Introduction
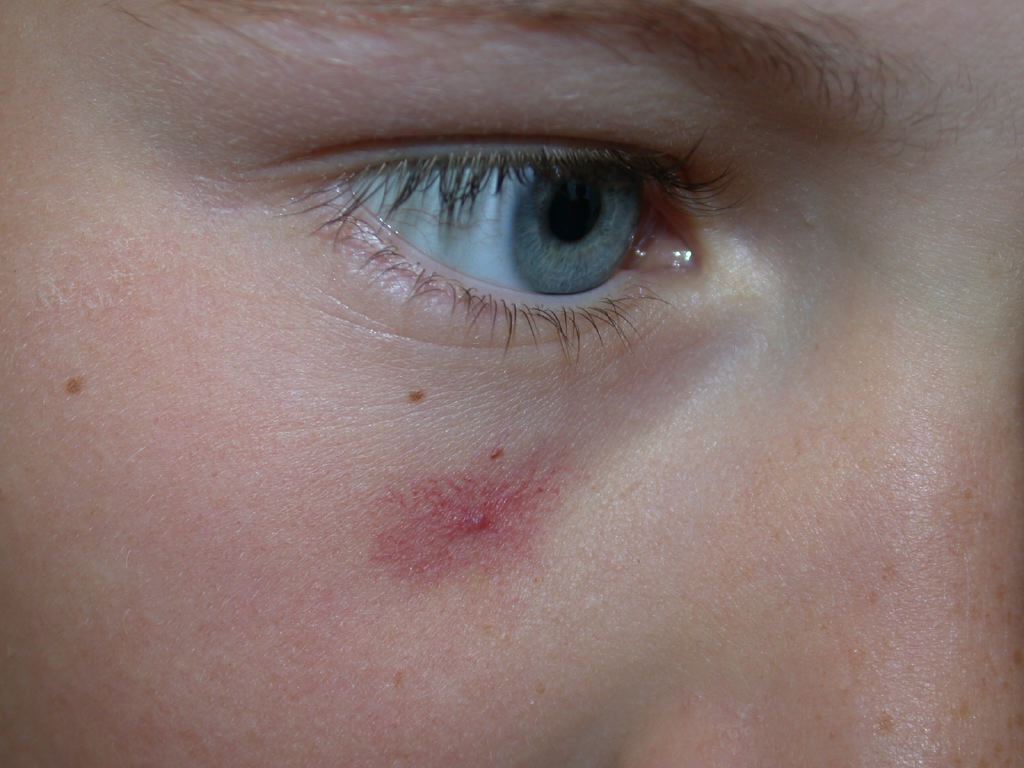
Spider veins, also known as telangiectasias, are prominent clusters of small, damaged, and superficial blood vessels visible in the skin. They appear as thin red, blue, or purple lines. Most commonly found on the legs, spider veins may occur elsewhere, particularly on the face (see Image. Spider Telangiectasis).
Telangiectasias were first described by von Graf in 1807. "Telangiectasias" is derived from the Greek words telos (end), angeion (vessel), and ektasis (dilatation).[1] Telangiectasias are also known as thread veins, venus flares, sunburst veins, stellate veins, and hyphen webs.[2]
Spider veins rarely cause health issues but may occasionally become painful. However, telangiectasias are often a cosmetic concern, and treatments primarily focus on improving appearance.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Spider veins result from abnormalities in the horizontal vascular plexus of capillary loops in the skin and can have either arterial or venous origins.[3] Venous spider veins are raised, appear blue or purple, and typically measure 1 to 3 mm in diameter. In contrast, arterial spider veins (originating from the arterial capillary loops) are flat, appear pink or red, and range from 0.1 to 1 mm in diameter.[4] In the legs, these veins are usually located 180 μm to 1 mm deep within the skin, consisting of a feeder vessel and ectatic venous sprouts found in the reticular dermis.[5]
The exact pathogenesis of spider veins is still unclear. Some theories suggest they may develop from varicose veins and are secondary to a similar mechanism of valvular incompetence seen in chronic venous disease or chronic venous insufficiency. However, a study found that only 22.9% of patients with spider veins showed evidence of venous incompetence.[6]
Goldman proposed that telangiectasias result from local anoxia, leading to endothelial inflammation and vascular neogenesis.[7] The pathophysiology may involve multiple factors, with spider veins representing the milder side of the spectrum of chronic venous insufficiency and varicose veins at the more severe end. Incompetent valves in the deep venous network allow retrograde blood flow, causing blood to pool in the small superficial vessels. The resulting inflammation and vascular neogenesis from local anoxia cause these vessels to bulge and branch out, giving them a spider-like appearance.
