Introduction
Squamous cell carcinoma is the second most common cutaneous malignancy in the United States. Risk factors include immunosuppression, chronic wounds, fair skin, male gender, older age, several genetic syndromes, environmental exposures such as UV radiation, and a previous history of squamous cell carcinoma. Although metastasis is rare, the most common site of metastasis is the lymph nodes. As the incidence steadily increases, posing a significant public health concern, timely surveillance, early diagnosis, and prompt treatment are essential to minimize morbidity and mortality risks. Photoprotection and frequent full-body skin exams are recommended. While most cases are treated with surgical excision, novel therapeutic modalities continue to emerge. Systemic oncologic therapy and radiation therapy may be warranted for more advanced cases.[1][2][3][4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The development of cutaneous squamous cell carcinoma is associated with the following risk factors and etiologies:
- UV radiation: UVA and UVB are the most significant risk factors.
- Environmental exposures other than UV radiation: This includes arsenic, polycyclic aromatic hydrocarbons, nitrosamines, alkylating agents, and ionizing radiation.
- Demographic factors: Fair skin, male, and older age.
- Immunosuppressed state: Iatrogenic, leukemia, and AIDS.
- Genetic syndromes: Huriez syndrome, xeroderma pigmentosa, oculocutaneous albinism, dyskeratosis congenita, Rothmund-Thomson syndrome, Werner syndrome, Bloom syndrome, dystrophic epidermolysis bullosa, epidermodysplasia verruciformis, Fanconi anemia, keratitis-ichthyosis-deafness syndrome, and genetic immunodeficiency syndromes.
- Preexisting lesions: Chronic wounds (Marjolin ulcer), human papillomavirus, actinic keratosis, porokeratosis, lichen sclerosus et atrophicus, hypertrophic or oral lichen planus, and discoid cutaneous lupus erythematosus.
