Introduction
Rubella, also known as German measles, is an acute viral exanthematous infection first described by German physicians in the mid-18th century.[1] The virus is primarily transmitted via respiratory droplets and can be spread by both asymptomatic and symptomatic individuals, including during the incubation period, which averages 14 days (range: 12-23 days).[2] Rubella typically causes a mild, self-limiting illness in children and adults, characterized by low-grade fever, malaise, lymphadenopathy, arthralgias, and a brief, generalized, erythematous maculopapular rash.[3][4] Approximately 25% to 50% of infections are subclinical.[2]
Rubella virus is a significant cause of vaccine-preventable congenital disabilities and can lead to epidemics. The primary public health concern is its teratogenic effect during the first trimester of pregnancy, which can cause miscarriage, fetal death, or a constellation of severe congenital disabilities collectively known as congenital rubella syndrome (CRS).[5][6]
The introduction of the live attenuated rubella vaccine in 1969 resulted in a dramatic decrease in rubella and CRS incidence in many countries. Rubella and CRS have been eliminated from the United States, the Western Hemisphere, and nearly all of Europe, primarily due to high vaccination coverage.[7] However, rubella remains a significant public health issue in regions with suboptimal vaccination strategies, limited diagnostic capacity, and inadequate infection control, particularly in parts of Africa and Asia.[4][7]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Rubella virus, the sole member of the genus Rubivirus in the Marnaviridae family, infects only humans.[8][9] The virus is a spherical, enveloped, single-stranded, positive-sense RNA virus, with a genome of approximately 9.7 kb (see Image. Paramyxovirus Virion, Transmission Electron Micrograph). The viral genome encodes 2 nonstructural proteins (p90 and p150) and 3 structural proteins: glycoproteins (E1 and E2) and the capsid protein (see Image. Rubella Virus, Transmission Electron Micrograph).
The viral protein contains surface glycoprotein spikes composed of E1 and E2. The E1 glycoprotein is essential for receptor-mediated endocytosis and is the primary immunodominant antigen; neutralizing antibodies against E1 correlate with protective immunity. The E2 protein is membrane-bound, interacts with E1, and is important for virion assembly and structural integrity. The capsid protein interacts with the viral RNA and the cytoplasmic tails of E1 and E2, facilitating virion assembly.[10][11][12]
Rubella virus is sensitive to heat (>56 °C), ultraviolet light, and extremes of pH (pH <6.8 or >8.1), which limits its environmental stability.[3] The E1 protein is a class II fusion protein and uniquely requires calcium for membrane fusion during cell entry. Immunity to rubella is mediated by neutralizing antibodies, primarily directed against E1, and is generally lifelong following infection or vaccination.[13]
Epidemiology
The rubella virus, which infects only humans, remains a significant cause of vaccine-preventable congenital disabilities. Transmission occurs through direct or droplet contact with nasopharyngeal secretions. Infected individuals can spread the virus from approximately 8 days before to 8 days after rash onset, with peak contagiousness occurring just before the rash's appearance.[4] This period of high transmissibility presents a significant risk to susceptible populations, particularly unvaccinated pregnant women, due to the potential for CRS in the fetus. After inhalation, the virus replicates locally within the respiratory epithelium, then in the cervical lymph nodes, before disseminating through primary viremia. While rubella affects both sexes equally in children, adult cases are more common in women.[3] Risk factors include lack of vaccination, travel to endemic areas, exposure to infected individuals, and immunodeficiency.[3]
Rubella is the leading vaccine-preventable cause of congenital disabilities due to its teratogenicity, which can lead to CRS. Before widespread vaccination, rubella caused major epidemics every 6 to 9 years, exposing and infecting large numbers of adults. The highest incidence occurred in children aged 5 to 9, with peak transmission in late winter and early spring. Sporadic cases were seen year-round in temperate climates.[3][4][12]
Since the introduction of the live-attenuated virus vaccine, outbreaks have shifted to older adolescents and young adults.[12][14] Widespread use of rubella-containing vaccines (RCV), particularly the measles-mumps-rubella (MMR) vaccine, has led to a dramatic global decline in rubella incidence.[15] Before the vaccine's licensure in the United States (US) in 1969, rubella was common among young children. Rubella was declared eliminated from the US in 2004.[16] Since then, fewer than 10 cases have been reported annually, most linked to international travel from endemic regions.[3][16][17]
Rubella remains a common and concerning public health issue in many parts of the world, especially in developing countries, where it continues to cause significant disability. Many cases go unrecognized or undiagnosed due to the rash's similarity to other illnesses and because up to 50% of infections are subclinical. Ongoing transmission is mainly due to inadequate vaccination coverage and poor infection control, increasing the risk for unvaccinated individuals, including pregnant women.[4]
As of 2023, 84% of World Health Organization (WHO) member states have included the rubella vaccine in their national immunization schedules.[18] In endemic countries, rubella occurs at an estimated 1.3 cases per 100,000 people.[4] Maternal rubella during pregnancy can cause miscarriage, fetal death, or CRS.[4] CRS remains a global public health concern, with over 100,000 cases reported annually. The rubella virus infects the placenta, disrupting organ development and causing inflammation in the fetus.[12] Common congenital disabilities associated with CRS include sensorineural deafness, visual impairments, congenital heart disease, developmental delays, microcephaly, and neurological complications.[12]
Infants with CRS can shed the rubella virus in nasopharyngeal secretions and urine for a year or more, posing a risk of transmission to vulnerable and unvaccinated individuals. Strict infection control measures are essential to prevent further spread, especially in healthcare and childcare settings.