Epidemiology
Numerous studies on the epidemiology of spider veins concluded that most adults will eventually develop some form of spider veins in their lifetime. The typical age of presentation is between the ages of 30 and 50.[2] In a study involving 1566 randomly selected adults in Scotland, Ruckley et al reported that 88% of women and 79% of men had spider veins in their right leg.[2] Of those affected, 98% experienced only very mild symptoms.
Chiesa et al studied 4288 patients and found that women were 4 times more likely than men to develop spider veins, with the risk increasing with the number of pregnancies.[8] They also reported that while men were less likely to be affected, their disease manifestations were more severe, and they were more likely to experience advanced chronic venous insufficiency. Additionally, the risk of developing spider veins increases with obesity and in occupations involving prolonged sitting or standing.[9][10]
Some evidence suggests that smoking is associated with spider veins, and other studies have found that local trauma or a history of venous thromboembolism increases the risk of developing spider veins in the affected limb.[11][12] Additional risk factors include genetics and ethnicity, with 90% of individuals with spider veins having a positive family history and non-Hispanic White people being at higher risk.[13][14] Other factors predisposing patients to spider veins are the use of topical steroids or female hormones and low-fiber diets.[15][16]
History and Physical
Spider veins are primarily an aesthetic issue. A survey reported that telangiectasias of the lower limbs are considered the most concerning cosmetic problem for American women.[17] While most patients are asymptomatic, a minority may experience symptoms such as burning, itching, pain, cramps, or leg fatigue.[7]
Patients may have one or more risk factors and a history of chronic venous insufficiency, including varicose veins or venous eczema. Physical examination may reveal spider veins that may be raised or flat and may appear as thin blue, red, or purple vessels, depending on whether they are arterial or venous in origin. Telangiectasia can form anywhere on the body and at any age, though most patients develop spider veins on their legs and face.
Evaluation
The diagnosis of spider veins is primarily clinical. There are no laboratory tests useful for diagnosing spider veins. However, imaging studies can help map the venous system if chronic venous insufficiency is suspected. These studies include duplex ultrasound, contrast venography, and magnetic resonance venography.
Spider veins are categorized in the Clinical, Etiological, Anatomical, and Pathological (CEAP) classification system for chronic venous insufficiency, which includes the following 7 categories: 0 through 6.[18]
- C0: Absence of venous disease
- C1: Telangiectasias and larger reticular veins
- C2: Varicose veins (3 mm or greater in diameter)
- C3: Edema
- C4: Changes in skin and subcutaneous tissue, including pigmentation (C4a), eczema (C4a), lipodermatosclerosis (C4b), or atrophie blanche (C4b)
- C5: Healed venous ulcer
- C6: Active venous ulcer
Telangiectasia is defined as a confluence of dilated intradermal venules less than 1 mm in caliber.[18] Redisch and Pelzer further classified telangiectasias into the following 4 types based on their clinical appearance.[19]
- Simple linear
- Spider: Radiating from a central locus
- Arborizing: Arranged like branches on a tree
- Papular: Discrete round spots
Treatment / Management
Spider veins are usually harmless and asymptomatic, with most patients pursuing treatment primarily for reduction, removal, or aesthetics. Treatment options include sclerotherapy, intense pulsed light (IPL) therapy, thermocoagulation, and microphlebectomy. The choice of treatment, whether used alone or in combination, depends on individual patient factors.[20](A1)
Sclerotherapy
Telangiectasias and reticular veins can be treated by injecting sclerosing agents into the vessels to obliterate them. Sclerotherapy is considered the first-line treatment for spider veins on the legs. The local vasculature is first assessed with ultrasonography to examine the deep, superficial, feeder, and perforating veins for obstruction and venous reflux or incompetence. Abnormal flow pathways are identified, and the target vessels for treatment are marked accordingly. The sclerosing agent, either in liquid or foam form, is injected with a fine 30-gauge needle under ultrasound guidance. This agent works by causing endothelial damage, leading to fibrosis of the vessel and halting blood flow. Various sclerosing substances are used in sclerotherapy, categorized as detergents, osmotic agents, or chemical irritants.
