Pulmonary Atresia With Intact Ventricular Septum
Pulmonary Atresia With Intact Ventricular Septum
Introduction
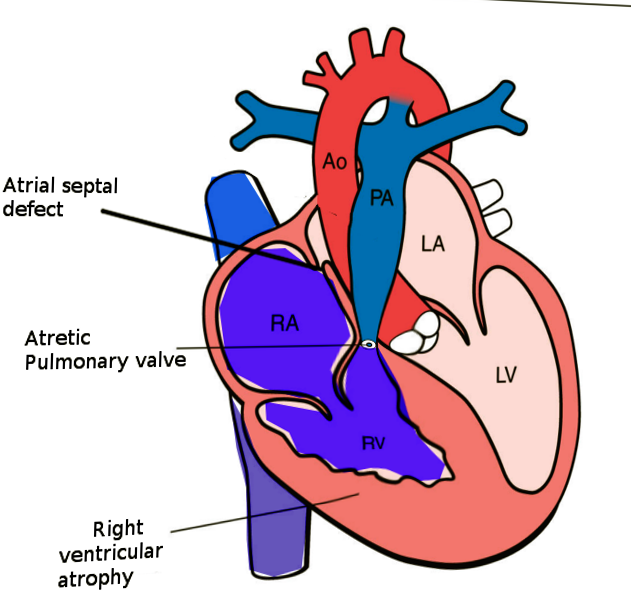
Pulmonary atresia with intact ventricular septum (PA-IVS) is one of the rare forms of congenital heart disease, accounting for <1% of total heart defects. PA-IVS is characterized by membranous or muscular atresia of the right ventricular outflow tract without any ventricular communication, often involving underdevelopment of the right ventricle and tricuspid valve (see Image. Pulmonary Atresia With Intact Ventricular Septum).
The anomaly was first described by Hunter in 1783 and later by Peacock in 1869. PA-IVS presents significant variability, influencing postoperative outcomes based on various factors, eg, the size of the right ventricle and tricuspid valve, as well as the presence of right ventricle-dependent coronary circulation (RVDCC).[1] Genetic research highlights that heterotaxy and right ventricular outflow tract obstruction are highly heritable congenital heart disease forms, but the precise cause of PA-IVS remains unknown. Theories suggest that potential causes may include issues with pulmonary valve development, tricuspid valve flow restriction, or coronary artery anomalies.
Newborns with PA-IVS often exhibit cyanosis and desaturation, especially after the closure of the ductus arteriosus, which is crucial for their pulmonary circulation. Physical examination may reveal single first and second heart sounds, a pansystolic murmur if the tricuspid valve regurgitates, and possibly a murmur from a patent ductus arteriosus. The diagnosis of this anomaly is predominantly made through echocardiography, which can detect PA-IVS prenatally in about 86% of cases. This imaging assesses the interatrial septum, tricuspid valve, right ventricle, and branch pulmonary arteries.
However, echocardiography alone cannot determine coronary circulation details, necessitating cardiac catheterization with angiography to assess coronary connections and RVDCC fully. Managing PA-IVS begins with prostaglandin infusion to maintain ductal patency, which is crucial for survival preoperatively. Intervention, either catheter-based or surgical, is necessary for neonates. Treatment is highly individualized, encompassing biventricular repair, systemic-to-pulmonary shunts, single-ventricular repair, or heart transplantation, depending on the characteristics of the right ventricle and tricuspid valve, as well as the anatomy of the coronary arteries.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Congenital heart disease categories are typically differentiated by their anatomical and physiological characteristics, but may also be distinguished by differences in heritability. Research indicates that heterotaxy and right ventricular outflow tract obstruction are the most heritable forms of congenital heart disease. For right ventricular outflow tract obstruction, the recurrence risk in first-degree relatives is 48.6%, compared to 12.9% for left ventricular outflow tract obstructive defects and 11.7% for conotruncal defects.[2] However, the precise abnormality that leads to PA-IVS remains unclear. Some theories postulated to explain the pathogenesis of this disorder include a primary insult to the pulmonary valve, leading to an atretic valve; an abnormal venous valve that limits flow through the tricuspid valve into the right ventricle; or abnormal coronary arterial development as a result.