- Medications: BRAF inhibitors, vismodegib, and voriconazole, immunosuppressive agents.[5][6][7][8][9]
Epidemiology
Squamous cell carcinoma is the second most common cutaneous malignancy in the United States. The incidence of this condition has steadily increased, with a nearly 3-fold rise reported from the 1970s to the early 2000s.[10] In 2012, the incidence of cutaneous squamous cell carcinoma was estimated at 140 per 100,000 American men and 50 per 100,000 American women. The mortality rate is approximately 1% to 2%.[11] However, in the southern and central regions of the United States, the mortality rate is similar to that of melanoma and renal and oropharyngeal carcinoma.[12] The prevalence is higher among men, fair-skinned individuals, and older age groups.
Pathophysiology
Cutaneous squamous cell carcinoma is derived from the keratinocytes. A mutation in the tp53 tumor suppressor gene is the most common genetic abnormality observed in both squamous cell carcinoma and its precursor, actinic keratosis. Decreased immunosurveillance in immunosuppressed patients may further potentiate tumor growth. For instance, patients with solid organ transplants on immunosuppressive therapy have a 65- to 250-fold increased risk of developing squamous cell carcinoma compared to the general population.[1]
Histopathology
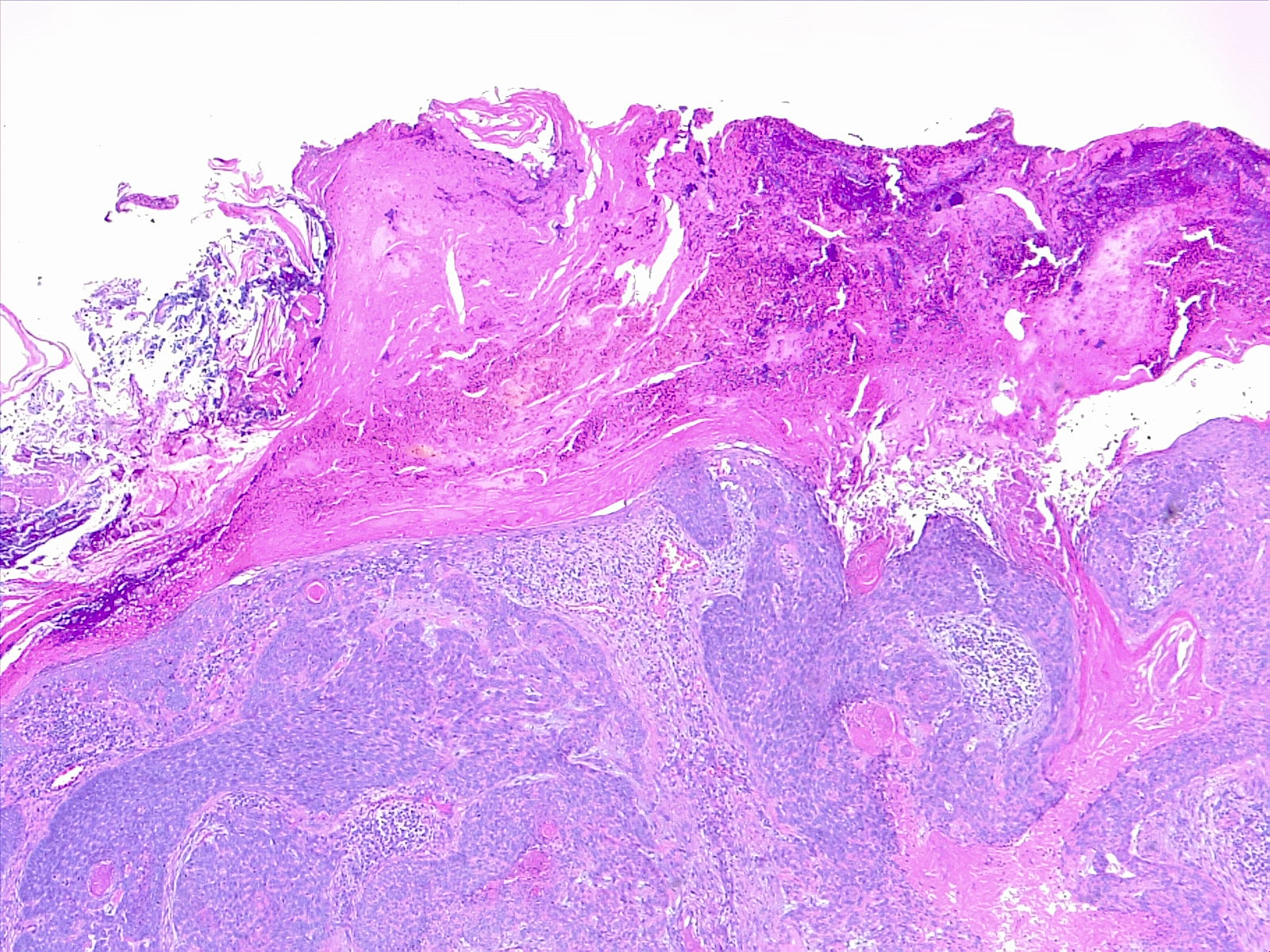
The histological subtypes of squamous cell carcinoma include squamous cell carcinoma in situ/Bowen disease, acantholytic/adenoid/pseudoglandular, clear cell, sarcomatoid/spindle cell, desmoplastic, keratoacanthoma, and verrucous carcinoma. Tumors classically consist of atypical keratinocytes with abundant eosinophilic or pink, glassy cytoplasm. Other common findings include parakeratosis, intercellular bridging, and keratin pearls (see Image. Histological Slide of Cutaneous Squamous Cell Carcinoma). High-risk histological subtypes include acantholytic, sarcomatoid, and desmoplastic.