Detergents such as polidocanol and sodium tetradecyl cause endothelial injury by disrupting surface tension around endothelial cells. Chemical irritants, such as chromated glycerin, achieve their effect through cauterization. Osmotic agents, including hypertonic saline and dextrose, dehydrate endothelial cells. For larger reticular veins, foam sclerotherapy may be more appropriate.[21] Foam sclerotherapy involves mixing a fluid such as polidocanol or sodium tetradecyl sulfate with gas to create foam. Over time, the treated vessels fade, are resorbed, and disappear. However, the treatment does not prevent the formation of new vessels from forming, and multiple treatments may be required to achieve the desired effect.(A1)
Sclerotherapy is a widely used treatment for spider veins, with some studies showing a 50% to 70% improvement in patients.[22] Common adverse effects of sclerotherapy include transient hyperpigmentation, ulceration, sloughing, allergy, thrombophlebitis, swelling, and pain. More severe but rare adverse effects include thromboembolic events, anaphylaxis, and transient visual disturbances. (A1)
Laser Treatment and Intense Pulsed Light Treatment
Laser and IPL therapy are noninvasive methods for treating spider veins with a diameter smaller than a 30-gauge needle. These treatments are especially useful for patients with spider veins on the face, which may not be suitable for sclerotherapy. This treatment is also recommended for patients allergic to sclerosing agents or those who are needle-phobic.
A basic appreciation of laser theory is essential to understand the suitability, limitations, and adverse effects of this treatment. Laser therapy is administered through a hand-held device, which works by heating the target as it absorbs energy from the laser's photons. The target absorbs this energy only if the laser's wavelength matches the chromophore of the target, meaning the target must be of a specific color. By adjusting the laser wavelength, different chromophores, and thus different structures, can be selectively targeted while minimizing harm to surrounding tissues.
In spider vein therapy, light energy is absorbed by hemoglobin in the damaged vessels. This absorption heats the vessels, causing them to occlude through thrombus formation via photocoagulation. The concept of selective photolysis, developed by Anderson and Parrish in 1983, underpins modern laser therapy in medicine today.[23](B3)
Types of Lasers for Spider Vein Treatment
Various lasers are available for treating spider veins, each with its advantages, drawbacks, and specific applications.
Argon laser: Argon lasers were among the first employed for vascular treatments. These lasers emit a blue-green wavelength in the range of 488 to 514 nm, which is well absorbed by hemoglobin and penetrates about 1 mm into the skin. Studies have reported success rates of up to 90% when used with sclerotherapy.[24][25] However, argon lasers carry a higher risk of scarring and pigmentation, likely due to their nonspecific thermal injury.[25]
Pulsed dye laser: Pulsed dye lasers, with long wavelengths of 595 nm and extended pulse durations of 40 ms, are effective in treating spider veins.[26] Modern versions of pulsed dye lasers feature integrated cooling devices and use pulses shorter than the relaxation time of small vessels (1 ms). These characteristics help reduce the risk of unintended thermal damage to surrounding tissues, reducing scarring and pigmentation. Pulsed dye lasers are effective for clearing small-caliber vessels, but their performance with larger-caliber vessels has not been as promising or successful.[27][28]
Nd:YAG laser: With a longer wavelength of 1064 nm, Nd:YAG lasers are effective for treating larger caliber veins. They are highly effective in spider vein therapy but may not be the most favored among patients due to the discomfort they can cause. Besides increased pain, Nd:YAG lasers can also cause hyperpigmentation.
