Preventing Cervical Cancer: Best Practices in Pap and HPV Testing
Preventing Cervical Cancer: Best Practices in Pap and HPV Testing
Introduction
Cervical cancer is a significant health concern for women worldwide. Many cases of cervical cancer occur in developing countries that have ineffective screening programs. The incidence and mortality of cervical cancer in the United States have been decreasing over the past 30 years due to widespread screening. In the United States, cervical cancer is typically diagnosed in women who have not undergone screening or have only received inadequate screening.[1] Screening guidelines for cervical cancer have been evaluated and adjusted considering the time required for disease progression. Screening methods for cervical cancer include Papanicolaou (Pap) testing through cytopathology and HPV testing.[2]
The Pap test, also known as a Pap smear or Pap, is primarily a routine screening test that serves as a secondary and tertiary prevention measure for cervical cancer. In addition, it can serve a diagnostic function in specific patient populations.[CMS. National Coverage Determination (NCD). Diagnostic Pap Smears] Primary prevention of cervical cancer is achieved through vaccination against the human papillomavirus (HPV), which is responsible for 99.7% of all cervical cancers.[3]
A Pap involves a speculum examination, direct visualization of the cervix, and collection of a sample from the cervix using a brush. The sample is then sent to pathology for cytopathological evaluation with or without testing for the presence of HPV. Pap cytopathology can also identify other cervical or endometrial pathologies, such as inflammation or infections.[3][NIH. Cervical Cancer Screening]
HPV-associated cervical cancer has an extended precancerous stage called cervical dysplasia, or cervical intraepithelial neoplasia (CIN), which can be identified on Pap cytology. This long precancerous stage, in combination with the anatomical accessibility of the cervix for direct visualization, makes the Pap an excellent test for early detection, monitoring, or excision of cervical dysplasia and cervical cancer.[4][5][6]
The Pap smear is named after Dr George Papanicolaou (1883–1962), a Greek-American physician-scientist widely regarded as the father of modern cytology or the father of exfoliative cytology.[7][8] Dr Papanicolaou was born in Greece, but moved to the United States with his wife, Andromache "Mary" Papanicolaou, in 1913. In the United States, he accepted a position at Cornell University. Dr Papanicolaou's research first focused on reproduction using animal models. After discovering that the timing of the reproductive cycle could be ascertained by examining vaginal secretions from guinea pigs, he began to study cytopathology in humans, beginning with his wife. In addition to assisting unpaid in the laboratory, Mary Papanicolaou supported her husband's research by providing daily samples from her cervix, enabling him to understand how tissue changes throughout the reproductive years to menopause.[9][10][11][12][13][Science History Institute. 21 Years, 7,600 Tests] In 1925, Dr Papanicolaou began systematically studying vaginal smears from volunteers beyond his wife, Mary,[11][13] and in 1928, he published the first association between cytopathological findings with cervical cancer,[14] 1 year after a similar discovery was reported by Romanian physician-scientists Dr Aurel Babes (1886–1962) and Dr Constantin Daniel (1876–1973).[11][12][15] By 1941, the efficacy of the Pap smear had been proven.[12] In 1943, Dr Papanicolaou published his landmark book in collaboration with Dr Herbert Traut, Diagnosis of Uterine Cancer by the Vaginal Smear, outlining the Pap technique and analysis in detail (see Images. George N Papanicolaou Drawings of Various Cells Found in Human Vaginal Smears I and George N Papanicolaou Drawings of Various Cells Found in Human Vaginal Smears II).[11][12][13]
In the 1960s, the United States and several other countries began formally recommending Pap tests and establishing organized screening programs, which led to significant decreases in the incidence and mortality rates of cervical cancer across multiple nations.[4][16][17][18][19] Cervical cancer screening combined with the recommended next steps in management are now believed to prevent up to 80% of cases of invasive cervical cancer in the developed world.[3][20][21]
Cervical cancer screening is generally considered to involve low-cost, reliable, and easy-to-perform tests with a high degree of sensitivity and specificity.[10][19][NIH. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition] Pap tests are widely recognized as the most significant advancement in controlling cancer in the 20th century [12] and have been identified as one of the most successful contributions to cancer screening and prevention of all time.[7][22]
Etiology and Epidemiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology and Epidemiology
Cervical cancer is the fourth most common cause of cancer-related death among women worldwide.[3][23][American Cancer Society. Cancer statistics, 2025] However, wide variations exist in the incidence and mortality rates of cervical cancer globally, in large part due to inequities in access to screening.[23][American Cancer Society. Cancer statistics, 2025] Even within the United States, the incidence of cervical cancer varies significantly by state, with an incidence of 5 per 100,000 in Massachusetts, New Hampshire, and Connecticut and an incidence of more than 10 per 100,000 in Texas, Kentucky, West Virginia, Oklahoma, and Puerto Rico.[American Cancer Society. Cancer statistics, 2025]
Cervical cancer is primarily caused by HPV, with approximately 99.7% of cases resulting from persistent genital infection with high-risk HPV strains.[3] Of these, about 70% are attributable to just 2 strains—HPV-16 and HPV-18.[24] Truly HPV-negative cervical cancers are nearly all adenocarcinomas with unclear etiologies, typically diagnosed at more advanced stages with poorer prognoses. False negatives can occur for a variety of reasons, such as latent HPV infection, non–high-risk HPV infection, sampling errors, testing method, and histological misclassification.[25]
There are more than 200 strains of HPV. Several strains are considered non-oncogenic or low-risk, whereas 15 strains have been identified as oncogenic or high-risk. Low-risk strains include HPV-6, -11, -42, -43, and -44, and are typically associated with benign genital warts; however, they are also occasionally found in cervical cancers. High-risk strains include HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -68, -73, and -82, and are associated with cervical cancer in addition to anal cancer, vaginal cancer, vulvar cancer, and oropharyngeal cancer.[3]
HPV is an extremely common virus, with the majority of people worldwide infected with at least one strain at some point in their lives.[3][26] [NIH. HPV and Cancer] The prevalence of high-risk HPV worldwide is about 10%; however, it is up to 36.5% in some developing countries.[3]
HPV is transmitted through direct contact. The primary mode of transmission is sexual contact, with the peak period of acquiring HPV occurring shortly after coitarche. HPV incidence is about 1% in sexually active adults and 3% in sexually active adolescents.[3] However, it is also transmitted through vertical transmission, fomites, and nonsexual skin-to-skin contact.[3][26]
Most HPV infections resolve spontaneously, with 90% of HPV infections clearing within 2 years.[3] However, HPV can also remain latent for months or years.[26] Persistent genital infections with high-risk strains can progress to cervical cancer. HPV can infect the squamous cells of the ectocervix, leading to squamous cell carcinoma of the cervix, or the glandular cells of the endocervix, resulting in adenocarcinoma of the cervix.[NIH. HPV and Cancer] Most HPV-associated cervical cancers are squamous cell carcinomas.[NIH. HPV and Cancer] Risk factors for progression from HPV infection to cervical cancer that have been identified include diet, tobacco use, a weakened immune system, HIV infection, high parity, long-term use of oral contraceptives, early coitarche, and multiple sexual partners.[23][American Cancer Society. Cancer Facts & Figures 2025] Please see StatPearls' companion resources, "Human Papillomavirus" and "Cervical Cancer," for more information.
Pathophysiology
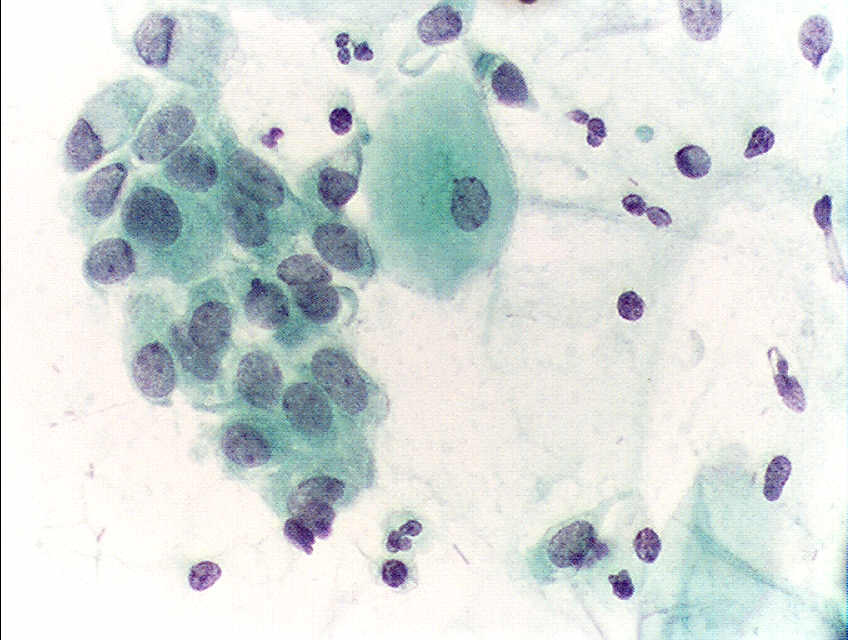
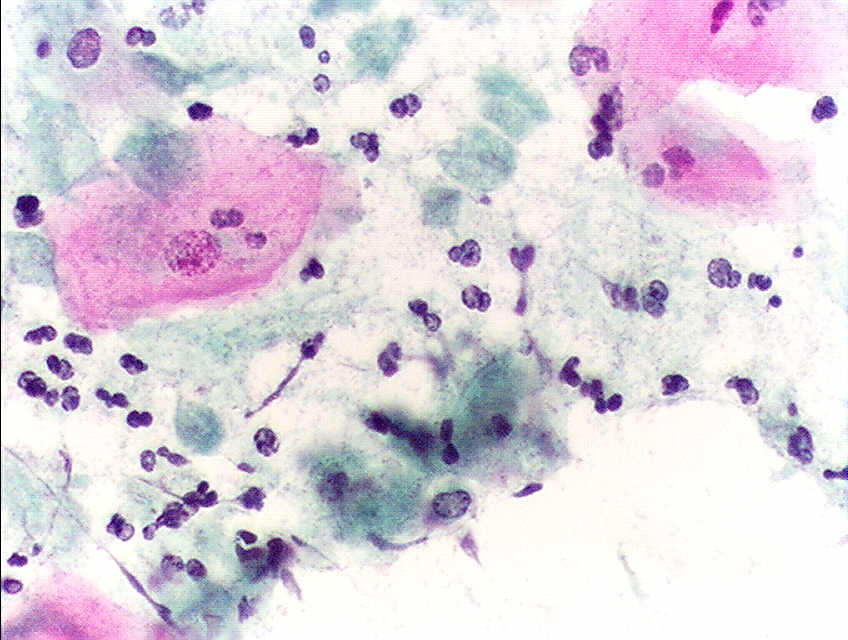
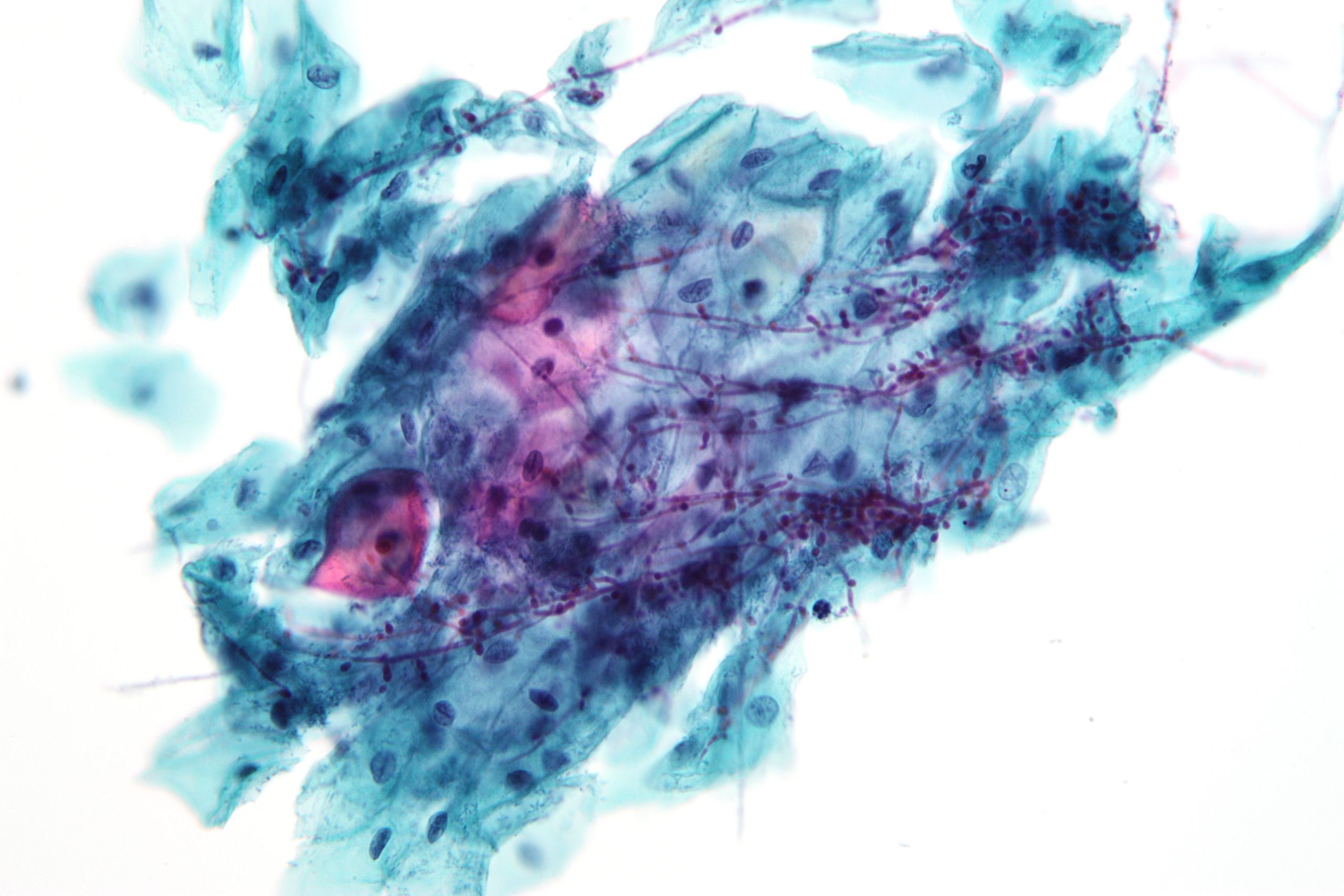
HPV infects cells of the mucocutaneous epithelium of the cervix, classically beginning with those at the transformation zone or squamocolumnar junction, where continuous metaplastic change occurs.[3][26] HPV that penetrates the basal layer of mature epithelial cells replicates within these cells during the initial phase of infection, before integrating into the host genome and upregulating the expression of oncogenes E6 and E7, which disrupts normal cell cycle control and induces the formation of koilocytes.[3][25][27] Koilocytes are squamous epithelial cells with characteristic changes caused by HPV-associated dysplasia, including an acentric, hyperchromatic nucleus displaced by a large perinuclear vacuole, and they are pathognomonic of HPV infection (see Images. Low-Grade Squamous Intraepithelial Lesion Pap with Features of Koilocytosis Compared to Unremarkable Intermediate Squamous Cell and Squamous Epithelial Cells With and Without Koilocytosis).[27]
Between HPV infection and cervical cancer is an extended precancerous stage called cervical dysplasia or CIN, which can be identified with the degree of dysplasia graded on Pap cytology. Progression from HPV infection to cervical cancer takes around 5 to 10 years at minimum and 20 to 25 years on average.[3] Please see StatPearls' companion resource, "Koilocytosis," for more information.
Specimen Requirements and Procedure
Specimen collection for cervical cancer screening involves a sterile speculum examination, visualization of the cervix, and collection of a sample from the cervix. Sample collection is typically completed with either an endocervical broom or an extended-tip spatula and an endocervical brush. The endocervical broom is inserted in the cervical os, ensuring the central bristles are in contact with the endocervix and the shorter bristles are in contact with the ectocervix, and then gently rotated 5 times in the same direction. The extended-tip spatula is inserted in the cervical os and then gently rotated to scrape the endocervix, ectocervix, and the transition zone in a 360° manner. The specimen can then be sent to pathology for cytopathological evaluation with or without testing for the presence of the HPV.[28]