The burden of CRS is typically low in countries with high coverage of RCVs. CRS cases decline significantly in these settings and can be eliminated once endemic rubella transmission is interrupted. Maintaining elimination requires sustained high immunization coverage. This underscores the importance of implementing the WHO's Immunization Agenda 2030 (IA2030) Framework for Action, which supports global efforts to strengthen vaccination programs.[18]
Pathophysiology
Humans are the only known reservoir for rubella. The virus is contracted through person-to-person contact via droplets and aerosolized particles from the respiratory tract secretions of affected individuals.[3][4][12] After infecting the cells of the susceptible host through receptor-mediated endocytosis, rubella replicates in the nasopharyngeal cells and then spreads to the regional lymphoid tissue of the nasopharynx and upper respiratory tract. This process is followed by a viremic phase, characterized by the hematogenous dissemination of the virus to multiple organs, typically occurring 5 to 7 days after inoculation.
The exanthem appears approximately 2 to 8 days after the onset of viremia and resolves 3 days later as the humoral immune response develops.[3] Infected individuals are contagious from 8 days before to 8 days after rash onset.[4] Immunity following natural infection or vaccination is generally lifelong, though reinfections have been reported after both wild-type infection and single-dose vaccinations.[4][12] These reinfections are usually mild or asymptomatic but can still pose a risk of viral transmission, particularly to unvaccinated or high-risk individuals.
In CRS, fetal infection occurs transplacentally during the maternal viremic phase.[3] The risk of transmission depends on the timing of maternal infection; if rubella occurs before 10 weeks gestation, up to 90% of fetuses may develop defects.[19] The risk decreases significantly with infections later in gestation.[12] The pathogenesis of CRS is complex and not fully understood.[4] Fetal damage may result from several mechanisms, including epithelial necrosis of the chorionic villi, apoptosis of infected cells due to direct viral damage, inhibition of mitosis through disrupted intracellular actin assembly, restricted precursor cell development, increased cytokine and interferon activity, and vascular damage leading to organ ischemia.[3][4]
History and Physical
Postnatal rubella infection can be asymptomatic in approximately 25% to 50% of cases.[20] The incubation period ranges from 14 to 21 days, followed by a mild prodrome of low-grade fever, malaise, anorexia, headache, sore throat, and adenopathy. Lymphadenopathy typically involves the postauricular, suboccipital, and anterior cervical lymph nodes.[3]
In children, the exanthem often serves as the first clinical sign, presenting as pinpoint, pink macules and papules that begin on the face and quickly spread to the trunk and extremities (see Image. German Measles, Rubella). In some cases, the rash may take on a scarlatiniform or purpuric appearance, typically lasting about 3 days and fading in the same sequence as its onset.[3] Petechiae on the soft palate, known as Forchheimer spots, appear in approximately 20% of cases. Congenital disabilities affect up to 85% of neonates when maternal rubella infection occurs within the first 12 weeks of gestation. The risk decreases to approximately 50% with infection between 13 and 16 weeks and further declines to 25% during the latter half of the second trimester.[21]
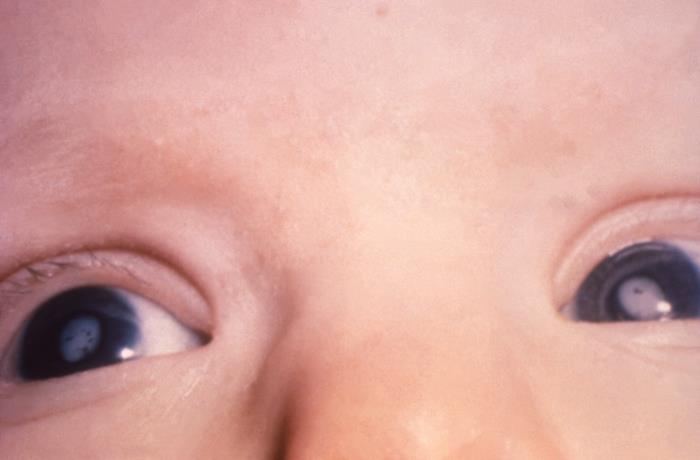
Rubella infection during embryogenesis typically results in the classic triad of cataracts, congenital heart defects, and sensorineural deafness, although many other abnormalities may also occur.[3] During the neonatal period, CRS has been associated with low birth weight, thrombocytopenic purpura, hemolytic anemia, hepatosplenomegaly, and meningoencephalitis. These manifestations are usually transient. Other clinical manifestations of CRS include ophthalmopathies (retinopathy, glaucoma, chorioretinitis, iris hypoplasia, and microphthalmia), cardiac defects (patent ductus arteriosus, pulmonary artery hypoplasia), psychomotor delay, and microcephaly.[21]
Deafness is the most common finding of CRS and may be the only defect present. Individuals who survive the neonatal period may face serious disabilities, eg, visual and hearing impairments, and are at increased risk for developmental disorders, including autism. Late-onset complications may also occur, including endocrine, cardiovascular, and neurological abnormalities.[3][4]
Serious birth defects associated with CRS include:
- Cardiac anomalies
- Patent ductus arteriosus
- Peripheral pulmonary artery stenosis
- Ventricular septal defects
- Atrial septal defects
- Auditory anomalies
- Sensorineural hearing impairment (most common)
- Ophthalmologic conditions
- Cataracts (see Image. Cataracts, Child, Pathology)
- Pigmentary retinopathy
- Microphthalmos
- Chorioretinitis
- Neurologic anomalies
- Microcephaly
- Cerebral calcifications
- Meningoencephalitis
- Behavioral disorders
- Intellectual disability or delay
- Hematologic conditions
- Thrombocytopenia
- Hemolytic anemia
- Ptechiae or purpura
- Dermal erythropoiesis causing “blueberry muffin” rash (see Image. Congenital Rubella)
- Neonatal conditions
- Low birth weight
- Interstitial pneumonitis
- Radiolucent bone disease causing “celery stalking” of long bone metaphyses
- Hepatosplenomegaly
Evaluation
Due to rubella's mild and nonspecific symptoms, the clinical diagnosis is often challenging. Therefore, a high index of suspicion is essential, and laboratory testing is required for confirmation.
