that combines immunotherapy, tumor management, and interprofessional rehabilitation offers the best chance for long-term recovery and improved quality of life in OMS patients.
Differential Diagnosis
The differential diagnosis of opsoclonus involves several conditions, including neurologic, toxicologic, and metabolic disorders, which should be carefully considered based on clinical presentation and examination findings.
Neurologic causes include the following:
- Benign paroxysmal positional vertigo
- Cerebral vascular events
- Lateral medullary syndrome
- Meniere disease
- Multiple sclerosis
- Tumors in the brain
- Wernicke encephalopathy [70]
Toxicologic causes should also be ruled out, including the following:
- Alcohol intoxication
- Amphetamines
- Barbiturates
- Benzodiazepines
- Ketamine
- Lithium
- Phencyclidine
- Phenytoin
- Salicylates
- SSRI toxicity
- Thiamine deficiency [71]
Accurately identifying the underlying cause of this rare neurological symptom and differentiating it from similar clinical entities allow for tailored treatment strategies and improved patient outcomes.
Pertinent Studies and Ongoing Trials
Research on OMS remains limited due to its rarity. However, several key studies and ongoing clinical trials are focused on improving the understanding and treatment of this complex condition.[72]
Notable studies include the following:
- Treatment approaches in neuroblastoma-associated OMS: A significant study assessed the efficacy of prednisone combined with risk-adapted chemotherapy in patients with neuroblastoma-associated OMS. The trial also explored whether adding IVIG could enhance patient outcomes. The findings underscored the potential advantages of immunosuppressive therapies in managing OMS symptoms.
- Clinical and immunological features in adults: A comprehensive analysis of adult patients with idiopathic and paraneoplastic OMS provided valuable insights into their clinical presentations and immunological profiles. The study highlighted the importance of identifying neuronal cell surface antibodies, which contribute to a deeper understanding of the autoimmune mechanisms driving OMS.
- Pediatric OMS Registry: The establishment of the OMSLife Foundation registry represented a significant step in collecting demographic, clinical, and treatment data from pediatric OMS patients. This registry serves as a crucial resource for studying the natural history of OMS and evaluating the long-term outcomes of various therapeutic interventions.
Ongoing clinical trials and research initiatives include the following:
- Phase III Randomized Trial of IVIG in OMS: This randomized phase III trial examines the efficacy of combining cyclophosphamide, prednisone, and IVIG versus cyclophosphamide and prednisone alone in treating patients with neuroblastoma-associated OMS. The study seeks to determine whether the addition of IVIG can improve therapeutic outcomes.[73]
- European OMS Database and Treatment Protocol: A Europe-wide initiative is underway to gather comprehensive data on children diagnosed with OMS. The project aims to standardize treatment protocols and deepen the understanding of OMS's impact on quality of life. The treatment regimen being investigated includes dexamethasone, cyclophosphamide, and rituximab.
- International Pediatric OMS Research Network: An international collaborative effort has led to the creation of a pediatric-onset OMS registry and clinical research network. This initiative focuses on advancing research, refining diagnostic criteria, and optimizing treatment strategies for pediatric OMS patients.[74]
While OMS remains a rare and complex disorder, ongoing research and clinical trials are vital for understanding its pathophysiology and improving treatment options. Continued collaboration among international research networks and the development of comprehensive patient registries are crucial for advancing outcomes for individuals affected by OMS.
Treatment Planning
Treatment of opsoclonus requires a comprehensive, interprofessional approach that focuses on symptom management, immunomodulation, and addressing the underlying cause. The treatment plan varies depending on the etiology and patient-specific factors such as age, disease severity, and response to initial therapy. Immediate management begins with stabilizing the patient and controlling symptoms. Benzodiazepines like clonazepam or diazepam are used for myoclonus, while valproic acid or levetiracetam may be administered for more severe cases. Supportive care is crucial for managing ataxia and cognitive dysfunction.
In paraneoplastic opsoclonus, such as that associated with neuroblastoma or SCLS, tumor resection is paramount, followed by chemotherapy and radiation for oncological control. Immunotherapy, including high-dose corticosteroids (eg, methylprednisolone 20–30 mg/kg/day for 3–5 days or prednisone 1–2 mg/kg/day with gradual tapering), IVIG (2 g/kg over 2 to 5 days), intravenous rituximab (375 mg/m² weekly for 4 weeks), and intravenous cyclophosphamide (500 to 750 mg/m² monthly), may be required to manage immune-mediated aspects of the disease.[75]
For autoimmune or postinfectious opsoclonus, first-line treatment typically includes high-dose methylprednisolone (1 g/day for 3–5 days) and IVIG (2g/kg over 2-5 days). Second-line agents such as rituximab, cyclophosphamide, MMF (600–1200 mg/m²/day), or PLEX are considered for refractory cases.[76]
In toxic or metabolic opsoclonus, discontinuation of offending agents like lithium, metronidazole, and aminoglycosides is essential, alongside supportive care such as hydration and renal support for clearance. Chelation therapy (EDTA, dimercaprol, penicillamine) is indicated for heavy metal toxicity, while vitamin B12 supplementation is recommended for deficiency-related opsoclonus. Correction of hepatic or renal dysfunction is also important.
Adjunct therapies for symptom control include physical therapy to improve coordination and gait instability, as well as the use of adaptive devices such as walkers or orthotic support. Neurocognitive rehabilitation is crucial, with cognitive and speech therapy targeting memory and language deficits. Psychiatric and behavioral management may include SSRIs or atypical antipsychotics for mood disturbances.[77] Long-term monitoring includes regular neurological follow-up to assess opsoclonus severity, tumor recurrence monitoring in paraneoplastic cases, and evaluation of the immune therapy response.
Thus, the treatment of OMS is determined by its underlying cause, with approaches varying based on the etiology. In paraneoplastic cases, treatment involves tumor resection, chemotherapy, IVIG, and rituximab. Adjunctive therapies such as physical therapy and cognitive rehabilitation are essential for addressing motor and cognitive impairments.
For autoimmune causes, the primary treatment includes intravenous steroids, IVIG, and PLEX. In refractory cases, rituximab and MMF are used as second-line therapies. In cases caused by toxic or metabolic factors, the primary treatment involves drug withdrawal and chelation therapy for heavy metal toxicity. Vitamin replacement and supportive care, such as hydration, are crucial for addressing metabolic imbalances. Early intervention with immunotherapy has been shown to improve neurological outcomes in OMS significantly.[78]
Toxicity and Adverse Effect Management
The management of opsoclonus-related toxicity and adverse effects depends on the underlying etiology, treatment regimen, and potential medication side effects. Immunosuppressive therapy, chemotherapy (in paraneoplastic cases), and symptomatic treatments can lead to significant toxicities requiring careful monitoring and intervention.
Corticosteroids, commonly used in opsoclonus management, are associated with osteoporosis, hyperglycemia, hypertension, and psychosis. To mitigate these risks, patients should receive calcium and vitamin D supplements, bisphosphonates for bone protection, and regular blood glucose monitoring. IVIG therapy, though effective, can result in thromboembolism, renal dysfunction, and aseptic meningitis. Strategies to manage these effects include maintaining adequate hydration, slowing the infusion rate, and monitoring renal function.
Chemotherapy agents like cyclophosphamide may cause myelosuppression, hemorrhagic cystitis, and infertility. Monitoring CBC changes, coadministering mesna to protect the bladder, and fertility preservation strategies are essential. Rituximab can lead to infusion reactions, infections, and progressive multifocal leukoencephalopathy (PML). Premedication with antihistamines and steroids, along with regular infection surveillance, can minimize these risks. PLEX is another therapeutic option, but it can cause hypotension, hypocalcemia, and an increased risk of infection. Monitoring electrolytes and providing calcium supplementation are necessary during PLEX therapy.
Long-term toxicity monitoring is crucial for optimizing patient outcomes. Chronic use of steroids and immunosuppressants can lead to neurological toxicities such as cognitive decline, neuropathy, and psychiatric disturbances. Regular neurological evaluations and cognitive rehabilitation are recommended.[79] Hematological toxicities, including pancytopenia, can result from immunosuppressive agents like cyclophosphamide and rituximab. Regular CBC monitoring and infection prophylaxis are necessary to address these concerns.[80] Additionally, renal or hepatic toxicity is a risk with long-term use of IVIG, cyclophosphamide, and rituximab, making regular monitoring of renal and liver function essential.[81]
Risk mitigation strategies include performing baseline assessments such as CBC, renal and liver function tests, and bone density scans before initiating therapy. Frequent laboratory monitoring and appropriate dose adjustments based on adverse effects should be implemented. Preventive measures, including prophylactic antibiotics and antifungals for immunosuppressed patients, can reduce the risk of infections. Early recognition and intervention for adverse drug reactions are key to preventing further complications. Notably, prolonged immunosuppression increases the risk of opportunistic infections, so prophylactic antimicrobial therapy should be considered for high-risk patients.[82]
Staging
OMS lacks a universally accepted staging system, but disease progression may be categorized based on severity, duration, and response to treatment. Clinicians often classify OMS into stages to guide treatment strategies and prognosis. Disease severity may be categorized into 4 stages as follows:
- Mild (Stage 1): Patients experience occasional opsoclonus, mild ataxia, subtle myoclonus, and no cognitive impairment.
