Mucinous Cystic Pancreatic Neoplasms
Mucinous Cystic Pancreatic Neoplasms
Introduction
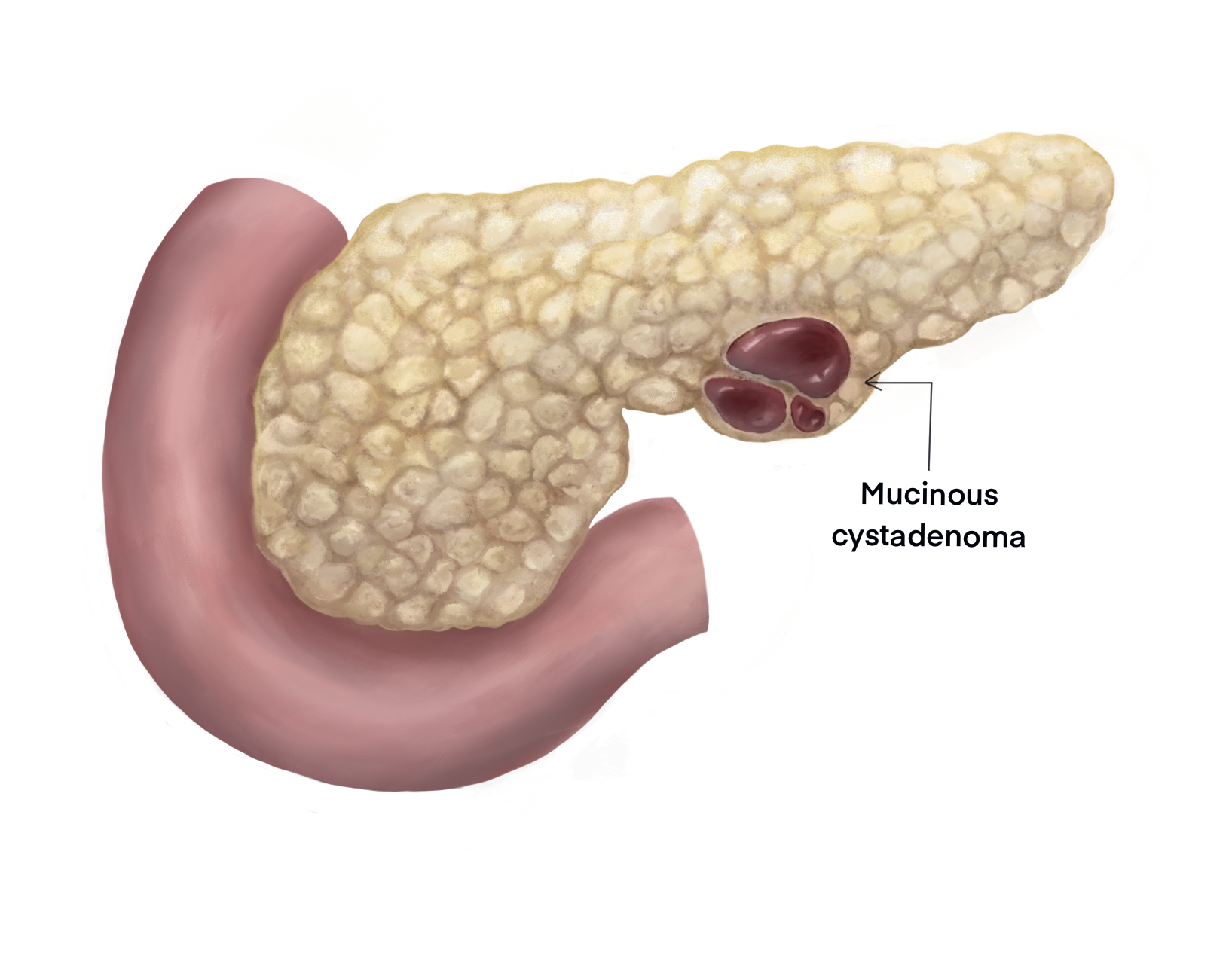
Mucinous cystic pancreatic neoplasms (MCPNs) are mucin-producing cystic lesions of the exocrine pancreas, usually located in the pancreatic body and tail.[1] While mostly benign, MCPNs can harbor foci of dysplasia and progress to invasive adenocarcinoma. MCPNs must be differentiated from other pancreatic cystic lesions, including benign entities such as serous cystadenomas and premalignant lesions such as intraductal pancreatic mucinous neoplasms. MCPNs are often asymptomatic and incidental findings on cross-sectional imaging. Improvements in diagnostic imaging and endoscopic techniques have increased the accuracy of diagnosis of MCPNs and helped clinicians formulate appropriate treatment plans.[2] Given their malignant potential, all MCPNs should be excised in patients fit to undergo surgery.