Epidemiology
PA-IVS is a rare and varied cardiac anomaly, occurring in 4 to 5 out of every 100,000 live births. Although outcomes have improved over time, they remain cautious, with reported survival rates of 70% to 75% at 1 year and 63% to 67% at 5 years, depending on the type of postnatal circulation.[3] A collaborative study in the United Kingdom and Eire published in 1998 reported an incidence of approximately 4 per 100,000 live births for this disorder.[4] In another study published in the United States in 1984, the incidence of PA-IVS was approximately 8 per 100,000 live births.[5]
With the widespread availability and use of fetal echocardiography, the number of patients with complex forms of congenital heart disease, including PA-IVS, being diagnosed during pregnancy across the world is increasing. Intrauterine fetal cardiac intervention is a complex and infrequently performed procedure requiring extensive experience and further studies to understand its prognosis and outcomes better. At present, the 3 most commonly performed fetal cardiac intervention procedures include catheter-based aortic stenosis in fetuses with evolving hypoplastic left heart syndrome (HLHS), HLHS with an intact or restrictive atrial septum, and PA-IVS. Catheter-based aortic stenosis performed during mid-gestation can lead to the development of HLHS.[6]
PA-IVS has no preference for either sex. In addition, no association with genetic disorders has been identified, although De Stefano et al reported PA-IVS in monozygotic twins.[7] The genetic evaluation of twins in the report was notable for a 55 kb deletion at WFDC8 and WFDC9; however, the clinical significance of this gene deletion is unknown. Similarly, Chitayat et al reported the incidence of PA-IVS in 2 siblings with no other associated cardiac anomalies.[8]
Developmental Considerations and Anatomical Characteristics
Regarding the timing of occurrence, Kusche and Van Mierop suggested that the insult leading to PA-IVS occurs later in gestation compared to pulmonary atresia with ventricular septal defect (PA-VSD).[9] They indicated that the insult leading to PA-VSD occurs before the complete formation of the ventricular septum, whereas PA-IVS occurs following the completion of ventricular septal formation. Atresia of the pulmonary valve is classified into membranous and muscular forms, and distinguishing between these forms is crucial. Membranous pulmonary atresia has a better long-term prognosis compared to muscular pulmonary atresia due to the higher incidence of abnormal connections between the right ventricle and the coronary arteries. Due to high right ventricular pressures, the tricuspid valve is generally abnormal. The tricuspid valve can be hypoplastic or dysplastic and has a malformed chordal apparatus.
Another characteristic feature of PA-IVS is the abnormal connections between the right ventricle and coronary arteries. The right ventricle, especially in patients with a competent tricuspid valve, is hypertensive due to the lack of egress for the blood. Due to this, the right ventricle develops these abnormal connections with the epicardial coronary arteries, which help to decompress the ventricle. Over time, these abnormal connections lead to progressive stenosis of the coronary arteries related to high-velocity blood flowing through these abnormal connections. Due to progressive stenosis of the coronary arteries over time, some parts of the myocardium become dependent on the right ventricle for perfusion, a condition known as RVDCC. The progressive nature of RVDCC correlates with a poor prognosis.[2]
Pathophysiology
Advancements in prenatal diagnostic and treatment methods, along with the advent of intrauterine interventions, have enabled treatments to be administered during the fetal period. Fetal cardiac intervention is a cutting-edge, invasive technique performed in utero, which helps decrease the mortality and morbidity associated with congenital heart disease and enhances the likelihood of achieving biventricular circulation.
Intrauterine valvuloplasty enhances ventricular growth and function. Fetal aortic valvuloplasty, first documented in 1991, is the most commonly performed fetal cardiac intervention in developed nations. Fetal pulmonary valvuloplasty, first performed in 2002 at the Children's Hospital of Linz, is anticipated to support right ventricular development and improve prognosis.[6]
History and Physical
The most prevalent presenting signs and symptoms of PA-IVS include cyanosis and desaturation. Neonates with PS-IVS become symptomatic following the closure of the ductus arteriosus, as their pulmonary circulation is dependent on it. They rarely present or have the symptoms of decreased cardiac output due to obligatory right-to-left shunting at the level of the atrial septum through the foramen ovale.
The presence of low cardiac output syndrome should raise suspicion for myocardial ischemia, especially in patients with coronary fistulae. As noted with many other forms of cyanotic congenital heart disease, these patients do not have an improvement in their cyanosis or desaturations with 100% oxygen delivery or failed hyperoxia tests. Clinicians also note single first and second heart sounds during cardiac auscultation. A pansystolic murmur is audible at the left lower sternal border if the tricuspid valve regurgitates.