- Moderate (Stage 2): This stage is characterized by persistent opsoclonus, significant ataxia affecting walking, and mild behavioral or cognitive impairment.
- Severe (Stage 3): The symptoms become severe, with continuous opsoclonus, disabling ataxia that may require wheelchair use, and profound cognitive and speech regression.
- Refractory (Stage 4): This stage occurs when no response to standard immunotherapy is observed, neurological function worsens, and severe autonomic instability develops.
Early intervention with immunotherapy can prevent progression to severe or refractory OMS.
OMS may also be classified based on disease duration as follows:
- Acute (< 3 months): Typically presents with a sudden onset of opsoclonus, ataxia, and myoclonus; most cases are postinfectious or paraneoplastic.
- Chronic Relapsing (> 6 months): Characterized by persistent symptoms with periods of remission and relapse, frequently seen in autoimmune cases.
- Chronic Progressive OMS (> 1 year): Involves gradual worsening despite treatment and is often seen in paraneoplastic cases associated with neuroblastoma.
In response-based staging, patients are categorized by their response to therapy.
- Complete remission: Full resolution of opsoclonus, myoclonus, and cognitive deficits following immunotherapy
- Partial remission: Symptoms improve; residual ataxia and mild cognitive impairment are observed
- Relapsing: Opsoclonus and myoclonus recur despite previous treatment
- Treatment-resistant: No significant improvement despite using steroids, IVIG, or rituximab
For children with OMS secondary to neuroblastoma, severity correlates with tumor burden and immune response.
- Stage A: Mild neurological symptoms, small neuroblastoma, good response to steroids
- Stage B: Moderate symptoms, medium-sized neuroblastoma, requires aggressive immunotherapy
- Stage C: Severe manifestations, high-risk neuroblastoma, often refractory to treatment
Staging OMS helps stratify disease severity, tailor treatment approaches, and predict outcomes. Early immunotherapy initiation can improve prognosis, reducing long-term cognitive deficits and motor disabilities.[83]
Prognosis
Intensive immunosuppression and early remission are crucial for improving long-term neurological outcomes in OMS. Approximately 75% of patients, particularly children, will experience relapses, often tied to the timing of tapering immunosuppressive therapy. These relapses are associated with worse long-term outcomes, including significant developmental sequelae in children. Therefore, the appropriate duration of treatment and careful tapering is vital.
Idiopathic opsoclonus generally has a better prognosis than paraneoplastic opsoclonus, and in children, neuroblastoma with opsoclonus typically offers a better prognosis. All patients presenting with opsoclonus or OMS require thorough investigation to exclude occult tumors. Immunotherapy plays a key role in preventing relapse and worsening neurological outcomes.
The prognosis of OMS depends on the underlying cause, severity at diagnosis, treatment response, and the presence of long-term neurological impairments. Early diagnosis and prompt intervention are critical for functional recovery. Several prognostic factors influence the outcome, including age at onset, the underlying cause of the disorder, the severity at diagnosis, and the patient's response to immunotherapy.
In general, younger patients, particularly infants, tend to experience better recovery with treatment. Paraneoplastic cases, especially those associated with neuroblastoma, have a more variable prognosis, while idiopathic and autoimmune forms of OMS typically respond better to therapy. Mild cases often resolve with treatment, but severe cases may leave patients with residual motor and cognitive deficits. A positive early response to immunotherapy agents, such as steroids, IVIG, or rituximab, is associated with a more favorable prognosis. Frequent relapses correlate with long-term neurological impairment, and in neuroblastoma cases, smaller tumor sizes tend to indicate a better prognosis with a lower risk of OMS recurrence.
Long-term outcomes vary widely. A favorable prognosis is seen in 30% to 50% of patients who receive early diagnosis and aggressive immunotherapy, resulting in near-complete recovery. These patients may have mild residual ataxia or opsoclonus but maintain independent function.[84] In contrast, 50% to 70% of survivors exhibit mild to moderate cognitive or behavioral deficits, such as attention issues, speech delay, learning disabilities, and emotional instability. Some children may require long-term physical and occupational therapy.[85]
A poor prognosis is marked by persistent opsoclonus, ataxia, and myoclonus despite treatment, along with severe cognitive dysfunction, speech regression, and intellectual disability. This presentation is more common in neuroblastoma-associated OMS and may follow a chronic relapsing-remitting course, necessitating prolonged immunosuppressive therapy.[86]
The relapse rate for OMS is between 30% and 50%, especially if immunotherapy is prematurely discontinued. Long-term follow-up is essential to monitor for neurological deterioration and recurrence. Survivors of neuroblastoma-associated OMS require oncologic surveillance for tumor recurrence.
Prognostic classification based on treatment response helps determine the likely outcome. Patients with an excellent response experience complete neurological recovery with minimal long-term deficits. A good response results in persistent mild ataxia or learning difficulties but allows for independent daily function. Partial response may involve significant motor or cognitive impairments despite therapy, while refractory disease indicates no improvement with immunotherapy, resulting in severe long-term disability.
Early and aggressive immunotherapy with steroids, IVIG, and rituximab can prevent long-term neurological impairment in OMS. Although many patients improve with timely intervention, a subset will experience chronic neurological sequelae. Long-term interprofessional care involving neurologists, oncologists, physiotherapists, and neuropsychologists is essential for optimizing patient outcomes.[87]
Complications
Opsoclonus is an ocular dyskinesia with an elusive underlying humoral and cell-mediated immunopathogenesis. This manifestation can lead to falls caused by imbalance, resulting in head and musculoskeletal injuries. Treatment with immunosuppressive agents may also increase the risk of opportunistic infections. In children with neuroblastoma and other tumors, developmental delay may occur as a consequence of the syndrome.
Neurological complications of opsoclonus include chronic opsoclonus and myoclonus, where persistent involuntary eye movements and myoclonic jerks continue despite treatment. Ataxia and motor dysfunction can lead to progressive loss of coordination, affecting gait and daily activities. Some patients may develop seizures and epilepsy, particularly in cases of chronic relapsing OMS. Peripheral neuropathy can also occur, either due to immune-mediated damage or as a side effect of treatment.
Cognitive and behavioral complications are also common. Cognitive impairment, including memory, attention, and executive function deficits, is especially prevalent in pediatric cases. Speech and language delays may affect children, leading to challenges in academic performance. Psychiatric disorders, such as anxiety, depression, emotional instability, and symptoms resembling those of attention-deficit/hyperactivity disorder (ADHD), are observed in some individuals. Learning disabilities, particularly difficulties in processing and retaining new information, may further complicate the condition.
Treatment-related complications are associated with various therapeutic agents. Corticosteroids, such as prednisone and methylprednisolone, can lead to osteoporosis, hyperglycemia, and mood disturbances. IVIG is associated with risks such as thromboembolism, renal dysfunction, and aseptic meningitis. Cyclophosphamide can cause myelosuppression, infertility, and hemorrhagic cystitis, while rituximab may lead to infusion-related reactions and an increased risk of infection. PLEX can result in hypotension and electrolyte imbalances.
The long-term course of OMS may include a chronic relapsing pattern, with 30% to 50% of cases relapsing, leading to progressive neurological deterioration. In severe, untreated, or refractory cases, OMS may result in permanent motor and cognitive disabilities. For patients with neuroblastoma-associated OMS, ongoing cancer surveillance is necessary to prevent tumor recurrence. Early aggressive immunotherapy significantly reduces the risk of long-term complications and improves functional outcomes in OMS.
Managing the complications of OMS requires early diagnosis, aggressive immunotherapy, and long-term neurological rehabilitation. Interprofessional care involving neurologists, oncologists, physiotherapists, and neuropsychologists is essential for optimizing patient outcomes.[88]
Postoperative and Rehabilitation Care
Postoperative and rehabilitation care plays a critical role in enhancing functional recovery, reducing the risk of relapses, and optimizing neurological outcomes in patients with OMS. This care involves an interprofessional approach, including medical management, physical therapy, cognitive rehabilitation, and ongoing monitoring.
For immediate posttreatment care, monitoring for relapse is essential, especially for patients who receive steroids, IVIG, rituximab, or chemotherapy, as these treatments may cause neurological worsening or immune suppression-related complications. Treatment response is assessed by evaluating improvements in opsoclonus, ataxia, and cognitive dysfunction. Addressing acute side effects is also important, such as managing steroid-induced hyperglycemia and mood disturbances, IVIG-related headaches, and the risk of thrombosis, as well as rituximab infusion reactions.
