Introduction
Peripheral nerve neuralgia or peripheral neuropathic pain may result from damage of a nerve due to various etiologies, including medical conditions such as diabetes, infections (eg, postherpetic neuralgia), kidney diseases, or nerve compressions such as entrapment, peripheral nerve injury due to trauma, cancer, or a combination of the above. Although treatment strategies for neuralgia usually start with pharmacotherapy with medications such as membrane-stabilizing agents (eg, gabapentin or pregabalin), anticonvulsants (eg, carbamazepine, topiramate, and lamotrigine), antidepressants (eg, amitriptyline), and muscle relaxants (eg, baclofen) to reduce the excitability of the peripheral nerve and central connections,[1] drug treatment often fails to obtain effective results and can expose the patient to adverse events with poor improvement in the quality of life. Therefore, it is often necessary to resort to nonpharmacological strategies such as neurolytic blocks. These approaches, however, are not only applicable when pharmacological strategies have failed but are to be integrated into the context of multimodal schemes.[2] Moreover, some types of painful conditions, such as pain from pancreatic neoplasia, require the early application of minimally invasive analgesic techniques to manage symptoms effectively.[3]
A neurolytic block involves the deliberate injury of a nerve by freezing, heating, or applying chemicals to cause a temporary degeneration of targeted nerve fibers, causing an interruption in the signal nerve transmission. In particular, neurolysis implies the destruction of neurons by placing a needle close to the nerve and either injecting neurodestructive chemical agents or producing damage with a physical method such as cold (ie, cryotherapy) or heat (ie, radiofrequency ablation).
Neurolytic blocks can be seen as a natural advancement from neurotomy. Neurotomy involves the transection or partial resection of a nerve, typically performed on small peripheral nerves that are exclusively sensory. This technique has historically been applied for treating conditions such as trigeminal neuralgia and pelvic pain syndrome (presacral neurotomy), as well as nonpainful conditions like spastic dysfunction of the elbow. However, as the surgical cutting of a nerve may lead to complications such as painful neuromas or differentiation over time, neurolytic approaches are generally preferred over surgical ones.
Neurolytic blocks are not a recent discovery. The first report of chemical neurolysis for treating pain was made in 1863 by Luton, who administered neurolytic agents into painful areas. Neural blockade with neurolytic agents has been documented for treating pain for over a century. In 1904, Schloesser was the first to report alcohol neurolysis for treating trigeminal neuralgia.[4] In 1928, Doppler used phenol neurolysis to destroy presacral sympathetic nerves for the treatment of pelvic pain.
Currently, the specialty of pain medicine defines neurolysis as the selective, iatrogenic destruction of neural tissue aimed at alleviating pain. As understanding of nervous system pathophysiology has deepened and techniques and tools have been refined, the applications for these techniques have expanded. For instance, advances in medical imaging have enhanced the precision and efficacy of interventional pain management. As a result, using peripheral neural blockade and neuro-destructive techniques has increased for the treatment of chronic intractable pain.[5] Additionally, peripheral nerve blockade is now recognized as a valuable treatment for muscle spasticity.[6]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Chemical and surgical neurolysis can have serious adverse events, so a thorough knowledge of the relevant anatomy and the mechanisms by which the neurolytic agent destroys nerve tissue is essential. The choice of anatomy for neurolysis depends on the sensory territory intended for the blockade. Common targets include peripheral nerves, sympathetic ganglia, and dorsal roots. The neurolytic blockade is typically guided by techniques such as ultrasonography, fluoroscopy, or nerve stimulation. Before proceeding with a neurolytic block, performing a diagnostic block using a local anesthetic is critical to assess the correct location of the intended neurolysis. Successful application of a neurolytic block requires a strong understanding of anatomy, meticulous management of potential side effects, and selecting appropriate block techniques.[7]
Indications
Proper selection of patients for neurolytic blocks is the key to the success of these potentially harmful procedures. Clear communication of alternative techniques, outcomes, complications, expectations, and disease progression with the patient and the family is important before a neurolytic procedure. An interprofessional approach, including an aggressive trial of opioids and adjuvant medications, along with temporary nerve blocks and psychological support, are the mainstays of therapy. A thorough medical examination, including laboratory testing and imaging studies, if appropriate, is necessary before performing this procedure. Neurolytic blocks are also valuable for differential diagnosis, as they help pinpoint the location and cause of pain and assess the patient's response to pain relief. When properly administered, these blocks can be used to predict the outcomes of sustained interruption, whether through the injection of neurolytic agents or neurosurgical transection.