Postnatal Rubella Diagnostic Evaluation
The most common diagnostic test for recent or acute postnatal rubella infection is the detection of rubella-specific immunoglobulin M (IgM) antibodies using enzyme immunoassay (EIA). These antibodies typically become detectable approximately 4 days after rash onset.[24] A 4-fold or greater rise in rubella-specific immunoglobulin G (IgG) titers between acute and convalescent serum samples also confirms infection or reinfection. Recent primary rubella infections can also be confirmed by rubella-specific low-avidity IgG.[12]
Maternal screening for rubella immunity is performed during early pregnancy by measuring IgG titers. Immediate serologic testing for rubella-specific IgG is recommended if a pregnant woman is exposed to suspected rubella. A positive result confirms immunity, while repeat IgG and IgM testing should follow a negative result after 3 weeks to assess for acute infection.
Congenital Rubella Diagnostic Evaluation
Maternal rubella screening with IgG titers in early pregnancy is the standard of care in the US. Suspected rubella-like illness in early pregnancy should be evaluated with laboratory testing to confirm the diagnosis. Diagnosis is based on seroconversion, as determined by IgG and IgM titers. Maternal counseling and, in some instances, consideration of pregnancy termination, may be appropriate depending on gestational age and diagnostic results.[3] Prenatal diagnosis of congenital rubella infection may be determined by detecting rubella-specific IgM in fetal blood or rubella RNA in amniotic fluid, fetal blood, or chorionic villus biopsy using reverse transcription-polymerase chain reaction (RT-PCR). These tests help assess fetal infection early, guiding clinical management and counseling.
During the newborn period, diagnosis is confirmed by the presence of rubella-specific IgM antibodies within the first 6 months of life. Persistent or rising rubella-specific IgG levels after 6 to 12 months also support the diagnosis. Detection of rubella RNA in nasopharyngeal swabs, urine, and oral fluid by RT-PCR provides additional confirmation of CRS.[4]
Postnatal diagnosis of congenital rubella infection is performed by detecting rubella-specific IgG antibodies in neonatal serum using enzyme-linked immunosorbent assay (ELISA), which has near 100% sensitivity and specificity in infants younger than 3 months. Confirmation is further supported by detecting rubella virus in nasopharyngeal swabs, urine, or oral fluid using PCR.[4]
Congenital infection can also be confirmed by stable or rising levels of rubella-specific IgG during the first year of life. Even without clinical signs of congenital rubella syndrome, postnatal confirmation is crucial, as it enables appropriate follow-up for early detection of long-term neurological and ocular complications.[25]
Treatment / Management
Perinatal Management
Supportive care remains the mainstay of treatment for postnatally acquired rubella in nonpregnant individuals, with nonsteroidal anti-inflammatory drugs (NSAIDs) used to alleviate fever, arthralgia, and arthritis.[3]
The diagnosis of CRS necessitates a coordinated, interprofessional approach involving physicians, nurses, infection control specialists, and allied health professionals due to the disease's multisystem involvement and the need for ongoing family education and support. In pregnant women, clinical management varies based on the gestational age at the time of infection.[4] Rubella infection before 18 weeks of gestation carries a high risk of fetal infection and congenital malformations. Under such circumstances, consideration of pregnancy termination may align with applicable ethical standards and local legal frameworks. Infections occurring after 18 weeks of gestation pose a lower risk of severe fetal complications. Continued pregnancy involves close ultrasound surveillance and appropriate neonatal evaluation, including testing for rubella virus-specific IgG.[4][22]
Infants with CRS can shed rubella virus in bodily secretions, particularly urine and nasopharyngeal secretions, for prolonged periods. Therefore, infants should be considered potentially infectious until 1 year of age unless 2 viral cultures are taken at least 1 month apart after 3 months of age and test negative for the rubella virus.[26][27][28] Treatment of children with CRS is symptomatic and organ-specific. Management typically requires an interprofessional approach with evaluations in pediatrics, ophthalmology, cardiology, audiology, and neurodevelopment. Long-term follow-up is essential to monitor for delayed or progressive manifestations of the condition.[3]
Infection prevention protocols should include immediate postnatal contact isolation to reduce the risk of nosocomial transmission, particularly in neonatal and pediatric units. Nursing care is vital to infection control, with strict adherence to hand hygiene emphasized as a key strategy to limit the transmission of virus-laden urine and respiratory secretions.[26][27][28]
Vaccination and Immunization
Beyond clinical care, healthcare professionals play a critical role in advancing public health initiatives by promoting the timely administration of rubella-containing vaccines (RCVs). Achieving high vaccination coverage across the population prevents maternal rubella infection and fetal transmission, thereby significantly reducing the incidence of congenital rubella syndrome (CRS). Universal immunization of all susceptible individuals remains essential for controlling rubella and, more importantly, preventing CRS. In the US, the rubella vaccine contains the live attenuated RA 27/3 strain and is typically administered as part of the MMR (measles, mumps, rubella) or MMRV (measles, mumps, rubella, and varicella) combination.