Rehabilitation strategies focus on neuromotor and cognitive recovery. Physical therapy emphasizes balance training to address ataxia and strength-building exercises for myoclonic jerks. Occupational therapy aids with fine motor skills, such as writing and hand-eye coordination, and provides adaptive devices for patients with severe motor impairments.[89]
Cognitive and speech therapy are also crucial, with neurocognitive training aimed at improving attention deficits, memory recall, and executive function. Speech-language therapy addresses speech regression, dysarthria, or articulation difficulties, particularly in pediatric patients. Psychiatric and behavioral support is also a key component of rehabilitation, offering counseling and behavioral therapy to manage anxiety, attention deficits, and mood instability. Medications like SSRIs for depression and stimulants for attention deficits may also be prescribed.[90]
Long-term follow-up and monitoring involve several key areas. Neurologically, routine EEG and MRI of the brain are recommended if relapses occur. For patients with neuroblastoma, oncological surveillance through CT or MRI every 6 months helps monitor for tumor recurrence. Regular CBC and liver and kidney function tests are necessary for patients receiving immunosuppressive therapy. Children should undergo academic and behavioral assessments to track developmental milestones, especially for school-aged children.
Patient and caregiver education is critical for relapse prevention, emphasizing early recognition of recurring opsoclonus and myoclonus. Ensuring adherence to long-term immunotherapy, such as steroids and IVIG, is vital for treatment success. Connecting families with OMS advocacy organizations and support groups can also provide valuable emotional support. Comprehensive postoperative and rehabilitation care, coordinated by a team of neurologists, physiotherapists, psychologists, oncologists, and caregivers, is essential for optimizing long-term functional outcomes in people with a history of OMS.[91]
Consultations
OMS requires an interprofessional approach involving consultations with various specialists to address neurological, oncological, immunological, and rehabilitative needs. The neurology consultation serves as the primary specialty for the diagnosis and management of OMS. EEG, MRI of the brain, and lumbar puncture may be necessary if autoimmune OMS is suspected. This consultation determines the need for immunotherapy, including steroids, IVIG, rituximab, or cyclophosphamide.[92]
An oncology consultation is essential if paraneoplastic OMS is suspected, such as in cases involving neuroblastoma or SCLC. Neuroblastoma screening involves an MRI or CT, an MIBG scan, and urine catecholamines. The oncology team coordinates chemotherapy, radiation, or surgical resection if a tumor is detected.[93] For autoimmune-mediated OMS cases requiring long-term immunosuppression, an immunology or rheumatology consultation is critical. These specialists monitor the response to immunotherapy and manage potential side effects associated with treatments like cyclophosphamide, MMF, and rituximab.[94]
Physical and occupational therapy focuses on addressing ataxia, myoclonus, and fine motor skill deficits. Regimens include balance training, coordination exercises, and adaptive movement strategies to improve mobility and functional abilities.[95] Speech and cognitive therapy is necessary for patients experiencing speech regression or learning disabilities, which are common in pediatric OMS cases. This therapy includes language therapy, cognitive rehabilitation, and support for school accommodations to address learning challenges.[96]
Psychiatry and psychology consultations are vital for managing behavioral issues, ADHD-like symptoms, and mood instability. Cognitive behavioral therapy (CBT) and medications, such as SSRIs and stimulants, can help address neuropsychiatric symptoms.[97] Rehabilitation and social work support assist patients in accessing support groups, disability accommodations, and financial aid. These specialists provide guidance on long-term care and necessary home adaptations for optimal recovery.
Early and coordinated consultations significantly improve treatment outcomes, reduce relapses, and enhance long-term recovery for individuals with OMS. Integrated care from neurologists, oncologists, immunologists, and rehabilitation specialists ensures the best possible neurological and functional recovery.[98]
Deterrence and Patient Education
Opsoclonus represents an ocular dyskinesia with an elusive underlying humoral and cell-mediated immunopathogenesis. Extensive investigation is required for all patients presenting with opsoclonus or OMS to exclude the presence of occult tumors. Paraneoplastic opsoclonus in adults is most commonly due to SCLC, breast carcinoma, or ovarian cancer, while neuroblastoma remains the most common etiology in children. Immunotherapy plays a crucial role in preventing relapse and preventing worsening neurological outcomes. Educating patients and caregivers is essential for early recognition, management adherence, and improved quality of life in individuals with OMS. Given the rarity of OMS and its frequent misdiagnosis, educating both healthcare providers and families is critical in reducing treatment delays and improving prognosis.
Recognizing symptoms of OMS is essential for early detection. Patients and caregivers should be alert to rapid, uncontrolled eye movements (opsoclonus), ataxia, and myoclonic jerks. Unexplained movement disorders, developmental regression, or suspected paraneoplastic symptoms warrant urgent neurology and oncology evaluation.
Understanding the disease, including its link to paraneoplastic syndromes such as neuroblastoma, is key for patients and caregivers. Timely immunotherapy with steroids, IVIG, rituximab, or cyclophosphamide is essential to prevent permanent neurological damage. Long-term follow-ups with neurology, oncology (if applicable), and rehabilitation teams are necessary for monitoring and ongoing care.[99]
For relapse prevention, recognizing signs such as the return of opsoclonus, ataxia, or cognitive decline is vital. Strict compliance with immunotherapy regimens, such as steroids, IVIG, or other immunosuppressants, is crucial in reducing the risk of relapse. Additionally, lifestyle modifications such as adequate rest, stress management, and balanced nutrition can support recovery.
Support systems are also fundamental in managing OMS. Physical therapy helps address ataxia and movement coordination, while cognitive and speech therapy supports children with language, memory, and attention training. Mental health support, including psychiatric counseling, is necessary to address emotional instability, anxiety, or depression. Connecting with OMS support networks and advocacy groups provides valuable emotional and practical support for families navigating the disease's long-term impact. Educating caregivers and primary care physicians about OMS significantly improves early detection and reduces delays in life-saving treatment.[100]
Pearls and Other Issues
OMS is a rare immune-mediated disorder with widespread neurological and systemic effects. The condition's paraneoplastic association, relapsing course, and long-term cognitive impact necessitate an interprofessional approach for optimal management and improved patient outcomes. The following outline presents essential clinical pearls and key considerations in OMS care:
- Multisystem involvement: OMS affects neurological, cognitive, behavioral, and systemic immune functions. Early diagnosis and aggressive immunotherapy help prevent long-term neurological impairment.[101]
- Paraneoplastic association: In children, OMS is frequently associated with neuroblastoma (50%-80% of cases). All pediatric OMS cases should undergo neuroblastoma screening with an MRI or CT of the abdomen, an MIBG scan for neuroblastoma detection, and urine catecholamines (HVA and VMA). In adults, OMS may be linked to SCLC, breast cancer, or ovarian teratomas. Paraneoplastic antibody panels are useful in identifying occult malignancies.
- High relapse risk: About 30% to 50% of OMS cases relapse, especially when immunotherapy is tapered too quickly. Relapse triggers include incomplete treatment or premature discontinuation of steroids, infections or immune reactivation, and recurrence of underlying malignancy (eg, neuroblastoma, SCLC). Long-term follow-up with immunotherapy adjustments is crucial.[102]
- Cognitive and developmental impact: Up to 70% of pediatric OMS cases result in long-term neurodevelopmental deficits, even with aggressive treatment. Common cognitive complications include attention deficits (ADHD-like symptoms), memory impairment and executive dysfunction, speech and language delay, and emotional instability with anxiety disorders. Early intervention with cognitive therapy, speech-language therapy, and behavioral support improves functional outcomes.[103]
- Immunotherapy considerations: Prolonged steroid therapy can cause osteoporosis, requiring calcium and vitamin D supplementation; hyperglycemia, necessitating blood glucose monitoring in long-term use; and mood instability with weight gain, which may warrant behavioral interventions and dietary adjustments. IVIG therapy risks include aseptic meningitis, renal dysfunction, and thromboembolic events, requiring careful infusion monitoring. Rituximab increases infection risk and may cause progressive multifocal leukoencephalopathy, so screening for JC virus before initiation is recommended.[104]
- Diagnostic challenges: OMS is often misdiagnosed as ataxia (from cerebellar dysfunction due to infections or toxins), viral encephalitis (due to altered mental status and movement disorders), or seizure disorders (due to myoclonus). Early antibody testing (anti-Hu, anti-Ri, anti-NMDA receptor antibodies) and imaging (MRI, MIBG scan) are critical for accurate diagnosis.[105]
- Emerging therapies and future research: Biologic agents targeting B-cell and T-cell pathways are promising for steroid-sparing immunotherapy. Interleukin 6 inhibitors (tocilizumab) are under investigation for refractory OMS. Janus kinase (JAK) inhibitors targeting the immune cascade are being explored for chronic relapsing cases. Gene expression profiling may identify genetic susceptibility to autoimmune OMS and aid in personalized medicine approaches.[106]
- Long-term care and quality of life considerations: Interprofessional rehabilitation is essential for improving long-term function. Neurology follow-up includes regular monitoring for symptom recurrence. Oncology follow-up is required for tumor recurrence, with surveillance imaging every 6 to 12 months when paraneoplastic OMS is present. Physical therapy helps maintain balance and coordination. Speech and cognitive therapy address developmental delays. Psychiatric support manages anxiety, depression, and behavioral issues. OMS support groups provide psychosocial support for families and caregivers.[107]
Key Takeaways
OMS is a complex, relapsing autoimmune disorder that requires early recognition, aggressive immunotherapy, and long-term comprehensive care. Early and intensive immunotherapy improves outcomes and helps prevent cognitive impairment.
