Introduction
Spina bifida is a congenital anomaly resulting from incomplete neural tube development (see Image. Infant With Spina Bifida). The term "spina bifida" is nonspecific and refers to any degree of neural tube closure defect. This condition can be divided into 2 categories—spina bifida occulta and spina bifida aperta.[1][2] Spina bifida occulta, also known as closed spinal dysraphism, is the mildest form of neural tube defect (NTD), characterized by a concealed vertebral defect with minimal neural involvement.
Spina bifida aperta, also known as open spinal dysraphism, refers to a defect in which neural tissue is exposed and communicates with the external environment, such as in meningocele, myelomeningocele, and myeloschisis (see Image. Types of Spina Bifida).[1] These conditions result in a wide range of neurological impairments. Spina bifida is often associated with several other developmental abnormalities, highlighting the importance of an interprofessional medical approach to optimize patient survival and outcomes.
Atypical variants of spinal bifida include segmental spinal dysgenesis, lipomyelomeningocele, human tail, membranous meningocele, and myeloschisis. These variants are associated with autonomic dysfunction, kyphotic deformity, and lower limb anomalies.[3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Spinal dysraphisms result from incomplete closure of the posterior spinal elements, typically occurring between days 17 and 30 of fetal development. The process of neurulation occurs in 2 phases—primary and secondary neurulation. Primary neurulation refers to the closure of the neural tube, which forms the brain and spinal cord. Secondary neurulation involves the development of the caudal structures of the neural tube, forming the sacral and coccygeal regions. These caudal structures begin to develop around day 26 of gestation, and failure to close them results in varying degrees of spinal dysraphism.[4] A complex genomic network governs neurulation.[5]
Defects in neural tube development are believed to be multifactorial, involving both environmental and genetic factors. The most common environmental cause is folate deficiency, with the majority of cases considered “folic acid-sensitive.”[6] Global dietary folate fortification programs have been implemented worldwide, leading to a 28% reduction in the prevalence of anencephaly and spina bifida.
Other environmental risk factors include maternal obesity, maternal diabetes, and exposure to teratogens, such as valproic acid. Valproic acid is strongly associated with the development of NTDs, increasing the risk by approximately 10-fold. The genomic landscape of spina bifida is diverse, following the omnigenic model.[7] Some genetic factors have also been correlated with poor neurulation, including several chromosomal syndromes and genetic polymorphism. Research has identified a polymorphism in the gene encoding the MTHFR enzyme, which has a role in folate metabolism, as a likely genetic risk factor. Although most NTDs occur in isolation, some are associated with chromosomal syndromes, most commonly trisomy 13 and trisomy 18.[4][8]
Epidemiology
The prevalence and incidence of NTDs have varied since they were first recognized, but overall, they have decreased due to various interventional programs and increased fetal screening. Studies show that approximately 1300 healthy babies born each year would have been affected by NTDs if folic acid fortification had not been introduced into standard prenatal care.[9] The global reported prevalence is 0.5 to 1 per 1000 live births, with 2 million cases annually in low- to middle-income countries.[1] However, as many parents in low- and middle-income nations opt to terminate pregnancies rather than consider fetal surgery, the true epidemiology of the condition is likely underreported.[10] The prevalence of spina bifida is 4.9 per 10,000 births in Europe, whereas in the United States, the overall prevalence of spina bifida between 1999 and 2007 was 3.17 per 10,000 live births.[11] The prevalence in the United States has decreased by 25% to 1 in 1500 babies following fortification.[2]
Hispanic women appear to have a higher rate of pregnancies affected by NTDs. Globally, Hispanic individuals have a prevalence of spina bifida of 3.8 per 10,000 live births, compared to non-Hispanic Black Americans, with a rate of 2.73 per 10,000, and non-Hispanic White individuals, who have a prevalence of 3.09 per 10,000.[8][9][12] The risk of recurrence is influenced by factors such as family history, geographic location, and the severity of the defect. An increased risk of recurrence of approximately 3% to 8% has been reported following one affected pregnancy or a maternal history of the defect, with the risk increasing as the number of affected children rises.[13]
History and Physical
Clinical History
Patients with NTDs should be evaluated during prenatal screening, and at-risk women should be counseled before conception to improve outcomes. However, in some areas, particularly underserved communities and those with low socioeconomic status, mothers may lack access to proper screening and may not present until birth or infancy. Standard prenatal screening includes measuring serum alpha-fetoprotein levels between 16 and 18 weeks to assess for NTDs. Follow-up can be performed with an ultrasound, with a sensitivity of 88% to 89%.[13] Patients may present with various symptoms and conditions due to the diverse range of neural tube malformations.