The first dose is administered at 12 to 15 months, and the second dose is given at ages 4 to 6 years.[3][4][29] Adolescents and adults without vaccination should receive at least 1 dose of the rubella vaccine. A single dose provides protective antibodies in over 95% of recipients, with nearly 100% seroconversion after 2 doses.
Adverse effects and contraindications
The vaccine is well-tolerated. Common adverse effects include mild fever, rash, and transient lymphadenopathy.[29] Contraindications to rubella vaccination include a history of an anaphylactic reaction to a previous dose or vaccine components such as neomycin and gelatin, as well as known immunodeficiency. The MMR vaccine is not contraindicated in individuals with an allergy to eggs. Additionally, the rubella vaccination should be deferred in individuals who have recently received high-dose corticosteroids, those who have received immunoglobulin or blood products, or those who have recently received another live vaccine (unless given simultaneously).[3]
Because the rubella vaccine is a live attenuated virus with theoretical teratogenic risk, it is contraindicated during pregnancy.[29] Women of childbearing age without documented rubella immunity should be vaccinated at least 1 month before conception or immediately postpartum.[30] If the vaccine is inadvertently given during pregnancy or conception occurs within 28 days of vaccination, termination is not recommended. Studies show no evidence of fetal or maternal harm from the vaccine.[3][4](A1)
Adverse effects of the rubella vaccine are uncommon in children. Following MMR administration, fever occurs in about 15% of recipients and rash in around 5%. Postpubertal women may develop transient arthralgia or arthritis.[4] Less common adverse reactions are transient lymphadenopathy, parotiditis, and febrile convulsions. Moderate to severe sequelae such as anaphylaxis, anterior uveitis, thrombocytopenic purpura, or encephalitis are rare.[3] No link has been found between MMR vaccination and autism.[31]
Rubella Immunoglobulin
Passive immunization with immunoglobulin is not recommended for nonpregnant contacts. However, for susceptible pregnant women with confirmed rubella exposure where pregnancy termination is not an option, immunoglobulin may be considered within 72 hours of exposure.[32]
Differential Diagnosis
Rubella should be distinguished from other infections that present with similar maculopapular rashes, including measles, human herpesvirus 6 and 7, infectious mononucleosis, cytomegalovirus, and arboviruses, eg, Zika virus, West Nile virus, Ross River fever, and Chikungunya virus. Other infectious mimics include enteroviruses, scarlet fever, parvovirus B19, and mycoplasma infection.
Noninfectious conditions in the differential diagnosis include Kawasaki disease, drug eruptions, and contact dermatitis.[3] A thorough clinical history, vaccination status, and laboratory testing are essential for differentiating rubella from these conditions.
Prognosis
Postnatal rubella infection is generally mild, self-limited, and associated with an excellent prognosis. In contrast, the prognosis for children with CRS is less favorable and depends on the severity and extent of organ involvement. Infants with thrombocytopenia, interstitial pneumonia, hepatosplenomegaly, or pulmonary hypertension are at higher risk of mortality.[3]
Long-term complications of CRS may include blindness, heart failure, developmental delays, and reduced life expectancy.[33] These children often require ongoing medical care and interprofessional support to manage chronic health issues and optimize developmental outcomes.
Complications
In adolescents and adults, polyarthritis and polyarthralgia are the most common complications of rubella, affecting up to 70% of cases. These symptoms typically involve symmetric joints, including the wrists, fingers, knees, and ankles.[34] Less common complications include thrombocytopenia, hemolytic anemia, myocarditis, pericarditis, hepatitis, orchitis, retinopathy, uveitis, Guillain-Barré syndrome, and postinfectious encephalitis.[35][36]
Rubella infection during pregnancy can result in miscarriage, intrauterine fetal demise, premature labor, intrauterine growth restriction, and CRS.[3] The risk of complications is highest when the infection is contracted within the first 12 weeks of gestation.
Deterrence and Patient Education
Deterrence and patient education regarding rubella focus on preventing transmission, ensuring immunity, and minimizing the risk of CRS. Healthcare practitioners must educate patients, especially women of childbearing age, about the importance of rubella immunity and the need for vaccination before conception. Routine screening for rubella-specific IgG antibodies during early pregnancy helps identify susceptible individuals, enabling timely counseling and postpartum immunization if necessary.