Potassium-titanyl-phosphate laser: Potassium-titanyl-phosphate (KTP) lasers double the frequency of Nd:YAG lasers by passing the light through a KTP crystal. The resultant light is in the wavelength range of 532 to 1064 nm. This higher frequency makes KTP lasers suitable for darker skin patients and smaller vessels. They are commonly used for facial spider veins due to their limited penetration but are less effective for treating spider veins on the legs.
Pulsed diode laser: Pulsed diode lasers emit photons within the range of 800 to 900 nm. These lasers have an excellent profile, largely due to their integrated cooling devices and minimal inflammatory effects. Numerous studies have reported high clearance rates for leg spider veins with pulsed diode lasers.[29][30](A1)
Overall, laser treatments are safe and effective methods of treating spider veins. They are noninvasive and are often preferred by patients with facial spider veins or those who are averse to needles. Adverse effects can include blistering, crusting, swelling, and spotting. Contraindications to laser therapy include pregnancy, blood-thinning medication, photosensitive or hypertrophic or keloidal scarring disorders, untreated varicose veins, and, in some cases, recent tanning or darker skin type. Patients are instructed to avoid aspirin, nonsteroidal anti-inflammatory drugs, and blood thinners before the procedure and for 48 hours afterward.
Microphlebectomy
Microphlebectomy involves the extraction of veins through minute skin incisions (1-3 mm) or needle punctures using specialized hooks. This technique minimizes the risk of complications associated with other treatments, including scarring, skin necrosis, and residual hyperpigmentation.[31]
Thermocoagulation
Similar to other treatment methods, thermocoagulation works by causing endothelial damage to the target vessel. This technique uses a high-frequency (radio frequency) pulse delivered through a fine needle inserted into the target vessel. When the temperature reaches 70 °C, the vessel is occluded through thermocoagulation. Over time, the vessel is resorbed and fades. Recent studies indicate that radiofrequency coagulation is more effective than sclerotherapy in eliminating small veins less than 0.3 mm in diameter.[32][33](B3)
Differential Diagnosis
Spider veins can be associated with chronic venous insufficiency and may occur concomitantly with varicose veins, venous eczema, stasis dermatitis, and leg ulcers. Please see StatPearls' companion resource, "Venous Insufficiency," for more information.[34] These conditions are more severe and require medical attention before addressing spider veins, which are primarily a cosmetic concern. As spider veins can be an early indicator of chronic venous insufficiency, clinicians should be vigilant for undiagnosed venous disease in patients presenting with spider veins.
Spider angiomas can sometimes resemble spider veins. Although generally harmless, spider angiomas may indicate more severe systemic conditions, such as rheumatoid arthritis, thyroid disease, or liver disease. Please see StatPearls' companion resource, "Spider Angioma," for more information. Unlike spider veins, spider angiomas feature a central red spot with radiating, web-like extensions.
Prognosis
Untreated spider veins are primarily a cosmetic issue and do not cause significant medical problems or functional impairment. However, in severe cases affecting the legs or face, they can impact a patient’s self-esteem and lead to psychological issues, especially in young females who are more body-conscious.
Complications
Complications from untreated spider veins are rare and generally do not pose significant health risks. However, in severe cases associated with varicose veins, there is a higher risk of developing sores, skin ulcers, and thrombophlebitis. As with any procedure, therapeutic treatments for spider veins carry some risk of complications, but these risks are very low.