Upper abdominal malignancies or chronic pancreatitis: Patients with intractable pain due to upper abdominal malignancies or chronic pancreatitis often experience a significant decrease in quality of life and may require high doses of opioids, leading to severe adverse events.[8] The percutaneous neurolytic celiac plexus block (NCPB) has been shown to reduce narcotic use by 70% to 90% in these patients.[9] Results from a meta-analysis covering 24 studies reported good to excellent pain relief in 89% of patients within the first 2 weeks after NCPB, with benefits persisting beyond 3 months.[10]
Trigeminal neuralgia: One of the most common forms of craniofacial pain, trigeminal neuralgia is characterized by sudden, brief, and excruciating facial pain attacks in 1 or more branches of the trigeminal nerve.[11] This pain may lead to a severe reduction in the quality of life of affected patients. Internal neurolysis is an effective surgical treatment for trigeminal neuralgia without neurovascular compression.[12]
Chest wall pain: Intercostal nerve block by chemical neurolysis with phenol shows promise as a therapeutic option in patients with intractable cancer-associated chest wall pain or postsurgical thoracic pain.[13]
Pelvic cancer pain: Pain associated with pelvic malignancies can be treated by superior hypogastric plexus blockade or neurolysis.[14]
Facetogenic and vertebral pain: Pain originating from the facet joints and vertebrae may be effectively managed with neurolysis of the medial branch of the primary dorsal ramus. This technique specifically targets the nerves supplying these structures, offering significant pain relief.[15]
Other indications: Ultrasound-guided alcohol ablation has been described to treat Morton neuroma, and several studies have been performed describing the safety and efficacy of alcohol ablation in these cases.[16] Additionally, alcohol neurolysis of the lateral femoral cutaneous nerve has been described to treat meralgia paresthetica.[17]
Contraindications
Absolute contraindications to a peripheral nerve blockade are rare. They include:
- Patient refusal
- Skin infection over the intended catheter or needle site
- Allergy to a chemical neurolytic agent
Relative contraindications include:
- Coagulopathy: Oral anticoagulants should be discontinued until the international normalized ratio is reduced to 1.5 or less. Correcting coagulopathy is especially important if the injection is planned at a site that cannot be easily compressed to control bleeding.
- Electronic implants: Patients with pacemakers or other electronic implants must be evaluated before procedures like radiofrequency ablation to prevent device interference or malfunction.
Equipment
The following equipment is necessary to perform a peripheral nerve blockade:
Antiseptic agents: Chlorhexidine gluconate or povidone-iodine for skin preparation
Guidance equipment: Based on the procedural type, an ultrasound probe with a sterile cover and gel, fluoroscopy equipment, and a nerve stimulator should be used as needed.
Injection tools:
- Local anesthetics: One pecent lidocaine for local anesthesia and 2% lidocaine or 1.5% mepivacaine for diagnostic blocks
- Syringes and needles: Ten to 20 mL syringe with extension tubing; block needles variable in length depending on the depth and location of the targeted nerve or spinal/epidural needles if a neuraxial approach is used.
- Chemical neurolytic agents: Includes 50% to 100% alcohol, 5% to 15% phenol, and other agents like hypertonic saline, glycerol, ammonium salt solutions, and chlorocresol.[18]
Ablation devices:
- Radiofrequency probes for nerve ablation procedures such as medial branch nerve ablation, percutaneous cordotomy, and thermocoagulation
- Cryomachines and cryoprobes with cooling agents like nitrous oxide or carbon dioxide are used for cryotherapy.
Monitoring equipment: Basic monitoring tools, including electrocardiogram, noninvasive blood pressure monitoring, pulse oximetry, and end-tidal carbon dioxide monitoring, are essential throughout the procedure.
Personnel
Neurolytic blocks should be performed in specialized interventional pain procedure rooms staffed by pain medicine specialists trained in ultrasound- and fluoroscopic-guided nerve injections. Additionally, trained nursing staff skilled in sedation anesthesia should be available to assist during the procedures.
Preparation
Patient preparation must be carried out considering that the procedure, although well-tolerated and safe in most cases, can still lead to serious complications:
- Informed consent is mandatory before conducting the intervention. The consent must contain details of the procedure and possible complications (possibly with frequency).
- An intravenous line should be placed.
- Aseptic technique should be maintained throughout the procedure.
- The position of the patient depends on the procedure to be performed.
- Sedation may be necessary for certain painful acts, such as chemical neurolysis. Although, excessive sedation of the patient should be avoided to allow patients to remain alert enough to report symptoms suggestive of any complication.[19]
Technique or Treatment
Neurolysis procedures generally follow a 3-phase approach:
- Preparation and initial identification: Under aseptic conditions and continuous cardiovascular monitoring, the target nerve is identified using ultrasound, nerve stimulation, or fluoroscopy. A local anesthetic is then administered to the skin and subcutaneous tissues over the target area to minimize patient discomfort during the procedure.