Squamous Cell Carcinoma In Situ
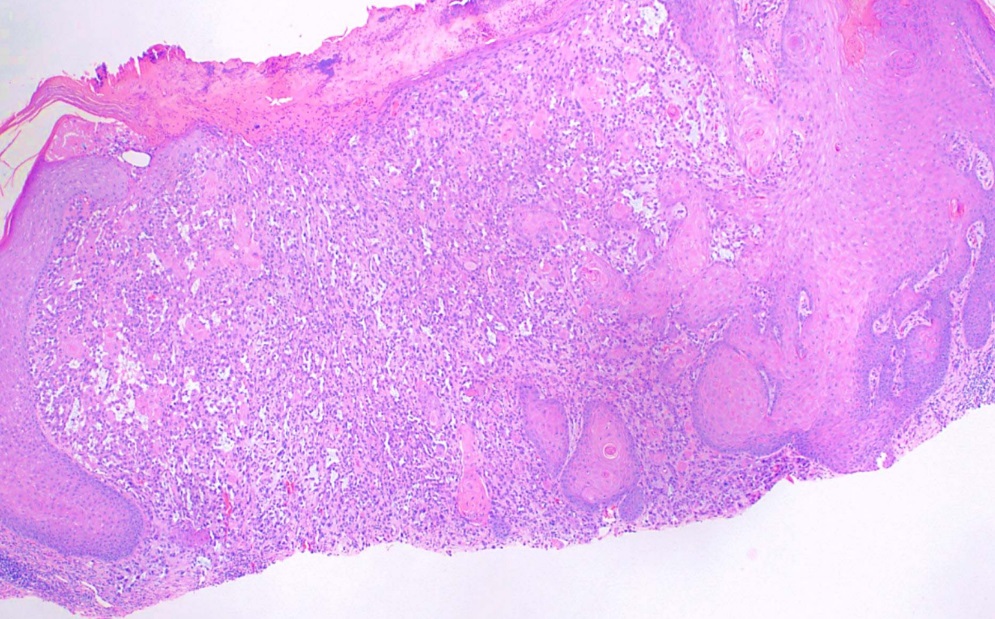
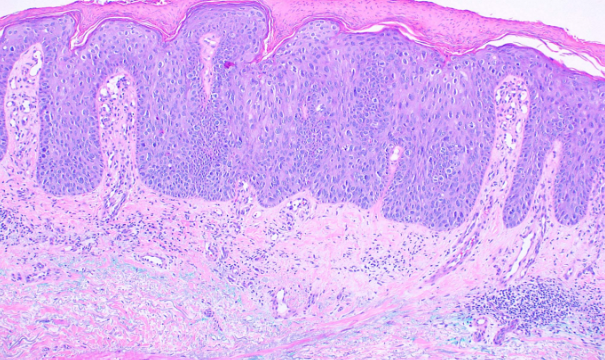
This subtype, also known as Bowen disease, is characterized by full-thickness keratinocyte atypia that has not invaded beyond the basal layer of the epidermis. In contrast, invasive squamous cell carcinoma has penetrated beyond the basal layer (see Image. Histological Slide of Squamous Cell Carcinoma, In Situ). The basal layer is often intact, forming the "eyeliner sign."
Acantholytic/Adenoid/Pseudoglandular
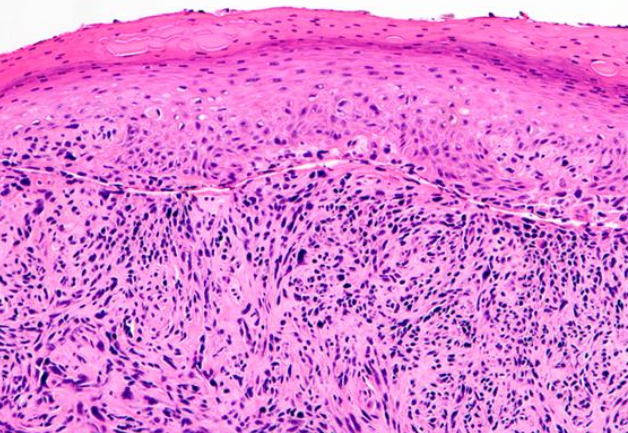
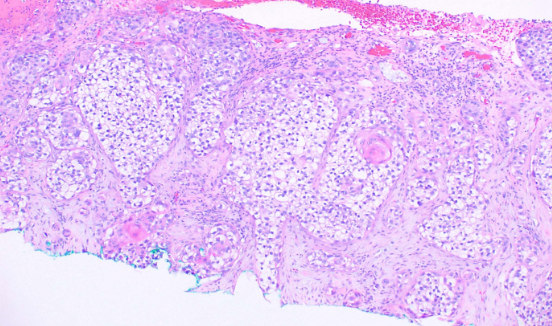
This subtype is characterized by clefting around nests or cords of plump, polygonal tumor cells, creating a glandular appearance (see Image. Histological Slide of Squamous Cell Carcinoma, Acantholytic). Clefting is due to desmosomal disruption. In contrast to tumors of true glandular origin, such as adenosquamous carcinoma, the carcinoembryonic antigen (CEA) staining is negative.
Clear Cell
This subtype is characterized by clear or pale tumor cells that may or may not demonstrate an epidermal connection (see Image. Histological Slide of Squamous Cell Carcinoma, Clear Cell). Histological differentials include clear cell acanthoma and renal cell carcinoma.
