Introduction
Vitamin D deficiency is the most common nutritional deficiency among children and adults. Osteomalacia describes a disorder of "bone softening" in adults that is usually due to prolonged vitamin D deficiency that can result in abnormal osteoid mineralization. In contrast, rickets, which usually occur at a young age, result from deficient mineralization at the cartilage of growth plates in children.
Bone comprises several cell types that participate in the coordinated process of bone remodeling. Osteoclasts, bone-resorbing cells, break down bone by secreting collagenase. Osteoblasts, the bone-forming cells, deposit the osteoid matrix, a collagen scaffold in which inorganic salts are deposited to form mineralized bone. This intricate process is directly and indirectly influenced by hormonal signals, namely parathyroid hormone (PTH) and calcitonin, which act in response to serum calcium levels.
In processes that decrease the amount of vitamin D or its bioproducts, normal serum calcium will be maintained by mobilizing calcium from the bones. Specifically, PTH will be secreted by the parathyroid glands in response to hypocalcemia from vitamin D deficiency in an attempt to restore calcium homeostasis. Bones are the primary site of calcium recruitment, and osteomalacia will ensue by extracting calcium from the bones. Therefore, adults affected by processes that disrupt vitamin D metabolism and production are at risk for eventually developing osteomalacia and its clinical manifestations.[1][2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Osteomalacia is a metabolic bone disease characterized by impaired mineralization of bone matrix.[4] Bone creation occurs by the deposition of hydroxyapatite crystals on the osteoid matrix. Details of the most common and sometimes overlooked causes are as follows:
Decreased Vitamin D Production
- Cold weather climates reduce skin sunlight exposure and cutaneous synthesis.
- Dark skin and relatively increased melanin compete with 7-dehydrocholesterol ultraviolet-B light absorption.
- Obesity can lead to increased adipose sequestration, which results in less calcidiol substrate available for activation.
- Vitamin D production decreases in older individuals, and the storage of vitamin D declines with age.
Decreased Vitamin D Absorption
- Nutritional deficiency can cause vitamin D deficiency even with adequate sunlight exposure.
- Malabsorptive syndromes such as Crohn disease, cystic fibrosis, celiac disease, cholestasis, and surgical alteration of the gastrointestinal tract, such as gastric bypass, are associated with deficient absorption of fat-soluble vitamins A, D, E, and K.
Altered Vitamin D Metabolism
- Chronic kidney disease leads to structural damage, loss of 1-alpha-hydroxylase, and suppressed enzymatic activity secondary to hyperphosphatemia.
- Nephrotic syndrome leads to pathologic excretion of vitamin D-binding protein, which binds to serum calcidiol.
- Liver disease, including cirrhosis and metabolic dysfunction-associated steatotic liver disease, leads to deficient production of calcidiol.
- Pregnancy is associated with decreased calcidiol levels, and the American College of Obstetricians and Gynecologists recommends treating with at least 1000 to 2000 international units daily when vitamin D deficiency is identified.
Hypophosphatemia or Hypocalcemia
- Renal tubular acidosis, such as in Fanconi syndrome, alters ion absorption and excretion.
- Multiple intravenous iron infusions have been found to cause hypophosphatemia and osteomalacia.[5]
- Tumor-induced osteomalacia, also known as oncogenic osteomalacia, is a rare acquired paraneoplastic disease characterized by hypophosphatemia, elevated or inappropriately normal levels of fibroblast growth factor 23, and renal phosphate wasting.[6][7][8][9]
- Tumor-induced osteomalacia is commonly caused by benign tumors involving the skin, muscles, bones of the extremities, or the paranasal sinuses.[10]
Medications
- Antiepileptic drugs, including phenobarbital, phenytoin, and carbamazepine, enhance the catabolism of calcidiol via induction of cytochrome P450 activity.[11]
- Isoniazid, rifampicin, and theophylline may also precipitate vitamin D deficiency in the same manner as antiepileptic medications.
- Antifungal agents such as ketoconazole increase vitamin D requirements by inhibiting 1-alpha-hydroxylase.