Deterrence and Patient Education
Naturally, little can be done to address non-modifiable risk factors such as age, sex, ethnicity, and gravidity. However, other factors can be managed through maintaining a healthy body mass index (BMI), quitting smoking, staying active, and avoiding tight clothing near the spider veins. Some evidence suggests that compression stockings may help prevent the formation of new spider veins after treatment.[35]
Enhancing Healthcare Team Outcomes
As a purely cosmetic issue, spider veins often involve an interprofessional team in aesthetic medicine, including dermatologists, aestheticians, and plastic surgeons. Patients may initially see their general practitioner, who might refer them subsequently to a dermatologist or plastic surgeon.
In many healthcare systems, spider vein treatments are categorized as aesthetic procedures, so government or insurance funding may be limited. A significant commercial market exists, and savvy practitioners should guide patients toward reputable aesthetic medicine providers. The treating practitioner should have an open discussion with the patient about the goals, risks, benefits, and out-of-pocket costs associated with any treatment plan, allowing the patient to make an informed decision. The interprofessional team members need to know when to refer to specialists when the condition warrants.
Media
(Click Image to Enlarge)
References
Merlen JF. [Red telangiectasis, blue telangiectasis]. Phlebologie. 1970 Apr-Jun:23(2):167-74 [PubMed PMID: 5448639]
Ruckley CV, Evans CJ, Allan PL, Lee AJ, Fowkes FG. Telangiectasia in the Edinburgh Vein Study: epidemiology and association with trunk varices and symptoms. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2008 Dec:36(6):719-24. doi: 10.1016/j.ejvs.2008.08.012. Epub 2008 Oct 9 [PubMed PMID: 18848475]
Level 2 (mid-level) evidenceBraverman IM. The cutaneous microcirculation. The journal of investigative dermatology. Symposium proceedings. 2000 Dec:5(1):3-9 [PubMed PMID: 11147672]
Level 3 (low-level) evidenceThomson L. Sclerotherapy of telangiectasias or spider veins in the lower limb: A review. Journal of vascular nursing : official publication of the Society for Peripheral Vascular Nursing. 2016 Jun:34(2):61-2. doi: 10.1016/j.jvn.2016.04.002. Epub [PubMed PMID: 27210454]
Böhler-Sommeregger K, Karnel F, Schuller-Petrovic S, Santler R. Do telangiectases communicate with the deep venous system? The Journal of dermatologic surgery and oncology. 1992 May:18(5):403-6 [PubMed PMID: 1607463]
Thibault PK, Lewis WA. Recurrent varicose veins. Part 1: Evaluation utilizing duplex venous imaging. The Journal of dermatologic surgery and oncology. 1992 Jul:18(7):618-24 [PubMed PMID: 1624636]
Schwartz L, Maxwell H. Sclerotherapy for lower limb telangiectasias. The Cochrane database of systematic reviews. 2011 Dec 7:2011(12):CD008826. doi: 10.1002/14651858.CD008826.pub2. Epub 2011 Dec 7 [PubMed PMID: 22161437]
Level 1 (high-level) evidenceChiesa R, Marone EM, Limoni C, Volonté M, Schaefer E, Petrini O. Demographic factors and their relationship with the presence of CVI signs in Italy: the 24-cities cohort study. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2005 Dec:30(6):674-80 [PubMed PMID: 16055355]
Level 2 (mid-level) evidenceJawien A. The influence of environmental factors in chronic venous insufficiency. Angiology. 2003 Jul-Aug:54 Suppl 1():S19-31 [PubMed PMID: 12934754]