Public health messaging should emphasize that rubella, though often mild or subclinical, poses serious risks during pregnancy, with potential lifelong consequences for the fetus. Promoting widespread MMR or MMRV vaccination, along with reinforcing infection control practices in clinical settings, further supports efforts to reduce rubella incidence and protect vulnerable populations.
Pearls and Other Issues
Key facts to keep in mind about rubella include:
- Rubella is caused by an RNA virus from the Togavirus family
- Transmitted via respiratory droplets and close person-to-person contact
- Humans are the only known reservoir
- The incubation period is 14 to 21 days
- Often mild or asymptomatic in children
- Typical symptoms include low-grade fever, lymphadenopathy (especially postauricular, suboccipital, and cervical), and a pink maculopapular rash that starts on the face and spreads
- Rash usually lasts around 3 days and may be the first sign in children
- Forchheimer spots (petechiae on the soft palate) can occur
- In adults, especially women, joint pain or arthritis is common
- Infection during early pregnancy (especially the first trimester) is most dangerous
- Can cause miscarriage, stillbirth, or congenital rubella syndrome
- Classic congenital rubella syndrome triad includes cataracts, congenital heart defects (like PDA), and sensorineural deafness
- Other CRS findings include blueberry muffin rash, low birth weight, hepatosplenomegaly, microcephaly, thrombocytopenia, and developmental delays
- Diagnosed by rubella-specific IgM antibodies or a 4-fold rise in IgG titers. In CRS, IgM is detected in the newborn; persistent or rising IgG after 6 months confirms infection
- RT-PCR can detect viral RNA in urine, throat swabs, or amniotic fluid
- Postnatal rubella is treated with supportive care, like NSAIDs
- CRS requires long-term interprofessional management
- Live vaccine is contraindicated in pregnancy and immunocompromised patients
- All women of childbearing age should have rubella immunity confirmed before pregnancy
- Rubella was eliminated in the US in 2004, but remains a concern globally where vaccine coverage is low
Enhancing Healthcare Team Outcomes
Effective management and prevention of rubella and CRS require strategic collaboration and communication across an interprofessional healthcare team. Physicians and advanced practitioners play a pivotal role in diagnosing rubella and CRS, initiating appropriate testing, and notifying public health authorities, such as the CDC, upon suspicion of infection. Nurses and infection control specialists are integral in enforcing standard and droplet precautions in hospitalized patients, especially neonates with CRS who may shed the virus for over a year. Infection prevention protocols, including strict hand hygiene and contact isolation immediately postnatally, are essential to minimizing nosocomial transmission. Pharmacists may contribute by educating patients and caregivers about the importance and timing of RCVs, thereby supporting both individual protection and broader public health initiatives.
The care of infants with CRS necessitates a comprehensive and sustained interprofessional approach. Physicians, nurses, developmental specialists, and social workers must collaborate to monitor multisystem complications, provide developmental support, and address the psychosocial needs of affected families. Early and continuous family education ensures caregivers understand the potential for prolonged viral shedding and the importance of ongoing precautions. Regular communication among team members ensures consistency in infection control measures and helps avoid care fragmentation. Allied health professionals and public health personnel further enhance outcomes by linking families to community resources and reinforcing vaccine advocacy. Through cohesive coordination and shared accountability, the interprofessional team can significantly improve patient safety, optimize developmental outcomes, and reduce future CRS incidence.
Media
(Click Image to Enlarge)
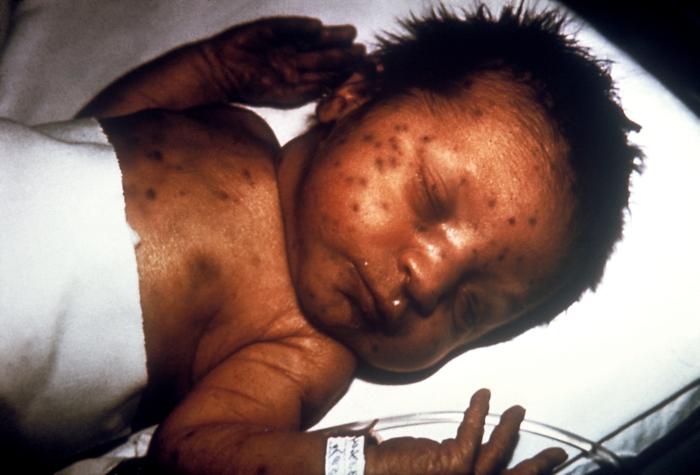
Congenital Rubella. This image demonstrates the blueberry muffin-like skin lesions indicative of congenital rubella.