- Long-term corticosteroid use also has implications for vitamin D deficiency, possibly by increasing 24-hydroxylase activity.[12][13][14][15]
Epidemiology
The prevalence of postmortem histological osteomalacia histologically is as high as 25% in adult Europeans have been reported. However, the true global incidence of osteomalacia remains vastly underestimated.[11] At-risk individuals include those with dark skin, limited sun exposure, low socioeconomic status, poor diet, and frequent wearers of full-body clothing. These risks vary worldwide and are contingent on geographic location, cultural preferences, and ethnicity. Healthcare professionals should take these factors, as well as other relevant clinical findings, into account when choosing to obtain further studies or recommending vitamin D supplementation.[16][17]
Pathophysiology
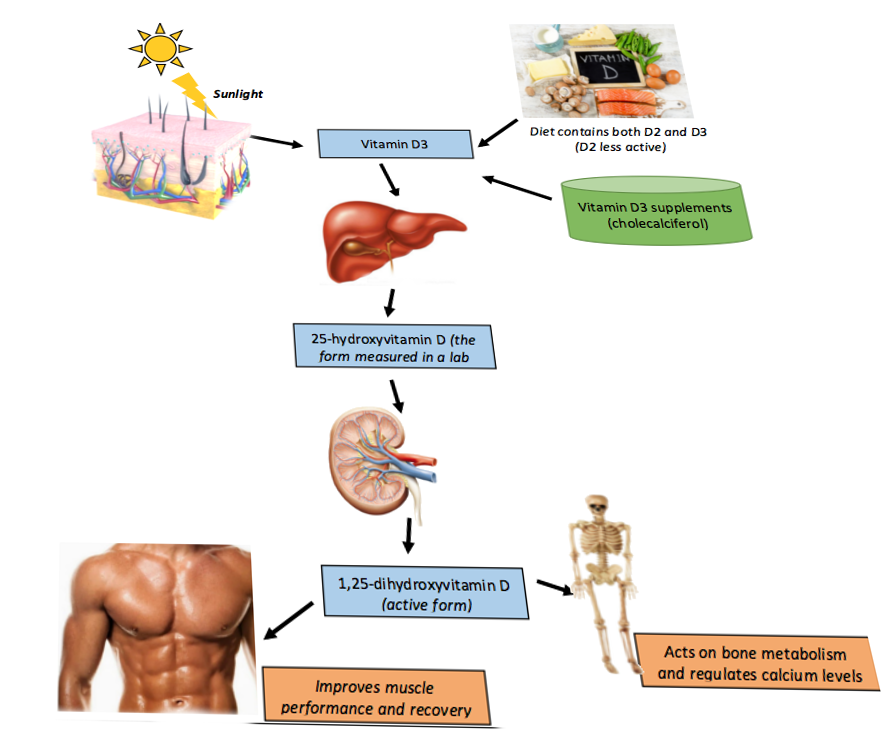
Reviewing vitamin D metabolism to understand the pathologic processes that result in vitamin D deficiency and its subsequent manifestations is essential (see Image. Vitamin D Metabolism). The synthesis of active vitamin D (calcitriol) organically begins in the skin, where cholecalciferol (vitamin D3) is formed by the action of ultraviolet B radiation in epidermal keratinocytes and dermal fibroblasts converting 7-dehydrocholesterol (provitamin D3) to pre-vitamin D, which spontaneously isomerizes to form cholecalciferol.
Subsequently, cholecalciferol is transported to the liver, where it is converted to calcidiol, 25-hydroxyvitamin D (25[OH]D), by 25-hydroxylase. Therefore, patients with chronic liver disease would have a higher risk of developing vitamin D deficiency. This particular form of vitamin D is partially water-soluble and has a short half-life. The vitamin 25(OH)D is also the best indicator of overall vitamin D status because this measurement accurately reflects total vitamin D levels from dietary intake, natural sunlight exposure, and converted adipose stores in the liver. The estimate is that approximately 40% to 50% of circulating 25(OH)D derives from skin conversion.[18]
Enzymatic conversion to calcitriol, 1,25(OH)D, occurs in the kidneys by 1-alpha-hydroxylase. Similarly, chronic renal disease, among other renal pathologies, can cause vitamin D deficiency, which is why secondary and, eventually, tertiary hyperparathyroidism can develop with long-term renal failure. The activity of 1-alpha-hydroxylase is strictly regulated.