Cervical Cancer Screening Guidelines
Cervical cancer screening guidelines regarding Pap and HPV testing, screening timing, frequency, and interpretation are developed by several major professional medical organizations, including the American College of Obstetricians and Gynecologists (ACOG), the American Society for Colposcopy and Cervical Pathology (ASCCP), the Society of Gynecologic Oncology (SGO), the American Society for Clinical Pathology (ASCP), the United States Preventive Services Task Force (USPSTF), and the American Cancer Society (ACS).
ACOG, ASCCP, and SGO endorsed the 2018 USPSTF guidelines for cervical cancer screening. USPSTF recommendations include:
- Grade A recommendation: Screening for cervical cancer every 3 years with cervical cytology alone in women aged 21 to 29.
- Grade A recommendation: Screening every 3 years with cervical cytology alone, every 5 years with high-risk HPV testing alone, or every 5 years with high-risk HPV testing combined with cytology (co-testing) in women aged 30 to 65.
- Grade D recommendation: No screening for cervical cancer in women younger than 21.
- Grade D recommendation: No screening for cervical cancer in women older than 65 who have had adequate prior screening and are not otherwise at high risk for the disease.
- Grade D recommendation: No screening for cervical cancer in women who have had a hysterectomy with removal of the cervix and do not have a history of a high-grade precancerous lesion or cervical cancer.
In 2020, the ACS updated its cervical cancer screening guidelines, recommending primary HPV testing as the preferred screening method for average-risk patients and raising the age to initiate screening to age 25.[29] The ASCCP supports but does not endorse the ACS guidelines.[ASCCP. Screening Guidelines] The ACOG has published a responsive Practice Advisory.[ACOG. Updated Cervical Cancer Screening Guidelines] The ASCP has also published commentary.[ASCP. Release of the 2020 American Cancer Society Cervical Cancer Screening Guidelines]
Special Populations
Certain patient populations should be screened more frequently and managed differently when abnormal screening results are obtained. These populations include patients living with HIV; immunocompromised patients, such as organ transplant patients; patients exposed to diethylstilbestrol while in utero; and those previously treated for CIN 2, CIN 3, or cervical cancer. Screening and management recommendations specific to patients living with HIV have been issued by the Centers for Disease Control and Prevention (CDC), the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America.[28]
Other special populations include pregnant patients and transgender patients. Various studies have demonstrated the safety of Paps in pregnancy. However, the pregnant state should be considered in the interpretation of results, as cervical hyperemia and inflammation are commonly present and create challenges to interpretation.[22][30] Although pregnancy is not ideal for cytopathological interpretation, it should be noted that prenatal visits may provide a window of increased opportunity for healthcare access.[31] Transgender men retaining uterine cervixes should be screened routinely.[32] Transgender women with neo-cervices after vaginoplasty do not require routine screening due to a lack of native cervical tissue.[33]
Diagnostic Tests
Positive screening Paps should be further managed with increased surveillance, diagnostic testing, and therapeutic measures. The ASCCP publishes risk-based management guidelines, most recently in 2019, to assist in recommendations for follow-up of abnormal screening results.[34]
Therapeutic measures may include:
- Colposcopy: This procedure can be performed with or without cervical biopsies to obtain samples for histopathological evaluation. Please see StatPearls' companion resource, "Colposcopy," for more information.
- Excisional procedure: This procedure includes cold knife conization and loop electrosurgical excision procedure. Please see StatPearls' companion resource, "Cold Knife Conization of the Cervix," for more information.
Testing Procedures
Collected specimens are examined by cervical cytopathology with or without HPV testing. Samples are prepared with Pap dye,[35][36] and cytopathology can be completed using 2 methods—the conventional Pap and liquid-based cytology.[5][37][AAFP. Conventional Pap Smear vs. Liquid-Based Cytology]
Pap cytopathology, whether conventional or liquid-based, examines collected specimens under the microscope, inspecting for signs of dysplasia. However, it can also detect the presence of inflammation or infection, thereby identifying other cervical or endometrial pathologies, such as cervicitis, endometritis, endometrial hyperplasia, or infections such as candidiasis or trichomoniasis.[3][NIH. Cervical Cancer Screening]
The conventional Pap consists of smearing and immediately fixing the sample on a glass slide. In contrast, liquid-based cytology involves rinsing the sample in a fixative suspension, which can later be prepared as a monolayer slide.[38][39][NIH. Cervical Cancer Screening]
Liquid-based cytology was introduced in 1996 as an evolution of conventional Pap smears to improve their diagnostic reliability.[5][39][40][AAFP. Conventional Pap Smear vs. Liquid-Based Cytology] As of 2022, several commercial systems have been developed, including 2 FDA-approved systems in the United States—ThinPrep, first approved in 1996, and SurePath, first approved in 1999.
Advantages of liquid-based cytology include rinsing specimens to remove obscuring cellular material and debris, and more evenly distributing diagnostically relevant cells in a single layer. In addition, residual fluid can be reserved for further investigations, such as HPV testing, sexually transmitted infection testing, and repeat smears.[39][41] However, liquid-based cytology is more expensive, both in terms of capital investment and ongoing operating costs, which compromises its utility in lower-resource settings.[39][41] In addition, comparative analyses with respect to sensitivity and specificity of the 2 methods have yielded conflicting results, especially with respect to higher-grade lesions.[41][AAFP. Conventional Pap Smear vs. Liquid-Based Cytology] Current evidence, according to the 2018 USPSTF practice guidelines, does not denote any clinically important cytopathological differences.[35]
In the early 2000s, the National Health Service (NHS) in England conducted a pilot study of liquid-based cytology. An interdisciplinary team of researchers, including pathologists, colposcopists, epidemiologists, and health economists, conducted an analysis that informed the NHS's decision to fully transition to liquid-based cytology. By the 2010s, liquid-based cytology had effectively replaced conventional Pap smears in the United States as well.
Notably, although HPV testing must be performed separately from conventional Pap testing, liquid-based cytology enables HPV testing from the same sample. HPV testing can be performed concurrently (co-testing), reflexively with abnormal results (reflex HPV testing), or independently (primary HPV screening). HPV testing can be performed using multiple techniques, most of which utilize signal or nucleic acid amplification methods, such as polymerase chain reaction.[3][35]
The latest ACS guidelines recommend primary HPV screening as the preferred method for individuals at average risk, while acknowledging a transition period before it becomes widely available across all clinical settings.[American Cancer Society. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society] Due to limitations in availability, accessibility, and ongoing research, current USPSTF guidelines endorsed by the ACOG, ASCCP, SGO, and ASCP continue to recommend dual testing. For individuals at average risk who are younger than 30, the recommendation is to perform cytology with reflex testing. For those aged 30 or older, the guidelines suggest cytology with co-testing.[29][ASCCP. Screening Guidelines][ACOG. Updated Cervical Cancer Screening Guidelines][ASCP. Release of the 2020 American Cancer Society Cervical Cancer Screening Guidelines] Primary HPV screening is recommended every 5 years, with direct referral to colposcopy for detection of HPV-16 and HPV-18 and cytology for other high-risk strains of HPV.[3]
As of May 2024, the Food and Drug Administration (FDA) has approved the use of 2 HPV detection kits for self-collection in a healthcare setting when a clinician is unable to collect a cervical specimen. The approved kits include the BD Onclarity HPV assay by Becton, Dickinson and Company and the Cobas HPV assay by Roche Molecular Systems. [NIH. FDA Approves HPV Tests That Allow for Self-Collection in a Health Care Setting]
Interfering Factors
Pap smear sample collection and interpretation can be limited by recent intercourse or use of douches, spermicidal foam, or vaginal medications within 48 hours before the examination.[28][CDC. Screening for Cervical Cancer] However, the use of water-based lubricants and menstruation do not interfere with cytopathological interpretation, especially when completed using liquid-based cytology.[42][43]
Pain and fear of pain associated with speculum examination and Pap collection, along with psychosocial factors such as health beliefs and health literacy, are consistently cited as common reasons for not adhering to screening guidelines.[43][44][45][46][47][48] The use of water-based lubricants, the selection of appropriate speculums, and a trauma-informed model of care can enhance patient experiences.[43][45][49] However, ongoing research efforts aimed at improving patient experiences with cervical cancer screening are needed and active.[47][48][47][50][51]
Results, Reporting, and Critical Findings
The Bethesda System for Reporting Cervical Cytology is a standardized classification system in the United States for communicating Pap smear cytopathology results. This system was established by a National Cancer Institute Workshop in Bethesda, Maryland, in 1988 in an attempt to provide a uniform format and standardized lexicon for cervical cytopathology reports.[15][52] The Bethesda System replaced the numerical Pap cytological class designations (Class I-V), which no longer reflected current medical understanding, as well as the three-tiered CIN classification (CIN I, II, III) based on tissue architecture with a two-tiered diagnostic terminology of low-grade squamous intraepithelial lesion and high-grade squamous intraepithelial lesion.[52][53][54][55] Although the three-tiered CIN terminology is still reflected in management guidelines, it is less histologically reproducible and less biologically relevant.[56][57][58][NLM: 101656343].