An additional murmur related to the flow across a patent ductus arteriosus might be audible in affected patients upon examination, especially following the initiation of prostaglandin infusion to maintain ductal patency. The peripheral pulses and capillary refill time are typically normal except in patients with severely restrictive right-to-left shunting at the level of the atria.
Evaluation
A readily available and accessible modality for cardiac evaluation is 2-dimensional echocardiography, which is frequently used in the diagnosis of PA-IVS. Due to the widespread availability and use of fetal echocardiography in conjunction with prenatal screening for congenital heart disease, the majority of patients with this disorder are diagnosed prenatally. In a recently published study on fetal echocardiography, the overall rate of prenatal diagnosis for this specific heart defect was approximately 86%.[10]
Echocardiogram alone can diagnose PA-IVS, but additional information regarding coronary circulation, a significant predictor of outcomes, and the type of repair cannot be discerned from this modality alone. Although echocardiography provides information regarding the anomalous connections between the right ventricle and coronary arteries, it does not diagnose RVDCC. Therefore, cardiac catheterization with angiograms is often necessary to arrive at a complete diagnosis, which includes assessing fistulous connections between the right ventricle and coronary arteries, as well as RVDCC.
Echocardiography
An apical 4-chamber sweep can diagnose PA-IVS. The diagnostic finding is the absence of an outflow tract from the right ventricle with an intact ventricular septum. When performing echocardiography on these patients, special attention should be given to the following anatomical structures:
- Interatrial septum: These patients depend on obligatory right-to-left shunting at the level of the interatrial septum to maintain cardiac output and systemic perfusion. Therefore, evaluating any obstruction across the interatrial septum is prudent. The subcostal views are generally optimal for imaging this part of the heart. A combination of 2-dimensional imaging, color Doppler imaging, and spectral Doppler imaging is required to assess the interatrial septum.
- Tricuspid valve: Assessing the anatomy of the tricuspid valve is crucial in these patients, as the adequacy of the tricuspid valve is 1 of the significant factors influencing the type of surgical repair. The assessment of the tricuspid valve should include the size of the tricuspid valve annulus, the morphology of the valve leaflets, and the functional status of the valve (atretic versus patent or competent versus regurgitate). Patients with regurgitant tricuspid valves have a lower incidence of coronary anomalies, as regurgitation through the valve helps to decompress the right ventricle.
- Right ventricle: The morphological characteristics of the right ventricle require detailed assessment during the echocardiogram. Generally, the size of the right ventricle is proportional to the size of the tricuspid valve. More than the absolute size of the right ventricle, evaluating the morphological characteristics of the right ventricle, which divides into inflow, apical, and outflow components, is critical. For instance, if the right ventricle is tripartite, with well-developed inflow, apical, and outflow components, neonates can undergo biventricular repair even if the ventricle is hypoplastic, provided all other characteristics favor the biventricular repair.
Cardiac Catheterization
Cardiac catheterization with angiocardiography is often used to diagnose patients with PA-IVS, as it provides additional information regarding coronary circulation; this procedure is critical in patients for whom decompression of the right ventricle is a consideration. The primary goal of cardiac catheterization is to assess for RVDCC. The diagnosis of RVDCC is made when a significant portion of the ventricular myocardium depends on the right ventricle for the blood supply.
The angiographic criteria for diagnosing RVDCC include stenosis of 2 or more major coronary arteries or atresia of the coronary ostia and the myocardium distal to the obstruction receiving the blood supply through fistulous connections from the right ventricle (see Image. Coronal Cardiac CT Angiogram). Loomba et al proposed that the aortic perfusion score, based on the angiographic characteristics of coronary perfusion, is a predictor of patient outcomes.[11] Galindo et al described various angiographic techniques that help obtain adequate or optimal coronary imaging.[Galindo et al] The methods described include right ventricle angiogram, aortogram, aortogram with balloon occlusion of the aorta, and selective coronary angiograms. The evidence of myocardial ischemia on the surface electrocardiogram following the placement of the catheter in the right ventricle (decompression of the right ventricle due to catheter-related tricuspid valve regurgitation) is highly suspicious for RVDCC.
Treatment / Management
Medical Management
Immediately following the diagnosis of PA-IVS, an attempt should be made to initiate prostaglandin infusion to maintain ductus arteriosus patency. This action is vital for the preoperative survival of these patients, as the ductus arteriosus is the sole source of pulmonary blood flow. Also, manipulating pulmonary and systemic vascular resistance is crucial for achieving optimal pulmonary and systemic blood flow.