Given the strong association with neuroblastoma, all pediatric OMS patients should undergo thorough tumor screening. Despite treatment, relapse occurs in 30% to 50% of cases, necessitating long-term immunomodulatory therapy. Cognitive and behavioral impairments persist in 70% of affected individuals, highlighting the need for lifelong neurorehabilitation. Emerging biologic therapies, including interleukin 6 and Janus kinase inhibitors, offer potential options for refractory cases, paving the way for improved management strategies.
Enhancing Healthcare Team Outcomes
Effective OMS management requires a well-integrated healthcare team to ensure timely diagnosis, effective immunotherapy, and long-term rehabilitation. Individuals with opsoclonus may not fully recover, making rehabilitation a key component of care. Early involvement of therapists helps optimize functional outcomes. Interprofessional collaboration among specialists, including neurologists, oncologists, immunologists, physiotherapists, speech therapists, and mental health professionals, enhances patient-centered care.
Neurologists play a central role in diagnosing OMS, coordinating immunotherapy, and monitoring relapses. Oncologists screen for paraneoplastic causes such as neuroblastoma and SCLC, providing tumor-directed therapy when necessary. Immunologists oversee immune modulation therapy, including steroids, IVIG, rituximab, and other immunosuppressants. Neuroophthalmologists evaluate ocular manifestations.
Physical therapists help improve balance, coordination, and motor function, while speech and cognitive therapists address neurodevelopmental challenges, particularly in pediatric patients. Mental health professionals manage behavioral symptoms, mood disturbances, and ADHD-like features commonly associated with OMS. Social workers assist families with disability accommodations, educational needs, and psychological counseling.
To enhance care coordination, early collaboration among neurology, oncology, and immunology specialists is essential for timely diagnosis and treatment initiation. Standardized tumor screening protocols should be implemented across hospitals and clinics.[108] Regular interprofessional meetings facilitate shared decision-making, ensuring continuous monitoring of disease progression and therapy effectiveness. Electronic health records improve real-time communication between specialties.[109]
Developing evidence-based treatment pathways streamlines OMS management, while clinical checklists help monitor relapse risk, medication side effects, and rehabilitation progress.[110] Continuous medical education initiatives, such as training pediatricians, general practitioners, and emergency physicians on early OMS symptoms, help reduce diagnostic delays. Conferences and workshops keep healthcare teams updated on emerging immunotherapies and rehabilitation techniques.[111]
Engaging patients and families in care improves adherence to treatment and rehabilitation. Providing accessible educational materials helps caregivers understand OMS, manage treatment expectations, and implement home care strategies. Involving caregivers in daily therapy exercises and medication adherence strengthens long-term outcomes, while support groups and advocacy organizations offer psychosocial assistance.
A well-coordinated team improves OMS outcomes by enabling early diagnosis, timely immunotherapy, and relapse prevention. Physical and cognitive therapy enhances recovery, reducing long-term disability. Regular neurological and oncological follow-up minimizes recurrence risk, while comprehensive rehabilitation and mental health support improve overall quality of life.
Enhancing interprofessional collaboration in OMS management facilitates earlier diagnosis, improves treatment outcomes, and supports long-term recovery. By implementing standardized care pathways, ongoing education, and patient-centered strategies, healthcare teams can optimize care and enhance patient prognosis.[112]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Kilgo GR, Schwartze GM. Opsoclonus. Update on clinical and pathologic associations. Journal of clinical neuro-ophthalmology. 1984 Jun:4(2):109-13 [PubMed PMID: 6233319]
Level 3 (low-level) evidenceArmangué T, Sabater L, Torres-Vega E, Martínez-Hernández E, Ariño H, Petit-Pedrol M, Planagumà J, Bataller L, Dalmau J, Graus F. Clinical and Immunological Features of Opsoclonus-Myoclonus Syndrome in the Era of Neuronal Cell Surface Antibodies. JAMA neurology. 2016 Apr:73(4):417-24. doi: 10.1001/jamaneurol.2015.4607. Epub [PubMed PMID: 26856612]
Wong A. An update on opsoclonus. Current opinion in neurology. 2007 Feb:20(1):25-31 [PubMed PMID: 17215685]
Level 3 (low-level) evidenceJen JC, Lopez I, Baloh RW. Opsoclonus: clinical and immunological features. Journal of the neurological sciences. 2012 Sep 15:320(1-2):61-5. doi: 10.1016/j.jns.2012.06.017. Epub 2012 Jul 18 [PubMed PMID: 22818114]
Level 3 (low-level) evidenceOh SY, Kim JS, Dieterich M. Update on opsoclonus-myoclonus syndrome in adults. Journal of neurology. 2019 Jun:266(6):1541-1548. doi: 10.1007/s00415-018-9138-7. Epub 2018 Nov 27 [PubMed PMID: 30483882]
Auconi M, Papetti L, Ruscitto C, Ferilli MAN, Ursitti F, Sforza G, Vigevano F, Valeriani M. Opsoclonus-Myoclonus Syndrome in Children and Adolescents: A Therapeutic Challenge. Children (Basel, Switzerland). 2021 Oct 26:8(11):. doi: 10.3390/children8110965. Epub 2021 Oct 26 [PubMed PMID: 34828678]
Gorman MP. Update on diagnosis, treatment, and prognosis in opsoclonus-myoclonus-ataxia syndrome. Current opinion in pediatrics. 2010 Dec:22(6):745-50. doi: 10.1097/MOP.0b013e32833fde3f. Epub [PubMed PMID: 20871403]
Level 3 (low-level) evidenceYıldırım M, Öncel İ, Bektaş Ö, Tanalı G, Şahin S, Kutluk T, Teber S, Anlar B. Clinical features and outcomes of opsoclonus myoclonus ataxia syndrome. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2022 Nov:41():19-26. doi: 10.1016/j.ejpn.2022.09.002. Epub 2022 Sep 20 [PubMed PMID: 36155293]
Finsterer J, Scorza FA. Opsoclonus Myoclonus Ataxia Syndrome Due to SARS-CoV-2. Neuro-ophthalmology (Aeolus Press). 2023:47(1):1-6. doi: 10.1080/01658107.2022.2128378. Epub 2022 Dec 15 [PubMed PMID: 36798867]
Gerstle K, Siddiqui A, Schulte JJ, Cohn SL. Paraneoplastic opsoclonus myoclonus syndrome associated with inflammatory myofibroblastic tumor in a pediatric patient. Pediatric blood & cancer. 2020 Aug:67(8):e28218. doi: 10.1002/pbc.28218. Epub 2020 May 30 [PubMed PMID: 32472953]
de Silva RN, Vallortigara J, Greenfield J, Hunt B, Giunti P, Hadjivassiliou M. Diagnosis and management of progressive ataxia in adults. Practical neurology. 2019 Jun:19(3):196-207. doi: 10.1136/practneurol-2018-002096. Epub 2019 May 2 [PubMed PMID: 31048364]
Leuzinger-Dias C, Ferrão T, Rebimbas S, Palavra F, Amaral J. Opsoclonus-Myoclonus-Ataxia Syndrome: A Rare Outcome Following Routine Vaccinations. Cureus. 2024 Nov:16(11):e74413. doi: 10.7759/cureus.74413. Epub 2024 Nov 25 [PubMed PMID: 39723301]