The Bethesda System has undergone revisions in 1991, 2001, and most recently in 2014.[15][59] In 2012, the College of American Pathologists and ASCCP cosponsored the Lower Anogenital Squamous Terminology Project. The goal of this initiative was to harmonize the histological and cytological terminology of HPV-related squamous lesions within and across anogenital sites, and extend the two-tiered terminology of low-grade squamous intraepithelial lesion and high-grade squamous intraepithelial lesion for reporting of all histopathology of the lower anogenital tract.[57][58][60] The Bethesda System recommends a systematic format, starting with the specimen type, a statement on specimen adequacy, a general categorization, and a more detailed results and interpretation section, followed by optional sections for adjunctive testing, computer-assisted interpretation, and educational comments, if applicable.[52][53]
Bethesda System Report
- Specimen type: Indicates whether the sample is conventional or liquid-based
- Specimen adequacy: Indicates whether the specimen is satisfactory or unsatisfactory for evaluation
- Any smear with abnormal cells is, by definition, satisfactory for evaluation.
- Presence of endocervical or transformation zone is an indicator of specimen adequacy, but is not required for specimen adequacy (see Image. Pap with Endocervical Cells).
- Specimens may be considered unsatisfactory for evaluation because too few cells are collected; cervical cells are obscured by mucus, inflammation, lubricants, and inappropriate fixation.
- General categorization: Indicates general findings
- Negative for intraepithelial lesion or malignancy
- Epithelial cell abnormality (concerning abnormal squamous or glandular epithelial interpretation or result)
- Other (concerning abnormal interpretation or result, including organisms or other non-neoplastic findings)
- Interpretation or results: Indicates detailed findings
- Cellular variations
- Squamous metaplasia
- Keratotic changes
- Tubal metaplasia
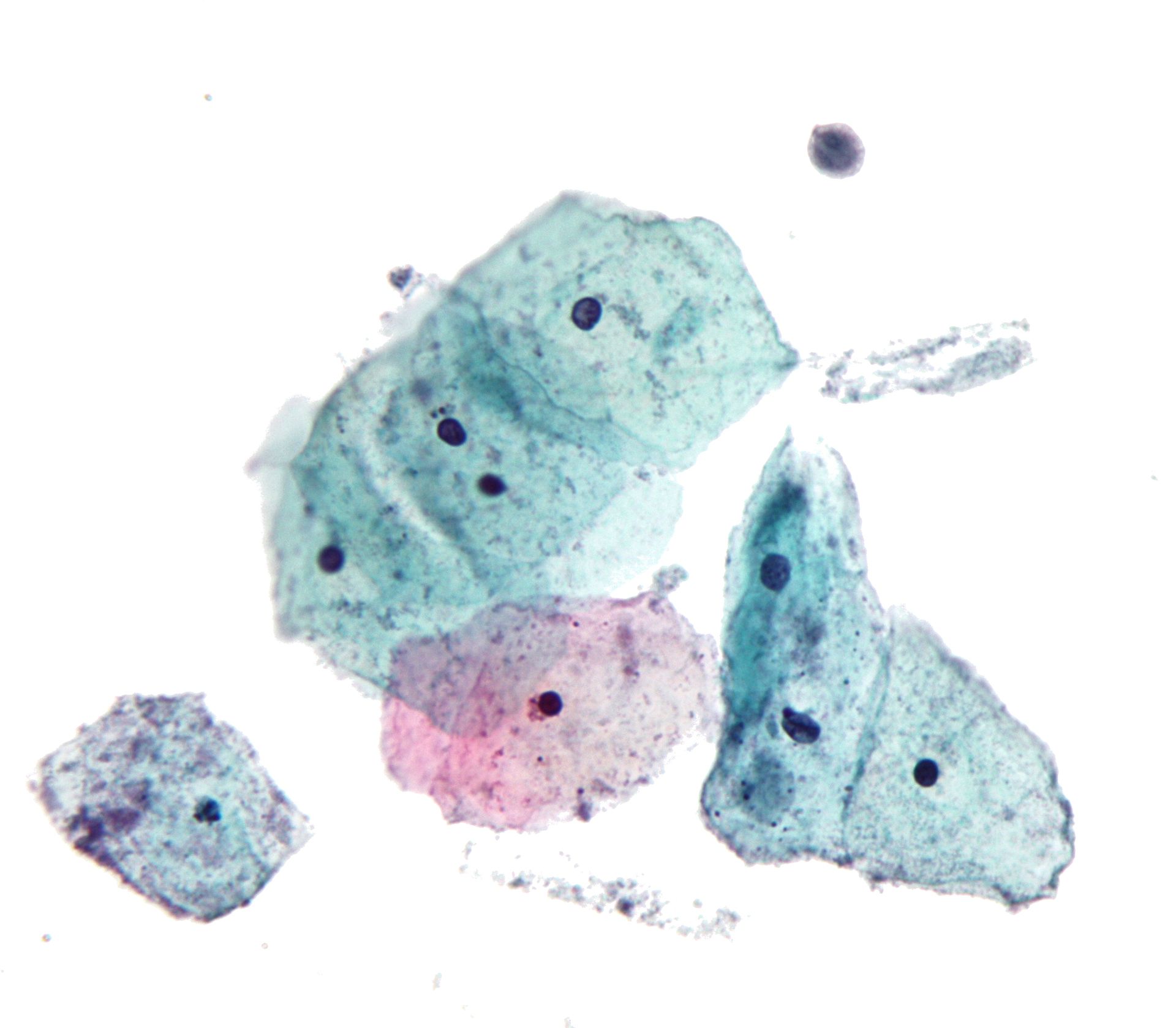
- Atrophy (see Image. Pap with Atrophic Squamous Cells)
- Pregnancy-associated changes
- Reactive cellular changes associated with
- Inflammation, including repair (see Image. Squamous Cells with Acute Inflammation)
- Lymphocytic cervicitis
- Radiation
- Intrauterine contraceptive device
- Glandular cell status post-hysterectomy
- Organisms
- Trichomonas vaginalis (see Images. Pap with Trichomonas vaginalis and Pap Test with Trichomonas vaginalis)
- Fungal organisms morphologically consistent with Candida spp. (see Image. Pap with Candida)
- Shift in flora suggestive of bacterial vaginosis
- Bacteria morphologically consistent with Actinomyces spp.
- Cellular changes consistent with herpes simplex virus (see Image. Pap with Herpes Simplex Virus)
- Cellular changes consistent with cytomegalovirus
- Other
- Endometrial cells in a patient older than 45, specify if negative for squamous intraepithelial lesion