Physical Examination Findings
During physical examination, the lower portions of the spine should be visualized to assess whether the patient has open or closed spinal dysraphism. Spina bifida occulta typically shows no obvious deformity, but may present with a hairy skin patch or dimple, which can indicate an underlying lesion. Meningocele involves a defect in the posterior elements of the spine, with extrusion of the meninges and cerebrospinal fluid (CSF) without involvement of the neural elements. Myelomeningocele involves the extrusion of the meninges, CSF, and functional neural elements, such as nerve or spinal cord tissue.
Myelomeningocele is typically associated with more long-term functional limitations due to the involvement of nervous tissue in the defect. Patients may present with symptoms such as spasticity, pain, motor deficits, neurogenic bowel or bladder dysfunction, cognitive impairment, seizures, and endocrine disorders (eg, precocious puberty).[4] Neurological dysfunction is common in open dysraphisms, such as meningocele and myelomeningocele, where neural tissue can extrude outside the defect and be affected. Additionally, patients with NTDs often present with latex allergies, with a prevalence ranging from 10% to 73%.[14] Upon encountering these signs or symptoms, a detailed gestational, birth, and family history should be obtained to better understand the contributing factors and guide family counseling.
Evaluation
Pregnant women should undergo routine screening to detect NTDs early, which helps guide therapeutic intervention and counseling. Initial screening is performed using serum alpha-fetoprotein levels, but amniocentesis may be considered for confirmation in cases of high suspicion. However, due to the risks associated with amniocentesis and the high accuracy of ultrasound, the latter has become the gold standard for in utero diagnosis and can even detect abnormalities as early as the first trimester.[15]
Several ultrasound signs have been identified as reliably diagnostic, as many patients also present with intracranial abnormalities.[16] A small biparietal diameter has been associated with NTDs. However, the most commonly cited signs are the "lemon and banana" signs. The "lemon" sign refers to the overlapping of the frontal bones caused by a posterior shift of intracranial contents. The "banana" sign describes a curved cerebellum due to its downward displacement, often leading to Arnold-Chiari II malformation at birth.[13]
Ventriculomegaly, even in the absence of hydrocephalus, may also be detected on prenatal ultrasound. Spinal ultrasound can help identify the affected vertebral levels and provide prognostic information regarding bowel and bladder function, as well as gait outcomes.[17] The functional level after birth correlates with the prenatal ultrasound-identified level of injury in 60% of patients.[18] Magnetic resonance imaging (MRI) can also be used after birth for prognostication within 1 to 2 vertebral levels in 89% of cases.[13]
Treatment / Management
Preventative Management
The mainstay of NTD treatment (eg, spina bifida) is prevention. Women of childbearing age are advised to supplement their diet with folate to reduce the risk of NTDs. In the United States, fortifying grains with folic acid was mandated to help decrease the incidence of these conditions.[8] Women attempting to conceive should take 0.4 mg of folic acid daily before conception.[9] In contrast, women with a history of NTDs or a previous affected pregnancy should take 4 mg of folic acid supplementation.[13] (A1)
Prenatal and Postnatal Surgical Repair
