Introduction
The 2 kidneys of the human body are typically drained of blood by a single vessel, the renal vein. Each renal vein courses through the abdominal cavity on either side of the body medially before reaching the inferior vena cava. Their paths parallel the trajectory of the renal arteries, and the left renal vein is longer than the right renal vein. There are many clinically significant anatomical variants of the course the renal veins may take and dissimilar distributions of the venous tributaries that drain into the renal veins. The renal veins are not symmetrical, as the left and right renal veins have considerably different paths as they travel away from the kidneys, draining the blood from each kidney as well as other organ systems, including the gonads, adrenal glands, and diaphragm. Owing to the increasing number of renal transplants, vascular surgeries, laparoscopic abdominal surgeries, and abdominal imaging being performed in the United States, having an understanding of the normal anatomic relationship of the renal vasculature and the prevalence of any possible variations is paramount.[1]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
In the fully developed human body, each kidney contains one renal vein and one renal artery.[2] The renal vasculature arises from the spinal vertebral levels of L1 to L2 or L3.[3][4] The venous system of the kidneys begins as the stellate veins in the renal cortex (see Image. Azygos Venous System). These veins coalesce into arcuate veins located near the base of the renal pyramids within the kidney. The arcuate veins drain into the interlobar veins as they near the renal calyces. The interlobar veins unify into segmental veins that finally unite to form the beginning of the renal vein in the hilum of each kidney. The renal vein is typically located anterior and inferior to the renal artery in the hilum of the kidney.[2]
Unlike the renal arterial system, the renal venous system has what anatomists refer to as a “free anastomosis” system in place. This system allows venous blood within the kidney to communicate and freely flow throughout all the segments of the kidney, allowing branches of the renal venous system to be severed or ligated without severely compromising the venous drainage of the renal parenchyma; this arrangement circumvents destruction of the renal tissue with the loss of minor branches of the renal venous system following injury.[2]
The right renal vein is distinctly unlike the left renal vein. It begins anteriorly to the renal artery in the hilum of the kidney and courses medially toward the lateral aspect of the inferior vena cava. The right renal vein is typically about 2 to 2.5 cm in length and courses in an anterior-superior direction until it reaches the inferior vena cava. The right renal vein most commonly does not have extrarenal vessels join its course before it enters the inferior vena cava, unlike the left renal vein.[2][5]
The left renal vein is typically 8.5 cm in length on average. It passes transversely toward the medial aspect of the inferior vena cava. Its course from the hilum of the left kidney to the inferior vena cava includes passing anteriorly to the aorta and inferiorly to the superior mesenteric artery close to each respective vessel. This anatomic relationship has important clinical implications, including whether or not the left or right kidney is preferable for transplantation. Owing to the longer length of the renal vein, it is commonly suggested that the left kidney be taken for transplant over the right kidney.[6] Other clinically relevant variations in the renal vasculature will be mentioned later in this article. The left renal vein is typically first joined by the left adrenal vein, draining into the superior aspect of the left renal vein. Shortly after, there is typically a branch of the inferior phrenic vein that joins the left renal vein on its superior aspect as well. The left gonadal vein usually joins the inferior aspect of the left renal vein before it crosses the aorta. In about 75% of the population, the posterior aspect of the left renal vein has additional tributaries (such as branches from the lumbar or hemiazygos veins) draining into it. These can be clinically significant in size and are highly variable.[2]
Physiologic Variants
There are a plethora of case reports citing anomalous vasculature originating from the kidneys, with some vessels branching at wide angles, others bifurcating before reaching their destination, and others encircling structures found within the abdominal cavity [3][7][8][1]. When compared to physiologic variants within the arterial system, those found within the renal venous system appear to have comparable prevalence. Multiple renal veins from each kidney are present in anywhere from 15% to 30% of patients, whereas variations in the number of renal arteries are present in as many as 33% of patients.[2]