Hero B, Schleiermacher G. Update on pediatric opsoclonus myoclonus syndrome. Neuropediatrics. 2013 Dec:44(6):324-9. doi: 10.1055/s-0033-1358604. Epub 2013 Nov 7 [PubMed PMID: 24203854]
Cannilla H, Messe M, Girardin F, Borruat FX, Bally JF. Drug- and Toxin-Induced Opsoclonus - a Systematized Review, including a Case Report on Amantadine-Induced Opsoclonus in Multiple System Atrophy. Tremor and other hyperkinetic movements (New York, N.Y.). 2024:14():23. doi: 10.5334/tohm.832. Epub 2024 May 8 [PubMed PMID: 38737300]
Level 3 (low-level) evidencePranzatelli MR, Slev PR, Tate ED, Travelstead AL, Colliver JA, Joseph SA. Cerebrospinal fluid oligoclonal bands in childhood opsoclonus-myoclonus. Pediatric neurology. 2011 Jul:45(1):27-33. doi: 10.1016/j.pediatrneurol.2011.02.012. Epub [PubMed PMID: 21723456]
Graus F, Ariño H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. 2014 May 22:123(21):3230-8. doi: 10.1182/blood-2014-03-537506. Epub 2014 Apr 4 [PubMed PMID: 24705493]
Chu CM, Rasalkar DD, Hu YJ, Cheng FW, Li CK, Chu WC. Clinical presentations and imaging findings of neuroblastoma beyond abdominal mass and a review of imaging algorithm. The British journal of radiology. 2011 Jan:84(997):81-91. doi: 10.1259/bjr/31861984. Epub [PubMed PMID: 21172969]
Emamikhah M, Babadi M, Mehrabani M, Jalili M, Pouranian M, Daraie P, Mohaghegh F, Aghavali S, Zaribafian M, Rohani M. Opsoclonus-myoclonus syndrome, a post-infectious neurologic complication of COVID-19: case series and review of literature. Journal of neurovirology. 2021 Feb:27(1):26-34. doi: 10.1007/s13365-020-00941-1. Epub 2021 Jan 25 [PubMed PMID: 33492608]
Level 2 (mid-level) evidenceLinnoila JJ, Rosenfeld MR, Dalmau J. Neuronal surface antibody-mediated autoimmune encephalitis. Seminars in neurology. 2014 Sep:34(4):458-66. doi: 10.1055/s-0034-1390394. Epub 2014 Nov 4 [PubMed PMID: 25369441]
Al-Jizani AS, Pathak S, Palit P, Achufusi N. Subacute Combined Degeneration of the Spinal Cord Caused by an Impairment in the Functional Vitamin B12 Metabolic Pathway. Cureus. 2024 Nov:16(11):e73617. doi: 10.7759/cureus.73617. Epub 2024 Nov 13 [PubMed PMID: 39552733]
Almudhry M, Wagner MW, Longoni G, Yea C, Vidarsson L, Ertl-Wagner B, Yeh EA. Brain Volumes in Opsoclonus-Myoclonus Ataxia Syndrome: A Longitudinal Study. Journal of child neurology. 2024 Mar:39(3-4):129-134. doi: 10.1177/08830738241240181. Epub 2024 Mar 27 [PubMed PMID: 38544431]
Dickson DW, Rademakers R, Hutton ML. Progressive supranuclear palsy: pathology and genetics. Brain pathology (Zurich, Switzerland). 2007 Jan:17(1):74-82 [PubMed PMID: 17493041]
Guedes ME, Almeida AC, Patricio MS, Costa JM. Opsoclonus-Myoclonus syndrome. BMJ case reports. 2011 Oct 28:2011():. doi: 10.1136/bcr.09.2011.4834. Epub 2011 Oct 28 [PubMed PMID: 22675112]
Level 3 (low-level) evidenceRossor T, Yeh EA, Khakoo Y, Angelini P, Hemingway C, Irani SR, Schleiermacher G, Santosh P, Lotze T, Dale RC, Deiva K, Hero B, Klein A, de Alarcon P, Gorman MP, Mitchell WG, Lim M, OMS Study Group. Diagnosis and Management of Opsoclonus-Myoclonus-Ataxia Syndrome in Children: An International Perspective. Neurology(R) neuroimmunology & neuroinflammation. 2022 May:9(3):. doi: 10.1212/NXI.0000000000001153. Epub 2022 Mar 8 [PubMed PMID: 35260471]
Level 3 (low-level) evidencePranzatelli MR, Tate ED, McGee NR. Demographic, Clinical, and Immunologic Features of 389 Children with Opsoclonus-Myoclonus Syndrome: A Cross-sectional Study. Frontiers in neurology. 2017:8():468. doi: 10.3389/fneur.2017.00468. Epub 2017 Sep 11 [PubMed PMID: 28959231]
Level 2 (mid-level) evidenceLino AMM, Spera RR, de Campos FPF, Freitas CHA, Garcia MRT, Lopes LDC, Prokopowitsch AS. Adult-onset opsoclonus-myoclonus-ataxia syndrome as a manifestation of brazilian lyme disease-like syndrome: a case report and review of literature. Autopsy & case reports. 2014 Jan-Mar:4(1):29-37. doi: 10.4322/acr.2014.005. Epub 2014 Mar 31 [PubMed PMID: 28652990]
Level 3 (low-level) evidencePranzatelli MR, Tate ED, McGee NR. Multifactorial analysis of opsoclonus-myoclonus syndrome etiology ("Tumor" vs. "No tumor") in a cohort of 356 US children. Pediatric blood & cancer. 2018 Aug:65(8):e27097. doi: 10.1002/pbc.27097. Epub 2018 May 4 [PubMed PMID: 29727049]
Tirthani E, Said MS, Smith RG, Jadhav N, Shanina E. Paraneoplastic Encephalomyelitis. StatPearls. 2025 Jan:(): [PubMed PMID: 33232100]
Tuchman RF, Alvarez LA, Kantrowitz AB, Moser FG, Llena J, Moshé SL. Opsoclonus-myoclonus syndrome: correlation of radiographic and pathological observations. Neuroradiology. 1989:31(3):250-2 [PubMed PMID: 2779775]
Chopra R, Shakkottai VG. Translating cerebellar Purkinje neuron physiology to progress in dominantly inherited ataxia. Future neurology. 2014 Mar 1:9(2):187-196 [PubMed PMID: 25221437]
Höftberger R, Lassmann H. Inflammatory demyelinating diseases of the central nervous system. Handbook of clinical neurology. 2017:145():263-283. doi: 10.1016/B978-0-12-802395-2.00019-5. Epub [PubMed PMID: 28987175]
Greenlee JE, Carlson NG, Abbatemarco JR, Herdlevær I, Clardy SL, Vedeler CA. Paraneoplastic and Other Autoimmune Encephalitides: Antineuronal Antibodies, T Lymphocytes, and Questions of Pathogenesis. Frontiers in neurology. 2021:12():744653. doi: 10.3389/fneur.2021.744653. Epub 2022 Jan 17 [PubMed PMID: 35111121]
Burcharth F, Castberg T, Vibe-Hansen H. Primary hyperparathyroidism. Acta chirurgica Scandinavica. Supplementum. 1973:433():169-77 [PubMed PMID: 4575143]
Averbuch-Heller L, Kori AA, Rottach KG, Dell'Osso LF, Remler BF, Leigh RJ. Dysfunction of pontine omnipause neurons causes impaired fixation: macrosaccadic oscillations with a unilateral pontine lesion. Neuro-ophthalmology (Aeolus Press). 1996 Apr:16(2):99-106 [PubMed PMID: 11539873]
Bhagavati S. Autoimmune Disorders of the Nervous System: Pathophysiology, Clinical Features, and Therapy. Frontiers in neurology. 2021:12():664664. doi: 10.3389/fneur.2021.664664. Epub 2021 Apr 14 [PubMed PMID: 33935958]
Radu RA, Terecoasă EO, Ene A, Băjenaru OA, Tiu C. Opsoclonus-Myoclonus Syndrome Associated With West-Nile Virus Infection: Case Report and Review of the Literature. Frontiers in neurology. 2018:9():864. doi: 10.3389/fneur.2018.00864. Epub 2018 Oct 16 [PubMed PMID: 30386288]
Level 3 (low-level) evidenceBimbato EM, Carvalho AG, Reis F. Toxic and metabolic encephalopathies: iconographic essay. Radiologia brasileira. 2015 Mar-Apr:48(2):121-5. doi: 10.1590/0100-3984.2013.1923. Epub [PubMed PMID: 25987753]
Ichikawa-Escamilla E, Velasco-Martínez RA, Adalid-Peralta L. Progressive Supranuclear Palsy Syndrome: An Overview. IBRO neuroscience reports. 2024 Jun:16():598-608. doi: 10.1016/j.ibneur.2024.04.008. Epub 2024 May 6 [PubMed PMID: 38800085]
Level 3 (low-level) evidenceMusunuru K, Kesari S. Paraneoplastic opsoclonus-myoclonus ataxia associated with non-small-cell lung carcinoma. Journal of neuro-oncology. 2008 Nov:90(2):213-6. doi: 10.1007/s11060-008-9650-1. Epub 2008 Jul 11 [PubMed PMID: 18618225]