Sarcomatoid/Spindle Cell
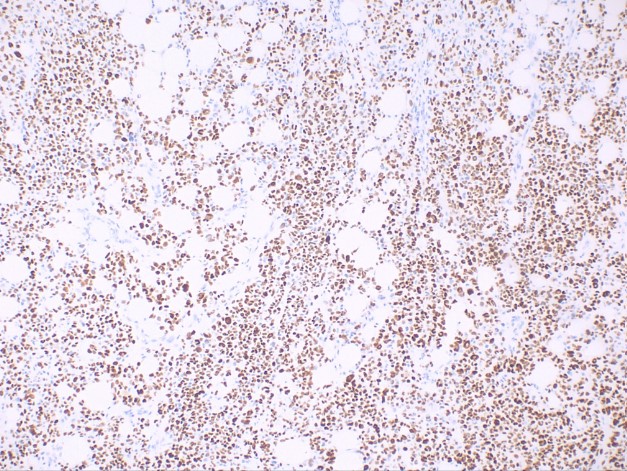
This subtype is characterized by spindle-shaped keratinocytes with pleomorphic nuclei arranged haphazardly throughout the dermis in a typically infiltrative pattern (see Image. Histological Slide of Squamous Cell Carcinoma, Sarcomatoid). Numerous mitotic figures may be observed. Atypical fibroxanthoma is an important histological differential that typically stains negative for p63 and p40, unlike sarcomatoid or spindle squamous cell carcinoma (see Image. Histological Slide of Squamous Cell Carcinoma, Positive p40 Stain). Immunohistochemistry with p40 is more specific than p63.
Desmoplastic
This subtype may appear similar to the sarcomatoid or spindle cell variant of squamous cell carcinoma. However, desmoplastic carcinoma is characterized by a desmoplastic (densely collagenous) stroma in more than 30% of the tumor. Perineural invasion is frequently reported. Desmoplastic melanoma is also considered in the histological differential diagnosis. Unlike desmoplastic melanoma, desmoplastic squamous cell carcinoma typically stains positive for p63 and negative for SOX10 and S100.
Keratoacanthoma
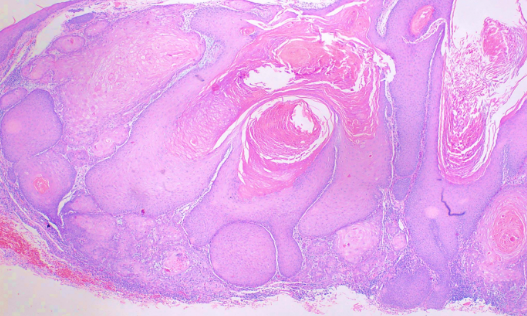
This subtype is characterized by a crateriform invagination filled with keratin (see Image. Histological Slide of Squamous Cell Carcinoma, Keratoacanthoma). The tumor cells are typically well-differentiated.
Verrucous Carcinoma
This subtype is characterized by verruciform acanthosis with blunt, broad projections that push into, rather than infiltrate, the dermis. Human papillomavirus-related cytomorphology in verrucous carcinoma is less pronounced compared to benign warts.[2][13][14][15]
History and Physical
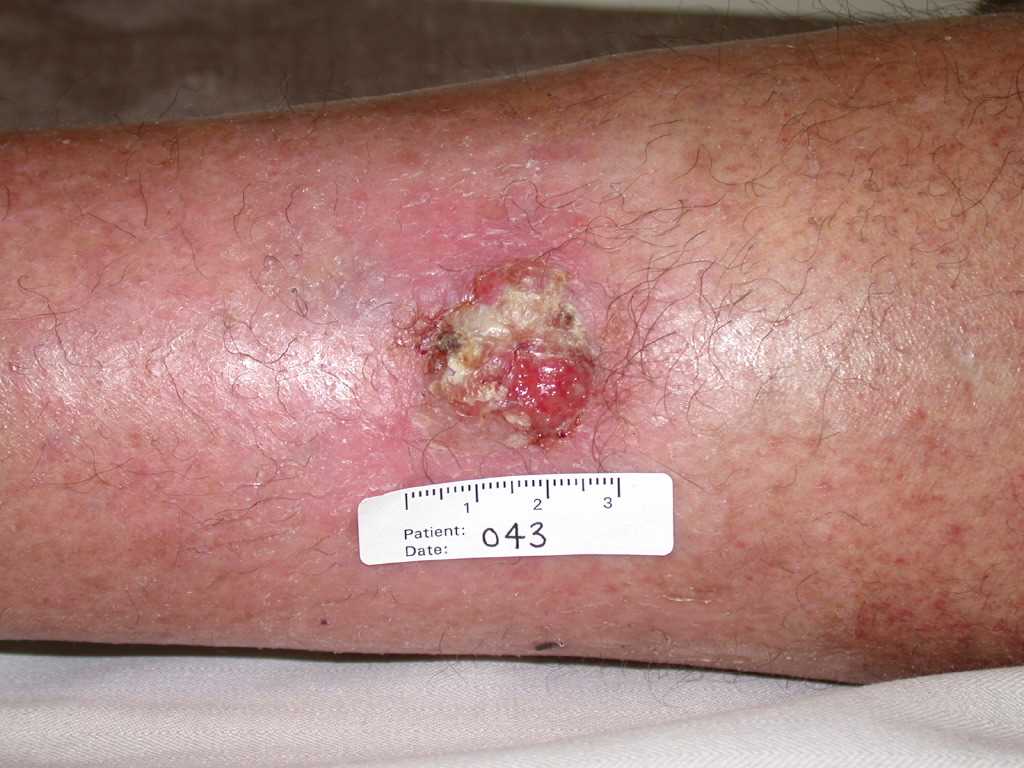
Cutaneous squamous cell carcinoma is typically characterized by a scaly, erythematous, or hyperpigmented papule or plaque. Some cases may exhibit ulceration, fungating features, or pain (see Image. Cutaneous Squamous Cell Carcinoma). Due to its strong association with UV radiation exposure, many cases arise in sun-damaged skin. This tumor can also develop from preexisting lesions such as actinic keratosis, chronic wounds (Marjolin ulcer), human papillomavirus infection, porokeratosis, lichen sclerosus et atrophicus, hypertrophic or oral lichen planus, and discoid cutaneous lupus erythematosus.[7][8][9][16][17]
Evaluation