This activity will review the epidemiology, etiology, pathophysiology, histologic characteristics, clinical presentation, evaluation, and management of patients with MCPNs and highlight the role of the interprofessional team caring for patients with these pancreatic lesions.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of MCPNs is unclear; an association with female reproductive hormones has been proposed. The preponderance of MCPNs in women, the presence of estrogen and progesterone receptors on the epithelial lining, and the classic histologic finding of columnar lining cells with dense ovarian-like stroma support this theory.[3] Several mutations have been associated with mucinous cystic neoplasms, especially in those that progress to invasive carcinoma. These include mutations in KRAS, CDKN2A, TP53, and SMAD4.[4][5]
Epidemiology
Mucinous cystic pancreatic neoplasms primarily affect middle-aged women, with a female-to-male ratio of 20:1 and a median age of diagnosis at 48 years. However, as most MCPNs are asymptomatic, the true incidence and prevalence of MCPNs are unknown. The increased use of high-quality cross-sectional imaging has led to a dramatic increase in the number of identified asymptomatic pancreatic cysts; a study in 2015 reported that up to 10% of all abdominal computed tomography (CT) scans revealed pancreatic cysts.[6]
MCPNs are the second-most commonly identified pancreatic cystic lesion after intraductal papillary mucinous neoplasms.[7] Approximately 93% of MCPNs are located in the body or tail of the pancreas as a solitary lesion with a median size of 5 cm.[8] Malignant MCPNs tend to be larger than their benign counterparts. Most (72%) MCPNs are benign; borderline (10.5%), in situ carcinoma (5.5%), and invasive carcinoma (12%) are less common.[9] Invasive MCPNs tend to present in older patients roughly a decade later than their benign counterparts.
Pathophysiology
MCPNs typically develop in the body or tail of the pancreas and enlarge slowly over time. Unlike intraductal papillary mucinous neoplasms, MCPNs rarely communicate with the pancreatic duct. While the development and growth of MCPNs are possibly related to hormonal stimulation, what instigates and promotes their growth is unclear.
Typical of pancreatic body and tail lesions, MCPNs are often asymptomatic until they are quite large.[10] The presenting symptoms of MCPNs are often due to compression of surrounding structures such as the pancreatic duct, duodenum, or stomach. Occasionally, MCPNs can induce pancreatic ductal obstruction secondary to extrinsic compression and precipitate acute pancreatitis.[2] When located in the pancreatic head, MCPNs may cause obstructive jaundice.
Worrisome features indicating the malignant transformation of MCPNs include increased lesional size, mural nodules, thickening or irregularity of the cyst wall, or calcification. Dysplastic cells confined to the capsule have a much better outcome with surgery than those with extracapsular invasion.[10]
Histopathology
Gross examination of MCPNs typically reveals encapsulated cysts with a fibrous capsule. While often multicystic with thin septae, MCPNs can be unilocular. Calcification within the cyst walls is common. When present, mural nodules and irregular capsular thickening are more commonly associated with malignancy. The cysts often contain varying amounts of viscous mucus and necrotic material.
Microscopic evaluation of an MCPN typically reveals lesions lined by tall mucin-producing columnar or cuboidal cells. The underlying dense stroma closely resembles typical ovarian tissue and is characterized by a highly vascular spindle cell matrix.[8] The World Health Organization (WHO) criteria deem the presence of ovarian stroma essential for diagnosing an MCPN. The lining epithelium may demonstrate varying degrees of atypia, ranging from low-grade dysplasia to invasive adenocarcinoma. Infiltration of dysplastic cells beyond the cyst epithelial lining is the hallmark of invasive adenocarcinoma.[11]
The 2019 WHO classification stratifies cystic pancreatic lesions as MCPNs with low-grade dysplasia, MCPNs with high-grade dysplasia, and MCPNs with associated adenocarcinoma.[12] Terms such as mucinous cystadenoma and mucinous cystadenocarcinoma were used historically to describe benign and malignant MCPNs; these lesions are now grouped under the WHO classification.