As with any synthetic biologic process, feedback loops exist to regulate calcitriol production and include the following:
- Positive feedback by PTH
- Positive feedback by decreased serum phosphate levels
- Negative feedback by fibroblast growth factor 23, secreted by osteocytes in the bone matrix, ultimately inhibits renal phosphate absorption.[8][6]
- Negative feedback by calcitriol inhibition of 1-alpha-hydroxylase, which in turn decreases calcitriol synthesis and also stimulates 24-hydroxylase activity; 24-hydroxylase effectively removes circulating calcitriol by converting it to biologically inactive 24,25-dihydroxy vitamin D 24,25(OH)D.[19]
History and Physical
When evaluating for osteomalacia, the comprehensive medical history should include a review of the patient's family and surgical history. Other pertinent questions should focus on activity level, hobbies, dietary restrictions, and an assessment of socioeconomic status. Patients with osteomalacia typically experience symptoms such as diffuse bone pain, particularly in the lower back, pelvis, hips, and legs, aggravated by activity and weight-bearing. They may also have muscle weakness, leading to difficulty walking or climbing stairs and increased falls. Myalgias, arthralgias, fatigue, and general malaise are common, and in severe cases, patients may experience fractures or bone deformities with minimal trauma. These symptoms often progress gradually, making early diagnosis challenging.
Physical signs in patients with osteomalacia are nonspecific and may include the following:
- Proximal muscle weakness and wasting
- Diffuse bone tenderness, particularly in weight-bearing areas such as the lower back, pelvis, hips, and legs
- Muscle spasms
- Altered or "waddling" gait
- Bowed legs (genu varum)
- Spinal, lower extremities, or pelvic deformities (in long-term osteomalacia)
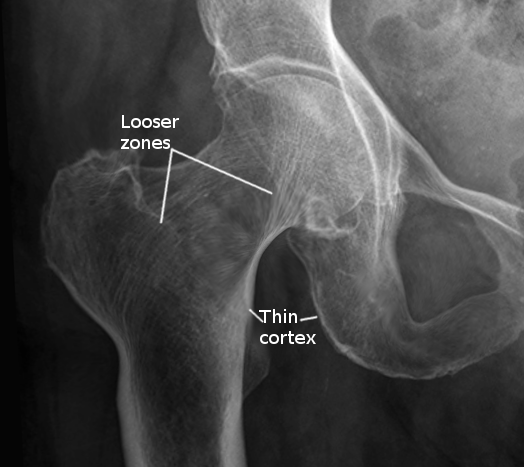
- Fractures or pseudofractures (Looser zones) with minimal trauma
- Positive Chvostek or Trousseau sign indicating associated hypocalcemia
- Hypocalcemic seizures or tetany [20]
Evaluation
No single laboratory finding is specific to osteomalacia. However, patients with osteomalacia typically have hypophosphatemia, hypocalcemia, or both. Additionally, increased alkaline phosphatase activity is typically characteristic of any disease with impaired osteoid mineralization. Some sources believe that hypophosphatemia or hypocalcemia and increased bone alkaline phosphatase levels are necessary to suspect osteomalacia. Low bone mineral density (BMD) and focal uptake at Looser zones can appear on plain x-rays and bone scintigraphy as the disease progresses. Fukumoto et al proposed the following findings of definite or possible osteomalacia, which require validation with further studies:
- Hypophosphatemia or hypocalcemia
- High bone alkaline phosphatase
- Muscle weakness or bone pain
- Less than 80% BMD of the young adult–mean
- Multiple uptake zones by bone scintigraphy or radiographic evidence of Looser zones (pseudofractures)[18][21]
Definite osteomalacia is defined as having all of the 5 above findings. Possible osteomalacia is defined as having the first 2 findings and 2 of the 3 remaining findings described above.