- Diagnostic and confirmatory phase: A needle attached to a radiofrequency probe or cryoprobe is advanced toward the identified nerve. A diagnostic block using a local anesthetic is performed to confirm the correct targeting of the nerve.
- Execution of neurolysis: Once the diagnostic block is confirmed, the neurolysis procedure is carried out. In the case of radiofrequency ablation (RFA), a microelectrode is inserted through the needle to begin the neurolysis. If chemical neurolysis is to be performed, the prepared chemical solution, like phenol, is injected. Because phenol has immediate local anesthetic effects, injection is typically painless.
Neurolysis Procedure Types
- Chemical neurolysis utilizes both nonselective agents and neuroselective toxins to manage pain. Nonselective agents such as phenol, ethyl alcohol, and glycerol 50% work by causing protein denaturation and Wallerian degeneration, which provide long-lasting analgesia typically lasting between 3 to 6 months.[20] These agents act by nonselectively denaturing proteins, which results in the loss of cellular contents and leads to structural disruption of the nerve. On the other hand, neuroselective toxins target specific nerve functions to alleviate pain. Capsaicin, a selective agonist for the transient receptor potential 1 receptor on unmyelinated C fiber nociceptors, induces desensitization by triggering an influx of calcium and sodium ions.[21] Similarly, botulinum toxins impede the release of neurotransmitters such as substance P, calctonin gene-related peptide, and glutamate, which effectively diminishes pain signaling.[22]
- Cryoneurotomy uses nitrogen or argon gas to generate extreme cold, selectively destroying diseased tissue while maintaining the structural integrity of the nerve.[23] Unlike surgical neurectomy, which amputates part of the nerve and often results in severe dysaesthetia, cryoneurotomy offers sustainable pain control, typically without permanent damage to the nerve structure.[24][25]
- Radiofrequency ablation encompasses various treatment methods that target nerve tissue to treat pain and other conditions. RFA uses medium-frequency alternating current to heat small areas of nerve tissue, effectively reducing pain transmission. This versatile technique is not only used for pain relief but also for treating conditions such as cardiac arrhythmias and tumors, and is used in cosmetic dermatology. The effectiveness of RFA largely depends on the application site, with over two-thirds of patients typically experiencing good results. While pain relief generally lasts less than 12 months, some patients report relief lasting several years.[26] Pulsed Radiofrequency, which delivers energy in short pulses to minimize heat production, is particularly effective for managing chronic neuropathic pain.[27][28] Additionally, cooled radiofrequency ablation incorporates active cooling to prevent excessive heat, thereby allowing for larger and more controlled lesions, enhancing its effectiveness and safety.
- Laser neurolysis, documented by Weintraub in 1997, focuses energy on specific nerve points. Laser neurolysis has been shown to alleviate symptoms in conditions like carpal tunnel syndrome and has a high rate of symptom resolution.
- Surgical neurolysis or neurectomy involves the direct severing of the nerve and is used sparingly. Neurectomy is generally reserved for severe cases due to the high risk of deafferentation pain, which can be more intense than the original symptoms being treated.
Complications
The following are potential complications following a peripheral nerve block:
- Infection: At the injection site
- Increased symptoms: Inflammation and pain may increase at the injection site
- Bleeding and bruising: Due to injury to blood vessels
- Nerve damage: Potential nerve injury
- Nerve injury can lead to neuritis, dysesthesia, or hyperesthesia, which may cause symptoms like tingling or increased sensitivity. Partial nerve destruction might result in procedural failure.[29]
- Allergic reactions: Local anesthetic or neurolytic medications
- Intravascular medication injection:
- Phenol: This may cause systemic effects such as tinnitus and flushing.
- Local anesthetic systemic toxicity: Initial symptoms often include nausea, vomiting, tinnitus, perioral numbness, and a metallic taste in the mouth. The central nervous system (CNS) is initially stimulated, leading to seizures, then CNS depression and coma. Additionally, toxic levels of local anesthetics can induce cardiac arrhythmias, which may escalate to cardiac arrest.
Clinical Significance
Neurolysis offers patients an alternative way to manage their pain with reduced reliance on systemic medications, significantly improving their quality of life. Neurolysis is primarily advocated for treating severe, intractable malignant pain, typically in cases of advanced terminal cancer. While it is less common, neurolysis can also address certain nonmalignant pain conditions, such as intractable postherpetic neuralgia and chronic pancreatitis. However, the use of these non-pharmacological approaches in managing chronic nonmalignant pain remains controversial and is often discouraged. The literature currently includes large-scale controlled studies on the use and beneficial effects of peripheral neurolytic block in the treatment of malignant and nonmalignant pain. These investigations include randomized controlled trials (RCTs), observational trials, and many case series.