The normal anatomical arrangement of renal vessels should include only one vein and one artery. Both arise from the renal hilum and extend medially before reaching the inferior vena cava. In approximately 6% of patients, the right gonadal vein drains into the right renal vein before it enters the lateral aspect of the inferior vena cava, mirroring the normal anatomy of the left renal venous system. In about 31% of individuals, an accessory vein from the right adrenal gland drains into the right renal vein before it reaches the inferior vena cava. In about 3% of cases, the retroperitoneal venous vessels, such as lumbar or hemiazygos vessels, drain into the right renal vein before it enters the inferior vena cava.[2]
The most common physiologic variant of the left renal vein is the “circumaortic left renal vein.” The circumaortic left renal vein is a variant that includes two branches of venous drainage from the left kidney. One branch courses anterior to the aorta, and one branch courses posterior to the aorta as they course toward the medial aspect of the inferior vena cava. Most of the time, the circumaortic branch begins as one renal vein at the hilum of the left kidney and then splits into the anterior and posterior branches; 25% of the time, a circumaortic left renal vein begins as 2 vessels at the hilum of the left kidney. In about 3% of the population, a retro-aortic left renal vein is present. This variant typically crosses posteriorly to the aorta and then drains into the inferior vena cava at a lower level than typical for anteriorly located left renal veins. A retro-aortic left renal vein has also rarely been known to drain into a common iliac vein instead of the inferior vena cava. About 25% of the time, there are no posterior tributaries of the left renal vein from the retroperitoneal space.[2]
Clinical Significance
The left renal vein transverses between the superior mesenteric artery and the aorta on its course toward the inferior vena cava. The superior mesenteric artery and the aorta typically form a 90-degree angle. However, specific anatomical variants of these arteries can reduce this angle. Constriction of the left renal vein between the superior mesenteric artery and the aorta leads to compression ischemia in a condition known as Nutcracker syndrome. The normal pressure gradient is 1 mm Hg between the renal vein and the inferior vena cava. In Nutcracker syndrome, the pressure gradient can reach as high as 3 mm Hg.[3] Nutcracker syndrome can lead to various manifestations of venous congestion within the pelvic cavity (for example, venous hypertension, left-sided varicocele, hematuria, and venous pelvic congestion syndrome in women). Another venous anatomical variant described in case studies is termed a retro-aortic left renal vein. With a retro-aortic renal vein, the vein courses posterior to the aorta. This anatomical variant is similarly susceptible to compression ischemia, but in this case, compression occurs between the aorta and vertebral body, resulting in posterior nutcracker syndrome.
The blood supply from the left adrenal, left inferior phrenic, and the left gonadal veins join the left renal vein at various sites along its trajectory before dumping into the inferior vena cava. These contributions may become clinically relevant when obstructed. Varicoceles in males can occur on the left side of the body owing to differences in venous drainage from the left and right gonadal veins. Because the left gonadal vein drains into the relatively high-pressure left renal vein at an acute angle, impairment in blood flow due to impingement of the renal vein distally results in venous valve failure, leading to reflux of blood back into the gonadal vein. Venous engorgement transmits blood backward into the scrotum and causes varicocele. A classic example includes the extension of renal cell carcinoma into the renal vein, obstructing venous drainage from the left gonadal vein and causing a left-sided varicocele.[9] Varicoceles in males tend to occur on the left side far more commonly than on the right side. In theory, this is because the left testicular vein drains into the relatively high-pressure left renal vein at an acute angle. This condition potentially results in venous valve failure, leading to reflux of blood back into the testicular vein.[4] Interestingly, 93% of cases occur on the left side, but cases of right-sided varicocele have incidentally led to a diagnosis of renal cell carcinoma.[10][11] In women, the reflux of blood backward from the left renal vein manifests with symptoms and signs of venous pelvic congestion syndrome.[12][13][14][15] The exact etiology is unclear but does include venous incompetence, dilatation, and reflux of blood from the gonadal veins. For this reason, the term "tubo-ovarian varicoceles" has been used as a female counterpart of testicular varicocele.[15]