In another review by Uday and Hogler, new criteria were proposed for the diagnosis of osteomalacia, and include the following:
- Elevated PTH levels
- Elevated alkaline phosphatase levels
- Low urine calcium levels
- Low calcium intake (usually <300 mg/day) or low calcidiol levels (<30 nmol/L) [22]
The use of the above criteria can help with the diagnosis of osteomalacia only in cases where kidney or liver problems are lacking.
The serum level of 25(OH)D is currently regarded as the best marker of vitamin D status and is usually severely low (<10 ng/mL) in patients with nutritional osteomalacia. Other sensitive biomarkers of early calcium deprivation include increased serum PTH and decreased urinary calcium. Radiographic findings may include Looser zones or pseudofractures, a classic finding in osteomalacia. These may represent poorly repaired insufficiency fractures and are visible as transverse lucencies perpendicular to the osseous cortex; they typically occur bilaterally and symmetrically at the femoral necks, shafts, pubic and ischial rami.
Additionally, radiographs can show decreased distinctness of vertebral body trabeculae due to the inadequate mineralization of osteoid. Although not required for diagnosis, study results have demonstrated reduced BMD in the spine, hip, and forearm (see Image. Osteomalacia in Hip Joint, Radiograph). Iliac crest bone biopsy is considered the gold standard for establishing the diagnosis of osteomalacia. Due to this test's invasive and more aggressive nature, it should be reserved for when the diagnosis is in doubt, or the cause of osteomalacia is equivocal by any of the noninvasive methods.[21][23]
Treatment / Management
After establishing the diagnosis of osteomalacia, it is crucial to evaluate the etiology. Treatment should focus on reversing the underlying disorder, if possible, and correcting the vitamin D and other nutritional or electrolyte deficiencies. When the clinician has determined that vitamin D deficiency is the underlying cause of certain symptomatology, treatment may significantly improve strength and relieve bone tenderness within weeks. Serum calcium and urine calcium levels should be monitored, initially after 1 and 3 months, and after that, every 6 to 12 months until 24-hour urine calcium excretion is normal. Serum 25(OH)D level can be measured 3 to 4 months after starting therapy. If hypercalcemia or hypercalciuria is present, the dose can be adjusted to prevent excessive vitamin D dosing.
For patients with severe vitamin D deficiency, a possible dosing approach is as follows:
- 50,000 IU (international units) ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) orally 1 day per week for 8 to 12 weeks, followed by
- 800 IU to 2000 IU vitamin D3 daily for maintenance therapy
Ergocalciferol is present in plant sources and fortified nutritional alternatives. Cholecalciferol is usually in fish, meat, and eggs. When using vitamin D supplements, accumulating evidence favors the use of cholecalciferol over ergocalciferol because cholecalciferol's side chain has a higher affinity for vitamin D binding protein, thus conferring a longer half-life and a more potent ability to increase vitamin D levels. Since inadequate calcium intake may contribute to the development of osteomalacia, patients should also take at least 1000 mg of calcium per day while being treated for vitamin D deficiency. This dose may need to be increased in patients with malabsorption syndromes, who may also have increased vitamin D dosing requirements. Patients with liver and renal disease may be unable to utilize vitamin D2 or D3 effectively, so calcidiol or calcitriol should be considered.[24] Healing from osteomalacia is achieved when there are increases in urine calcium excretion as well as in BMD. Serum calcium and phosphate may normalize after a few weeks of treatment, but normalization of bone alkaline phosphatase usually lags, and those levels may stay elevated for months.[25]
Differential Diagnosis
Depending on the presenting symptoms, the initial differential diagnosis of osteomalacia can be extensive. Clinical history, physical examination, laboratory values, and imaging may be necessary to help narrow down the possibilities. However, specific diagnoses may present with similar symptoms and lab values and will require exclusion.
These conditions include metastatic disease, primary hyperparathyroidism, and renal osteodystrophy. Osteoblastic bone metastases have similar lab findings and may also show multiple zones of uptake by bone scintigraphy. Further evaluation may be warranted to exclude malignancy. Multiple myeloma can present with similar clinical symptoms (ie, bone pain and weakness) but will often reveal lytic lesions on radiographs. Patients with multiple myeloma may also have anemia and decreased renal function.