To evaluate the efficacy of cryoneurolysis in reducing pain associated with knee osteoarthritis, a randomized, double-blind, sham-controlled, multicenter trial was conducted. The study followed patients over 6 months, focusing on those who received cryoneurolysis targeting the infrapatellar branch of the saphenous nerve. Results showed a statistically significant improvement in the WOMAC (Western Ontario and McMaster Osteoarthritis Index) pain subscale scores at 30, 60, and 90 days post-treatment compared to the sham group.[30] Cryoneurolysis led to notable decreases in knee pain and enhanced symptom management for up to 150 days, proving safe and well-tolerated. Notably, the effects of cryoneurolysis are not permanent as cryoinjured axons typically regenerate, with the median duration of pain relief lasting from 2 weeks to 5 months. This makes cryoneurolysis particularly suitable for conditions requiring short- to medium-term analgesia, such as neuroma, entrapment neuropathies, and postoperative pain.[31]
Radiofrequency ablation for the lumbar medial branch has been extensively studied as a treatment for chronic low back pain (LBP). A notable study involving 85 patients with zygapophyseal joint (z-joint)-mediated LBP provided results showing that 70.6% of participants reported reduced pain after at least 6 months. These findings underscore RFA's effectiveness and durability in managing persistent lumbar z-joint pain.[32] Additionally, a systematic review analyzing 1063 papers, which included 11 sham-controlled RCTs, further supports RFA efficacy. This review encompassed studies on discogenic back pain, lumbar facet joint pain, and sacroiliac joint pain, with the majority demonstrating significant pain relief in lumbar facet and sacroiliac joint treatments.[33]
The long-term effectiveness of RFA for chronic LBP remains controversial. One RCT involving 681 participants with chronic LBP originating from facet joints, sacroiliac joints, or a combination including intervertebral disks showed that combining radiofrequency denervation with exercise did not significantly reduce pain intensity after 3 months compared to exercise alone.[34] Despite these findings, radiofrequency denervation continues to be a common treatment for chronic facet joint pain resistant to more conservative treatments. A meta-analysis of 7 trials involving 454 patients found that a subgroup who responded well to diagnostic block procedures experienced significant improvements in back pain compared to controls.[35] However, this effectiveness is debated. Results from a Cochrane analysis by Maas et al, which included 23 RCTs with 1309 participants, found no high-quality evidence that RF denervation provides substantial pain relief in patients with chronic LBP who responded positively to a diagnostic block or discography.[36]
Endoscopic ultrasound-guided neurolytic celiac plexus block (NCPB) should be considered as an adjunct to standard pain management for pancreatic cancer, as NCPB has been shown to reduce pain effectively. Results from a systematic review of 27 RCTs demonstrated that NCPB not only improves analgesia but also decreases opioid consumption and mitigates opioid-induced adverse effects, compared to conventional analgesic treatments.[37] Although the evidence is robust regarding its analgesic efficacy, the data on reducing opioid consumption and side effects are less conclusive. Nonetheless, current research largely supports the use of NCPB; this procedure is more effective than standard pharmacotherapy in reducing pain, lowering opioid requirements, and consequently, preventing a decline in the quality of life for patients.
Of all peripheral nerve neurectomies, ilioinguinal and iliohypogastric neurectomies are the most extensively studied. Inguinal neurectomy at the time of hernia repair may reduce the risk of postoperative pain. Results from a meta-analysis (11 studies on 1031 patients, including Lichtenstein hernia repair) showed a significant reduction in pain with neurectomy for short and midterm.[38]
Interventional techniques for managing pain can be highly effective for patients who are unable to tolerate systemic opioids or those with limited life expectancy and localized pain. However, outcomes data for pain relief are inconsistent, primarily due to variations in patient prognosis. Therefore, carefully selecting patients, considering their specific pain location and prognosis, is essential. Moreover, choosing the appropriate interventional technique requires a depth of knowledge that is still developing among clinicians. Peripheral neurolytic blocks are generally recommended only after other treatment modalities—including pharmacologic, physical, psychiatric, and non-destructive interventional therapies—have failed. In particular cases, such as severe pain from pancreatic neoplasia, minimally invasive techniques like NCPB should be considered early in the treatment pathway.