History and Physical
Patients with MCPNs are often asymptomatic. Those patients presenting with symptoms most commonly have abdominal pain (62.2%). Less common presenting symptoms include weight loss, abdominal fullness, loss of appetite, and fatigue. Large MCPNs can compress surrounding organs and structures, leading to abdominal pain and a sensation of fullness. Occasionally, they can cause ductal obstruction and acute pancreatitis.
The physical examination of patients with MCPNs is often unrevealing. In the presence of a large MCPN, a fullness or vague upper abdominal mass may be palpable. As MCPNs are rarely located in the pancreatic head, jaundice is uncommon.[13]
Evaluation
The evaluation of a patient with a suspected MCPN should include a complete blood count, comprehensive metabolic panel, serum lipase, and a cancer antigen 19-9 (CA 19-9).
High-quality cross-sectional abdominal imaging is the backbone of diagnosis. Triple-phase pancreas-protocol CT with arterial, venous, and portal venous phases or magnetic resonance imaging (MRI) with contrast provides excellent visualization of the retroperitoneal space. Radiological findings suggestive of an MCPN include a single, multiloculated lesion in the pancreatic body or tail, often with areas of calcification and without communication with the pancreatic duct (85%).[14] An MRI is useful in distinguishing an MCPN from an intraductal papillary mucinous neoplasm by discerning cyst communication with the pancreatic duct.[15] Imaging findings that are suggestive of malignant transformation include dense calcifications, the presence of mural nodules, infiltration of the capsule, and lymphadenopathy.
Endoscopic ultrasound with cyst wall biopsy and cyst fluid cytology may be employed and is especially useful in cases where high-risk radiological features are seen; a preoperative diagnosis of malignant transformation guides the timing and type of surgical intervention. Current American Gastroenterological Association (AGA) guidelines for the management of cystic pancreatic neoplasms recommend an endoscopic ultrasound with fine needle aspiration and potential cyst wall biopsy for lesions with a minimum of 2 high-risk features, such as a mural nodule, lesional size greater than 3 cm, or a dilated pancreatic duct.[16]
The cyst fluid analysis from an MCPN typically reveals elevated carcinoembryonic antigen (CEA) levels, a positive mucin stain, low glucose levels, and normal amylase levels.[17] Molecular analysis is used increasingly for more precise diagnostics. Specifically, KRAS mutations have a 97 to 100% specificity for detecting mucinous differentiation and can be helpful in equivocal cases.[18]
Treatment / Management
The size of the neoplasm dictates the management of MCPNs, the presence of high-risk features, presenting symptoms, and the patient's ability to tolerate surgical intervention.
Guidelines for the management of MCPNs differ among international associations. The AGA and the American College of Gastroenterology recommend surveillance in asymptomatic cysts < 3 cm without high-risk features such as mural nodules, pancreatic duct dilation, or elevated CA 19-9. AGA guidelines recommend an MRI 1 year from diagnosis and scans every 2 years for 5 years if no significant change occurs.[16] Follow-up or surveillance is not recommended in patients with cystic pancreatic neoplasms who are not surgical candidates.
In contrast, the 2017 International consensus recommends surgical resection for all MCPNs.[19] Retrospective data suggests that the malignancy rate for MCPNs less than 3 cm with a normal CA 19-9 is almost zero, making observation a reasonable strategy in these patients.[20] (B3)
For MCPNs with high-risk features or lesions that are symptomatic, surgical resection is the standard of care. The extent of surgery depends on the size and location of the tumor. For most lesions in the body or the tail of the pancreas, a distal pancreatectomy or a distal subtotal pancreatectomy is usually sufficient. Splenic preservation may be attempted in patients without features suggestive of invasive disease. A distal pancreatectomy with splenectomy and regional lymph node dissection is recommended for lesions with high-risk features. In the rare instances where the tumor arises in the pancreatic head, pancreaticoduodenectomy may be required.[21]
Differential Diagnosis
The differential diagnosis of cystic pancreatic neoplasms includes, but is not limited to the following:
- Intraductal papillary mucinous neoplasm
- Pancreatic pseudocyst
- Serous cystadenoma
- Pancreatic ductal adenocarcinoma
- Pancreatic neuroendocrine tumor
Staging
Preoperative staging imaging should be performed for patients with suspected MCPN-associated invasive cancer, including a CT scan of the chest, abdomen, and pelvis. An intraoperative diagnostic laparoscopy at the start of the surgical procedure may also be indicated in these patients to rule out metastatic disease.