Public Health Image Library, Public Domain, Centers for Disease Control and Prevention
(Click Image to Enlarge)
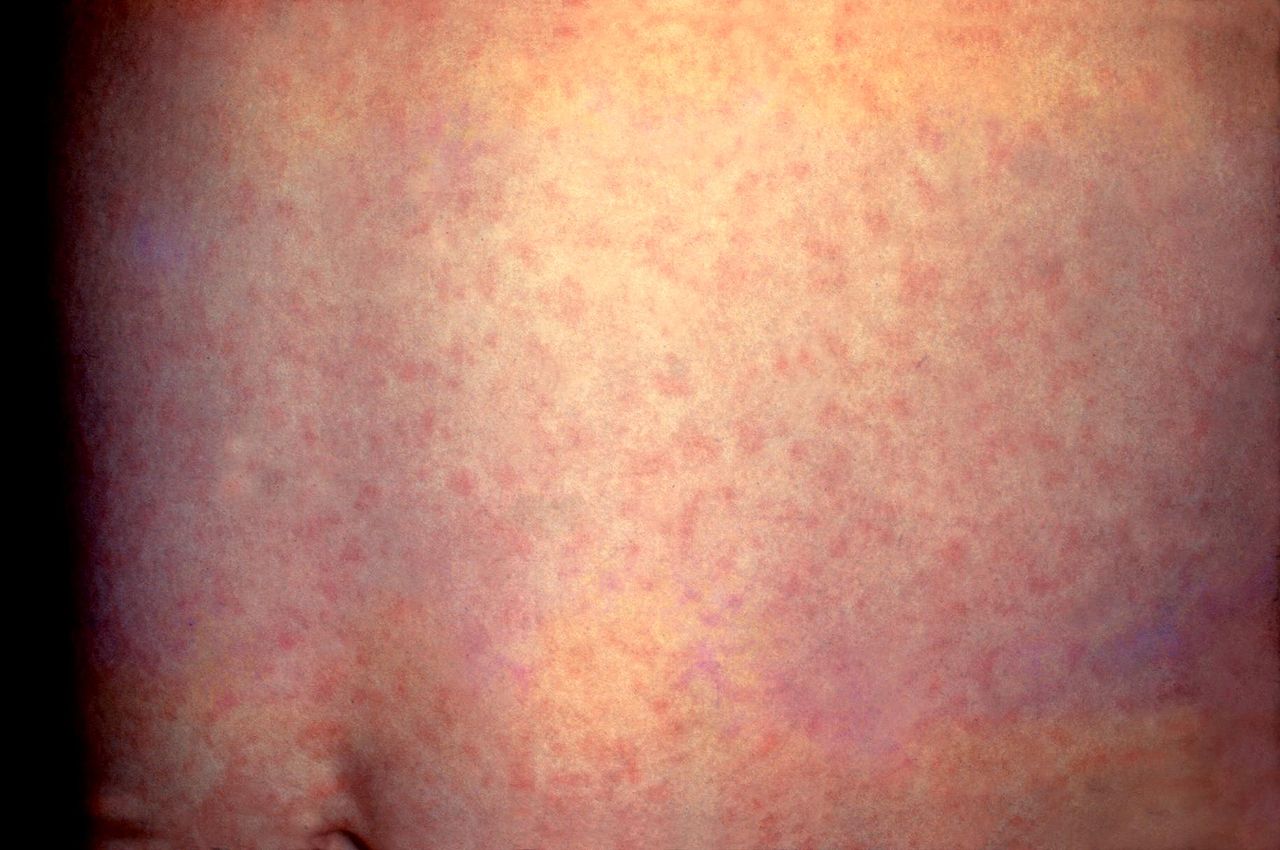
German Measles, Rubella. This patient presented with a generalized rash on the abdomen caused by German measles (rubella). The rash usually lasts about 3 days and may be accompanied by a low-grade fever.
Centers for Disease Control and Prevention, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
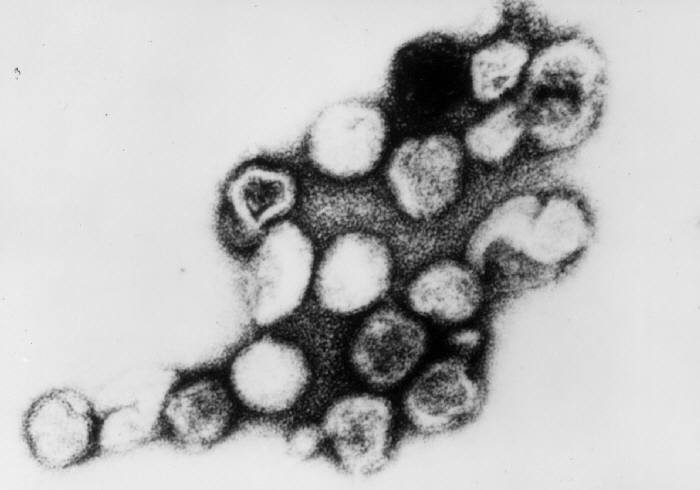
Rubella Virus, Transmission Electron Micrograph. Persons with measles can transmit the virus 4 days before and 4 days after the onset of the rash.
Erskine Palmer, MD, Public Domain, via Wikimedia Commons.
(Click Image to Enlarge)
(Click Image to Enlarge)
Paramyxovirus Virion, Transmission Electron Micrograph. The image displays the viral nucleocapsid of a paramyxovirus virion as visualized under a transmission electron microscope.