Enhancing Healthcare Team Outcomes
An interprofessional approach is essential for successful therapy management, especially in complex interventions like neurolytic blocks. The healthcare team should ideally include a pain specialist, an oncologist, a nurse trained in minimally invasive procedures, and an x-ray technologist. Together, they develop a tailored treatment plan that respects the patient's medical and personal needs while ensuring that ethical considerations such as informed consent and patient autonomy are prioritized.
The interventional pain specialist assesses pain and plans the block. The oncologist ensures that pain management strategies are compatible with the overall cancer treatment plans. The procedural nurse administers care and monitors the patient during the procedure, while the x-ray technologist guarantees optimal imaging results.
Effective interprofessional communication is facilitated through regular team meetings and the use of shared electronic health records, maintaining a unified approach to patient care. This coordination is crucial not only for procedural success but also for monitoring patient safety and responding promptly to any adverse events. The patient-centered approach aims to reduce reliance on systemic medications and enhance quality of life.[39] Ethical practices guarantee that interventions are carried out with the full consent and understanding of the patient. Regular treatment outcomes and team performance reviews are conducted to refine practices and enhance overall patient safety.
Media
(Click Image to Enlarge)
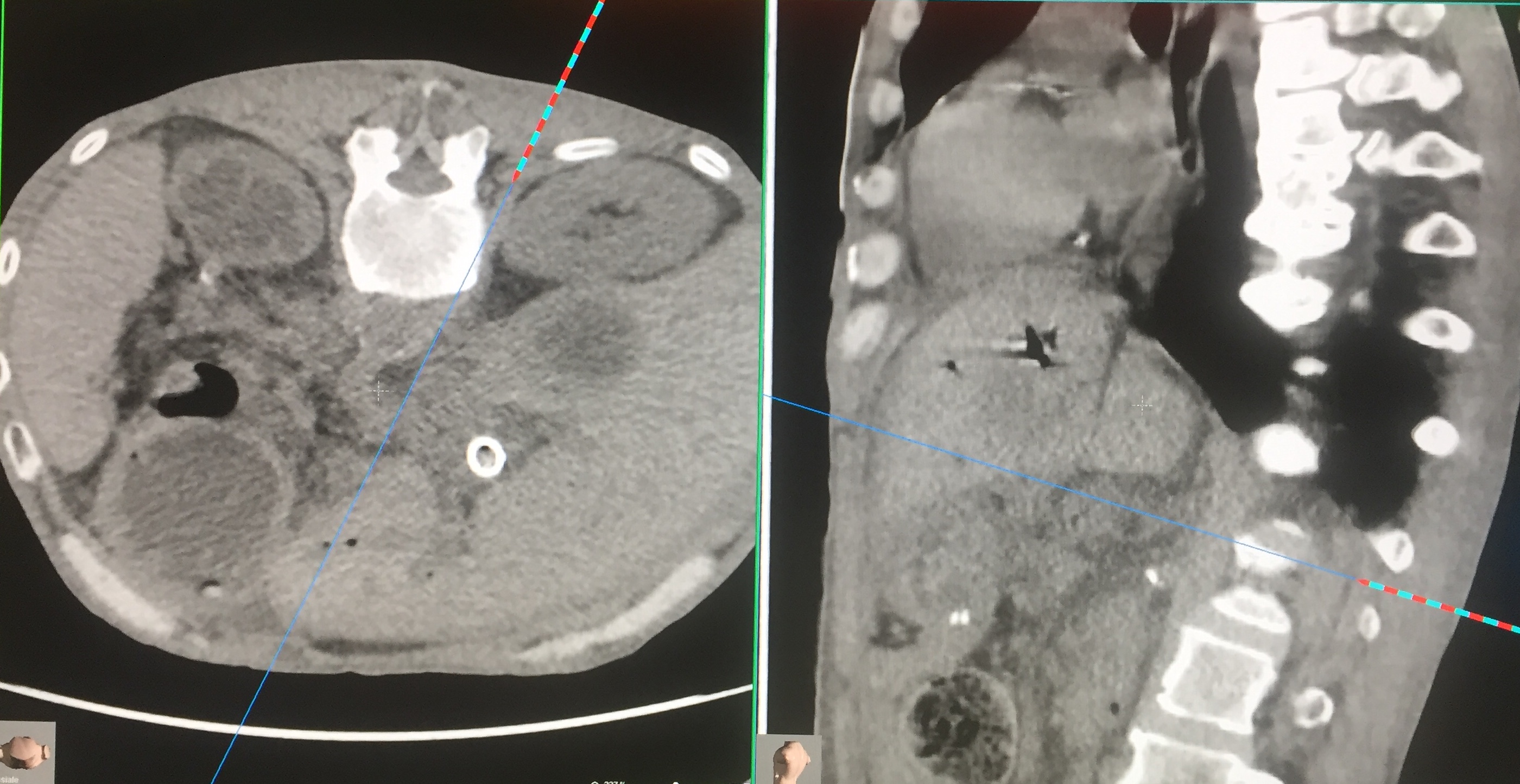
Celiac Ganglion Block. Performed through posterior access with the patient prone. CT-guided centering system (navigator). The celiac ganglion is found at the front of the Aorta near the emergence of the superior mesenteric artery. The procedure is bilateral. After injection of contrast medium, the procedure is conducted by chemical neurolysis or physical energy. In the photo, the needle is following the path indicated by the navigator to reach the block site. Contributed by Marco Cascella, MD
References
Cruccu G, Truini A. A review of Neuropathic Pain: From Guidelines to Clinical Practice. Pain and therapy. 2017 Dec:6(Suppl 1):35-42. doi: 10.1007/s40122-017-0087-0. Epub 2017 Nov 24 [PubMed PMID: 29178033]
Cuomo A, Bimonte S, Forte CA, Botti G, Cascella M. Multimodal approaches and tailored therapies for pain management: the trolley analgesic model. Journal of pain research. 2019:12():711-714. doi: 10.2147/JPR.S178910. Epub 2019 Feb 19 [PubMed PMID: 30863143]
O'Reilly D, Fou L, Hasler E, Hawkins J, O'Connell S, Pelone F, Callaway M, Campbell F, Capel M, Charnley R, Corrie P, Elliot D, Goodburn L, Jewell A, Joharchi S, McGeeney L, Mukherjee S, Oppong K, Whelan P, Primrose J, Neoptolemos J. Diagnosis and management of pancreatic cancer in adults: A summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.]. 2018 Dec:18(8):962-970. doi: 10.1016/j.pan.2018.09.012. Epub 2018 Sep 28 [PubMed PMID: 30292643]
Cole CD, Liu JK, Apfelbaum RI. Historical perspectives on the diagnosis and treatment of trigeminal neuralgia. Neurosurgical focus. 2005 May 15:18(5):E4 [PubMed PMID: 15913280]