Prognosis
The final pathology dictates the prognosis for patients with MCPNs. Noninvasive MCPNs have an excellent prognosis with a 5-year overall survival rate of 100%. Interestingly, there is no reported difference in survival rates between noninvasive lesions with low-grade or high-grade dysplasia.[22] Invasive neoplasms have a significantly poorer prognosis, with a 5-year overall survival rate of 26%.[10] Advanced age, degree of tumor invasion, and multifocality have been identified as poor prognostic factors.
Complications
Most complications of MCPNs are related to the procedures required for diagnosis and treatment. Interventional diagnostic studies such as endoscopic ultrasound have a relatively low complication rate, although serious complications such as gastric or duodenal perforation, acute pancreatitis, and hemorrhage may occur.[23]
Distal pancreatectomy or subtotal pancreatectomy are the most common operative procedures performed in the treatment of MCPNs. Operative complications include pancreatic stump leak with fistula, hemorrhage, injury to surrounding organs, infections secondary to splenectomy, and more general complications associated with lengthy operations under general anesthesia, such as deep vein thrombosis.[24]
Postoperative pancreatic leaks are relatively common and are diagnosed by elevated amylase levels in the pancreatic drain fluid. Persistent leaks are known as fistulas. Most reported fistulas are grade A, and management consists of continued drainage as most of these fistulas will heal without other intervention. Occasionally, a patient may require percutaneous drainage of a small fluid collection (grade B). Rarely patients will require intensive care for sepsis and multiple organ failure secondary to a persistent pancreatic leak (grade C); such patients require swift surgical intervention.
Postoperative impaired glucose tolerance and diabetes may occur, depending on the amount of pancreatic parenchyma removed. Infections secondary to surgical splenia can be mitigated by appropriate preoperative vaccination.
Deterrence and Patient Education
There are no known modifiable risk factors for the development of MCPNs.
Patients with suspected MCPN and high-risk features should be evaluated for definitive surgical treatment, as lesions larger than 3 cm and those with high-risk features are at increased risk for malignant pathology.
The prognosis for most MCPNs is excellent, and management at a center of excellence for pancreatic surgery is essential for accurate diagnosis and optimum management.
Pearls and Other Issues
It is imperative to distinguish intraductal papillary mucinous neoplasm from MCPN. MCPNs are mucin-producing cystic lesions with an ovarian stroma and no communication with the pancreatic duct, unlike intraductal papillary mucinous neoplasms, which lack ovarian stroma and always communicate with the pancreatic duct. Surgical resection of mucinous cystic pancreatic neoplasms is recommended in fit patients, given the potential for malignant transformation.
Enhancing Healthcare Team Outcomes
Patient-centered care for individuals with MPCN requires collaboration among healthcare professionals, including physicians, advanced practice providers, nurses, pharmacists, and others. These neoplasms are often incidentally discovered during imaging performed for another purpose. The necessary skills involve interpreting radiological findings, identifying potential complications, effectively communicating these findings to the patient and their care team, and understanding the intricacies of managing pancreatic cystic neoplasms. Gastroenterology, interventional radiology, pathology, general surgery, and primary care practitioners typically play a role in coordinating and delivering care to patients with suspected MCPNs.
The entire healthcare team also plays a crucial role in ensuring that patients who are not candidates for surgical intervention or those who qualify for or elect surveillance of their condition receive appropriate follow-up. MPCNs carry a low but genuine risk of malignant transformation. Timely recognition of concerning features relies on adherence to monitoring.
The complexity of the surgical procedure necessitates teamwork and communication among operating room staff, anesthesia providers, nurses, and other allied team members for patients undergoing surgery.