A skin biopsy is necessary to confirm the diagnosis of cutaneous squamous cell carcinoma. Additionally, sentinel lymph node biopsy and/or radiological evaluation for lymph node metastasis with computed tomography or ultrasound is recommended in cases classified as stage T2B-T3 according to the Brigham and Women's Hospital (BWH) staging system or stage T4 according to the American Joint Committee on Cancer 8th ed. (AJCC-8) staging system. Case-by-case consideration is needed for AJCC-8 stage T2-3. For patients presenting with palpable lymphadenopathy, fine-needle aspiration or biopsy of the lymph node(s) is advised.[1][3]
Treatment / Management
The preferred therapeutic intervention for cutaneous squamous cell carcinoma is surgical excision. Mohs micrographic surgery is preferred in cases that meet appropriate use criteria (AUC). Factors listed in the AUC include, but are not limited to, clinical diameter of the apparent lesion greater than 2 cm, high-risk histological features, recurrent versus primary lesions, cosmetically sensitive and/or high-risk anatomical locations such as the ears, lips, nose, and periocular regions, as well as immunosuppression.[18] The reported 5-year recurrence rate for Mohs micrographic surgery is approximately 3.1%, whereas standard excision with 4 to 6 mm margins has a reported recurrence rate of approximately 8.1%. Mohs micrographic surgery offers a significantly greater risk reduction compared to standard excision, particularly in cases with high-risk features. For example, locally recurrent (previously treated) lesions showed a 10% recurrence rate with Mohs micrographic surgery and a 23.3% recurrence rate with standard excision.[19] Electrodessication and curettage are alternative options for in situ cases, albeit with slightly higher recurrence rates compared to Mohs micrographic surgery and standard excision.[20]
For patients who are not suitable for surgery, options for treating cutaneous squamous cell carcinoma include superficial radiation therapy, 5-fluorouracil cream, imiquimod cream, cryotherapy, photodynamic therapy, and/or ablative laser. However, these treatments often result in higher recurrence rates and lack histologically confirmed clearance.[21][22] Lymphadenectomy of the associated nodal basin is recommended for cases with positive lymph nodes. Radiation therapy is typically used for cases involving large-caliber nerve invasion, lymphovascular invasion, multiple lymph nodes, or extracapsular extension. Adjuvant systemic oncologic therapy for advanced cases may include chemotherapy, epidermal growth factor inhibitors, or immunotherapy.[3] Recently, novel immunotherapeutic agents have emerged, showing superior clinical outcomes compared to traditional systemic therapies.[23][24](A1)
Differential Diagnosis
Differential diagnoses include but are not limited to:
Staging
The staging categories for cutaneous squamous cell carcinoma as per BWH and AJCC-8 are listed below.