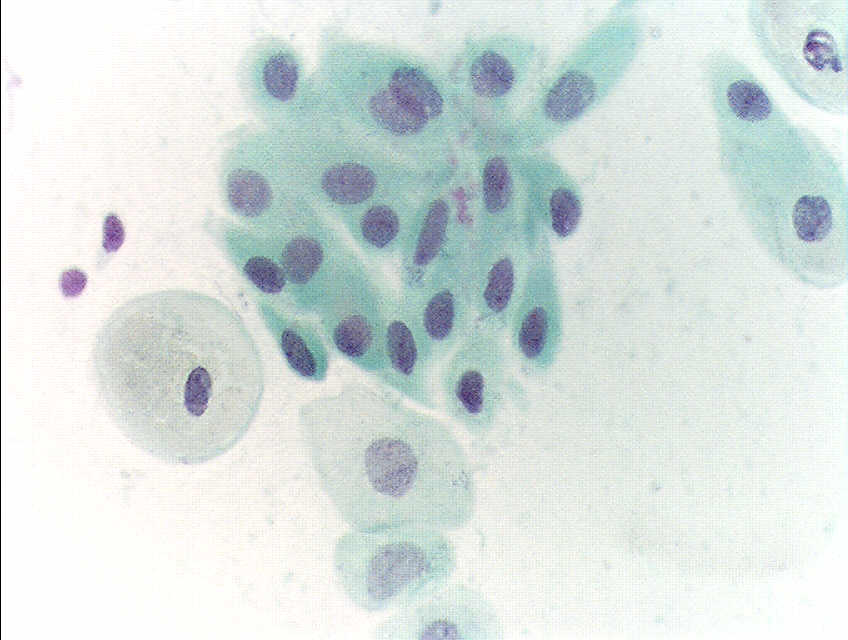
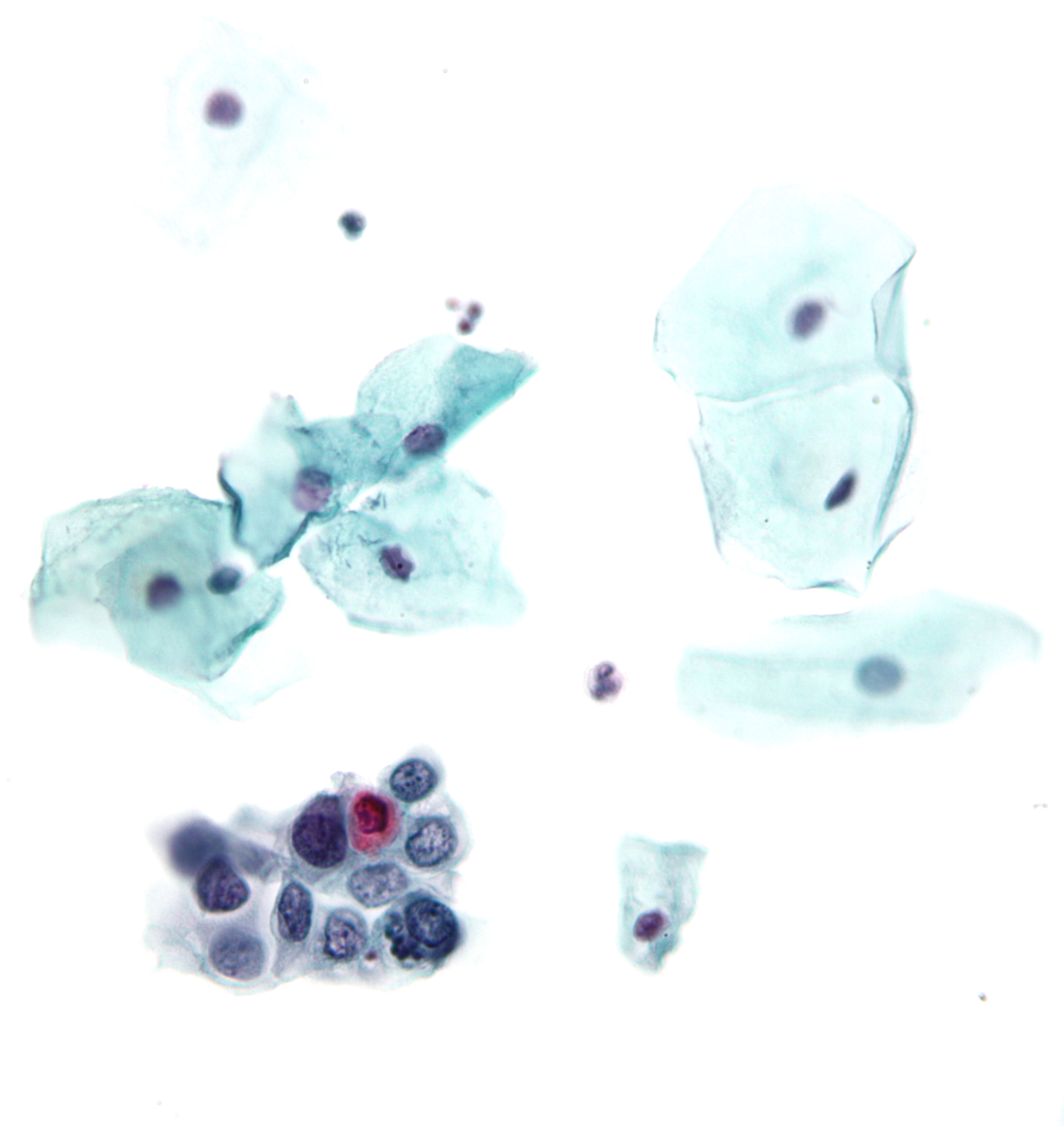
- Negative for intraepithelial lesion or malignancy (see Images. Pap with Negative for Intraepithelial Lesion or Malignancy and Second Pap with Negative for Intraepithelial Lesion or Malignancy)
- Non-neoplastic findings: Includes cellular variations, reactive cellular changes, organisms, and the presence of endometrial cells in a perimenopausal or postmenopausal patient
- Epithelial cell abnormalities
- Squamous cell abnormalities
- Atypical squamous cells of undetermined significance
- Atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion
- Low-grade squamous intraepithelial lesion: Encompassing HPV, mild dysplasia, and CIN I (see Image. Pap with Low-Grade Squamous Intraepithelial Lesion and Benign Endocervical Cells)
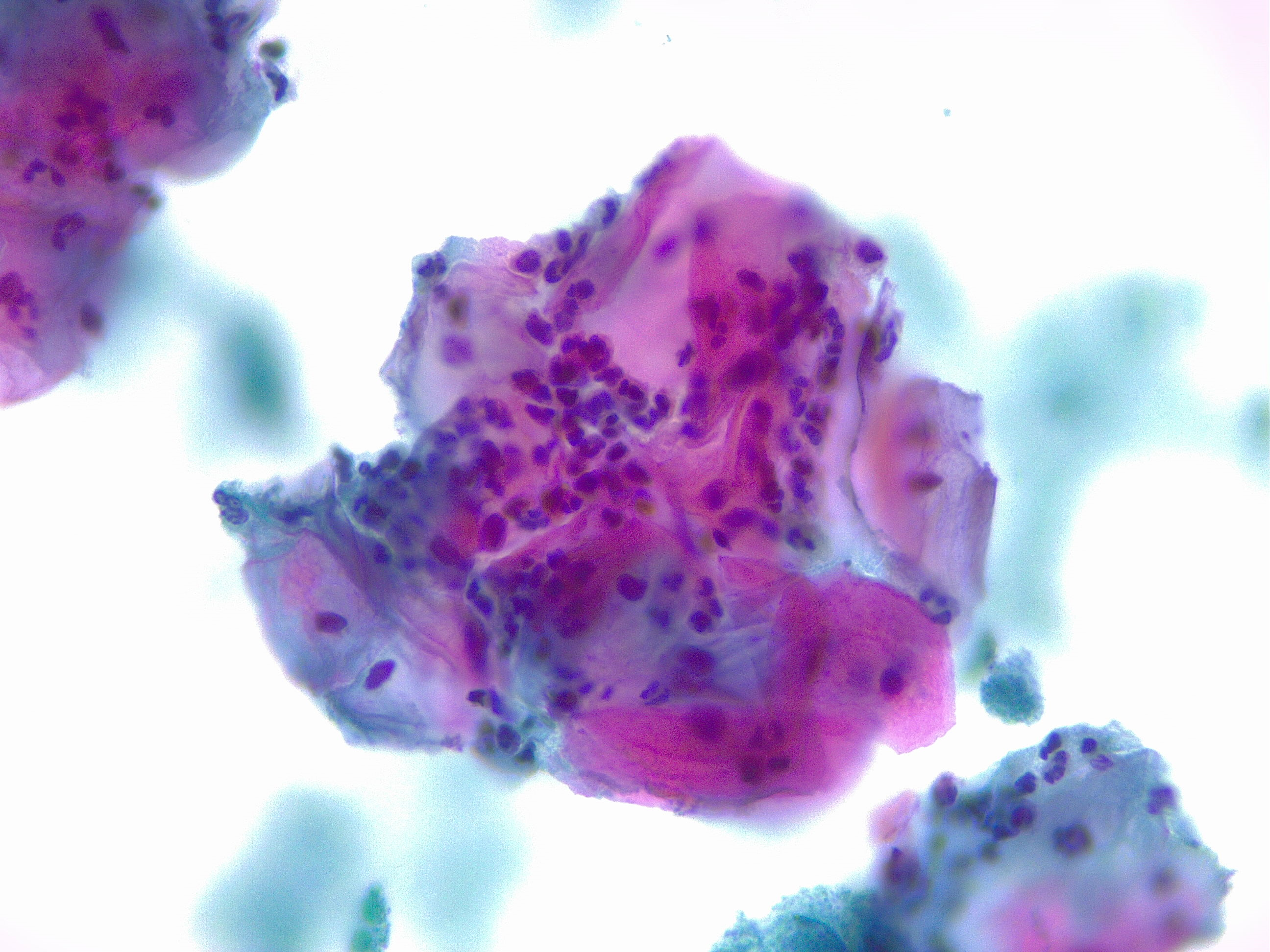
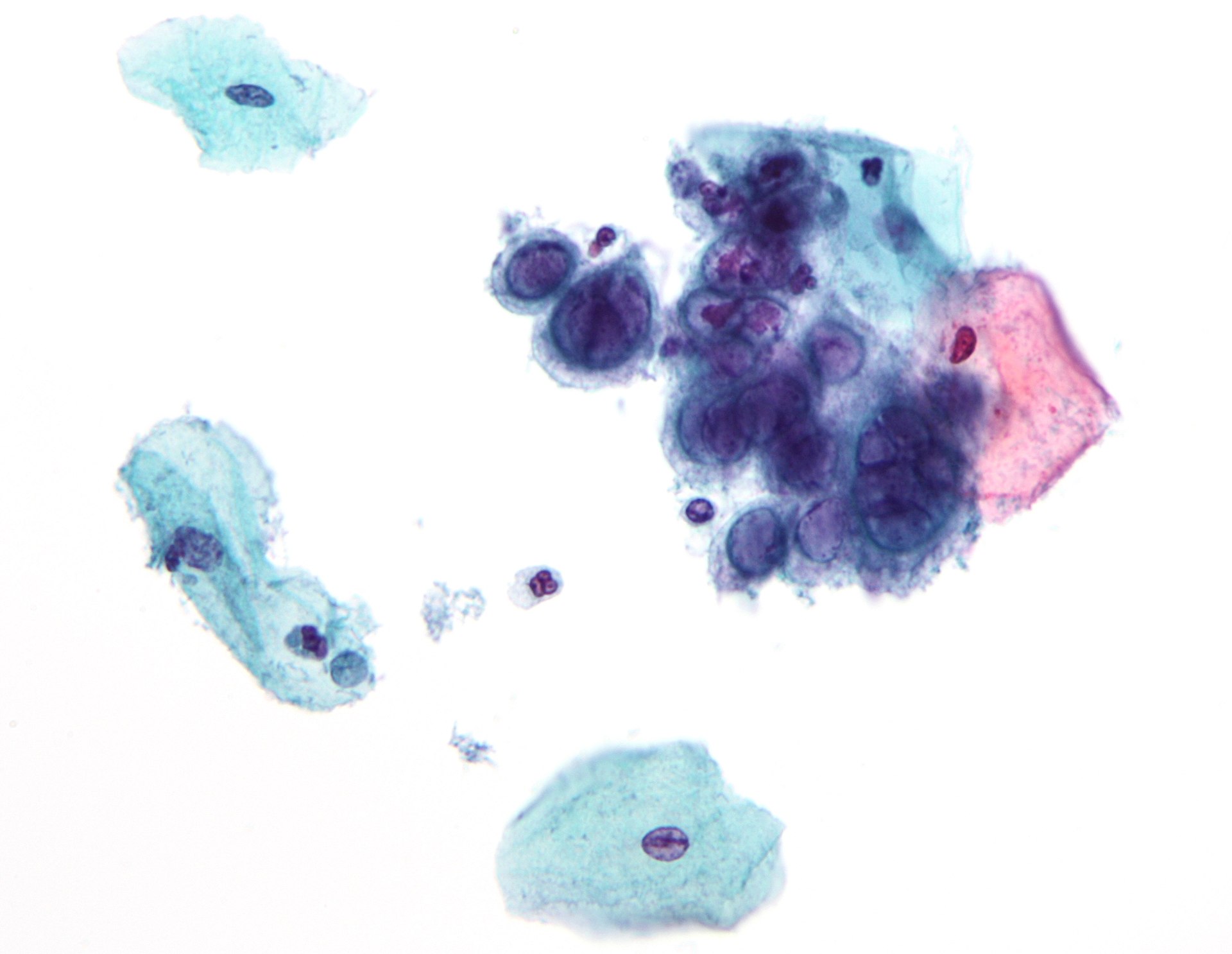
- High-grade squamous intraepithelial lesion: Encompassing moderate and severe dysplasia, CIS, CIN 2, and CIN 3 (see Image. Pap with High-Grade Squamous Intraepithelial Lesion)
- High-grade squamous intraepithelial lesion with features suspicious for invasion
- Squamous cell carcinoma
- Glandular cell abnormalities
- Atypical glandular endocervical cells (not otherwise specified or specify)
- Atypical glandular endometrial cells (not otherwise specified or specify)
- Atypical glandular cells (not otherwise specified or specify)
- Atypical glandular endocervical cells, favor neoplastic
- Atypical glandular cells, favor neoplastic
- Endocervical adenocarcinoma in situ
- Endocervical adenocarcinoma (see Image. Pap with Adenocarcinoma)
- Endometrial adenocarcinoma
- Extrauterine adenocarcinoma
- Adenocarcinoma not otherwise specified
- Other malignant neoplasms (specify)
- Adjunctive testing: Provides a brief description of the test method(s) and reports the results, if applicable
- Squamous cell abnormalities
- Computer-assisted interpretation of cervical cytology: If the patient is examined using an automated device, the report should specify the device and the result
- Educational notes and comments: Provide optional suggestions that should be concise and consistent with clinical follow-up guidelines published by professional organizations
Please see StatPearls' companion resource, "Cervical Intraepithelial Neoplasia," for more information.
Clinical Significance
After implementing organized cervical cancer screening programs using Pap tests, the incidence and mortality rates of cervical cancer significantly decreased. In the United States, the American Medical Association began recommending Pap screening in 1960 [4][Science History Institute. 21 Years, 7,600 Tests] and subsequently saw a more than 70% decline in incidence and mortality of cervical cancer.[4][16][19] Similar programs introduced in Finland, Sweden, Denmark, and Iceland in the 1960s [4][17][61] and in England in the 1980s [4][18][61] resulted in similar dramatic reductions in the incidence of cervical cancer.