Media
References
Perri G, Marchegiani G, Frigerio I, Dervenis CG, Conlon KC, Bassi C, Salvia R. Management of Pancreatic Cystic Lesions. Digestive surgery. 2020:37(1):1-9. doi: 10.1159/000496509. Epub 2019 Jan 11 [PubMed PMID: 30636253]
Ozcan K, Klimstra DS. A Review of Mucinous Cystic and Intraductal Neoplasms of the Pancreatobiliary Tract. Archives of pathology & laboratory medicine. 2022 Mar 1:146(3):298-311. doi: 10.5858/arpa.2021-0399-RA. Epub [PubMed PMID: 35192699]
Nilsson LN, Keane MG, Shamali A, Millastre Bocos J, Marijinissen van Zanten M, Antila A, Verdejo Gil C, Del Chiaro M, Laukkarinen J. Nature and management of pancreatic mucinous cystic neoplasm (MCN): A systematic review of the literature. Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.]. 2016 Nov-Dec:16(6):1028-1036. doi: 10.1016/j.pan.2016.09.011. Epub 2016 Sep 20 [PubMed PMID: 27681503]
Level 1 (high-level) evidenceFischer CG, Wood LD. From somatic mutation to early detection: insights from molecular characterization of pancreatic cancer precursor lesions. The Journal of pathology. 2018 Dec:246(4):395-404. doi: 10.1002/path.5154. Epub [PubMed PMID: 30105857]
Conner JR, Mariño-Enríquez A, Mino-Kenudson M, Garcia E, Pitman MB, Sholl LM, Srivastava A, Doyle LA. Genomic Characterization of Low- and High-Grade Pancreatic Mucinous Cystic Neoplasms Reveals Recurrent KRAS Alterations in "High-Risk" Lesions. Pancreas. 2017 May/Jun:46(5):665-671. doi: 10.1097/MPA.0000000000000805. Epub [PubMed PMID: 28196015]
Zanini N, Giordano M, Smerieri E, Cipolla d'Abruzzo G, Guidi M, Pazzaglini G, De Luca F, Chiaruzzi G, Vitullo G, Piva P, Lombardi R, Jovine E, Gatti M, Landolfo G. Estimation of the prevalence of asymptomatic pancreatic cysts in the population of San Marino. Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.]. 2015 Jul-Aug:15(4):417-22. doi: 10.1016/j.pan.2015.05.461. Epub 2015 May 21 [PubMed PMID: 26028332]
Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012 Sep:152(3 Suppl 1):S4-12. doi: 10.1016/j.surg.2012.05.033. Epub 2012 Jul 6 [PubMed PMID: 22770958]
Reddy RP, Smyrk TC, Zapiach M, Levy MJ, Pearson RK, Clain JE, Farnell MB, Sarr MG, Chari ST. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2004 Nov:2(11):1026-31 [PubMed PMID: 15551256]
Level 2 (mid-level) evidenceWarshaw AL, Compton CC, Lewandrowski K, Cardenosa G, Mueller PR. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Annals of surgery. 1990 Oct:212(4):432-43; discussion 444-5 [PubMed PMID: 2171441]
Jang KT, Park SM, Basturk O, Bagci P, Bandyopadhyay S, Stelow EB, Walters DM, Choi DW, Choi SH, Heo JS, Sarmiento JM, Reid MD, Adsay V. Clinicopathologic characteristics of 29 invasive carcinomas arising in 178 pancreatic mucinous cystic neoplasms with ovarian-type stroma: implications for management and prognosis. The American journal of surgical pathology. 2015 Feb:39(2):179-87. doi: 10.1097/PAS.0000000000000357. Epub [PubMed PMID: 25517958]
Level 2 (mid-level) evidenceFarrell JJ. Prevalence, Diagnosis and Management of Pancreatic Cystic Neoplasms: Current Status and Future Directions. Gut and liver. 2015 Sep 23:9(5):571-89. doi: 10.5009/gnl15063. Epub [PubMed PMID: 26343068]
Level 3 (low-level) evidenceFukushima N, Zamboni G. Mucinous cystic neoplasms of the pancreas: update on the surgical pathology and molecular genetics. Seminars in diagnostic pathology. 2014 Nov:31(6):467-474. doi: 10.1053/j.semdp.2014.08.007. Epub 2014 Sep 2 [PubMed PMID: 25441310]