BWH Staging System
- Stage T1: 0 high-risk features
- Stage T2A: 1 high-risk feature
- Stage T2B: 2 to 3 high-risk features
- Stage T3: 4 or more high-risk features or bone invasion
- High-risk features, as defined by BWH, include:
- Tumor diameter of at least 2 cm
- Histologically poor differentiation
- Perineural invasion of at least 0.1 mm
- Invasion beyond subcutaneous tissue
AJCC-8 Staging System
- Stage Ti: In situ
- Stage T1: Tumor diameter less than 2 cm
- Stage T2: tumor diameter 2 to 3.9 cm
- Stage T3:
- Tumor diameter of at least 4 cm
- Minor bone erosion
- Perineural invasion at least 0.1 mm or invading a nerve located deeper than the dermis
- Invasion beyond subcutaneous tissue
- Invasion greater than 6 mm depth
- Stage T4A: Gross cortical bone or marrow invasion
- Stage T4B: Skull invasion or skull base foramen involvement [1]
Prognosis
The prognosis for localized disease is generally excellent. The overall mortality rate for cutaneous squamous cell carcinoma is approximately 1% to 2%,[11] with approximately 3% of cases metastasizing.[12] The lymph nodes are the most common site of metastasis.[2] Cases involving single node metastasis up to 3 cm are associated with a 90% disease-specific 5-year survival.[1]
Complications
Complications of cutaneous squamous cell carcinoma include:
- Metastases
- Local invasion
- Pain
- Loss of function
- Poor cosmesis
- Death
Deterrence and Patient Education
The American Academy of Dermatology (AAD) recommends the following preventative measures for patients:
- Individuals should limit sun exposure during peak UV index hours.
- Sunscreen with at least SPF 30 should be regularly used and reapplied every 2 hours and even after swimming or excessive sweating.
- Protective clothing and sunglasses should always be worn when exposed to sunlight.
- Annual full-body skin exams are recommended for the general adult population, with more frequent exams suggested for patients with significant risk factors (AAD, Shade, Clothing, and Sunscreen).
Enhancing Healthcare Team Outcomes
Due to the strong association with UV radiation, photoprotection is crucial in reducing the risk of developing cutaneous squamous cell carcinoma. Healthcare professionals should advise patients on photoprotective measures. Annual full-body skin exams are recommended, with more frequent exams advised for patients with significant risk factors. Referral to radiation or surgical oncology, urology, gynecology, or otolaryngology may be necessary for comprehensive management in advanced cases.[28]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. Journal of the American Academy of Dermatology. 2018 Feb:78(2):237-247. doi: 10.1016/j.jaad.2017.08.059. Epub [PubMed PMID: 29332704]
Caudill J, Thomas JE, Burkhart CG. The risk of metastases from squamous cell carcinoma of the skin. International journal of dermatology. 2023 Apr:62(4):483-486. doi: 10.1111/ijd.16164. Epub 2022 Mar 24 [PubMed PMID: 35324009]
Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Management of advanced and high-stage tumors. Journal of the American Academy of Dermatology. 2018 Feb:78(2):249-261. doi: 10.1016/j.jaad.2017.08.058. Epub [PubMed PMID: 29332705]
Omland SH, Ahlström MG, Gerstoft J, Pedersen G, Mohey R, Pedersen C, Kronborg G, Larsen CS, Kvinesdal B, Gniadecki R, Obel N, Omland LH. Risk of skin cancer in patients with HIV: A Danish nationwide cohort study. Journal of the American Academy of Dermatology. 2018 Oct:79(4):689-695. doi: 10.1016/j.jaad.2018.03.024. Epub 2018 Mar 26 [PubMed PMID: 29588249]