Most patients with spina bifida occulta do not require surgical correction of the defect. However, surgical intervention is warranted in cases of open spinal dysraphism.[19] In select cases, intrauterine repair may be performed to close the defect earlier and improve outcomes. Prenatal repair helps prevent neuronal injury from exposure to amniotic fluid and reduces mechanical trauma.[11] Different strategies of prenatal repair of open spina bifida include open repair (the most common) and hybrid techniques, such as mini-hysterotomy and laparotomy-assisted fetoscopic and percutaneous fetoscopic procedures.[20] Transverse laparotomy and hysterotomy, while commonly used, are associated with a high risk of preterm delivery and necessitate cesarean section in subsequent pregnancies due to the risk of uterine dehiscence.[11](A1)
Laparoscopic-assisted strategies have the lowest risk of preterm deliveries, whereas laparotomy-assisted fetoscopic surgery offers the highest chances for vaginal deliveries.[20] The 3-layer closure of the defect in hybrid models, using a bovine dura patch, muscles, and skin, reduces the risk of uterine dehiscence, premature rupture of membranes, and preterm delivery, thus facilitating the possibility of vaginal deliveries.[11] (A1)
Postnatal repair within 48 to 72 hours is the conventional management for myelomeningocele, which prevents significant neurological decline and reduces the risk of infection or injury to exposed neural tissue.[19] Human umbilical cord mesenchymal stromal cells (hUC-MSCs) patches and cryopreserved decellularized human umbilical cord matrix allografts have been utilized for duraplasty during myelomeningocele repair in the ovine model.[21][22](B3)
Intraoperative neurophysiological monitoring, including alert criteria such as a greater than 80% reduction in motor evoked potentials, a 30% to 50% reduction in sensory evoked potentials, and the absence of the bulbocavernosus reflex, significantly reduces the risk of neurological complications.[23][24] Electromyography (EMG) contributes to identifying the nonfunctional motor rootlets and ensures the safe release of the placode.[23]
The Management of Myelomeningocele Study (MOMS) was a randomized clinical trial that compared prenatal versus standard postnatal intervention for repairing myelomeningocele. The results of the study showed that prenatal repair reduced the incidence of hydrocephalus and hindbrain herniation, lowered the need for ventriculoperitoneal shunt placement in the future, and improved leg function and ambulation in children aged 12 to 30 months.[4][25] However, intrauterine repair carries certain risks, including an increased risk of prematurity and maternal complications.[26] The MOMS 2 trial, which reevaluated the patients at school age, found that the functional benefits of prenatal repair persisted into school age. Children who underwent prenatal repair were more independent in self-care tasks, more likely to be community ambulators, and demonstrated better mobility skills than those who had postnatal repair.[27](A1)
Several implications and sequela must be monitored and managed in the neonatal stage.[16] Many patients require placement of a ventriculoperitoneal shunt for hydrocephalus following closure of the defect. Arnold-Chiari malformations are common and may necessitate surgical intervention if symptomatic.
Long-Term Management
Long-term management requires an interprofessional approach, as multiple organ systems may be affected. Neurogenic bowel and bladder dysfunction are common manifestations of the condition. Neurogenic bladder often presents with detrusor-sphincter dyssynergia, which can lead to renal failure if not properly managed. Patients should undergo biannual renal ultrasounds for surveillance and may require intermittent catheterization as part of long-term care. Additionally, neurogenic bowel results from impaired sensation and poor sphincter control. To manage this, patients may need to establish a consistent bowel program that includes stool softeners, motility agents, and digital stimulation.