Fred Murphy, MD, Public Health Image Library, Public Domain, Centers for Disease Control and Prevention
References
Forbes JA. Rubella: historical aspects. American journal of diseases of children (1960). 1969 Jul:118(1):5-11 [PubMed PMID: 4892774]
Winter AK, Moss WJ. Rubella. Lancet (London, England). 2022 Apr 2:399(10332):1336-1346. doi: 10.1016/S0140-6736(21)02691-X. Epub [PubMed PMID: 35367004]
Leung AKC, Hon KL, Leong KF. Rubella (German measles) revisited. Hong Kong medical journal = Xianggang yi xue za zhi. 2019 Apr:25(2):134-141. doi: 10.12809/hkmj187785. Epub 2019 Apr 10 [PubMed PMID: 30967519]
Bouthry E, Picone O, Hamdi G, Grangeot-Keros L, Ayoubi JM, Vauloup-Fellous C. Rubella and pregnancy: diagnosis, management and outcomes. Prenatal diagnosis. 2014 Dec:34(13):1246-53. doi: 10.1002/pd.4467. Epub 2014 Sep 16 [PubMed PMID: 25066688]
Lee JY, Bowden DS. Rubella virus replication and links to teratogenicity. Clinical microbiology reviews. 2000 Oct:13(4):571-87 [PubMed PMID: 11023958]
Lazar M, Abernathy E, Chen MH, Icenogle J, Janta D, Stanescu A, Pistol A, Santibanez S, Mankertz A, Hübschen JM, Mihaescu G, Necula G, Lupulescu E. Epidemiological and molecular investigation of a rubella outbreak, Romania, 2011 to 2012. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2016 Sep 22:21(38):. doi: 10.2807/1560-7917.ES.2016.21.38.30345. Epub [PubMed PMID: 27684329]
Level 2 (mid-level) evidenceReef SE, Icenogle JP, Plotkin SA. The path to eradication of rubella. Vaccine. 2023 Dec 7:41(50):7525-7531. doi: 10.1016/j.vaccine.2023.11.014. Epub 2023 Nov 14 [PubMed PMID: 37973510]
Woyessa AB, Ali MS, Korkpor TK, Tuopileyi R 2nd, Kohar HT, Dogba J, Baller A, Monday J, Abdullahi S, Nagbe T, Mulbah G, Kromah M, Sesay J, Yealue K, Nyenswah T, Gebrekidan MZ. Rubella transmission and the risk of congenital rubella syndrome in Liberia: a need to introduce rubella-containing vaccine in the routine immunization program. BMC infectious diseases. 2019 Sep 18:19(1):813. doi: 10.1186/s12879-019-4464-7. Epub 2019 Sep 18 [PubMed PMID: 31533658]
Frey TK. Molecular biology of rubella virus. Advances in virus research. 1994:44():69-160 [PubMed PMID: 7817880]
Level 3 (low-level) evidencePukuta E, Waku-Kouomou D, Abernathy E, Illunga BK, Obama R, Mondonge V, Dahl BA, Maresha BG, Icenogle J, Muyembe JJ. Genotypes of rubella virus and the epidemiology of rubella infections in the Democratic Republic of the Congo, 2004-2013. Journal of medical virology. 2016 Oct:88(10):1677-84. doi: 10.1002/jmv.24517. Epub 2016 Apr 5 [PubMed PMID: 27479298]
Dominguez G, Wang CY, Frey TK. Sequence of the genome RNA of rubella virus: evidence for genetic rearrangement during togavirus evolution. Virology. 1990 Jul:177(1):225-38 [PubMed PMID: 2353453]
Level 3 (low-level) evidenceLambert N, Strebel P, Orenstein W, Icenogle J, Poland GA. Rubella. Lancet (London, England). 2015 Jun 6:385(9984):2297-307. doi: 10.1016/S0140-6736(14)60539-0. Epub 2015 Jan 8 [PubMed PMID: 25576992]
DuBois RM, Vaney MC, Tortorici MA, Kurdi RA, Barba-Spaeth G, Krey T, Rey FA. Functional and evolutionary insight from the crystal structure of rubella virus protein E1. Nature. 2013 Jan 24:493(7433):552-6. doi: 10.1038/nature11741. Epub 2013 Jan 6 [PubMed PMID: 23292515]
McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS, Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2013 Jun 14:62(RR-04):1-34 [PubMed PMID: 23760231]
Vynnycky E, Adams EJ, Cutts FT, Reef SE, Navar AM, Simons E, Yoshida LM, Brown DW, Jackson C, Strebel PM, Dabbagh AJ. Using Seroprevalence and Immunisation Coverage Data to Estimate the Global Burden of Congenital Rubella Syndrome, 1996-2010: A Systematic Review. PloS one. 2016:11(3):e0149160. doi: 10.1371/journal.pone.0149160. Epub 2016 Mar 10 [PubMed PMID: 26962867]
Level 1 (high-level) evidence. Achievements in public health: elimination of rubella and congenital rubella syndrome-US, 1969-2004. The Annals of pharmacotherapy. 2005 Jun:39(6):1151-2 [PubMed PMID: 15886287]
Al Hammoud R, Murphy JR, Pérez N. Imported Congenital Rubella Syndrome, United States, 2017. Emerging infectious diseases. 2018 Apr:24(4):800-801. doi: 10.3201/eid2404.171540. Epub [PubMed PMID: 29553333]
Durrheim DN, Andrus JK, Tabassum S, Githanga D, Kojouharova M, Talab N. Accelerating Global Measles and Rubella Eradication-Saving Millions of Lives, Preventing Disability, and Averting the Next Pandemic. Vaccines. 2024 Jun 20:12(6):. doi: 10.3390/vaccines12060699. Epub 2024 Jun 20 [PubMed PMID: 38932428]