Dixit R, Riviere J, Krishnan K, Andersen ME. Toxicokinetics and physiologically based toxicokinetics in toxicology and risk assessment. Journal of toxicology and environmental health. Part B, Critical reviews. 2003 Jan-Feb:6(1):1-40 [PubMed PMID: 12587252]
Bellettato CM, Scarpa M. Possible strategies to cross the blood-brain barrier. Italian journal of pediatrics. 2018 Nov 16:44(Suppl 2):131. doi: 10.1186/s13052-018-0563-0. Epub 2018 Nov 16 [PubMed PMID: 30442184]
Farrell G, Baird-Lambert J, Cvejic M, Buchanan N. Disposition and metabolism of metronidazole in patients with liver failure. Hepatology (Baltimore, Md.). 1984 Jul-Aug:4(4):722-6 [PubMed PMID: 6745863]
Mason RW, McQueen EG, Keary PJ, James NM. Pharmacokinetics of lithium: elimination half-time, renal clearance and apparent volume of distribution in schizophrenia. Clinical pharmacokinetics. 1978 May-Jun:3(3):241-6 [PubMed PMID: 657687]
Strupp M, Kremmyda O, Adamczyk C, Böttcher N, Muth C, Yip CW, Bremova T. Central ocular motor disorders, including gaze palsy and nystagmus. Journal of neurology. 2014 Sep:261 Suppl 2(Suppl 2):S542-58. doi: 10.1007/s00415-014-7385-9. Epub [PubMed PMID: 25145891]
Zhang X, Yan W, Song Y, Zhu H, Sun Y. Adult-onset idiopathic opsoclonus-myoclonus syndrome. Arquivos brasileiros de oftalmologia. 2023 Mar 24:87(4):. pii: S0004-27492023005002304. doi: 10.5935/0004-2749.2022-0024. Epub 2023 Mar 24 [PubMed PMID: 36995813]
Barahman M, Shamsaei G, Kashipazha D, Bahadoram M, Akade E. Paraneoplastic neurological syndromes of small cell lung cancer. Postepy psychiatrii neurologii. 2024 Jun:33(2):80-92. doi: 10.5114/ppn.2024.141157. Epub 2024 Jul 11 [PubMed PMID: 39119541]
Terao Y, Fukuda H, Hikosaka O. What do eye movements tell us about patients with neurological disorders? - An introduction to saccade recording in the clinical setting. Proceedings of the Japan Academy. Series B, Physical and biological sciences. 2017:93(10):772-801. doi: 10.2183/pjab.93.049. Epub [PubMed PMID: 29225306]
Rossi M, van der Veen S, Merello M, Tijssen MAJ, van de Warrenburg B. Myoclonus-Ataxia Syndromes: A Diagnostic Approach. Movement disorders clinical practice. 2021 Jan:8(1):9-24. doi: 10.1002/mdc3.13106. Epub 2020 Nov 3 [PubMed PMID: 33426154]
Alavi S. Paraneoplastic neurologic syndromes in children: a review article. Iranian journal of child neurology. 2013 Summer:7(3):6-14 [PubMed PMID: 24665300]
Altabakhi IW, Babiker HM, Lui F. Paraneoplastic Limbic Encephalitis. StatPearls. 2025 Jan:(): [PubMed PMID: 30137808]
Grossman SN, Rucker JC. Opsoclonus and ocular flutter: evaluation and management. Current opinion in ophthalmology. 2023 Nov 1:34(6):465-469. doi: 10.1097/ICU.0000000000000998. Epub 2023 Aug 21 [PubMed PMID: 37603546]
Level 3 (low-level) evidenceChiu D, Rhee J, Gonzalez Castro LN. Diagnosis and Treatment of Paraneoplastic Neurologic Syndromes. Antibodies (Basel, Switzerland). 2023 Jul 31:12(3):. doi: 10.3390/antib12030050. Epub 2023 Jul 31 [PubMed PMID: 37606434]
Benninger F, Steiner I. CSF in acute and chronic infectious diseases. Handbook of clinical neurology. 2017:146():187-206. doi: 10.1016/B978-0-12-804279-3.00012-5. Epub [PubMed PMID: 29110770]
Haidar Z, Fatema K, Shoily SS, Sajib AA. Disease-associated metabolic pathways affected by heavy metals and metalloid. Toxicology reports. 2023:10():554-570. doi: 10.1016/j.toxrep.2023.04.010. Epub 2023 Apr 24 [PubMed PMID: 37396849]
Shadmani G, Simkins TJ, Assadsangabi R, Apperson M, Hacein-Bey L, Raslan O, Ivanovic V. Autoimmune diseases of the brain, imaging and clinical review. The neuroradiology journal. 2022 Apr:35(2):152-169. doi: 10.1177/19714009211042879. Epub 2021 Sep 7 [PubMed PMID: 34490814]
Bresler R, Schroeder HW, Chow DZ, Lim R. (18)F-fluorodeoxyglucose positron emission tomography/ computed tomography in the diagnosis of suspected paraneoplastic syndromes: A retrospective analysis. World journal of nuclear medicine. 2020 Apr-Jun:19(2):124-130. doi: 10.4103/wjnm.WJNM_48_19. Epub 2020 Jan 17 [PubMed PMID: 32939199]
Level 2 (mid-level) evidenceGwinn KA, Caviness JN. Electrophysiological observations in idiopathic opsoclonus-myoclonus syndrome. Movement disorders : official journal of the Movement Disorder Society. 1997 May:12(3):438-42 [PubMed PMID: 9159744]
Coleman JJ, Ferner RE, Evans SJ. Monitoring for adverse drug reactions. British journal of clinical pharmacology. 2006 Apr:61(4):371-8 [PubMed PMID: 16542197]
Stingl C, Cardinale K, Van Mater H. An Update on the Treatment of Pediatric Autoimmune Encephalitis. Current treatment options in rheumatology. 2018 Mar:4(1):14-28. doi: 10.1007/s40674-018-0089-z. Epub 2018 Feb 17 [PubMed PMID: 29780690]
Dalakas MC. Update on Intravenous Immunoglobulin in Neurology: Modulating Neuro-autoimmunity, Evolving Factors on Efficacy and Dosing and Challenges on Stopping Chronic IVIg Therapy. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2021 Oct:18(4):2397-2418. doi: 10.1007/s13311-021-01108-4. Epub 2021 Nov 11 [PubMed PMID: 34766257]
Allen MJ, Sharma S. Physiology, Adrenocorticotropic Hormone (ACTH). StatPearls. 2025 Jan:(): [PubMed PMID: 29763207]
Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, Johnson P, Lister A, Feuring-Buske M, Radford JA, Capdeville R, Diehl V, Reyes F. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998 Sep 15:92(6):1927-32 [PubMed PMID: 9731049]
Gezgin Yildirim D, Orulluoglu EY, Yildiz C, Acari C, Dundar HA, Akaci O, Akinci N, Aliyev E, Alpman BN, Altug Gucenmez O, Arslanoglu Aydin E, Atmis B, Avar Aydin PO, Aydin F, Baba O, Baglan E, Bagrul I, Barut K, Basaran O, Bayrakci US, Belder N, Yucel BB, Buyukkaragoz B, Caglayan S, Cakan M, Celikel E, Demir F, Demir S, Demir Yigit Y, Demirkan FG, Dincel N, Dogantan S, Ekici Tekin Z, Genc E, Haslak F, Isguder R, Kara A, Kasap Cuceoglu M, Kaya Akca U, Kisaoglu H, Kisla Ekinci RM, Kızıldag Z, Kurt T, Kucukali B, Leventoglu E, Nalcacioglu H, Yener GO, Ozdel S, Ozdemir Atikel Y, Ozdemir Cicek S, Pektas Leblebiciler S, Serdaroglu E, Sonmez HE, Sunar Yayla EN, Surmeli Doven S, Sahin S, Sener S, Tanatar A, Tanidir M, Taskin SN, Tiryaki B, Tuncez S, Turkucar S, Uzun Kenan B, Yildiz N, Yilmaz K, Tabel Y, Dursun I, Canpolat N, Mir S, Peru H, Topaloglu R, Kaya Gurgoze M, Balat A, Bilginer Y, Celikel Acar B, Sozeri B, Unsal E, Kasapcopur O, Bakkaloglu SA. Cyclophosphamide treatment with a comparison in both pediatric rheumatology and pediatric nephrology practices. Pediatric rheumatology online journal. 2025 Mar 11:23(1):24. doi: 10.1186/s12969-025-01080-9. Epub 2025 Mar 11 [PubMed PMID: 40069880]
Ioannides D, Apalla Z, Lazaridou E, Rigopoulos D. Evaluation of mycophenolate mofetil as a steroid-sparing agent in pemphigus: a randomized, prospective study. Journal of the European Academy of Dermatology and Venereology : JEADV. 2012 Jul:26(7):855-60. doi: 10.1111/j.1468-3083.2011.04170.x. Epub 2011 Jul 14 [PubMed PMID: 21752101]