Jaju PD, Ransohoff KJ, Tang JY, Sarin KY. Familial skin cancer syndromes: Increased risk of nonmelanotic skin cancers and extracutaneous tumors. Journal of the American Academy of Dermatology. 2016 Mar:74(3):437-51; quiz 452-4. doi: 10.1016/j.jaad.2015.08.073. Epub [PubMed PMID: 26892653]
Coggshall K, Farsani T, Ruben B, McCalmont TH, Berger TG, Fox LP, Shinkai K. Keratitis, ichthyosis, and deafness syndrome: a review of infectious and neoplastic complications. Journal of the American Academy of Dermatology. 2013 Jul:69(1):127-34. doi: 10.1016/j.jaad.2012.12.965. Epub 2013 Feb 4 [PubMed PMID: 23384797]
Williams GM, Dreyer MA, Fillman EP. Porokeratosis. StatPearls. 2024 Jan:(): [PubMed PMID: 30335323]
Bleeker MC, Visser PJ, Overbeek LI, van Beurden M, Berkhof J. Lichen Sclerosus: Incidence and Risk of Vulvar Squamous Cell Carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016 Aug:25(8):1224-30. doi: 10.1158/1055-9965.EPI-16-0019. Epub 2016 Jun 2 [PubMed PMID: 27257093]
de Lanna CA, da Silva BNM, de Melo AC, Bonamino MH, Alves LDB, Pinto LFR, Cardoso AS, Antunes HS, Boroni M, Cohen Goldemberg D. Oral Lichen Planus and Oral Squamous Cell Carcinoma share key oncogenic signatures. Scientific reports. 2022 Nov 30:12(1):20645. doi: 10.1038/s41598-022-24801-6. Epub 2022 Nov 30 [PubMed PMID: 36450755]
Muzic JG, Schmitt AR, Wright AC, Alniemi DT, Zubair AS, Olazagasti Lourido JM, Sosa Seda IM, Weaver AL, Baum CL. Incidence and Trends of Basal Cell Carcinoma and Cutaneous Squamous Cell Carcinoma: A Population-Based Study in Olmsted County, Minnesota, 2000 to 2010. Mayo Clinic proceedings. 2017 Jun:92(6):890-898. doi: 10.1016/j.mayocp.2017.02.015. Epub 2017 May 15 [PubMed PMID: 28522111]
Wehner MR, Cidre Serrano W, Nosrati A, Schoen PM, Chren MM, Boscardin J, Linos E. All-cause mortality in patients with basal and squamous cell carcinoma: A systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2018 Apr:78(4):663-672.e3. doi: 10.1016/j.jaad.2017.11.026. Epub 2017 Nov 13 [PubMed PMID: 29146125]
Level 1 (high-level) evidenceKaria PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. Journal of the American Academy of Dermatology. 2013 Jun:68(6):957-66. doi: 10.1016/j.jaad.2012.11.037. Epub 2013 Feb 1 [PubMed PMID: 23375456]
Parekh V, Seykora JT. Cutaneous Squamous Cell Carcinoma. Clinics in laboratory medicine. 2017 Sep:37(3):503-525. doi: 10.1016/j.cll.2017.06.003. Epub [PubMed PMID: 28802498]
Neagu TP, Ţigliş M, Botezatu D, Enache V, Cobilinschi CO, Vâlcea-Precup MS, GrinŢescu IM. Clinical, histological and therapeutic features of Bowen's disease. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2017:58(1):33-40 [PubMed PMID: 28523295]
Henderson SA, Torres-Cabala CA, Curry JL, Bassett RL, Ivan D, Prieto VG, Tetzlaff MT. p40 is more specific than p63 for the distinction of atypical fibroxanthoma from other cutaneous spindle cell malignancies. The American journal of surgical pathology. 2014 Aug:38(8):1102-10. doi: 10.1097/PAS.0000000000000245. Epub [PubMed PMID: 25029117]
Idriss MH, Barbosa N, Chang MB, Gibson L, Baum CL, Vidal NY. Concomitant hypertrophic lichen planus and squamous cell carcinoma: Clinical features and treatment outcomes. International journal of dermatology. 2022 Dec:61(12):1527-1531. doi: 10.1111/ijd.16321. Epub 2022 Jun 29 [PubMed PMID: 35766459]
Mufti A, Sachdeva M, Maliyar K, Sibbald RG. Squamous cell carcinoma arising within discoid lupus erythematous lesions: A systematic review. JAAD international. 2021 Mar:2():1-4. doi: 10.1016/j.jdin.2020.10.001. Epub 2020 Nov 30 [PubMed PMID: 34409345]