Fernández-del Castillo C. Mucinous cystic neoplasms. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2008 Mar:12(3):411-3 [PubMed PMID: 17955316]
Ethun CG, Postlewait LM, McInnis MR, Merchant N, Parikh A, Idrees K, Isom CA, Hawkins W, Fields RC, Strand M, Weber SM, Cho CS, Salem A, Martin RCG, Scoggins CR, Bentrem D, Kim HJ, Carr J, Ahmad SA, Abbott DE, Wilson G, Kooby DA, Maithel SK. The diagnosis of pancreatic mucinous cystic neoplasm and associated adenocarcinoma in males: An eight-institution study of 349 patients over 15 years. Journal of surgical oncology. 2017 Jun:115(7):784-787. doi: 10.1002/jso.24582. Epub 2017 Feb 17 [PubMed PMID: 28211072]
Joshi U, Poudel P, Ghimire RK, Basnet B. Pancreatic pseudocyst or mucinous cystadenocarcinoma of pancreas? A diagnostic dilemma. Clinical case reports. 2017 Apr:5(4):501-504. doi: 10.1002/ccr3.887. Epub 2017 Mar 6 [PubMed PMID: 28396777]
Level 3 (low-level) evidenceVege SS, Ziring B, Jain R, Moayyedi P, Clinical Guidelines Committee, American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015 Apr:148(4):819-22; quize12-3. doi: 10.1053/j.gastro.2015.01.015. Epub [PubMed PMID: 25805375]
van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointestinal endoscopy. 2005 Sep:62(3):383-9 [PubMed PMID: 16111956]
Level 1 (high-level) evidenceNikiforova MN, Khalid A, Fasanella KE, McGrath KM, Brand RE, Chennat JS, Slivka A, Zeh HJ, Zureikat AH, Krasinskas AM, Ohori NP, Schoedel KE, Navina S, Mantha GS, Pai RK, Singhi AD. Integration of KRAS testing in the diagnosis of pancreatic cystic lesions: a clinical experience of 618 pancreatic cysts. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013 Nov:26(11):1478-87. doi: 10.1038/modpathol.2013.91. Epub 2013 Jun 7 [PubMed PMID: 23743931]
Buerlein RCD, Shami VM. Management of pancreatic cysts and guidelines: what the gastroenterologist needs to know. Therapeutic advances in gastrointestinal endoscopy. 2021 Jan-Dec:14():26317745211045769. doi: 10.1177/26317745211045769. Epub 2021 Sep 23 [PubMed PMID: 34589706]
Level 3 (low-level) evidenceGoh BK, Tan YM, Chung YF, Chow PK, Cheow PC, Wong WK, Ooi LL. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World journal of surgery. 2006 Dec:30(12):2236-45 [PubMed PMID: 17103100]
D'Haese JG, Werner J. Surgery of Cystic Tumors of the Pancreas - Why, When, and How? Visceral medicine. 2018 Jul:34(3):206-210. doi: 10.1159/000489234. Epub 2018 Jun 14 [PubMed PMID: 30182025]
Hui L, Rashid A, Foo WC, Katz MH, Chatterjee D, Wang H, Fleming JB, Tamm EP, Wang H. Significance of T1a and T1b Carcinoma Arising in Mucinous Cystic Neoplasm of Pancreas. The American journal of surgical pathology. 2018 May:42(5):578-586. doi: 10.1097/PAS.0000000000001040. Epub [PubMed PMID: 29462092]
Kadiyala V, Lee LS. Endosonography in the diagnosis and management of pancreatic cysts. World journal of gastrointestinal endoscopy. 2015 Mar 16:7(3):213-23. doi: 10.4253/wjge.v7.i3.213. Epub [PubMed PMID: 25789091]
Chincarini M, Zamboni GA, Pozzi Mucelli R. Major pancreatic resections: normal postoperative findings and complications. Insights into imaging. 2018 Apr:9(2):173-187. doi: 10.1007/s13244-018-0595-4. Epub 2018 Feb 15 [PubMed PMID: 29450852]