Level 1 (high-level) evidenceTolbert VP, Matthay KK. Neuroblastoma: clinical and biological approach to risk stratification and treatment. Cell and tissue research. 2018 May:372(2):195-209. doi: 10.1007/s00441-018-2821-2. Epub 2018 Mar 23 [PubMed PMID: 29572647]
Obeso JA, Artieda J, Rothwell JC, Day B, Thompson P, Marsden CD. The treatment of severe action myoclonus. Brain : a journal of neurology. 1989 Jun:112 ( Pt 3)():765-77 [PubMed PMID: 2499399]
Bland DC, Zampieri C, Damiano DL. Effectiveness of physical therapy for improving gait and balance in individuals with traumatic brain injury: a systematic review. Brain injury. 2011:25(7-8):664-79. doi: 10.3109/02699052.2011.576306. Epub 2011 May 11 [PubMed PMID: 21561297]
Level 1 (high-level) evidenceRinaldi S, Caselli MC, Cofelice V, D'Amico S, De Cagno AG, Della Corte G, Di Martino MV, Di Costanzo B, Levorato MC, Penge R, Rossetto T, Sansavini A, Vecchi S, Zoccolotti P. Efficacy of the Treatment of Developmental Language Disorder: A Systematic Review. Brain sciences. 2021 Mar 23:11(3):. doi: 10.3390/brainsci11030407. Epub 2021 Mar 23 [PubMed PMID: 33806938]
Level 1 (high-level) evidenceHuckans M, Hutson L, Twamley E, Jak A, Kaye J, Storzbach D. Efficacy of cognitive rehabilitation therapies for mild cognitive impairment (MCI) in older adults: working toward a theoretical model and evidence-based interventions. Neuropsychology review. 2013 Mar:23(1):63-80. doi: 10.1007/s11065-013-9230-9. Epub 2013 Mar 8 [PubMed PMID: 23471631]
Palmeri R, Kumar A. Benign Paroxysmal Positional Vertigo. StatPearls. 2025 Jan:(): [PubMed PMID: 29261987]
Baldaçara L, Pettersen AG, Leite VDS, Ismael F, Motta CP, Freitas RA, Fasanella NA, Pereira LA, Barros MEL, Barbosa L, Teles ALS, Palhano R, Guimaraes HP, Braga MA, Castaldelli-Maia JM, Bicca C, Gligliotti A, Marques ACPR, da Silva AG. Brazilian Psychiatric Association Consensus for the Management of Acute Intoxication: general management and specific interventions for drugs of abuse. Trends in psychiatry and psychotherapy. 2024:46():e20220571. doi: 10.47626/2237-6089-2022-0571. Epub 2022 Dec 2 [PubMed PMID: 36463505]
Level 3 (low-level) evidenceKoziorowska-Gawron E, Koszewicz M, Bladowska J, Ejma M, Budrewicz S. Opsoclonus-myoclonus syndrome with severe clinical course and beneficial outcome: A case report. Medicine. 2021 Apr 9:100(14):e25261. doi: 10.1097/MD.0000000000025261. Epub [PubMed PMID: 33832088]
Level 3 (low-level) evidencede Alarcon PA, Matthay KK, London WB, Naranjo A, Tenney SC, Panzer JA, Hogarty MD, Park JR, Maris JM, Cohn SL. Intravenous immunoglobulin with prednisone and risk-adapted chemotherapy for children with opsoclonus myoclonus ataxia syndrome associated with neuroblastoma (ANBL00P3): a randomised, open-label, phase 3 trial. The Lancet. Child & adolescent health. 2018 Jan:2(1):25-34. doi: 10.1016/S2352-4642(17)30130-X. Epub 2017 Nov 3 [PubMed PMID: 29376112]
Level 1 (high-level) evidenceKerr LM, Ryan ME, Lim M, Hearn S, Klein A, Deiva K, Hopkins SE, Bacchus MK, Sokol EA, Waanders AJ, Mitchell WG, Khakoo Y, Lotze TE, Zhang B, Gorman MP. An International Pediatric-Onset Opsoclonus-Myoclonus Ataxia Syndrome Registry and Clinical Research Network: Development, Progress, and Vision. Pediatric neurology. 2023 Nov:148():145-147. doi: 10.1016/j.pediatrneurol.2023.05.006. Epub 2023 May 16 [PubMed PMID: 37716108]
Ertle F, Behnisch W, Al Mulla NA, Bessisso M, Rating D, Mechtersheimer G, Hero B, Kulozik AE. Treatment of neuroblastoma-related opsoclonus-myoclonus-ataxia syndrome with high-dose dexamethasone pulses. Pediatric blood & cancer. 2008 Mar:50(3):683-7 [PubMed PMID: 17226843]
Adrichem ME, Bus SR, Wieske L, Mohammed H, Verhamme C, Hadden R, van Schaik IN, Eftimov F. Combined intravenous immunoglobulin and methylprednisolone as induction treatment in chronic inflammatory demyelinating polyneuropathy (OPTIC protocol): a prospective pilot study. European journal of neurology. 2020 Mar:27(3):506-513. doi: 10.1111/ene.14096. Epub 2019 Nov 7 [PubMed PMID: 31571349]
Level 3 (low-level) evidenceChien HF, Zonta MB, Chen J, Diaferia G, Viana CF, Teive HAG, Pedroso JL, Barsottini OGP. Rehabilitation in patients with cerebellar ataxias. Arquivos de neuro-psiquiatria. 2022 Mar:80(3):306-315. doi: 10.1590/0004-282X-ANP-2021-0065. Epub [PubMed PMID: 35239817]
Hsu M, Tejani I, Shah N, Olaosebikan R, Kumar A, Naik S. Review of Opsoclonus-Myoclonus Ataxia Syndrome in Pediatric Patients. Children (Basel, Switzerland). 2024 Mar 19:11(3):. doi: 10.3390/children11030367. Epub 2024 Mar 19 [PubMed PMID: 38539402]
Fischer S, von Bonin M, Bornhäuser M, Beste C, Ziemssen T. Neurological complications in oncology and their monitoring and management in clinical practice: a narrative review. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2024 Sep 25:32(10):685. doi: 10.1007/s00520-024-08894-5. Epub 2024 Sep 25 [PubMed PMID: 39317778]
Level 3 (low-level) evidenceKroll MH, Rojas-Hernandez C, Yee C. Hematologic complications of immune checkpoint inhibitors. Blood. 2022 Jun 23:139(25):3594-3604. doi: 10.1182/blood.2020009016. Epub [PubMed PMID: 34610113]
Kobayashi RH, Rigas MT. Immune globulin therapy and kidney disease: Overview and screening, monitoring, and management recommendations. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2022 Aug 19:79(17):1415-1423. doi: 10.1093/ajhp/zxac139. Epub [PubMed PMID: 35595720]
Level 3 (low-level) evidenceLiu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, Brown JP, Cohen A, Kim H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 2013 Aug 15:9(1):30. doi: 10.1186/1710-1492-9-30. Epub 2013 Aug 15 [PubMed PMID: 23947590]
Mizia-Malarz A, Stolpa W, Sobol-Milejska G. The Treatment of Opsoclonus-Myoclonus Syndrome Secondary to Neuroblastic Tumours-Single-Centre Experience and Literature Review. Medicina (Kaunas, Lithuania). 2020 Aug 14:56(8):. doi: 10.3390/medicina56080412. Epub 2020 Aug 14 [PubMed PMID: 32823831]
Galstyan A, Wilbur C, Selby K, Hukin J. Opsoclonus-Myoclonus Syndrome: A New Era of Improved Prognosis? Pediatric neurology. 2017 Jul:72():65-69. doi: 10.1016/j.pediatrneurol.2017.03.011. Epub 2017 Mar 27 [PubMed PMID: 28479124]
Marrus N, Hall L. Intellectual Disability and Language Disorder. Child and adolescent psychiatric clinics of North America. 2017 Jul:26(3):539-554. doi: 10.1016/j.chc.2017.03.001. Epub [PubMed PMID: 28577608]
Sun Q, Wang Y, Xie Y, Wu P, Li S, Zhao W. Long-term neurological outcomes of children with neuroblastoma with opsoclonus-myoclonus syndrome. Translational pediatrics. 2022 Mar:11(3):368-374. doi: 10.21037/tp-21-519. Epub [PubMed PMID: 35378965]
Sathian K, Buxbaum LJ, Cohen LG, Krakauer JW, Lang CE, Corbetta M, Fitzpatrick SM. Neurological principles and rehabilitation of action disorders: common clinical deficits. Neurorehabilitation and neural repair. 2011 Jun:25(5 Suppl):21S-32S. doi: 10.1177/1545968311410941. Epub [PubMed PMID: 21613535]
Wilken B, Baumann M, Bien CG, Hero B, Rostasy K, Hanefeld F. Chronic relapsing opsoclonus-myoclonus syndrome: combination of cyclophosphamide and dexamethasone pulses. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2008 Jan:12(1):51-5 [PubMed PMID: 17625938]