Procedural Management
Neonates with PA-IVS require interventional treatment, either through a catheter-based intervention or a surgical procedure. Due to the complexity and heterogeneity of this disorder, no single method is effective for all patients. Even though biventricular repair is the ideal and preferred surgical approach, managing this disorder must be highly individualized. The preferred surgical or transcatheter intervention is influenced by several variables, which include the size and function of the tricuspid valve, the anatomy of the right ventricle and coronary arteries, and the type of pulmonary atresia. The currently available therapeutic algorithms are pretty diverse. Chikkabyrappa et al published an article discussing the following therapeutic options for this disorder:
- Single-ventricular repair (bidirectional Glenn procedure followed by Fontan) should be considered in patients with severe right ventricular hypoplasia and RVDCC.
- Patients with adequate and functionally tripartite right ventricle size, a tricuspid valve z-score >2.5, and normal coronary artery anatomy benefit from biventricular repair, radiofrequency perforation of the pulmonary valve for pulmonary atresia, and surgical right ventricular outflow tract reconstruction for long-segment atresia.
- Patients with borderline bipartite hypoplastic right ventricle and tricuspid valve z-scores between −2.5 and −4.5 might benefit from attempted biventricular repair with or without a systemic-to-pulmonary shunt. These patients require close surveillance for right ventricular growth; If the right ventricle fails to grow, they may benefit from one-and-a-half ventricular repairs.
- Primary heart transplantation might be a reasonable approach in patients with myocardial ischemia related to coronary artery abnormalities.[12]
Clinicians should also recall that patients in categories 2, 3, and 4 require a systemic-to-pulmonary artery shunt or stenting of the PDA in the immediate newborn period.
Differential Diagnosis
Differential diagnoses of PA-IVS that should also be considered are conditions that may also cause oxygen desaturation and cyanosis, including:
- Tetralogy of Fallot
- Transposition of the great arteries with pulmonary stenosis
- A single ventricle with severe pulmonary stenosis
- Tricuspid atresia
Prognosis
Several studies have demonstrated a gradual improvement in the survival rates of patients with this disorder, attributed to advancements in pediatric cardiology and cardiothoracic surgery. A population-based study from the United Kingdom and Ireland, published in 2005, reported 1-year survival rates of approximately 71% and 5-year survival rates of about 64%.[13] The same study identified low birth weight and unipartite right ventricle as independent risk factors for death. RVDCC, especially atresia of the coronary ostia, is an independent risk factor for poor outcomes.[14]
A recent study sought to describe outcomes in a recent multicenter cohort, identify factors associated with different clinical outcomes, and assess the impact of right ventricular coronary dependency and coronary atresia on transplant-free survival. Data were collected from neonates treated between 2009 and 2019 across 19 centers in the United States. Using competing risks analysis, they estimated the cumulative risk for each clinical outcome, and multivariable regression analyses helped identify factors linked to each outcome and transplant-free survival. A total of 295 patients were reviewed. The median tricuspid valve z-score was −3.06. The outcomes included biventricular repair for 45 patients (15.2%), one-and-a-half ventricle repair for 16 patients (5.4%), Fontan procedure for 75 patients (25.4%), cardiac transplantation for 29 patients (9.8%), and death for 54 patients (18.3%). A reported 76 patients (25.7%) remained in mixed circulation. The cumulative risk estimates for death were 10.9% at 1 month, 16.1% at 6 months, 16.9% at 1 year, and 18.8% at 5 years.[15]
Complications
PA-IVS is incompatible with life in the absence of ductal patency and extensive aortopulmonary collaterals. Although echocardiography is frequently the primary, and sometimes sole, imaging method for pre-surgical or interventional diagnosis in complex neonatal congenital heart diseases with borderline right ventricles, the echocardiographic indicators available for risk prediction in these conditions remain limited. Various echocardiographic parameters have been suggested for assessing the risk of biventricular repair in patients with PA-IVS. However, determining which parameters should be routinely recommended is a challenging task.