Primary hyperparathyroidism should present with hypophosphatemia, increased bone alkaline phosphatase, and increased zones of uptake. However, it usually presents with hypercalcemia, which is atypical in osteomalacia. In renal osteodystrophy, hyperphosphatemia, rather than hypophosphatemia, is most frequently observed.
Prognosis
Osteomalacia is a preventable metabolic bone disorder. Most cases are related to vitamin D deficiency, so they can usually be treated appropriately and even considered cured. If other clinical factors have contributed to the development of osteomalacia, then treatment will need to be tailored and adjusted as necessary depending on the other findings.
Once identified and an appropriate treatment plan is in place, laboratory values may begin to normalize within weeks of initiation. Symptom improvement is also appreciable in a similar period. Patients will require interval lab monitoring after starting therapy. Overall, complete healing of osteomalacia may vary and can take many months to over a year, depending on the initial cause.
Complications
Due to poor osteoid mineralization, several complications may occur if osteomalacia is left untreated. Insufficiency fractures, also known as Looser zones, can present as bone pain and occur with little or no trauma. They are typically bilateral, perpendicular to the cortex, and usually involve the femoral neck, pubic and ischial rami. Reports also exist of Looser zones in the ribs, scapulae, and clavicles.
Spinal compression fractures are less common and are usually associated with osteoporosis. Researchers have also reported kyphoscoliosis in patients with long-standing osteomalacia.[26]
Consultations
Whenever osteomalacia is suspected, the patient should be evaluated and treated by any specialty, from primary care and family medicine clinicians to endocrinologists or rheumatologists who may see the patient for other bone-related diseases. Radiology providers can help clarify the relevant findings in plain x-rays or any other imaging studies, while orthopedic surgeons should consider osteomalacia during the checkups of patients presenting with fractures. The collaboration among different medical professionals can help optimize the management of patients with diagnosed osteomalacia.
Deterrence and Patient Education
Deterrence and patient education are crucial in managing and preventing osteomalacia. Educating patients about the importance of adequate vitamin D, calcium, and phosphate intake is essential for bone health. Patients should be informed about dietary sources of these nutrients and the benefits of safe sun exposure to maintain optimal vitamin D levels. For those at risk, such as individuals with malabsorption syndromes or limited sun exposure, supplementation may be necessary. Patients should also be encouraged to engage in regular weight-bearing exercises to strengthen bones and prevent fractures. Educating patients about recognizing early symptoms, such as bone pain and muscle weakness, can lead to prompt medical attention and treatment, ultimately improving outcomes and preventing complications.
Certain populations are at greater risk for developing osteomalacia. Although insufficient data recommends obtaining serum 25(OH)D levels in asymptomatic patients, clinicians should be aware of the factors that may put their patients at risk, which include the following:
- Dark skin
- Decreased sunlight-to-skin exposure
- Diets deficient in vitamin D
- Medications that may precipitate vitamin D deficiency
- Obesity
- Older individuals
- Malabsorptive syndromes
- Renal or hepatic disease
Educating patients about these specific risks and, if possible, making helpful suggestions on how to make tolerable lifestyle changes is important. In patients who come from more conservative cultures, a vitamin D-deficient diet, and inadequate direct sunlight exposure may be overlooked by clinicians. Foods with the highest naturally occurring vitamin D content are usually meat- or fish-based. Since patients with vegetarian diets will not consume these foods, educating them on alternative sources of vitamin D-enriched nutrition is important. These food choices include fortified milk, yogurt, cheese, orange juice, bread, and ultraviolet B light-enhanced mushrooms. The biological significance of consuming these foods requires further assessment.[27][28]
Enhancing Healthcare Team Outcomes
Patient-centered care for individuals with osteomalacia necessitates an interprofessional approach involving physicians, advanced care practitioners, nurses, pharmacists, and other healthcare professionals. Medical professionals should possess the clinical skills to recognize and diagnose osteomalacia. These skills include understanding the clinical manifestations, interpreting relevant laboratory results (eg, vitamin D levels), and performing x-rays and bone density scans when needed. Clinicians should be skilled in designing and implementing patient-specific treatment plans, such as prescribing appropriate vitamin D supplementation, recommending dietary adjustments, and managing underlying conditions contributing to osteomalacia.