Children with NTDs can exhibit various motor impairments, including weakness, flaccidity, spasticity, and contractures. Tendon lengthening procedures may be considered for patients with severe contractures that interfere with ambulation, hygiene, or positioning. Common foot deformities in NTDs include equinovarus, calcaneus, and rocker-bottom deformities. Management strategies such as splinting, passive stretching, and serial casting can help alleviate significant spasticity and contractures. Machine learning algorithms have also been developed to support ambulation and facilitate wheelchair transfers.[28] Additionally, it is important to address the transition challenges faced by individuals with spina bifida as they enter adulthood.[29]
Differential Diagnosis
The differential diagnoses of spina bifida are generally straightforward based on the physical presentation. However, when clinical or radiographic findings are equivocal, several differential diagnoses should be considered, including:
- Cord compression
- Diastematomyelia
- Isolated Chiari malformations
- Mass lesions
- Tethered cord
Prognosis
Few recent longitudinal studies have examined the long-term outcomes of spina bifida, making it challenging to predict patient prognosis, especially given the significant advances in spina bifida management. Most studies combine data from patients with both open and closed dysraphisms. Overall, prognosis is primarily influenced by the presence of hydrocephalus, the level of the defect, and the severity of Chiari malformation.
The MOMS Trial demonstrated that intrauterine repair significantly reduced the need for shunting by nearly 50% and improved motor function.[11][30][31] Prenatal surgery has also been associated with improved memory performance.[32] Hybrid surgery offers similar neurodevelopmental improvements (in nearly two-thirds of cases) when compared to open fetal surgery.[33]
With advancements in medical care, the survival rate for spina bifida has increased from less than 50% to 85%.[34] Among patients with and without hydrocephalus, 56% and 88% survive beyond age 1, respectively. Survival rates slightly decline as patients with hydrocephalus age, dropping to about 50% by age 20.[35] Most deaths beyond age 5 are attributed to seizures, pulmonary emboli, hydrocephalus, and acute renal failure or sepsis.[13] However, significant morbidity is associated with NTDs if patients are not adequately managed.
Urinary tract infections (UTIs) are the most common complication in individuals with NTDs due to neurogenic bladder, affecting approximately 48% of patients, with 6% ultimately developing renal failure.[13] The severity of Chiari malformation is a significant risk factor for mortality in infants with NTDs and may lead to complications such as sleep apnea, vocal cord paralysis, and bradycardia. Patients are often concerned about functional outcomes, specifically the prognosis regarding ambulation. In the MOMS trial, only 7% of participants were wheelchair-dependent for ambulation, while the remaining participants could ambulate with assistive devices, and about 29% were independent.[36] Recent studies have also shown that 60% of individuals are ambulators and 80% are socially continent.[7] Spina bifida places significant financial, physical, and socioeconomic burdens on both survivors and their clinicians.[9]
Complications
Spina Bifida Complications
Bladder and bowel dysfunction is the most common complication associated with acute renal failure and urosepsis, often resulting from ureteral reflux caused by a neurogenic bladder. Affected patients have an approximately 7- to 11-fold increased risk of renal failure, double the risk of bladder cancer, and a 46-fold increased risk of UTIs. Aggressive treatment and surveillance of urologic dysfunction are essential. Many patients will require intermittent catheterization for urinary management. Neurogenic bladder has a prevalence of up to 80%. A video-urodynamic study is recommended to rule out vesicoureteric reflux. Bladder augmentation is indicated for patients with medically refractory detrusor overactivity.
Tethered cord and hydromyelia are additional complications associated with spina bifida, which can present as sudden neurological decline, spasticity, or pain. Patients with neural tube defects also have an increased risk of fractures, likely due to osteopenia, contractures, decreased sensation, and immobilization. Vertebral column resection may be warranted in cases of retethering.