Level 1 (high-level) evidenceAd Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, Fazio MJ, Storrs PA, Vidimos AT, Zalla MJ, Brewer JD, Smith Begolka W, Ratings Panel, Berger TG, Bigby M, Bolognia JL, Brodland DG, Collins S, Cronin TA Jr, Dahl MV, Grant-Kels JM, Hanke CW, Hruza GJ, James WD, Lober CW, McBurney EI, Norton SA, Roenigk RK, Wheeland RG, Wisco OJ. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Journal of the American Academy of Dermatology. 2012 Oct:67(4):531-50. doi: 10.1016/j.jaad.2012.06.009. Epub 2012 Sep 5 [PubMed PMID: 22959232]
Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. Journal of the American Academy of Dermatology. 1992 Jun:26(6):976-90 [PubMed PMID: 1607418]
Chren MM, Linos E, Torres JS, Stuart SE, Parvataneni R, Boscardin WJ. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. The Journal of investigative dermatology. 2013 May:133(5):1188-96. doi: 10.1038/jid.2012.403. Epub 2012 Nov 29 [PubMed PMID: 23190903]
Stewart JR, Lang ME, Brewer JD. Efficacy of nonexcisional treatment modalities for superficially invasive and in situ squamous cell carcinoma: A systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2022 Jul:87(1):131-137. doi: 10.1016/j.jaad.2021.07.067. Epub 2021 Aug 8 [PubMed PMID: 34375669]
Level 1 (high-level) evidenceNestor MS, Berman B, Goldberg D, Cognetta AB Jr, Gold M, Roth W, Cockerell CJ, Glick B. Consensus Guidelines on the Use of Superficial Radiation Therapy for Treating Nonmelanoma Skin Cancers and Keloids. The Journal of clinical and aesthetic dermatology. 2019 Feb:12(2):12-18 [PubMed PMID: 30881578]
Level 3 (low-level) evidenceMager L, Gardeen S, Carr DR, Shahwan KT. Cemiplimab for the Treatment of Advanced Cutaneous Squamous Cell Carcinoma: Appropriate Patient Selection and Perspectives. Clinical, cosmetic and investigational dermatology. 2023:16():2135-2142. doi: 10.2147/CCID.S381471. Epub 2023 Aug 9 [PubMed PMID: 37581012]
Level 3 (low-level) evidenceHarrington KJ, Burtness B, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Brana I, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesia R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Lin J, Gumuscu B, Swaby RF, Rischin D. Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2023 Feb 1:41(4):790-802. doi: 10.1200/JCO.21.02508. Epub 2022 Oct 11 [PubMed PMID: 36219809]
Peng L, Wang Y, Hong Y, Ye X, Shi P, Zhang J, Zhao Q. Incidence and relative risk of cutaneous squamous cell carcinoma with single-agent BRAF inhibitor and dual BRAF/MEK inhibitors in cancer patients: a meta-analysis. Oncotarget. 2017 Oct 10:8(47):83280-83291. doi: 10.18632/oncotarget.21059. Epub 2017 Sep 19 [PubMed PMID: 29137342]
Level 1 (high-level) evidenceMohan SV, Chang J, Li S, Henry AS, Wood DJ, Chang AL. Increased Risk of Cutaneous Squamous Cell Carcinoma After Vismodegib Therapy for Basal Cell Carcinoma. JAMA dermatology. 2016 May 1:152(5):527-32. doi: 10.1001/jamadermatol.2015.4330. Epub [PubMed PMID: 26914338]
D'Arcy ME, Pfeiffer RM, Rivera DR, Hess GP, Cahoon EK, Arron ST, Brownell I, Cowen EW, Israni AK, Triplette MA, Yanik EL, Engels EA. Voriconazole and the Risk of Keratinocyte Carcinomas Among Lung Transplant Recipients in the United States. JAMA dermatology. 2020 Jul 1:156(7):772-779. doi: 10.1001/jamadermatol.2020.1141. Epub [PubMed PMID: 32401271]
Waldman RA, Grant-Kels JM. The role of sunscreen in the prevention of cutaneous melanoma and nonmelanoma skin cancer. Journal of the American Academy of Dermatology. 2019 Feb:80(2):574-576.e1. doi: 10.1016/j.jaad.2018.06.069. Epub [PubMed PMID: 30012373]