The tricuspid valve-to-mitral valve and right ventricle-to-left ventricle diameter ratios are easily measurable and reproducible indices that demonstrate good accuracy in predicting successful biventricular repair or the need for pulmonary blood flow augmentation following percutaneous balloon pulmonary valvuloplasty. Studies have shown that a higher degree of tricuspid regurgitation is favorable for biventricular repair. However, estimating tricuspid regurgitation in neonates is highly subjective and significantly influenced by load and pressure conditions, the pulmonary vascular bed, and ventilatory support.[16]
Deterrence and Patient Education
PA-IVS is a rare and complex form of congenital heart defect with a broad spectrum of presentation. An insult during sensitive stages of embryological development is proposed to lead to PA-IVS. This entity's presentation is similar to that of other complex cyanotic congenital heart defects with decreased pulmonary blood flow, eg, tetralogy of Fallot. The echocardiogram plays a vital role in diagnosing PA-IVS. Cardiac catheterization provides additional information regarding the status of the coronary circulation. Treating patients with this heart defect is complex, with many therapeutic algorithms guiding the interprofessional team through various management approaches.
Enhancing Healthcare Team Outcomes
An interprofessional approach is crucial for achieving optimal outcomes in patients with PA-IVS. The healthcare team typically includes a pediatric cardiologist, a cardiothoracic surgeon, an obstetrician, a maternal-fetal specialist for fetal diagnosis, a neonatal-perinatal specialist, neonatal and intensive care unit nurses, and a cardiac intensivist. For prenatally diagnosed cases, establishing a management plan before delivery is crucial. Delivering the neonate at a tertiary care center with pediatric cardiothoracic surgical services is often advisable. Every effort should be made to avoid deliveries during weekends and holidays, when staffing levels are reduced.
Clinicians collaborate closely with families to ensure optimal care, offering counseling and answering questions. Surgical nurses play a vital role during delivery, neonatal surgery, and postoperative care in the intensive care unit, ensuring continuous communication with managing physicians. This interprofessional collaboration is crucial for achieving the best possible outcomes for mother and child. Patients and their families receive detailed counseling regarding heart defects and surgical strategies, understanding the implications for future pregnancies. This coordinated, interprofessional team-based approach significantly enhances patient-centered care, safety, and team performance.
Media
(Click Image to Enlarge)
(Click Video to Play)
Coronal Cardiac CT Angiogram. This angiogram in a patient status post Glenn and Fontan procedures demonstrates an atretic pulmonary valve. The main pulmonary artery is not visualized and is also likely atretic. The branch pulmonary arteries are widely patent. The right pulmonary artery opacifies through the Glenn conduit. The left branch pulmonary artery opacifies through the Fontan conduit.
Contributed by A Thomas, MD
References
Luo G, Liu A, Sun H, Wang K, Pan S. A case report of pulmonary atresia with intact ventricular septum: an extraordinary finding of subsystemic right ventricle. Frontiers in pediatrics. 2024:12():1251274. doi: 10.3389/fped.2024.1251274. Epub 2024 May 1 [PubMed PMID: 38751746]
Level 3 (low-level) evidenceWeaver KN, Chen J, Shikany A, White PS, Prada CE, Gelb BD, Cnota JF, Pediatric Cardiac Genomics Consortium Investigators*. Prevalence of Genetic Diagnoses in a Cohort With Valvar Pulmonary Stenosis. Circulation. Genomic and precision medicine. 2022 Aug:15(4):e003635. doi: 10.1161/CIRCGEN.121.003635. Epub 2022 Jun 6 [PubMed PMID: 35666834]
Wolter A, Markert N, Wolter JS, Kurkevych A, Degenhardt J, Ritgen J, Stressig R, Enzensberger C, Bedei I, Vorisek C, Schenk J, Graupner O, Khalil M, Thul J, Jux C, Axt-Fliedner R. Natural history of pulmonary atresia with intact ventricular septum (PAIVS) and critical pulmonary stenosis (CPS) and prediction of outcome. Archives of gynecology and obstetrics. 2021 Jul:304(1):81-90. doi: 10.1007/s00404-020-05929-0. Epub 2021 Feb 14 [PubMed PMID: 33585987]