Media
(Click Image to Enlarge)
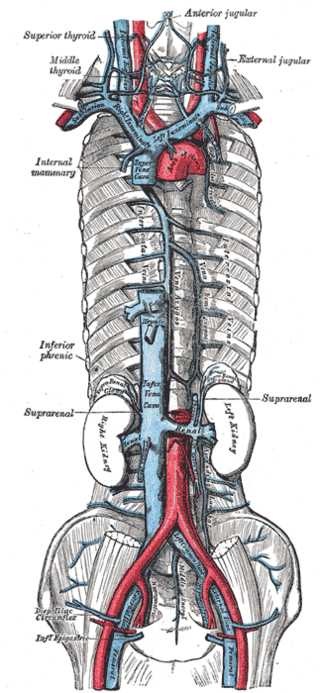
Azygos Venous System. The azygos venous system includes the internal mammary vein, inferior phrenic vein, suprarenal vein, and the left and right kidneys.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Pollak R, Prusak BF, Mozes MF. Anatomic abnormalities of cadaver kidneys procured for purposes of transplantation. The American surgeon. 1986 May:52(5):233-5 [PubMed PMID: 3518559]
Arévalo Pérez J, Gragera Torres F, Marín Toribio A, Koren Fernández L, Hayoun C, Daimiel Naranjo I. Angio CT assessment of anatomical variants in renal vasculature: its importance in the living donor. Insights into imaging. 2013 Apr:4(2):199-211. doi: 10.1007/s13244-012-0217-5. Epub 2013 Jan 26 [PubMed PMID: 23355302]
Anjamrooz SH, Azari H, Abedinzadeh M. Abnormal patterns of the renal veins. Anatomy & cell biology. 2012 Mar:45(1):57-61. doi: 10.5115/acb.2012.45.1.57. Epub 2012 Mar 31 [PubMed PMID: 22536553]
Level 3 (low-level) evidenceReginelli A, Somma F, Izzo A, Urraro F, D'Andrea A, Grassi R, Cappabianca S. Renovascular anatomic variants at CT angiography. International angiology : a journal of the International Union of Angiology. 2015 Dec:34(6 Suppl 1):36-42 [PubMed PMID: 26498890]
Pozniak MA, Balison DJ, Lee FT Jr, Tambeaux RH, Uehling DT, Moon TD. CT angiography of potential renal transplant donors. Radiographics : a review publication of the Radiological Society of North America, Inc. 1998 May-Jun:18(3):565-87 [PubMed PMID: 9599383]
Phelan PJ, Shields W, O'Kelly P, Pendergrass M, Holian J, Walshe JJ, Magee C, Little D, Hickey D, Conlon PJ. Left versus right deceased donor renal allograft outcome. Transplant international : official journal of the European Society for Organ Transplantation. 2009 Dec:22(12):1159-63 [PubMed PMID: 19891044]
Level 2 (mid-level) evidenceAli Mohammed AM, Elseed Abdalrasol RG, Alamin Abdalhai K, Gommaa Hamad M. Accessory renal vessels. Acta informatica medica : AIM : journal of the Society for Medical Informatics of Bosnia & Herzegovina : casopis Drustva za medicinsku informatiku BiH. 2012 Sep:20(3):196-7. doi: 10.5455/aim.2012.20.196-197. Epub [PubMed PMID: 23322980]
Level 3 (low-level) evidenceEldefrawy A, Arianayagam M, Kanagarajah P, Acosta K, Manoharan M. Anomalies of the inferior vena cava and renal veins and implications for renal surgery. Central European journal of urology. 2011:64(1):4-8. doi: 10.5173/ceju.2011.01.art1. Epub 2011 Mar 18 [PubMed PMID: 24578852]
PINALS RS, KRANE SM. Medical aspects of renal carcinoma. Postgraduate medical journal. 1962 Sep:38(443):507-19 [PubMed PMID: 14486698]
Hadad Z, Norup K, Petersen C. [Right-sided varicocele testis as the only sign of right-sided renal tumour]. Ugeskrift for laeger. 2016 Feb 1:178(5):V05140307 [PubMed PMID: 26857303]
Ryan JW, Sugrue G, Graham S, Cronin C. A rare testicular vein anatomical variant contributes to right-sided varicocoele formation and leads to the diagnosis of renal cell carcinoma. BMJ case reports. 2017 Jun 13:2017():. pii: bcr-2017-219519. doi: 10.1136/bcr-2017-219519. Epub 2017 Jun 13 [PubMed PMID: 28611135]
Level 3 (low-level) evidenceGavorník P, Holomáň K, Gašpar Ľ, Dukát A, Komorníková A, Gavorník E. [Pelvic venous congestion syndrome - diagnosis and management. Guidelines of the angiology section of slovak medical chamber (2015)]. Vnitrni lekarstvi. 2015 Mar:61(3):244-50 [PubMed PMID: 25873121]
Alonso V, Sánchez-Abuín A, Velasco JJ, Marugán de Miguelsanz JM. Pelvic Congestion Syndrome Secondary to a Circumaortic Left Renal Vein. The Journal of pediatrics. 2020 May:220():261-262. doi: 10.1016/j.jpeds.2020.01.005. Epub 2020 Feb 6 [PubMed PMID: 32037151]
Antignani PL, Lazarashvili Z, Monedero JL, Ezpeleta SZ, Whiteley MS, Khilnani NM, Meissner MH, Wittens CH, Kurstjens RL, Belova L, Bokuchava M, Elkashishi WT, Jeanneret-Gris C, Geroulakos G, Gianesini S, de Graaf R, Krzanowski M, Al Tarazi L, Tessari L, Wikkeling M. Diagnosis and treatment of pelvic congestion syndrome: UIP consensus document. International angiology : a journal of the International Union of Angiology. 2019 Aug:38(4):265-283. doi: 10.23736/S0392-9590.19.04237-8. Epub 2019 Jul 24 [PubMed PMID: 31345010]
Level 3 (low-level) evidenceBeard RW, Highman JH, Pearce S, Reginald PW. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet (London, England). 1984 Oct 27:2(8409):946-9 [PubMed PMID: 6149342]