A patient-centered approach involves tailoring care to individual needs, preferences, and values. Healthcare professionals should engage patients in shared decision-making, ensuring they understand their condition and treatment options. Interprofessional teams should follow evidence-based guidelines and stay updated with the latest research to provide the best care for osteomalacia patients. The key to achieving successful results is patient education; thus, all team members have a vital role in preventing the high morbidity of the disorder. The interprofessional team is responsible for educating patients about the importance of vitamin D, sunlight exposure, and adherence to treatment plans to prevent recurrence.
Fortified foods are those that have been modified to include essential nutrients. In randomized controlled trials, results showed fortified vitamin D foods, including dairy products, bread, orange juice, and ultraviolet B light-enhanced mushrooms, to be effective at increasing circulating levels of 25(OH)D without adverse events. Most countries have individual national policies regarding food enhancement. However, current fortification levels may not satisfy physiologic requirements; this particularly applies to patients already at risk for vitamin D or calcium nutritional deficiencies. Successful enhancement of animal products has also been demonstrated in pigs, hens, and fish by utilizing vitamin D3-enriched feeding. Current results are promising, but further research is necessary on the impact of widespread introduction, particularly in developing countries.
Patients need long-term follow-up as the resolution can take months. Dietitians and nurses should continue to educate patients about the importance of a healthy diet, which is also reinforced by vitamin D supplements.[29] If the treating clinician decides to supplement with exogenous vitamin D, a pharmacist should be consulted to vet the precise agent and assist with appropriate dosing for the condition.
Nurses can counsel the patient on supplement administration and assist in monitoring and evaluating results on follow-up visits, reporting their findings and concerns to the prescriber. Close communication among interprofessional healthcare team members is vital for comprehensive care and improved patient outcomes. Physicians, advanced care clinicians, nurses, pharmacists, and other healthcare professionals should communicate openly, sharing insights and updates on patient progress, medication adjustments, and potential complications.
Collaborative care coordination among healthcare professionals ensures that all aspects of a patient's health are considered, including comorbidities and medication interactions. Regular follow-up appointments and assessments are essential to monitor treatment efficacy, address adverse effects, and prevent complications. The interprofessional team should emphasize the importance of compliance with treatment plans and lifestyle modifications. Clinicians should facilitate referrals to specialists such as endocrinologists or dietitians to address complex cases or nutritional deficiencies when necessary. By integrating these skills, strategies, and responsibilities, the interprofessional team can enhance patient-centered care, improve outcomes, ensure patient safety, and optimize team performance in managing individuals with osteomalacia. This collaborative approach is essential for addressing the multifaceted nature of the condition and achieving the best possible results for patients.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Vaishya R, Vijay V, Agarwal AK, Jahangir J. Resurgence of vitamin D: Old wine in new bottle. Journal of clinical orthopaedics and trauma. 2015 Sep:6(3):173-83. doi: 10.1016/j.jcot.2015.02.002. Epub 2015 Mar 26 [PubMed PMID: 26155053]