Pap smears are generally considered to be a low-cost, reliable, and easy-to-perform test with a high degree of sensitivity and specificity.[10][19][NIH. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition] When combined with the recommended follow-up steps in management, Pap smears are now believed to prevent up to 80% of cases of invasive cervical cancer in the developed world.[3][20][21]
The success and utility of Pap smears can be demonstrated through the expansion of exfoliative cytopathology and cytological fluid analysis as a reliable technique to detect cancers and pre-cancers throughout the body, including the thyroid, lungs, bladder, and gastrointestinal tract.[19][62][NIH. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition]
Pap smears are now widely recognized as the most significant advancement in controlling cancer in the 20th century [12] and have been identified as the single most successful contribution to cancer screening and prevention of all time.[7] Dr Papanicolaou is considered one of the most eminent figures of 20th-century medicine.[8]
The widespread use of Pap tests identified the connection between cervical dysplasia and HPV, significantly influencing the drive for HPV vaccine research and development.[NIH. Preventing Cervical Cancer: The Development of HPV Vaccines][Cancer Research UK. Into the archives: the story of HPV and cervical cancer] Bivalent, quadrivalent, and nonavalent vaccines have since been developed. The 9-valent Gardasil 9 vaccine has eclipsed the bivalent (Cervarix) and quadrivalent (Gardasil) and has been the only HPV vaccine used in the United States since 2016. Gardasil 9 provides protection against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58, and it is FDA-approved for patients aged 9 to 45, regardless of biological sex.[63] Please see StatPearls' companion resource, "Human Papilloma Virus Vaccine," for more information.
Quality Control and Lab Safety
Maintaining rigorous quality control and laboratory safety is essential to ensure the accuracy and reliability of Pap test results. This approach involves standardized protocols for specimen collection, proper handling and transport, and stringent procedures during cytological analysis.
Quality of Collection
Key components of an effective cervical cancer screening program include patient access to care, trained healthcare professionals relevant to the screening, adequately collected and processed specimens, reliable systems for transporting specimens to laboratories, and mechanisms to ensure timely follow-up and treatment for patients with abnormal results. Common pitfalls include an overreliance on maternal and child health services for screening, opportunistic screening that often targets low-risk patients, inexperienced healthcare workers, which can result in a high risk of false-negative or false-positive results, and evaluating the number of screenings completed rather than the number of patients screened.[64][65]
Quality of the Specimen
The Bethesda System's criteria for adequacy encompass the quality and cellularity of the sample, with additional guidance for special situations, such as assessing cellularity in specimens obtained after radiation and the effects of interfering substances, including blood or lubricants. The presence of the transformation zone is an indicator of specimen adequacy, but it is not required for specimen adequacy. The recommended management after an unsatisfactory Pap is most commonly a repeat testing, typically within 2 to 4 months.[41][66][67]
Quality of Analysis
Automated, computer-assisted, and artificial intelligence–assisted cytopathological analysis is a growing area of research. Computer-assisted interpretation of cytology has been successfully attempted since the late 1990s. As of 1997, 2 systems have received regulatory approval by the FDA, including Papnet, a computerized tool that uses neural-network technology to identify slides initially read as negative to be rescreened due to likelihood of containing abnormal cells, and AutoPap, which uses an algorithm-based technology to identify slides likely to contain abnormal cells in contrast to random rescreening.[68]
Enhancing Healthcare Team Outcomes
The interprofessional team plays a vital role in improving patient care and outcomes related to Pap smears, cervical cancer screening, pelvic examinations, and overall gynecologic care. This collaborative approach involves various healthcare professionals, including obstetrician-gynecologists, family medicine physicians, outpatient internal medicine physicians, mid-level clinicians, nurses, laboratory technicians, and other healthcare professionals. Each member contributes unique expertise, enhancing the quality of care provided to patients.
Collaboration begins with clear communication among team members regarding screening guidelines, patient history, and individual risk factors for cervical cancer. This collaboration is fostered through the shared use of resources for existing screening recommendations and management. For example, the ASCCP has published a smartphone application titled ASCCP Management Guidelines App, which is updated regularly. This application has a user-friendly algorithm for screening guidelines and management recommendations. The application is available for Android and iOS platforms for a minimal fee.
All clinicians, including those who may not feel comfortable collecting Pap smears, should be able to discuss cervical cancer screening guidelines and management protocols. All healthcare team members can aid in implementing a reliable system for follow-up, especially for abnormal results. This system should include a combination of verbal and written notifications regarding the evaluation process and the importance of appropriate follow-up. Regular interdisciplinary meetings allow the team to review and discuss cases, share insights on interpreting Pap smear results, develop shared protocols for follow-up care based on the findings, and offer consistent communication with patients and families regarding the findings and next steps.
In addition, ongoing interprofessional continuing education initiatives enable healthcare professionals to stay current on best practices and emerging evidence. This shared learning experience fosters an environment where each team member understands their role in the care continuum and the importance of their contributions. By recognizing and valuing each team member's contributions, the interprofessional team can effectively flatten the hierarchy in health care, leading to improved patient outcomes and a more integrated approach to cervical cancer prevention and care.
Patient Safety and Education
Patients should receive education regarding the limitations of cervical cancer screening, including recommendations for Paps with cytopathology and HPV testing, screening timelines, and appropriate follow-up intervals. Annual gynecologic exams are recommended even if cervical cancer screening is not performed at the visit.[69][70]
Protection from cervical cancer is the primary goal of cervical cancer screening. However, patients should receive comprehensive counseling on additional strategies for reducing cervical cancer risk. This counseling includes education on modifiable risk factors, such as smoking, with emphasis on smoking cessation; safe sexual practices to reduce exposure to sexually transmitted infections; and adherence to routine HPV vaccination for all adolescents aged 9 to 12, in accordance with the CDC Advisory Committee on Immunization Practices guidelines.[71]
Media
(Click Image to Enlarge)
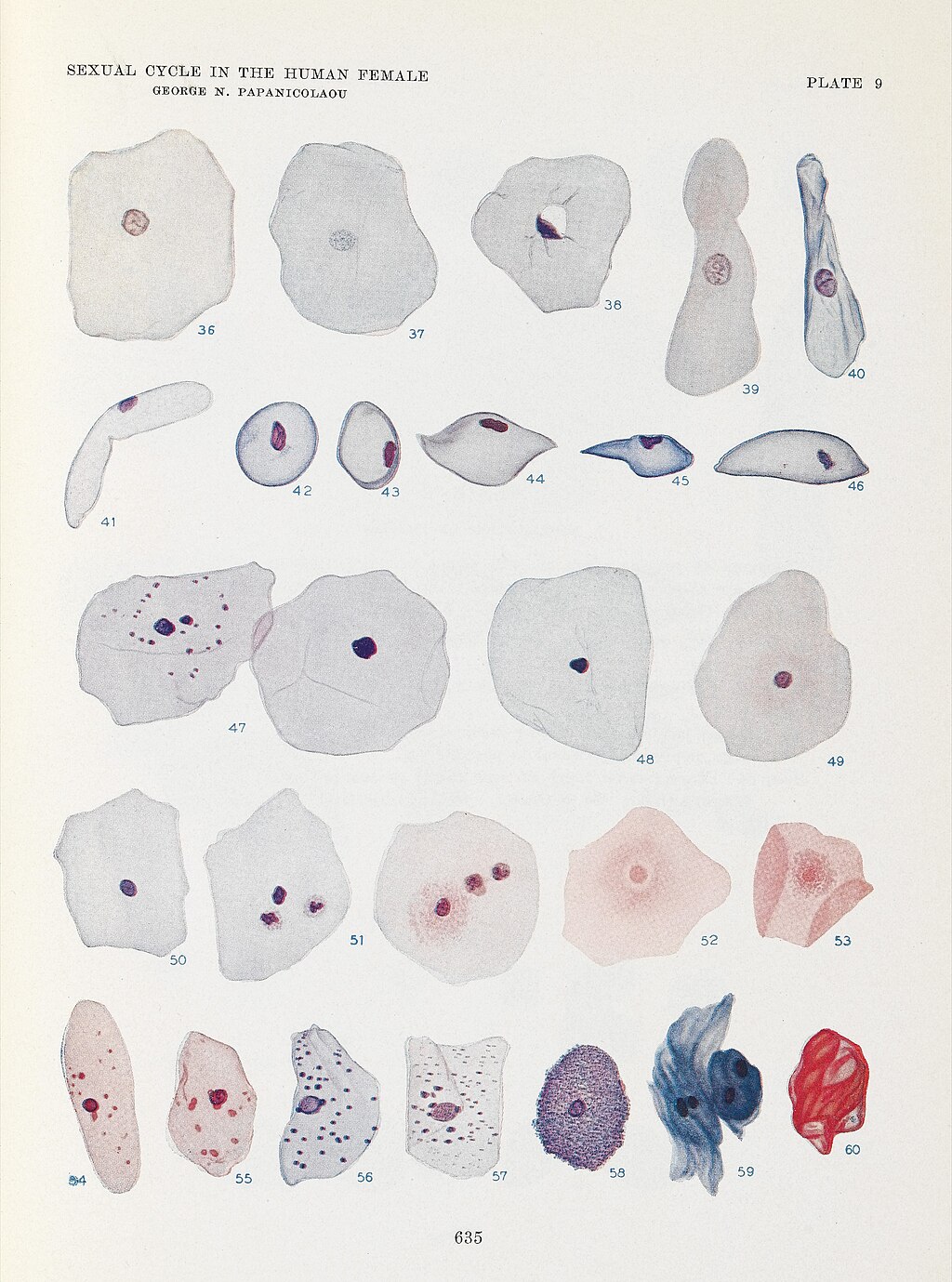
George N Papanicolaou Drawings of Various Cells Found in Human Vaginal Smears I.
Wellcome Collection, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
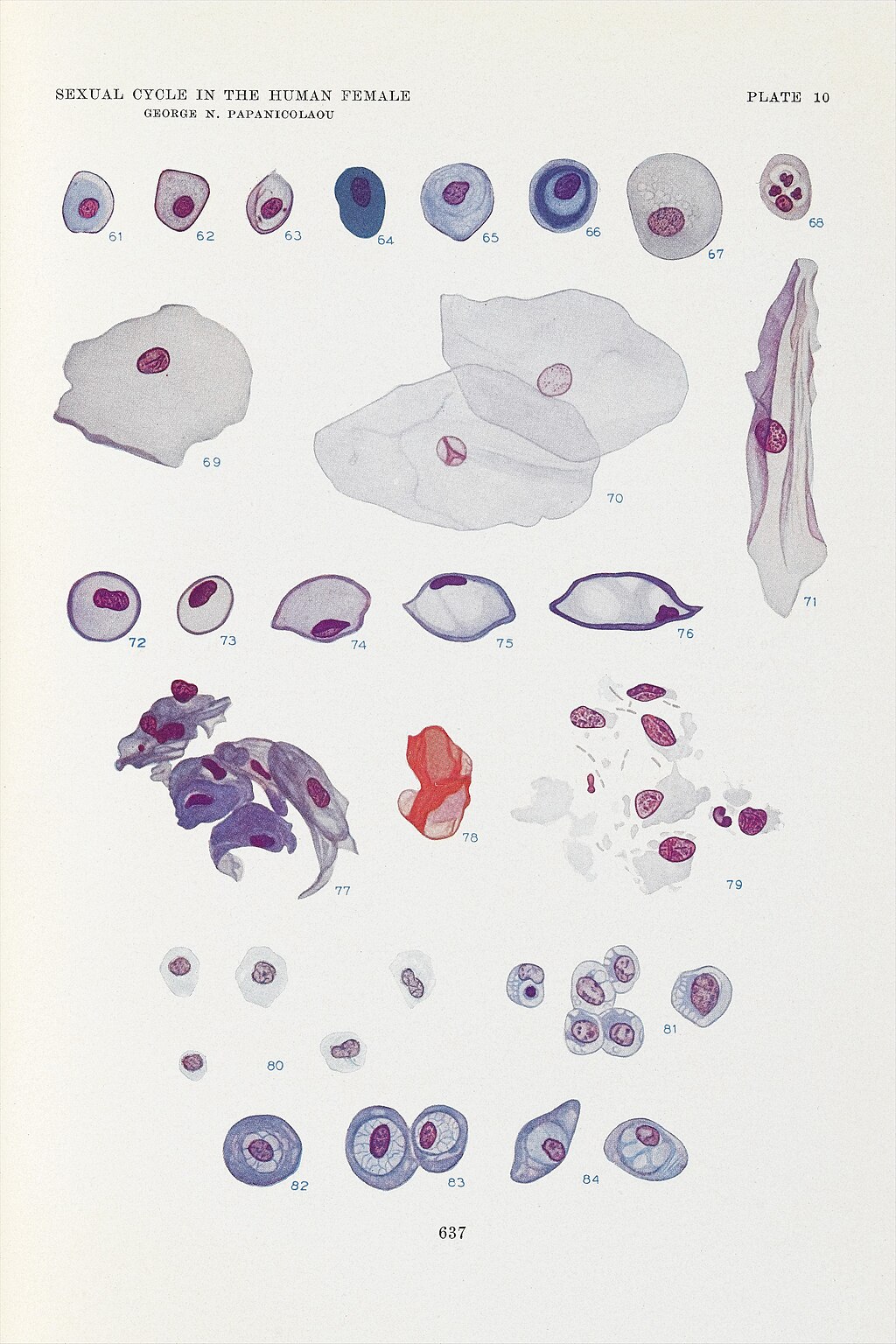
George N Papanicolaou Drawings of Various Cells Found in Human Vaginal Smears II.