Level 2 (mid-level) evidenceLacroix P, Aboyans V, Preux PM, Houlès MB, Laskar M. Epidemiology of venous insufficiency in an occupational population. International angiology : a journal of the International Union of Angiology. 2003 Jun:22(2):172-6 [PubMed PMID: 12865883]
Level 2 (mid-level) evidenceGourgou S, Dedieu F, Sancho-Garnier H. Lower limb venous insufficiency and tobacco smoking: a case-control study. American journal of epidemiology. 2002 Jun 1:155(11):1007-15 [PubMed PMID: 12034579]
Level 2 (mid-level) evidenceKahn SR, Solymoss S, Lamping DL, Abenhaim L. Long-term outcomes after deep vein thrombosis: postphlebitic syndrome and quality of life. Journal of general internal medicine. 2000 Jun:15(6):425-9 [PubMed PMID: 10886478]
Level 2 (mid-level) evidenceBernstein EF. Clinical characteristics of 500 consecutive patients presenting for laser removal of lower extremity spider veins. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2001 Jan:27(1):31-3 [PubMed PMID: 11231238]
Criqui MH, Jamosmos M, Fronek A, Denenberg JO, Langer RD, Bergan J, Golomb BA. Chronic venous disease in an ethnically diverse population: the San Diego Population Study. American journal of epidemiology. 2003 Sep 1:158(5):448-56 [PubMed PMID: 12936900]
Level 2 (mid-level) evidenceGoldman MP. Treatment of varicose and telangiectatic leg veins: double-blind prospective comparative trial between aethoxyskerol and sotradecol. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2002 Jan:28(1):52-5 [PubMed PMID: 11991271]
Level 1 (high-level) evidenceSadick NS. Predisposing factors of varicose and telangiectatic leg veins. The Journal of dermatologic surgery and oncology. 1992 Oct:18(10):883-6 [PubMed PMID: 1430543]
De Maeseneer MG, Kakkos SK, Aherne T, Baekgaard N, Black S, Blomgren L, Giannoukas A, Gohel M, de Graaf R, Hamel-Desnos C, Jawien A, Jaworucka-Kaczorowska A, Lattimer CR, Mosti G, Noppeney T, van Rijn MJ, Stansby G, Esvs Guidelines Committee, Kolh P, Bastos Goncalves F, Chakfé N, Coscas R, de Borst GJ, Dias NV, Hinchliffe RJ, Koncar IB, Lindholt JS, Trimarchi S, Tulamo R, Twine CP, Vermassen F, Wanhainen A, Document Reviewers, Björck M, Labropoulos N, Lurie F, Mansilha A, Nyamekye IK, Ramirez Ortega M, Ulloa JH, Urbanek T, van Rij AM, Vuylsteke ME. Editor's Choice - European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2022 Feb:63(2):184-267. doi: 10.1016/j.ejvs.2021.12.024. Epub 2022 Jan 11 [PubMed PMID: 35027279]
Level 1 (high-level) evidenceEklöf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, Meissner MH, Moneta GL, Myers K, Padberg FT, Perrin M, Ruckley CV, Smith PC, Wakefield TW, American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. Journal of vascular surgery. 2004 Dec:40(6):1248-52 [PubMed PMID: 15622385]
Level 3 (low-level) evidenceREDISCH W, PELZER RH. Localized vascular dilatations of the human skin, capillary microscopy and related studies. American heart journal. 1949 Jan:37(1):106-13 [PubMed PMID: 18104384]
Nakano LC, Cacione DG, Baptista-Silva JC, Flumignan RL. Treatment for telangiectasias and reticular veins. The Cochrane database of systematic reviews. 2021 Oct 12:10(10):CD012723. doi: 10.1002/14651858.CD012723.pub2. Epub 2021 Oct 12 [PubMed PMID: 34637138]
Level 1 (high-level) evidenceRai A, Porsalman M, Khatony A, Sobhiyeh M. Comparison of foam sclerotherapy versus radiofrequency ablation in the treatment of primary varicose veins due to incompetent great saphenous vein: Randomized clinical trial. Journal of vascular nursing : official publication of the Society for Peripheral Vascular Nursing. 2019 Dec:37(4):226-231. doi: 10.1016/j.jvn.2019.10.002. Epub 2019 Nov 18 [PubMed PMID: 31847976]