Rokan Z, Kealey WD. Osteomalacia: a forgotten cause of fractures in the elderly. BMJ case reports. 2015 Feb 9:2015():. doi: 10.1136/bcr-2014-207184. Epub 2015 Feb 9 [PubMed PMID: 25666245]
Level 3 (low-level) evidenceEmini-Sadiku M, Morina-Kuqi N. Concealing Clothing Leading to Severe Vitamin D Deficiency, Osteomalacia and Muscle Weakness. Open access Macedonian journal of medical sciences. 2019 Jul 15:7(13):2146-2149. doi: 10.3889/oamjms.2019.584. Epub 2019 Jul 14 [PubMed PMID: 31456842]
Level 2 (mid-level) evidenceCianferotti L, Osteomalacia Is Not a Single Disease. International journal of molecular sciences. 2022 Nov 28; [PubMed PMID: 36499221]
Vilaca T, Velmurugan N, Smith C, Abrahamsen B, Eastell R. Osteomalacia as a Complication of Intravenous Iron Infusion: A Systematic Review of Case Reports. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2022 Jun:37(6):1188-1199. doi: 10.1002/jbmr.4558. Epub 2022 May 7 [PubMed PMID: 35426179]
Level 1 (high-level) evidenceJan de Beur SM, Minisola S, Xia WB, Abrahamsen B, Body JJ, Brandi ML, Clifton-Bligh R, Collins M, Florenzano P, Houillier P, Imanishi Y, Imel EA, Khan AA, Zillikens MC, Fukumoto S. Global guidance for the recognition, diagnosis, and management of tumor-induced osteomalacia. Journal of internal medicine. 2023 Mar:293(3):309-328. doi: 10.1111/joim.13593. Epub 2022 Dec 13 [PubMed PMID: 36511653]
Cianferotti L, Delli Poggi C, Bertoldo F, Caffarelli C, Crotti C, Gatti D, Giannini S, Gonnelli S, Mazzantini M, Ombretta V, Sella S, Setti A, Varenna M, Zucchi F, Brandi ML. Persistence and recurrence in tumor-induced osteomalacia: A systematic review of the literature and results from a national survey/case series. Endocrine. 2022 Jun:76(3):709-721. doi: 10.1007/s12020-022-03039-2. Epub 2022 Apr 5 [PubMed PMID: 35381903]
Level 1 (high-level) evidenceMinisola S, Fukumoto S, Xia W, Corsi A, Colangelo L, Scillitani A, Pepe J, Cipriani C, Thakker RV. Tumor-induced Osteomalacia: A Comprehensive Review. Endocrine reviews. 2023 Mar 4:44(2):323-353. doi: 10.1210/endrev/bnac026. Epub [PubMed PMID: 36327295]
Rendina D, Abate V, Cacace G, D'Elia L, De Filippo G, Del Vecchio S, Galletti F, Cuocolo A, Strazzullo P. Tumor-induced Osteomalacia: A Systematic Review and Individual Patient's Data Analysis. The Journal of clinical endocrinology and metabolism. 2022 Jul 14:107(8):e3428-e3436. doi: 10.1210/clinem/dgac253. Epub [PubMed PMID: 35468192]
Level 1 (high-level) evidenceBrandi ML, Clunie GPR, Houillier P, Jan de Beur SM, Minisola S, Oheim R, Seefried L. Challenges in the management of tumor-induced osteomalacia (TIO). Bone. 2021 Nov:152():116064. doi: 10.1016/j.bone.2021.116064. Epub 2021 Jun 18 [PubMed PMID: 34147708]
Minisola S, Colangelo L, Pepe J, Diacinti D, Cipriani C, Rao SD. Osteomalacia and Vitamin D Status: A Clinical Update 2020. JBMR plus. 2021 Jan:5(1):e10447. doi: 10.1002/jbm4.10447. Epub 2020 Dec 21 [PubMed PMID: 33553992]
Khan MA, Dar HA, Baba MA, Shah AH, Singh B, Shiekh NA. Impact of Vitamin D Status in Chronic Liver Disease. Journal of clinical and experimental hepatology. 2019 Sep-Oct:9(5):574-580. doi: 10.1016/j.jceh.2019.03.001. Epub 2019 Mar 13 [PubMed PMID: 31695247]
Gou M, Ma Z. Osteomalacia, renal Fanconi syndrome, and bone tumor. The Journal of international medical research. 2018 Aug:46(8):3487-3490. doi: 10.1177/0300060518763708. Epub 2018 Apr 3 [PubMed PMID: 29614898]
Liu S, Zhou X, Song A, Huo Z, Wang Y, Xia W, Liu Y. Successful treatment of tumor-induced osteomalacia causing by phosphaturic mesenchymal tumor of the foot. Medicine. 2019 Jul:98(27):e16296. doi: 10.1097/MD.0000000000016296. Epub [PubMed PMID: 31277164]