Wellcome Collection, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
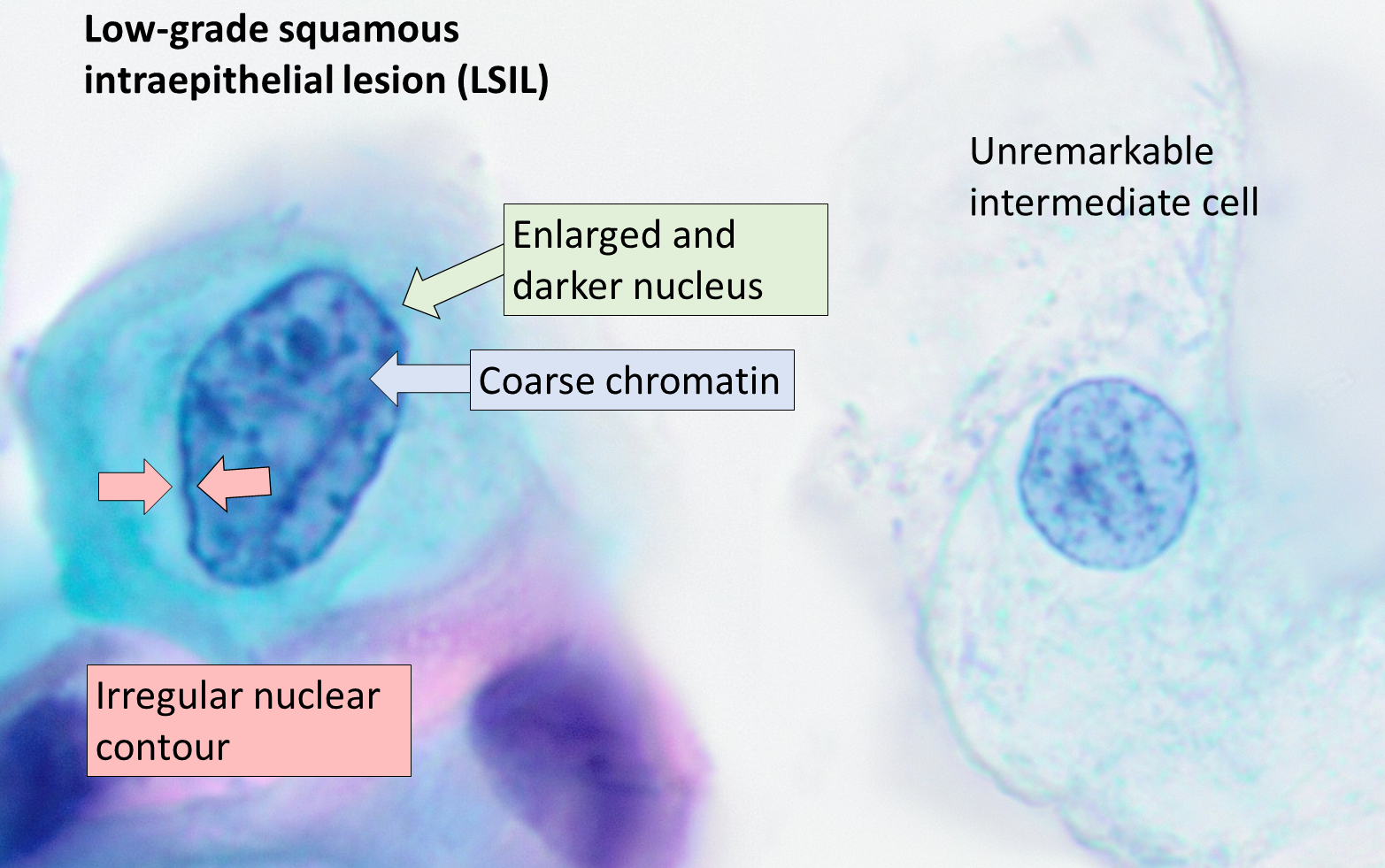
Low-Grade Squamous Intraepithelial Lesion Pap With Features of Koilocytosis Compared to Unremarkable Intermediate Squamous Cell.
Mikael Häggström, MD, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
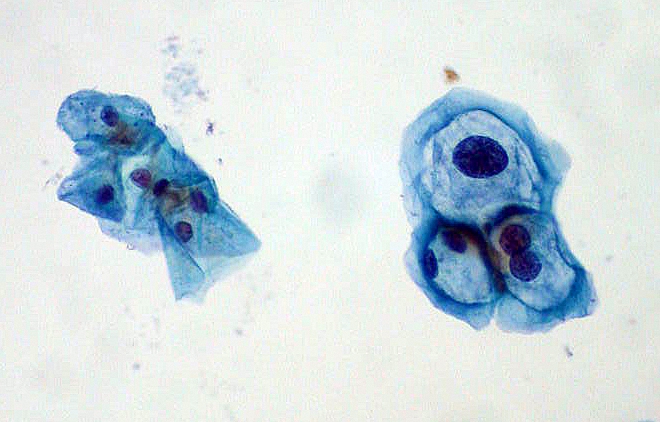
Squamous Epithelial Cells With and Without Koilocytosis.
Ed Uthman, MD, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
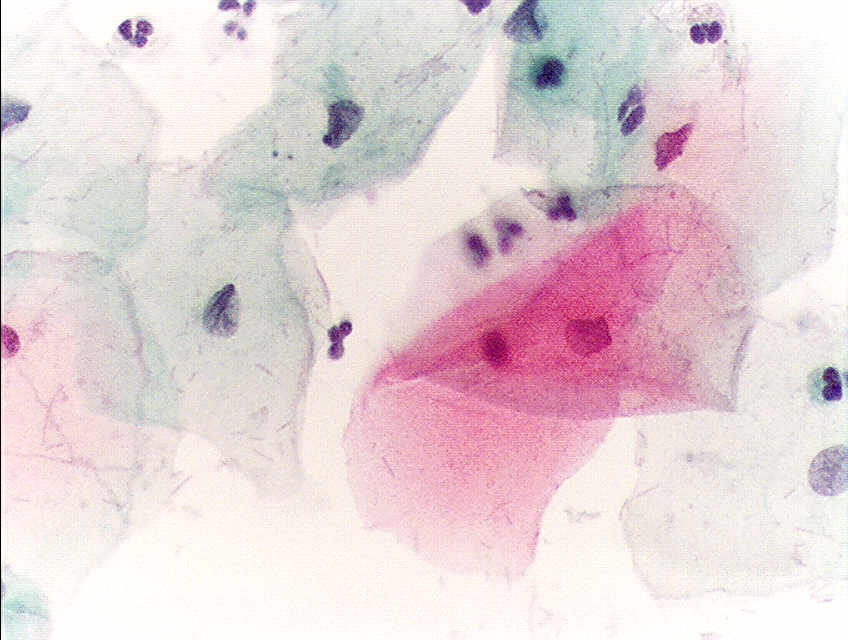
Pap With Negative for Intraepithelial Lesion or Malignancy. A Papainicolau stain at 400× magnification, revealing normal squamous epithelial cells in a premenopausal woman.
Alex Brollo, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
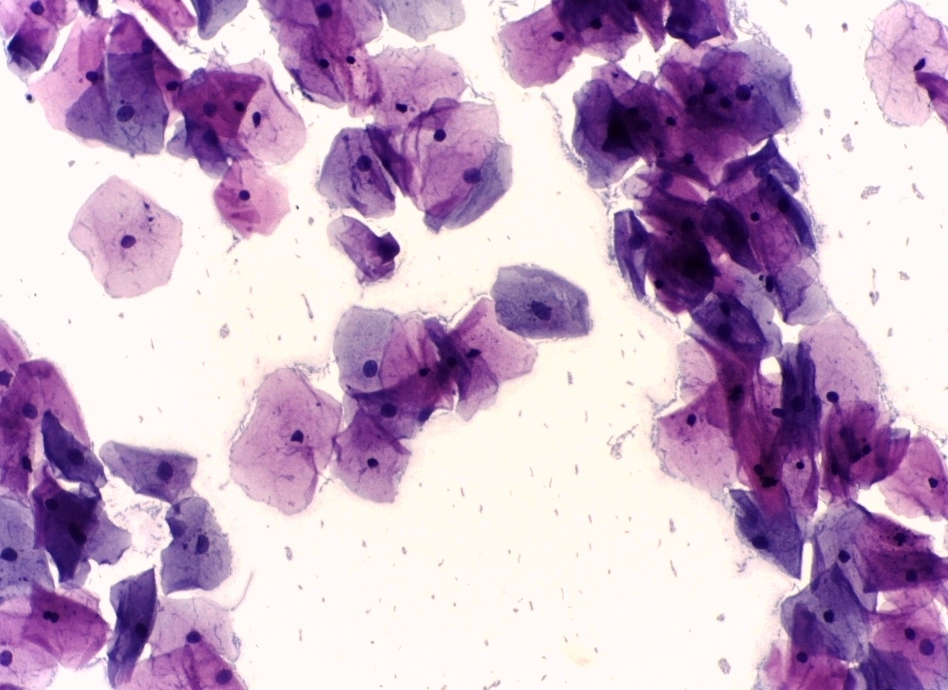
Second Pap With Negative for Intraepithelial Lesion or Malignancy.