Aman JE, Elangovan N, Yeh IL, Konczak J. The effectiveness of proprioceptive training for improving motor function: a systematic review. Frontiers in human neuroscience. 2014:8():1075. doi: 10.3389/fnhum.2014.01075. Epub 2015 Jan 28 [PubMed PMID: 25674059]
Level 1 (high-level) evidencePasqualotto A, Mazzoni N, Bentenuto A, Mulè A, Benso F, Venuti P. Effects of Cognitive Training Programs on Executive Function in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. Brain sciences. 2021 Sep 27:11(10):. doi: 10.3390/brainsci11101280. Epub 2021 Sep 27 [PubMed PMID: 34679345]
Level 1 (high-level) evidenceCacciotti C, Lenzen A, Self C, Pillay-Smiley N. Recurrence Patterns and Surveillance Imaging in Pediatric Brain Tumor Survivors. Journal of pediatric hematology/oncology. 2024 Apr 1:46(3):e227-e232. doi: 10.1097/MPH.0000000000002850. Epub 2024 Mar 5 [PubMed PMID: 38447113]
Shindo K, Onohara A, Hata T, Kobayashi F, Nagasaka K, Nagasaka T, Takiyama Y. Opsoclonus-myoclonus syndrome associated with multiple system atrophy. Cerebellum & ataxias. 2014:1():15. doi: 10.1186/s40673-014-0015-6. Epub 2014 Nov 1 [PubMed PMID: 26331039]
Balamurugan S, Kaur K, Gurnani B, Agrawal A. Bilateral acute vision loss as the initial presentation of chronic myeloid leukemia in a young female. Indian journal of cancer. 2023 Oct 1:60(4):578-582. doi: 10.4103/ijc.ijc_573_21. Epub 2024 Jan 9 [PubMed PMID: 38206079]
Murugan SRB, Sanjay S, Somanath A, Mahendradas P, Patil A, Kaur K, Gurnani B. Artificial Intelligence in Uveitis: Innovations in Diagnosis and Therapeutic Strategies. Clinical ophthalmology (Auckland, N.Z.). 2024:18():3753-3766. doi: 10.2147/OPTH.S495307. Epub 2024 Dec 14 [PubMed PMID: 39703602]
Kaur K, Gurnani B. Revisiting color vision standards and testing methods in various occupational groups. Indian journal of ophthalmology. 2022 Jan:70(1):329-331. doi: 10.4103/ijo.IJO_1222_21. Epub [PubMed PMID: 34937273]
Burrow MK, Gurnani B, Patel BC. Keratoconjunctivitis. StatPearls. 2025 Jan:(): [PubMed PMID: 31194419]
Okoye GS, Bonabe D, Obasi CU, Munikrishna D, Osho F, Mutali M, Ogwumu K, Oke-Ifidon EO, Nathan IG, Enaholo ES, Suleman AI, Chukwuyem C, Enang AE, Oji RC, Ogechukwu VN, Chidera SP, Ogechukwu HC, Kaur K, Gurnani B. Visual outcomes and complications after phacoemulsification and small incision manual cataract surgery in two eye hospitals. Journal francais d'ophtalmologie. 2025 Jan:48(1):104353. doi: 10.1016/j.jfo.2024.104353. Epub 2024 Nov 18 [PubMed PMID: 39561679]
Velez M, Lugo-Agudelo LH, Patiño Lugo DF, Glenton C, Posada AM, Mesa Franco LF, Negrini S, Kiekens C, Spir Brunal MA, Roberg AB, Cruz Sarmiento KM. Factors that influence the provision of home-based rehabilitation services for people needing rehabilitation: a qualitative evidence synthesis. The Cochrane database of systematic reviews. 2023 Feb 10:2(2):CD014823. doi: 10.1002/14651858.CD014823. Epub 2023 Feb 10 [PubMed PMID: 36780267]
Level 1 (high-level) evidenceDuong SL, Prüss H. Paraneoplastic Autoimmune Neurological Syndromes and the Role of Immune Checkpoint Inhibitors. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2022 Apr:19(3):848-863. doi: 10.1007/s13311-022-01184-0. Epub 2022 Jan 18 [PubMed PMID: 35043373]
Hartley H, Cassidy E, Bunn L, Kumar R, Pizer B, Lane S, Carter B. Exercise and Physical Therapy Interventions for Children with Ataxia: A Systematic Review. Cerebellum (London, England). 2019 Oct:18(5):951-968. doi: 10.1007/s12311-019-01063-z. Epub [PubMed PMID: 31392562]
Level 1 (high-level) evidenceMoreira I, Vilas-Boas I, Cassiano Neves M. Paraneoplastic Opsoclonus-Myoclonus Syndrome as a Rare Presentation of Small-Cell Lung Cancer. Cureus. 2022 Nov:14(11):e32066. doi: 10.7759/cureus.32066. Epub 2022 Nov 30 [PubMed PMID: 36600862]
He F, Kessi M, Zhang C, Peng J, Yin F, Yang L. The utilization of the multimodal immunotherapy for the opsoclonus-myoclonus syndrome can reduce relapses and permanent neurological sequelae. Italian journal of pediatrics. 2025 Feb 7:51(1):33. doi: 10.1186/s13052-025-01875-2. Epub 2025 Feb 7 [PubMed PMID: 39920749]
Otterman DL, Koopman-Verhoeff ME, White TJ, Tiemeier H, Bolhuis K, Jansen PW. Executive functioning and neurodevelopmental disorders in early childhood: a prospective population-based study. Child and adolescent psychiatry and mental health. 2019:13():38. doi: 10.1186/s13034-019-0299-7. Epub 2019 Oct 22 [PubMed PMID: 31649749]
Ly S, Nedosekin D, Wong HK. Review of an Anti-CD20 Monoclonal Antibody for the Treatment of Autoimmune Diseases of the Skin. American journal of clinical dermatology. 2023 Mar:24(2):247-273. doi: 10.1007/s40257-022-00751-7. Epub 2023 Jan 11 [PubMed PMID: 36630066]
Venkatesh MH, Viswanathan S, Selvaraj J, Pillai V. Acute cerebellar ataxia and peripheral neuropathy due to an atypical infection. BMJ case reports. 2021 Mar 17:14(3):. doi: 10.1136/bcr-2021-242229. Epub 2021 Mar 17 [PubMed PMID: 33731395]
Level 3 (low-level) evidenceEmery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, Alecock E, Lee J, Kremer J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Annals of the rheumatic diseases. 2008 Nov:67(11):1516-23. doi: 10.1136/ard.2008.092932. Epub 2008 Jul 14 [PubMed PMID: 18625622]
Level 1 (high-level) evidenceHassan KA, Kalemkerian GP, Trobe JD. Long-term survival in paraneoplastic opsoclonus-myoclonus syndrome associated with small cell lung cancer. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2008 Mar:28(1):27-30. doi: 10.1097/WNO.0b013e318167550a. Epub [PubMed PMID: 18347455]
Rodriguez SA, Lee SC, Higashi RT, Chen PM, Eary RL, Sadeghi N, Santini N, Balasubramanian BA. Factors influencing implementation of a care coordination intervention for cancer survivors with multiple comorbidities in a safety-net system: an application of the Implementation Research Logic Model. Implementation science : IS. 2023 Dec 4:18(1):68. doi: 10.1186/s13012-023-01326-8. Epub 2023 Dec 4 [PubMed PMID: 38049844]
Rosen MA, DiazGranados D, Dietz AS, Benishek LE, Thompson D, Pronovost PJ, Weaver SJ. Teamwork in healthcare: Key discoveries enabling safer, high-quality care. The American psychologist. 2018 May-Jun:73(4):433-450. doi: 10.1037/amp0000298. Epub [PubMed PMID: 29792459]
Level 2 (mid-level) evidenceTenny S, Varacallo MA. Evidence-Based Medicine. StatPearls. 2025 Jan:(): [PubMed PMID: 29262040]
Kalnow A, Beck-Esmay J, Riddell J, Casey J, Carlson JN, Rezaie SR, Little A. Continuing Medical Education Delivery Preferences Among Physicians and Advanced Practice Providers in Emergency Medicine. Cureus. 2021 Dec:13(12):e20406. doi: 10.7759/cureus.20406. Epub 2021 Dec 14 [PubMed PMID: 35047249]
Level 3 (low-level) evidenceDukhanin V, Dy SM, Sharma R, Vass M, Zhang A, Bass EB, Rosen M. Patient and Family Engagement: Rapid Response. Making Healthcare Safer IV: A Continuous Updating of Patient Safety Harms and Practices. 2023 Jul:(): [PubMed PMID: 38011302]