Rigante M, Loperfido A, Paludetti G. Oncogenic Osteomalacia with Elevated Fibroblast Growth Factor 23: A Rare Case of Paranasal Sinus Tumor Onset. Cureus. 2019 Jun 17:11(6):e4919. doi: 10.7759/cureus.4919. Epub 2019 Jun 17 [PubMed PMID: 31423395]
Level 3 (low-level) evidenceUday S, Högler W. Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised Concepts, Public Health, and Prevention Strategies. Current osteoporosis reports. 2017 Aug:15(4):293-302. doi: 10.1007/s11914-017-0383-y. Epub [PubMed PMID: 28612338]
Uday S, Högler W. Nutritional rickets & osteomalacia: A practical approach to management. The Indian journal of medical research. 2020 Oct:152(4):356-367. doi: 10.4103/ijmr.IJMR_1961_19. Epub [PubMed PMID: 33380700]
Arboleya L, Braña I, Pardo E, Loredo M, Queiro R. Osteomalacia in Adults: A Practical Insight for Clinicians. Journal of clinical medicine. 2023 Apr 5:12(7):. doi: 10.3390/jcm12072714. Epub 2023 Apr 5 [PubMed PMID: 37048797]
Rosen CJ. Clinical practice. Vitamin D insufficiency. The New England journal of medicine. 2011 Jan 20:364(3):248-54. doi: 10.1056/NEJMcp1009570. Epub [PubMed PMID: 21247315]
Hill TR, Aspray TJ. The role of vitamin D in maintaining bone health in older people. Therapeutic advances in musculoskeletal disease. 2017 Apr:9(4):89-95. doi: 10.1177/1759720X17692502. Epub 2017 Feb 14 [PubMed PMID: 28382112]
Level 3 (low-level) evidenceFukumoto S, Ozono K, Michigami T, Minagawa M, Okazaki R, Sugimoto T, Takeuchi Y, Matsumoto T. Pathogenesis and diagnostic criteria for rickets and osteomalacia--proposal by an expert panel supported by the Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research, and the Japan Endocrine Society. Journal of bone and mineral metabolism. 2015 Sep:33(5):467-73. doi: 10.1007/s00774-015-0698-7. Epub 2015 Jul 22 [PubMed PMID: 26197863]
Uday S, Högler W. Spot the silent sufferers: A call for clinical diagnostic criteria for solar and nutritional osteomalacia. The Journal of steroid biochemistry and molecular biology. 2019 Apr:188():141-146. doi: 10.1016/j.jsbmb.2019.01.004. Epub 2019 Jan 14 [PubMed PMID: 30654108]
Chang CY, Rosenthal DI, Mitchell DM, Handa A, Kattapuram SV, Huang AJ. Imaging Findings of Metabolic Bone Disease. Radiographics : a review publication of the Radiological Society of North America, Inc. 2016 Oct:36(6):1871-1887 [PubMed PMID: 27726750]
Manson JE, Brannon PM, Rosen CJ, Taylor CL. Vitamin D Deficiency - Is There Really a Pandemic? The New England journal of medicine. 2016 Nov 10:375(19):1817-1820 [PubMed PMID: 27959647]
Allen SC, Raut S. Biochemical recovery time scales in elderly patients with osteomalacia. Journal of the Royal Society of Medicine. 2004 Nov:97(11):527-30 [PubMed PMID: 15520146]
Motosuneya T, Asazuma T, Yasuoka H, Tsuji T, Fujikawa K. Severe kyphoscoliosis associated with osteomalacia. The spine journal : official journal of the North American Spine Society. 2006 Sep-Oct:6(5):587-90 [PubMed PMID: 16934733]
Level 3 (low-level) evidenceBikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chemistry & biology. 2014 Mar 20:21(3):319-29. doi: 10.1016/j.chembiol.2013.12.016. Epub 2014 Feb 13 [PubMed PMID: 24529992]
Arabi A, El Rassi R, El-Hajj Fuleihan G. Hypovitaminosis D in developing countries-prevalence, risk factors and outcomes. Nature reviews. Endocrinology. 2010 Oct:6(10):550-61. doi: 10.1038/nrendo.2010.146. Epub [PubMed PMID: 20852586]
O'Mahony L, Stepien M, Gibney MJ, Nugent AP, Brennan L. The potential role of vitamin D enhanced foods in improving vitamin D status. Nutrients. 2011 Dec:3(12):1023-41. doi: 10.3390/nu3121023. Epub 2011 Dec 16 [PubMed PMID: 22292109]