Calicut Medical College Department of Pathology, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
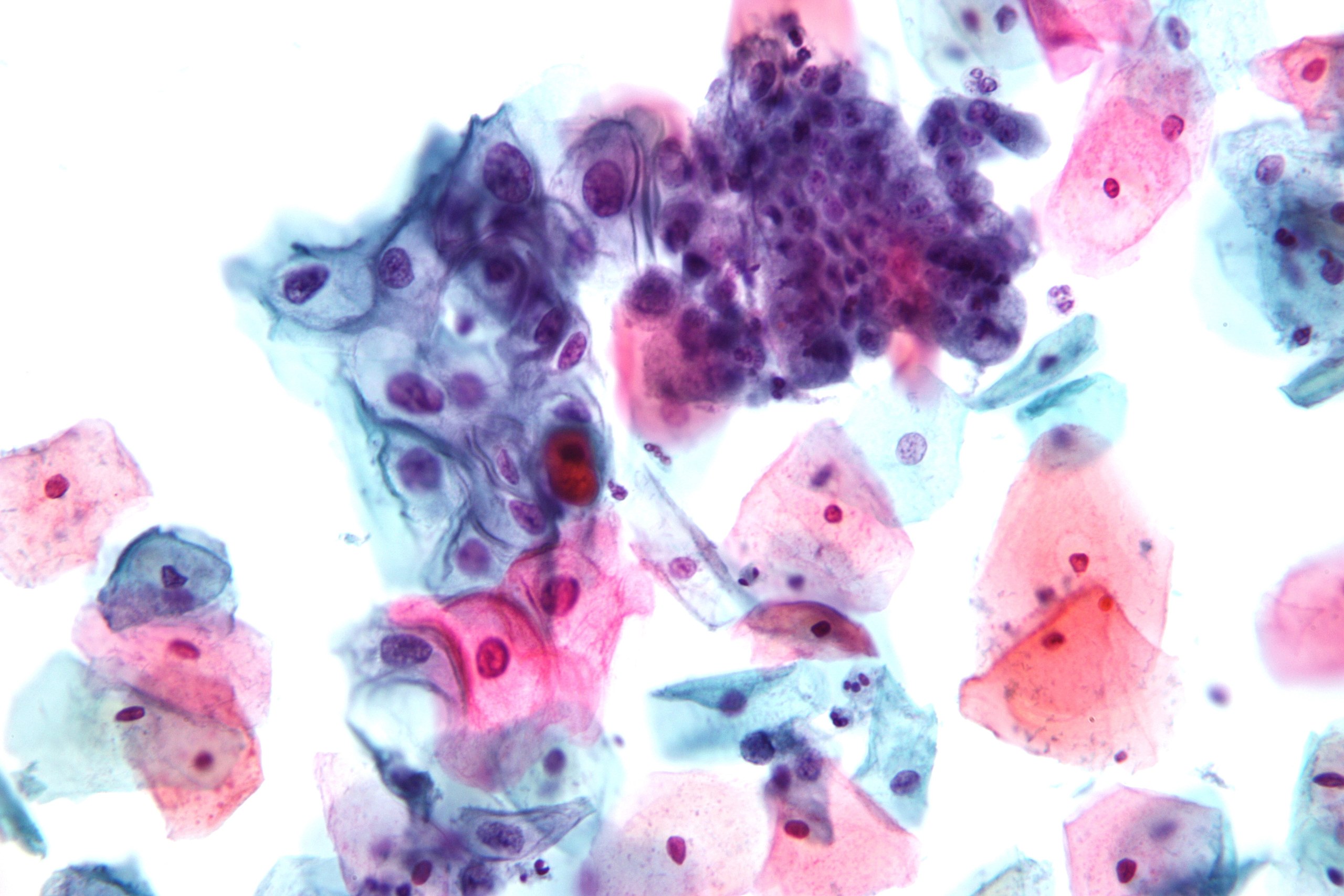
Pap With Low-Grade Squamous Intraepithelial Lesion and Benign Endocervical Cells.
Nephron, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Leyden WA, Manos MM, Geiger AM, Weinmann S, Mouchawar J, Bischoff K, Yood MU, Gilbert J, Taplin SH. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. Journal of the National Cancer Institute. 2005 May 4:97(9):675-83 [PubMed PMID: 15870438]
Level 2 (mid-level) evidenceChrysostomou AC, Stylianou DC, Constantinidou A, Kostrikis LG. Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses. 2018 Dec 19:10(12):. doi: 10.3390/v10120729. Epub 2018 Dec 19 [PubMed PMID: 30572620]
Okunade KS. Human papillomavirus and cervical cancer. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2020 Jul:40(5):602-608. doi: 10.1080/01443615.2019.1634030. Epub 2019 Sep 10 [PubMed PMID: 31500479]
Safaeian M, Solomon D, Castle PE. Cervical cancer prevention--cervical screening: science in evolution. Obstetrics and gynecology clinics of North America. 2007 Dec:34(4):739-60, ix [PubMed PMID: 18061867]
Dasgupta S. The Efficiency of Cervical Pap and Comparison of Conventional Pap Smear and Liquid-Based Cytology: A Review. Cureus. 2023 Nov:15(11):e48343. doi: 10.7759/cureus.48343. Epub 2023 Nov 6 [PubMed PMID: 38060751]
Dobbs SP, Asmussen T, Nunns D, Hollingworth J, Brown LJ, Ireland D. Does histological incomplete excision of cervical intraepithelial neoplasia following large loop excision of transformation zone increase recurrence rates? A six year cytological follow up. BJOG : an international journal of obstetrics and gynaecology. 2000 Oct:107(10):1298-301 [PubMed PMID: 11028584]
Dugas A, Persaud NA, Ganti L. Dr. George Papanicolaou: Inventor of the Pap Smear and Father of Modern Cytology. Cureus. 2024 Dec:16(12):e75106. doi: 10.7759/cureus.75106. Epub 2024 Dec 4 [PubMed PMID: 39759704]
Diamantis A, Magiorkinis E, Koutselini H. 50 years after the death of George Nicholas Papanicolaou (1883-1962): evaluation of his scientific work. Acta medico-historica adriatica : AMHA. 2014:12(1):181-8 [PubMed PMID: 25310617]
Diamantis A, Beloukas AI, Kalogeraki AM, Magiorkinis E. A brief chronicle of cytology: from Janssen to Papanicolaou and beyond. Diagnostic cytopathology. 2013 Jun:41(6):555-64. doi: 10.1002/dc.22887. Epub 2012 Jul 16 [PubMed PMID: 22807413]
Tan SY, Tatsumura Y. George Papanicolaou (1883-1962): Discoverer of the Pap smear. Singapore medical journal. 2015 Oct:56(10):586-7. doi: 10.11622/smedj.2015155. Epub [PubMed PMID: 26512152]
Chatterjee T, Gill SS, Rac R. STANDARDIZATION OF CERVICAL/VAGINAL CYTOPATHOLOGY REPORTING: THE BETHESDA SYSTEM (TBS) FOR REPORTING CERVICAL/VAGINAL CYTOLOGIC DIAGNOSES. Medical journal, Armed Forces India. 2000 Jan:56(1):45-49. doi: 10.1016/S0377-1237(17)30090-4. Epub 2017 Jun 8 [PubMed PMID: 28790644]
Vilos GA. The history of the Papanicolaou smear and the odyssey of George and Andromache Papanicolaou. Obstetrics and gynecology. 1998 Mar:91(3):479-83 [PubMed PMID: 9491881]
Koss LG. The Papanicolaou test for cervical cancer detection. A triumph and a tragedy. JAMA. 1989 Feb 3:261(5):737-43 [PubMed PMID: 2642983]
. Classes in oncology: George Nicholas Papanicolaou's new cancer diagnosis presented at the Third Race Betterment Conference, Battle Creek, Michigan, January 2-6, 1928, and published in the Proceedings of the Conference. CA: a cancer journal for clinicians. 1973 May-Jun:23(3):174-9 [PubMed PMID: 4196138]
Virtej P, Vasiliu C. Cytodiagnosis in cervical neoplasia: from the Babes/Papanicolaou smear to the actual Bethesda System. Clinical and experimental obstetrics & gynecology. 2003:30(4):173-7 [PubMed PMID: 14664403]
Wingo PA, Cardinez CJ, Landis SH, Greenlee RT, Ries LA, Anderson RN, Thun MJ. Long-term trends in cancer mortality in the United States, 1930-1998. Cancer. 2003 Jun 15:97(12 Suppl):3133-275 [PubMed PMID: 12784323]
Lăără E, Day NE, Hakama M. Trends in mortality from cervical cancer in the Nordic countries: association with organised screening programmes. Lancet (London, England). 1987 May 30:1(8544):1247-9 [PubMed PMID: 2884378]
Sasieni P, Adams J. Effect of screening on cervical cancer mortality in England and Wales: analysis of trends with an age period cohort model. BMJ (Clinical research ed.). 1999 May 8:318(7193):1244-5 [PubMed PMID: 10231253]
Palatianos GM, Cintron JR, Narula T. George N. Papanicolaou, M.D. Father of modern cytology. A 30-year commemorative. The Journal of the Florida Medical Association. 1992 Dec:79(12):837-8 [PubMed PMID: 1335475]
Gichangi P, Estambale B, Bwayo J, Rogo K, Ojwang S, Opiyo A, Temmerman M. Knowledge and practice about cervical cancer and Pap smear testing among patients at Kenyatta National Hospital, Nairobi, Kenya. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2003 Nov-Dec:13(6):827-33 [PubMed PMID: 14675320]
Level 2 (mid-level) evidenceKivistik A, Lang K, Baili P, Anttila A, Veerus P. Women's knowledge about cervical cancer risk factors, screening, and reasons for non-participation in cervical cancer screening programme in Estonia. BMC women's health. 2011 Sep 28:11():43. doi: 10.1186/1472-6874-11-43. Epub 2011 Sep 28 [PubMed PMID: 21951661]
Michael CW. The Papanicolaou smear and the obstetric patient: a simple test with great benefits. Diagnostic cytopathology. 1999 Jul:21(1):1-3 [PubMed PMID: 10405797]
Pimple S, Mishra G. Cancer cervix: Epidemiology and disease burden. CytoJournal. 2022:19():21. doi: 10.25259/CMAS_03_02_2021. Epub 2022 Mar 29 [PubMed PMID: 35510109]
Porras C, Rodríguez AC, Hildesheim A, Herrero R, González P, Wacholder S, Burk RD, Schiffman M. Human papillomavirus types by age in cervical cancer precursors: predominance of human papillomavirus 16 in young women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009 Mar:18(3):863-5. doi: 10.1158/1055-9965.EPI-08-0951. Epub [PubMed PMID: 19273486]
Xing B, Guo J, Sheng Y, Wu G, Zhao Y. Human Papillomavirus-Negative Cervical Cancer: A Comprehensive Review. Frontiers in oncology. 2020:10():606335. doi: 10.3389/fonc.2020.606335. Epub 2021 Feb 17 [PubMed PMID: 33680928]
Petca A, Borislavschi A, Zvanca ME, Petca RC, Sandru F, Dumitrascu MC. Non-sexual HPV transmission and role of vaccination for a better future (Review). Experimental and therapeutic medicine. 2020 Dec:20(6):186. doi: 10.3892/etm.2020.9316. Epub 2020 Oct 13 [PubMed PMID: 33101476]
Krawczyk E, Suprynowicz FA, Liu X, Dai Y, Hartmann DP, Hanover J, Schlegel R. Koilocytosis: a cooperative interaction between the human papillomavirus E5 and E6 oncoproteins. The American journal of pathology. 2008 Sep:173(3):682-8. doi: 10.2353/ajpath.2008.080280. Epub 2008 Aug 7 [PubMed PMID: 18688031]
Level 3 (low-level) evidenceSaslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, Cohen C, American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA: a cancer journal for clinicians. 2002 Nov-Dec:52(6):342-62 [PubMed PMID: 12469763]
Fontham ETH, Wolf AMD, Church TR, Etzioni R, Flowers CR, Herzig A, Guerra CE, Oeffinger KC, Shih YT, Walter LC, Kim JJ, Andrews KS, DeSantis CE, Fedewa SA, Manassaram-Baptiste D, Saslow D, Wender RC, Smith RA. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA: a cancer journal for clinicians. 2020 Sep:70(5):321-346. doi: 10.3322/caac.21628. Epub 2020 Jul 30 [PubMed PMID: 32729638]
Kumari S. Screening for Cervical Cancer in Pregnancy. Oncology reviews. 2023:17():11429. doi: 10.3389/or.2023.11429. Epub 2023 Oct 17 [PubMed PMID: 37915464]
Monteiro PB, Monteiro Filho MP, de Figueirêdo JT, Saintrain MVL, Bruno ZV, Carvalho FHC. Cytology-Based Screening During Antenatal Care as a Method for Preventing Cervical Cancer. Asian Pacific journal of cancer prevention : APJCP. 2017 Sep 27:18(9):2513-2518 [PubMed PMID: 28952289]
Dhillon N, Oliffe JL, Kelly MT, Krist J. Bridging Barriers to Cervical Cancer Screening in Transgender Men: A Scoping Review. American journal of men's health. 2020 May-Jun:14(3):1557988320925691. doi: 10.1177/1557988320925691. Epub [PubMed PMID: 32489142]
Level 2 (mid-level) evidenceLabanca T, Mañero I, Pannunzio M. Transgender patients: considerations for routine gynecologic care and cancer screening. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2020 Dec:30(12):1990-1996. doi: 10.1136/ijgc-2020-001860. Epub 2020 Oct 27 [PubMed PMID: 33109526]
Level 2 (mid-level) evidencePerkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, Huh WK, Kim JJ, Moscicki AB, Nayar R, Saraiya M, Sawaya GF, Wentzensen N, Schiffman M, 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines: Updates Through 2023. Journal of lower genital tract disease. 2024 Jan 1:28(1):3-6. doi: 10.1097/LGT.0000000000000788. Epub [PubMed PMID: 38117563]
Level 3 (low-level) evidenceUS Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 Aug 21:320(7):674-686. doi: 10.1001/jama.2018.10897. Epub [PubMed PMID: 30140884]
Sathawane P, Kamal MM, Deotale PR, Mankar H. Nuances of the Papanicolaou stain. CytoJournal. 2022:19():43. doi: 10.25259/CMAS_03_18_2021. Epub 2022 Jun 14 [PubMed PMID: 35928529]