Level 3 (low-level) evidenceCandido KD, Kusper TM, Knezevic NN. New Cancer Pain Treatment Options. Current pain and headache reports. 2017 Feb:21(2):12. doi: 10.1007/s11916-017-0613-0. Epub [PubMed PMID: 28265859]
Viel E, Pellas F, Ripart J, Pélissier J, Eledjam JJ. [Peripheral neurolytic blocks and spasticity]. Annales francaises d'anesthesie et de reanimation. 2005 Jun:24(6):667-72 [PubMed PMID: 15950114]
Level 3 (low-level) evidenceAhmed DG, Mohamed MF, Mohamed SA. Superior hypogastric plexus combined with ganglion impar neurolytic blocks for pelvic and/or perineal cancer pain relief. Pain physician. 2015 Jan-Feb:18(1):E49-56 [PubMed PMID: 25675070]
Miceli L, Bednarova R, Rizzardo A, Cuomo A, Riccardi I, Vetrugno L, Bove T, Cascella M. Opioids prescriptions in pain therapy and risk of addiction: a one-year survey in Italy. Analysis of national opioids database. Annali dell'Istituto superiore di sanita. 2018 Oct-Dec:54(4):370-374. doi: 10.4415/ANN_18_04_15. Epub [PubMed PMID: 30575575]
Level 3 (low-level) evidenceKambadakone A, Thabet A, Gervais DA, Mueller PR, Arellano RS. CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics : a review publication of the Radiological Society of North America, Inc. 2011 Oct:31(6):1599-621. doi: 10.1148/rg.316115526. Epub [PubMed PMID: 21997984]
Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesthesia and analgesia. 1995 Feb:80(2):290-5 [PubMed PMID: 7818115]
Level 1 (high-level) evidenceGambeta E, Chichorro JG, Zamponi GW. Trigeminal neuralgia: An overview from pathophysiology to pharmacological treatments. Molecular pain. 2020 Jan-Dec:16():1744806920901890. doi: 10.1177/1744806920901890. Epub [PubMed PMID: 31908187]
Level 3 (low-level) evidenceKo AL, Ozpinar A, Lee A, Raslan AM, McCartney S, Burchiel KJ. Long-term efficacy and safety of internal neurolysis for trigeminal neuralgia without neurovascular compression. Journal of neurosurgery. 2015 May:122(5):1048-57. doi: 10.3171/2014.12.JNS14469. Epub 2015 Feb 13 [PubMed PMID: 25679283]
Level 2 (mid-level) evidenceCappellari AM, Tiberio F, Alicandro G, Spagnoli D, Grimoldi N. Intercostal Neurolysis for The Treatment of Postsurgical Thoracic Pain: a Case Series. Muscle & nerve. 2018 Nov:58(5):671-675. doi: 10.1002/mus.26298. Epub 2018 Oct 2 [PubMed PMID: 29995980]
Level 2 (mid-level) evidenceHou S, Novy D, Felice F, Koyyalagunta D. Efficacy of Superior Hypogastric Plexus Neurolysis for the Treatment of Cancer-Related Pelvic Pain. Pain medicine (Malden, Mass.). 2020 Jun 1:21(6):1255-1262. doi: 10.1093/pm/pnz151. Epub [PubMed PMID: 31343689]
Van Zundert J, Vanelderen P, Kessels A, van Kleef M. Radiofrequency treatment of facet-related pain: evidence and controversies. Current pain and headache reports. 2012 Feb:16(1):19-25. doi: 10.1007/s11916-011-0237-8. Epub [PubMed PMID: 22090264]
Pasquali C, Vulcano E, Novario R, Varotto D, Montoli C, Volpe A. Ultrasound-guided alcohol injection for Morton's neuroma. Foot & ankle international. 2015 Jan:36(1):55-9. doi: 10.1177/1071100714551386. Epub 2014 Nov 3 [PubMed PMID: 25367249]