Pandolfi E, Chiaradia G, Moncada M, Rava L, Tozzi AE. Prevention of congenital rubella and congenital varicella in Europe. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2009 Mar 5:14(9):16-20 [PubMed PMID: 19317971]
Vauloup-Fellous C, Grangeot-Keros L. Humoral immune response after primary rubella virus infection and after vaccination. Clinical and vaccine immunology : CVI. 2007 May:14(5):644-7 [PubMed PMID: 17344342]
Al Beloushi M, Saleh H, Ahmed B, Konje JC. Congenital and Perinatal Viral Infections: Consequences for the Mother and Fetus. Viruses. 2024 Oct 30:16(11):. doi: 10.3390/v16111698. Epub 2024 Oct 30 [PubMed PMID: 39599813]
Yazigi A, De Pecoulas AE, Vauloup-Fellous C, Grangeot-Keros L, Ayoubi JM, Picone O. Fetal and neonatal abnormalities due to congenital rubella syndrome: a review of literature. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2017 Feb:30(3):274-278 [PubMed PMID: 27002428]
Kaushik A, Verma S, Kumar P. Congenital rubella syndrome: A brief review of public health perspectives. Indian journal of public health. 2018 Jan-Mar:62(1):52-54. doi: 10.4103/ijph.IJPH_275_16. Epub [PubMed PMID: 29512566]
Level 3 (low-level) evidenceHübschen JM, Bork SM, Brown KE, Mankertz A, Santibanez S, Ben Mamou M, Mulders MN, Muller CP. Challenges of measles and rubella laboratory diagnostic in the era of elimination. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2017 Aug:23(8):511-515. doi: 10.1016/j.cmi.2017.04.009. Epub 2017 Apr 13 [PubMed PMID: 28412379]
Vauloup-Fellous C. Standardization of rubella immunoassays. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2018 May:102():34-38. doi: 10.1016/j.jcv.2018.02.006. Epub 2018 Feb 15 [PubMed PMID: 29486385]
Dinede G, Wondimagegnehu A, Enquselassie F. Rubella outbreak in the school children, Addis Ababa, Ethiopia: February-April 2018. BMC infectious diseases. 2019 Mar 18:19(1):267. doi: 10.1186/s12879-019-3873-y. Epub 2019 Mar 18 [PubMed PMID: 30885148]
Obam Mekanda FM, Monamele CG, Simo Nemg FB, Sado Yousseu FB, Ndjonka D, Kfutwah AKW, Abernathy E, Demanou M. First report of the genomic characterization of rubella viruses circulating in Cameroon. Journal of medical virology. 2019 Jun:91(6):928-934. doi: 10.1002/jmv.25445. Epub 2019 Mar 12 [PubMed PMID: 30822356]
Dewan P, Gupta P. 50 Years Ago in The Journal of Pediatrics: Maternal and Congenital Rubella before 1964: Frequency, Clinical Features, and Search for Isoimmune Phenomena. The Journal of pediatrics. 2019 Feb:205():82. doi: 10.1016/j.jpeds.2018.08.045. Epub [PubMed PMID: 30684990]
Issa AN, Wodi AP, Moser CA, Cineas S. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2025. MMWR. Morbidity and mortality weekly report. 2025 Jan 16:74(2):26-29. doi: 10.15585/mmwr.mm7402a2. Epub 2025 Jan 16 [PubMed PMID: 39819853]
Laris-González A, Bernal-Serrano D, Jarde A, Kampmann B. Safety of Administering Live Vaccines During Pregnancy: A Systematic Review and Meta-Analysis of Pregnancy Outcomes. Vaccines. 2020 Mar 11:8(1):. doi: 10.3390/vaccines8010124. Epub 2020 Mar 11 [PubMed PMID: 32168941]
Level 1 (high-level) evidenceDeStefano F, Shimabukuro TT. The MMR Vaccine and Autism. Annual review of virology. 2019 Sep 29:6(1):585-600. doi: 10.1146/annurev-virology-092818-015515. Epub 2019 Apr 15 [PubMed PMID: 30986133]
Young MK. The indications and safety of polyvalent immunoglobulin for post-exposure prophylaxis of hepatitis A, rubella and measles. Human vaccines & immunotherapeutics. 2019:15(9):2060-2065. doi: 10.1080/21645515.2019.1621148. Epub 2019 Jun 19 [PubMed PMID: 31116633]
Dammeyer J. Congenital rubella syndrome and delayed manifestations. International journal of pediatric otorhinolaryngology. 2010 Sep:74(9):1067-70. doi: 10.1016/j.ijporl.2010.06.007. Epub 2010 Jul 8 [PubMed PMID: 20619470]
McCormick JN, Duthie JJ, Gerber H, Hart H, Baker S, Marmion BP. Rheumatoid polyarthritis after rubella. Annals of the rheumatic diseases. 1978 Jun:37(3):266-72 [PubMed PMID: 686860]
Frey TK. Neurological aspects of rubella virus infection. Intervirology. 1997:40(2-3):167-75 [PubMed PMID: 9450233]
Level 3 (low-level) evidenceAtkins MC, Esmonde TF. Guillain-Barré syndrome associated with rubella. Postgraduate medical journal. 1991 Apr:67(786):375-6 [PubMed PMID: 2068032]