Hashmi AA, Naz S, Ahmed O, Yaqeen SR, Irfan M, Asif MG, Kamal A, Faridi N. Comparison of Liquid-Based Cytology and Conventional Papanicolaou Smear for Cervical Cancer Screening: An Experience From Pakistan. Cureus. 2020 Dec 26:12(12):e12293. doi: 10.7759/cureus.12293. Epub 2020 Dec 26 [PubMed PMID: 33520497]
Makde MM, Sathawane P. Liquid-based cytology: Technical aspects. CytoJournal. 2022:19():41. doi: 10.25259/CMAS_03_16_2021. Epub 2022 Jun 14 [PubMed PMID: 35928530]
Patel N, Bavikar R, Buch A, Kulkarni M, Dharwadkar A, Viswanathan V. A Comparison of Conventional Pap Smear and Liquid-Based Cytology for Cervical Cancer Screening. Gynecology and minimally invasive therapy. 2023 Apr-Jun:12(2):77-82. doi: 10.4103/gmit.gmit_118_22. Epub 2023 May 18 [PubMed PMID: 37416097]
Siebers AG, Klinkhamer PJ, Grefte JM, Massuger LF, Vedder JE, Beijers-Broos A, Bulten J, Arbyn M. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: a randomized controlled trial. JAMA. 2009 Oct 28:302(16):1757-64. doi: 10.1001/jama.2009.1569. Epub [PubMed PMID: 19861667]
Level 1 (high-level) evidenceArbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, Wiener H, Herbert A, von Karsa L. European Guidelines for Quality Assurance in Cervical Cancer Screening. Second edition--summary document. Annals of oncology : official journal of the European Society for Medical Oncology. 2010 Mar:21(3):448-458. doi: 10.1093/annonc/mdp471. Epub [PubMed PMID: 20176693]
Level 2 (mid-level) evidenceHathaway JK, Pathak PK, Maney R. Is liquid-based pap testing affected by water-based lubricant? Obstetrics and gynecology. 2006 Jan:107(1):66-70 [PubMed PMID: 16394041]
Pawlik M, Martin FJ. Does a water-based lubricant affect Pap smear and cervical microbiology results? Canadian family physician Medecin de famille canadien. 2009 Apr:55(4):376-7 [PubMed PMID: 19366945]
Arrivillaga M, Bermúdez PC, García-Cifuentes JP, Rodríguez-López M, Neira D, Vargas-Cardona HD. Women's critical experiences with the pap smear for the development of cervical cancer screening devices. Heliyon. 2023 Mar:9(3):e14289. doi: 10.1016/j.heliyon.2023.e14289. Epub 2023 Mar 7 [PubMed PMID: 36938419]
Hoyo C, Yarnall KS, Skinner CS, Moorman PG, Sellers D, Reid L. Pain predicts non-adherence to pap smear screening among middle-aged African American women. Preventive medicine. 2005 Aug:41(2):439-45 [PubMed PMID: 15917039]
Limmer K, LoBiondo-Wood G, Dains J. Predictors of cervical cancer screening adherence in the United States: a systematic review. Journal of the advanced practitioner in oncology. 2014 Jan:5(1):31-41 [PubMed PMID: 25032031]
Level 1 (high-level) evidenceWentzensen N, Clarke MA, Perkins RB. Impact of COVID-19 on cervical cancer screening: Challenges and opportunities to improving resilience and reduce disparities. Preventive medicine. 2021 Oct:151():106596. doi: 10.1016/j.ypmed.2021.106596. Epub 2021 Jun 30 [PubMed PMID: 34217415]
Moshkovich O, Lebrun-Harris L, Makaroff L, Chidambaran P, Chung M, Sripipatana A, Lin SC. Challenges and Opportunities to Improve Cervical Cancer Screening Rates in US Health Centers through Patient-Centered Medical Home Transformation. Advances in preventive medicine. 2015:2015():182073. doi: 10.1155/2015/182073. Epub 2015 Jan 21 [PubMed PMID: 25685561]
Level 3 (low-level) evidenceAmerican College of Obstetricians and Gynecologists’ Committee on Adolescent Health Care. The Initial Reproductive Health Visit: ACOG Committee Opinion, Number 811. Obstetrics and gynecology. 2020 Oct:136(4):e70-e80. doi: 10.1097/AOG.0000000000004094. Epub [PubMed PMID: 32976378]
Level 3 (low-level) evidenceNijhuis ER, Reesink-Peters N, Wisman GB, Nijman HW, van Zanden J, Volders H, Hollema H, Suurmeijer AJ, Schuuring E, van der Zee AG. An overview of innovative techniques to improve cervical cancer screening. Cellular oncology : the official journal of the International Society for Cellular Oncology. 2006:28(5-6):233-46 [PubMed PMID: 17167177]
Level 3 (low-level) evidenceSingla AA, Komesaroff P. Self-collected Pap smears may provide an acceptable and effective method of cervical cancer screening. Health science reports. 2018 May:1(5):e33. doi: 10.1002/hsr2.33. Epub 2018 Apr 6 [PubMed PMID: 30623069]
. The 1988 Bethesda System for reporting cervical/vaginal cytological diagnoses. National Cancer Institute Workshop. JAMA. 1989 Aug 18:262(7):931-4 [PubMed PMID: 2754794]
Pangarkar MA. The Bethesda System for reporting cervical cytology. CytoJournal. 2022:19():28. doi: 10.25259/CMAS_03_07_2021. Epub 2022 Apr 30 [PubMed PMID: 35673697]
Kurman RJ, Malkasian GD Jr, Sedlis A, Solomon D. From Papanicolaou to Bethesda: the rationale for a new cervical cytologic classification. Obstetrics and gynecology. 1991 May:77(5):779-82 [PubMed PMID: 1849626]
Nayar R, Wilbur DC. The Bethesda System for Reporting Cervical Cytology: A Historical Perspective. Acta cytologica. 2017:61(4-5):359-372. doi: 10.1159/000477556. Epub 2017 Jul 11 [PubMed PMID: 28693017]
Level 3 (low-level) evidenceHsieh MC, Van Dyne E, Lefante C, Shapiro JA, Pordell P, Lynch MA, Gomez N, Mumphrey B, Maniscalco L, Jetly-Shridhar R, Saraiya M, Wu XC. Evaluating the Use of LAST 2-Tiered Nomenclature and Its Impact on Reporting Cervical Lesions in a Population-Based Cancer Registry. Journal of registry management. 2019 Winter:46(4):120-127 [PubMed PMID: 32822336]
Darragh TM, Colgan TJ, Thomas Cox J, Heller DS, Henry MR, Luff RD, McCalmont T, Nayar R, Palefsky JM, Stoler MH, Wilkinson EJ, Zaino RJ, Wilbur DC, Members of the LAST Project Work Groups. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2013 Jan:32(1):76-115. doi: 10.1097/PGP.0b013e31826916c7. Epub [PubMed PMID: 23202792]
Level 2 (mid-level) evidenceWaxman AG, Chelmow D, Darragh TM, Lawson H, Moscicki AB. Revised terminology for cervical histopathology and its implications for management of high-grade squamous intraepithelial lesions of the cervix. Obstetrics and gynecology. 2012 Dec:120(6):1465-71 [PubMed PMID: 23168774]
Wang T, Zhang H, Liu Y, Zhao C. Updates in Cervical Cancer Screening Guidelines, The Bethesda System for Reporting Cervical Cytology, and Clinical Management Recommendations. Journal of clinical and translational pathology. 2023 Jun:3(2):75-83. doi: 10.14218/jctp.2023.00004. Epub 2023 Apr 21 [PubMed PMID: 37456763]
Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, Huh WK, Kim JJ, Moscicki AB, Nayar R, Saraiya M, Sawaya GF, Wentzensen N, Schiffman M, 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. Journal of lower genital tract disease. 2020 Apr:24(2):102-131. doi: 10.1097/LGT.0000000000000525. Epub [PubMed PMID: 32243307]
Level 3 (low-level) evidenceKitchener HC, Castle PE, Cox JT. Chapter 7: Achievements and limitations of cervical cytology screening. Vaccine. 2006 Aug 31:24 Suppl 3():S3/63-70 [PubMed PMID: 16950019]
Avdulla CS, Tachirai N. George N. Papanicolaou (1883-1962): The Pioneer of Cytology and Early Cancer Detection. Cureus. 2024 Sep:16(9):e68999. doi: 10.7759/cureus.68999. Epub 2024 Sep 9 [PubMed PMID: 39385916]
Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR. Morbidity and mortality weekly report. 2019 Aug 16:68(32):698-702. doi: 10.15585/mmwr.mm6832a3. Epub 2019 Aug 16 [PubMed PMID: 31415491]
Cronjé HS. Cervical screening strategies in resourced and resource-constrained countries. Best practice & research. Clinical obstetrics & gynaecology. 2011 Oct:25(5):575-84. doi: 10.1016/j.bpobgyn.2011.05.002. Epub [PubMed PMID: 21689987]
zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nature reviews. Cancer. 2002 May:2(5):342-50 [PubMed PMID: 12044010]
Jordan J, Arbyn M, Martin-Hirsch P, Schenck U, Baldauf JJ, Da Silva D, Anttila A, Nieminen P, Prendiville W. European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology : official journal of the British Society for Clinical Cytology. 2008 Dec:19(6):342-54. doi: 10.1111/j.1365-2303.2008.00623.x. Epub [PubMed PMID: 19040546]
Level 2 (mid-level) evidenceDavey DD, Austin RM, Birdsong G, Buck HW, Cox JT, Darragh TM, Elgert PA, Hanson V, Henry MR, Waldman J. ASCCP Patient Management Guidelines: Pap Test Specimen Adequacy and Quality Indicators. Journal of lower genital tract disease. 2002 Jul:6(3):195-9 [PubMed PMID: 17051020]
Level 2 (mid-level) evidenceMcCrory DC, Matchar DB, Bastian L, Datta S, Hasselblad V, Hickey J, Myers E, Nanda K. Evaluation of cervical cytology. Evidence report/technology assessment (Summary). 1999 Jan:(5):1-6 [PubMed PMID: 11925972]
. Practice Bulletin No. 168: Cervical Cancer Screening and Prevention. Obstetrics and gynecology. 2016 Oct:128(4):e111-e130. doi: 10.1097/AOG.0000000000001708. Epub [PubMed PMID: 27661651]
Committee on Gynecologic Practice. Committee opinion No. 534: well-woman visit. Obstetrics and gynecology. 2012 Aug:120(2 Pt 1):421-4. doi: 10.1097/AOG.0b013e3182680517. Epub [PubMed PMID: 22825111]
Level 3 (low-level) evidencePetrosky E, Bocchini JA Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE, Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR. Morbidity and mortality weekly report. 2015 Mar 27:64(11):300-4 [PubMed PMID: 25811679]