Level 1 (high-level) evidenceParlar B, Blazek C, Cazzaniga S, Naldi L, Kloetgen HW, Borradori L, Buettiker U. Treatment of lower extremity telangiectasias in women by foam sclerotherapy vs. Nd:YAG laser: a prospective, comparative, randomized, open-label trial. Journal of the European Academy of Dermatology and Venereology : JEADV. 2015 Mar:29(3):549-54. doi: 10.1111/jdv.12627. Epub 2014 Jul 28 [PubMed PMID: 25069999]
Level 1 (high-level) evidenceAnderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science (New York, N.Y.). 1983 Apr 29:220(4596):524-7 [PubMed PMID: 6836297]
Level 3 (low-level) evidenceApfelberg DB, Maser MR, Lash H, White DN, Flores JT. Use of the argon and carbon dioxide lasers for treatment of superficial venous varicosities of the lower extremity. Lasers in surgery and medicine. 1984:4(3):221-31 [PubMed PMID: 6438415]
Dixon JA, Gilbertson JJ. Cutaneous laser therapy. The Western journal of medicine. 1985 Dec:143(6):758-63 [PubMed PMID: 4090490]
Bernstein EF. The new-generation, high-energy, 595 nm, long pulse-duration, pulsed-dye laser effectively removes spider veins of the lower extremity. Lasers in surgery and medicine. 2007 Mar:39(3):218-24 [PubMed PMID: 17096414]
Dover JS, Arndt KA. New approaches to the treatment of vascular lesions. Lasers in surgery and medicine. 2000:26(2):158-63 [PubMed PMID: 10685088]
Bernstein EF, Lee J, Lowery J, Brown DB, Geronemus R, Lask G, Hsia J. Treatment of spider veins with the 595 nm pulsed-dye laser. Journal of the American Academy of Dermatology. 1998 Nov:39(5 Pt 1):746-50 [PubMed PMID: 9810891]
Varma S, Lanigan SW. Laser therapy of telangiectatic leg veins: clinical evaluation of the 810 nm diode laser. Clinical and experimental dermatology. 2000 Jul:25(5):419-22 [PubMed PMID: 11012600]
Eremia S, Li C, Umar SH. A side-by-side comparative study of 1064 nm Nd:YAG, 810 nm diode and 755 nm alexandrite lasers for treatment of 0.3-3 mm leg veins. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2002 Mar:28(3):224-30 [PubMed PMID: 11896773]
Level 1 (high-level) evidenceRamelet AA. Phlebectomy. Technique, indications and complications. International angiology : a journal of the International Union of Angiology. 2002 Jun:21(2 Suppl 1):46-51 [PubMed PMID: 12515980]
Korolova K, Korolova Z, Teplyi V, Sydorenko R. MINI-INVASIVE TREATMENT METHODS OF SPIDER VEINS: SCLEROTHERAPY AND RADIOFREQUENCY THERMOCOAGULATION. Wiadomosci lekarskie (Warsaw, Poland : 1960). 2023:76(9):1992-1999. doi: 10.36740/WLek202309113. Epub [PubMed PMID: 37898935]
Diken Aİ, Alemdaroğlu U, Özyalçın S, Hafez İ, Tünel HA, Yalçınkaya A, Ecevit AN. Adjuvant radiofrequency thermocoagulation improves the outcome of liquid sclerotherapy in the treatment of spider veins of the leg: A pilot study. Phlebology. 2021 Sep:36(8):620-626. doi: 10.1177/02683555211006534. Epub 2021 Apr 4 [PubMed PMID: 33813962]
Level 3 (low-level) evidencePartsch H. Varicose veins and chronic venous insufficiency. VASA. Zeitschrift fur Gefasskrankheiten. 2009 Nov:38(4):293-301. doi: 10.1024/0301-1526.38.4.293. Epub [PubMed PMID: 19998250]
Kern P, Ramelet AA, Wütschert R, Hayoz D. Compression after sclerotherapy for telangiectasias and reticular leg veins: a randomized controlled study. Journal of vascular surgery. 2007 Jun:45(6):1212-6 [PubMed PMID: 17467226]
Level 1 (high-level) evidence