Level 2 (mid-level) evidenceChen CK, Phui VE, Saman MA. Alcohol neurolysis of lateral femoral cutaneous nerve for recurrent meralgia paresthetica. Agri : Agri (Algoloji) Dernegi'nin Yayin organidir = The journal of the Turkish Society of Algology. 2012:24(1):42-4. doi: 10.5505/agri.2012.47450. Epub [PubMed PMID: 22399128]
Level 3 (low-level) evidenceSwerdlow M. Intrathecal neurolysis. Anaesthesia. 1978 Sep:33(8):733-40 [PubMed PMID: 581435]
Benzoni T, Cascella M. Procedural Sedation. StatPearls. 2024 Jan:(): [PubMed PMID: 31869149]
Liu CW, Flamer D. Supraclavicular Brachial Plexus Neurolysis for a Malignant Peripheral Nerve Sheath Tumor: A Case Report. A&A practice. 2018 Dec 1:11(11):309-311. doi: 10.1213/XAA.0000000000000815. Epub [PubMed PMID: 29894352]
Level 3 (low-level) evidenceHaanpää M, Treede RD. Capsaicin for neuropathic pain: linking traditional medicine and molecular biology. European neurology. 2012:68(5):264-75. doi: 10.1159/000339944. Epub 2012 Sep 28 [PubMed PMID: 23037991]
Level 3 (low-level) evidenceMittal SO, Jabbari B. Botulinum Neurotoxins and Cancer-A Review of the Literature. Toxins. 2020 Jan 5:12(1):. doi: 10.3390/toxins12010032. Epub 2020 Jan 5 [PubMed PMID: 31948115]
SUNDERLAND S. A classification of peripheral nerve injuries producing loss of function. Brain : a journal of neurology. 1951 Dec:74(4):491-516 [PubMed PMID: 14895767]
Quinn JH. Repetitive peripheral neurectomies for neurolgia of second and third divisions of trigeminal nerve. Journal of oral surgery (American Dental Association : 1965). 1965 Nov:23(7):600-8 [PubMed PMID: 5214121]
D'Rozario R, Ito K, Goss AN. Effect of peripheral cryoneurotomy and nerve transection on the trigeminal ganglion in rats. The British journal of oral & maxillofacial surgery. 2019 May:57(4):341-344. doi: 10.1016/j.bjoms.2018.11.023. Epub 2019 Apr 2 [PubMed PMID: 30952375]
Shah RJ, Dixon B, Padalia D. Sphenopalatine Ganglion Radiofrequency Thermocoagulation. StatPearls. 2024 Jan:(): [PubMed PMID: 30725629]
Sluijter ME, Imani F. Evolution and mode of action of pulsed radiofrequency. Anesthesiology and pain medicine. 2013 Spring:2(4):139-41. doi: 10.5812/aapm.10213. Epub 2013 Mar 26 [PubMed PMID: 24223349]
Cahana A, Vutskits L, Muller D. Acute differential modulation of synaptic transmission and cell survival during exposure to pulsed and continuous radiofrequency energy. The journal of pain. 2003 May:4(4):197-202 [PubMed PMID: 14622704]
Level 3 (low-level) evidenceD'Souza RS, Warner NS. Phenol Nerve Block. StatPearls. 2024 Jan:(): [PubMed PMID: 30247853]
Radnovich R, Scott D, Patel AT, Olson R, Dasa V, Segal N, Lane NE, Shrock K, Naranjo J, Darr K, Surowitz R, Choo J, Valadie A, Harrell R, Wei N, Metyas S. Cryoneurolysis to treat the pain and symptoms of knee osteoarthritis: a multicenter, randomized, double-blind, sham-controlled trial. Osteoarthritis and cartilage. 2017 Aug:25(8):1247-1256. doi: 10.1016/j.joca.2017.03.006. Epub 2017 Mar 20 [PubMed PMID: 28336454]