Daubeney PE, Sharland GK, Cook AC, Keeton BR, Anderson RH, Webber SA. Pulmonary atresia with intact ventricular septum: impact of fetal echocardiography on incidence at birth and postnatal outcome. UK and Eire Collaborative Study of Pulmonary Atresia with Intact Ventricular Septum. Circulation. 1998 Aug 11:98(6):562-6 [PubMed PMID: 9714114]
Ferencz C, Rubin JD, McCarter RJ, Brenner JI, Neill CA, Perry LW, Hepner SI, Downing JW. Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. American journal of epidemiology. 1985 Jan:121(1):31-6 [PubMed PMID: 3964990]
Level 2 (mid-level) evidenceZhao L, Wang L, Xia H, Wu Y, Jiao X, Zhu H, Chen S, Sun K. Prognosis and outcome of intrauterine treatment of fetuses with critical congenital heart disease. Chinese medical journal. 2024 Jun 20:137(12):1431-1436. doi: 10.1097/CM9.0000000000002796. Epub 2023 Jul 24 [PubMed PMID: 37488672]
De Stefano D, Li P, Xiang B, Hui P, Zambrano E. Pulmonary atresia with intact ventricular septum (PA-IVS) in monozygotic twins. American journal of medical genetics. Part A. 2008 Feb 15:146A(4):525-8. doi: 10.1002/ajmg.a.32160. Epub [PubMed PMID: 18203206]
Level 3 (low-level) evidenceChitayat D, McIntosh N, Fouron JC. Pulmonary atresia with intact ventricular septum and hypoplastic right heart in sibs: a single gene disorder? American journal of medical genetics. 1992 Feb 1:42(3):304-6 [PubMed PMID: 1536166]
Level 3 (low-level) evidenceKutsche LM, Van Mierop LH. Pulmonary atresia with and without ventricular septal defect: a different etiology and pathogenesis for the atresia in the 2 types? The American journal of cardiology. 1983 Mar 15:51(6):932-5 [PubMed PMID: 6829467]
Gorla SR, Chakraborty A, Garg A, Gugol RA, Kardon RE, Swaminathan S. Emerging trends in the prenatal diagnosis of complex CHD and its influence on infant mortality in this cohort. Cardiology in the young. 2019 Mar:29(3):270-276. doi: 10.1017/S1047951118002147. Epub 2018 Dec 26 [PubMed PMID: 30585560]
Loomba RS, Pelech AN. Aortic perfusion score for pulmonary atresia with intact ventricular septum: An antegrade coronary perfusion scoring system that is predictive of need for transplant and mortality. Congenital heart disease. 2018 Jan:13(1):92-97. doi: 10.1111/chd.12510. Epub 2017 Jun 27 [PubMed PMID: 28653340]
Chikkabyrappa SM, Loomba RS, Tretter JT. Pulmonary Atresia With an Intact Ventricular Septum: Preoperative Physiology, Imaging, and Management. Seminars in cardiothoracic and vascular anesthesia. 2018 Sep:22(3):245-255. doi: 10.1177/1089253218756757. Epub 2018 Feb 7 [PubMed PMID: 29411679]
Daubeney PE, Wang D, Delany DJ, Keeton BR, Anderson RH, Slavik Z, Flather M, Webber SA, UK and Ireland Collaborative Study of Pulmonary Atresia with Intact Ventricular Septum. Pulmonary atresia with intact ventricular septum: predictors of early and medium-term outcome in a population-based study. The Journal of thoracic and cardiovascular surgery. 2005 Oct:130(4):1071 [PubMed PMID: 16214522]
Cheung EW, Richmond ME, Turner ME, Bacha EA, Torres AJ. Pulmonary atresia/intact ventricular septum: influence of coronary anatomy on single-ventricle outcome. The Annals of thoracic surgery. 2014 Oct:98(4):1371-7. doi: 10.1016/j.athoracsur.2014.06.039. Epub 2014 Aug 22 [PubMed PMID: 25152382]
Iliopoulos I, Mastropietro CW, Flores S, Cheung E, Amula V, Radman M, Kwiatkowski D, Puente BN, Buckley JR, Allen KY, Loomba R, Karki KB, Chiwane S, Cashen K, Piggott K, Kapileshwarkar Y, Gowda KMN, Badheka A, Raman R, Zang H, Costello JM, Collaborative Research from the Pediatric Cardiac Intensive Care Society Investigators. Pulmonary Atresia with Intact Ventricular Septum: Midterm Outcomes from a Multicenter Cohort. Pediatric cardiology. 2024 Apr:45(4):847-857. doi: 10.1007/s00246-022-02954-5. Epub 2022 Jun 25 [PubMed PMID: 35751685]
Cantinotti M, McMahon CJ, Marchese P, Köstenberger M, Scalese M, Franchi E, Santoro G, Assanta N, Jacquemyn X, Kutty S, Giordano R. Echocardiographic Parameters for Risk Prediction in Borderline Right Ventricle: Review with Special Emphasis on Pulmonary Atresia with Intact Ventricular Septum and Critical Pulmonary Stenosis. Journal of clinical medicine. 2023 Jul 10:12(14):. doi: 10.3390/jcm12144599. Epub 2023 Jul 10 [PubMed PMID: 37510714]