Level 1 (high-level) evidenceIlfeld BM, Gabriel RA, Trescot AM. Ultrasound-guided percutaneous cryoneurolysis providing postoperative analgesia lasting many weeks following a single administration: a replacement for continuous peripheral nerve blocks?: a case report. Korean journal of anesthesiology. 2017 Oct:70(5):567-570. doi: 10.4097/kjae.2017.70.5.567. Epub 2017 Feb 3 [PubMed PMID: 29046778]
Level 3 (low-level) evidenceConger A, Burnham T, Salazar F, Tate Q, Golish M, Petersen R, Cunningham S, Teramoto M, Kendall R, McCormick ZL. The Effectiveness of Radiofrequency Ablation of Medial Branch Nerves for Chronic Lumbar Facet Joint Syndrome in Patients Selected by Guideline-Concordant Dual Comparative Medial Branch Blocks. Pain medicine (Malden, Mass.). 2020 May 1:21(5):902-909. doi: 10.1093/pm/pnz248. Epub [PubMed PMID: 31609391]
Level 2 (mid-level) evidenceLeggett LE, Soril LJ, Lorenzetti DL, Noseworthy T, Steadman R, Tiwana S, Clement F. Radiofrequency ablation for chronic low back pain: a systematic review of randomized controlled trials. Pain research & management. 2014 Sep-Oct:19(5):e146-53 [PubMed PMID: 25068973]
Level 1 (high-level) evidenceKapural L, Provenzano D, Narouze S. RE: Juch JNS, et al. Effect of Radiofrequency Denervation on Pain Intensity Among Patients With Chronic Low Back Pain: The Mint Randomized Clinical Trials. JAMA 2017;318(1):68-81. Neuromodulation : journal of the International Neuromodulation Society. 2017 Dec:20(8):844. doi: 10.1111/ner.12729. Epub [PubMed PMID: 29220124]
Level 1 (high-level) evidenceLee CH, Chung CK, Kim CH. The efficacy of conventional radiofrequency denervation in patients with chronic low back pain originating from the facet joints: a meta-analysis of randomized controlled trials. The spine journal : official journal of the North American Spine Society. 2017 Nov:17(11):1770-1780. doi: 10.1016/j.spinee.2017.05.006. Epub 2017 May 30 [PubMed PMID: 28576500]
Level 1 (high-level) evidenceMaas ET, Ostelo RW, Niemisto L, Jousimaa J, Hurri H, Malmivaara A, van Tulder MW. Radiofrequency denervation for chronic low back pain. The Cochrane database of systematic reviews. 2015 Oct 23:2015(10):CD008572. doi: 10.1002/14651858.CD008572.pub2. Epub 2015 Oct 23 [PubMed PMID: 26495910]
Level 1 (high-level) evidenceMercadante S, Klepstad P, Kurita GP, Sjøgren P, Giarratano A, European Palliative Care Research Collaborative (EPCRC). Sympathetic blocks for visceral cancer pain management: A systematic review and EAPC recommendations. Critical reviews in oncology/hematology. 2015 Dec:96(3):577-83. doi: 10.1016/j.critrevonc.2015.07.014. Epub 2015 Aug 1 [PubMed PMID: 26297518]
Level 1 (high-level) evidenceBarazanchi AW, Fagan PV, Smith BB, Hill AG. Routine Neurectomy of Inguinal Nerves During Open Onlay Mesh Hernia Repair: A Meta-analysis of Randomized Trials. Annals of surgery. 2016 Jul:264(1):64-72. doi: 10.1097/SLA.0000000000001613. Epub [PubMed PMID: 26756767]
Level 1 (high-level) evidenceCascella M, Thompson NS, Muzio MR, Forte CA, Cuomo A. The underestimated role of psychological and rehabilitation approaches for management of cancer pain. A brief commentary. Recenti progressi in medicina. 2016 Aug:107(8):418-21. doi: 10.1701/2332.25064. Epub [PubMed PMID: 27571557]
Level 3 (low-level) evidence