Scoliosis is a common complication, affecting approximately 33% of patients, followed by chronic pain in 29% and epilepsy in 12%. Additional complications associated with spina bifida include:
- Motor deficits
- Hydrocephalus
- Arnold–Chiari II malformation
- Clubfoot (almost 50%)
- Latex allergy (prevalence 10% and 73%)
- Poor working memory, with difficulties particularly in English, mathematics, and history
- Higher propensity for eating disorders and body dissatisfaction
- Psychosocial impacts
- Barriers to transitioning into adult healthcare
- Need for lifelong self-management
- Negative experiences and challenges faced by caregivers [3][11][34][37][38][39][40][41]
Surgical Complications
Patients undergoing surgery for spina bifida may face several potential complications, including:
Postoperative and Rehabilitation Care
Early rehabilitative intervention is crucial for improving functional outcomes and quality of life. Early mobilization and stretching can help patients maintain a range of motion for future applications with gait, hygiene, and activities of daily living (ADLs). Most patients present with a combination of upper and lower motor neuron involvement and varying degrees of motor control, necessitating individualized therapy plans. Orthotic devices are commonly required to provide joint stabilization, prevent deformities, enhance gait, and improve overall function. Additionally, patients often need assistive devices such as crutches, canes, walkers, or wheelchairs for mobility support. Some may also require assistive technologies to aid in ADLs.
Patients should engage in long-term physical and occupational therapy to support their functional development. Some may also require speech therapy to address dysphagia, dysarthria, vocal cord paralysis, and cognitive training. Neuropsychological support is critical during the early stages of adolescence to help adjust to emerging complications. Despite the potential for significant challenges, research indicates that only 5% of patients develop depression.[13]
Classifying a patient’s functional capacity based on the level of injury and clinical presentation helps healthcare professionals, allied health staff, families, and patients understand expected outcomes and set appropriate therapy goals. Several scales used in rehabilitation practice generally describe a patient’s functional mobility. Dias et al proposed a classification system specific to myelomeningocele that integrates the Functional Mobility Scale (FMS) with clinical and ambulatory characteristics of the condition, providing a comprehensive framework for functional levels, anticipated mobility outcomes, and expected bracing requirements.[44]
The FMS rates a patient's mobility on a scale from 1 to 6, where 1 indicates the need for the most support and 6 the least. The scale assesses mobility across 3 distances—5 meters (eg, home), 50 meters (eg, school), and 500 meters (eg, community). Although this scale was originally designed for pediatric patients with cerebral palsy, it has been applied to other childhood-onset conditions with neuromuscular involvement.[45] The Swaroop and Dias Spina Bifida Classification scale outlines the clinical and ambulatory characteristics of patients with spina bifida, linking the level of vertebral defect to the likely affected muscle groups and corresponding ambulatory capacity.[46] Together, Dias et al introduced the Myelomeningocele Functional Classification (MMFC), which integrates the level of injury, muscle activation patterns, ambulatory capacity, and the FMS. This classification helps predict the expected level of ambulation and identifies the assistive devices needed to achieve these goals.[44]
Consultations
Important consultations for spina bifida management include:
- Neurosurgery
- Endocrinology
- Orthopedics
- Urology
- Rehabilitation or therapy
- Neurology
- Maternal-fetal medicine
Deterrence and Patient Education
Women of childbearing age should receive counseling on the importance of folic acid supplementation to help prevent NTDs. Consultation with specialized genetics clinicians may also be beneficial.[47] As recommended, women planning to become pregnant should take 0.4 mg of folic acid daily. Those with a personal or family history of NTDs, or who have previously had an affected child, should take 4 mg daily, beginning at least 1 month before conception.[2] This approach can reduce the risk of NTDs by approximately 50% to 70%. Fortification efforts targeting serum folate levels of 44 ng/mL could potentially prevent up to 83% of NTD cases—equating to 250,000 annually—compared to the 20% prevention rate associated with current average levels of 10 and 16 ng/mL.[48]
Serial ultrasound imaging is recommended, even for presumed "closed" spina bifida, to rule out sac rupture that could lead to Chiari II malformation.[49] Prenatal screening allows for timely intervention, helping to prevent neuronal damage caused by toxic amniotic fluid (in utero) or infection (after delivery).[1]
In the early stages of the condition, education should focus primarily on caretakers, equipping them to effectively advocate for the patient during childhood. Increasing autonomy should be encouraged based on their cognitive development as the patient matures. Both patients and their caregivers should be engaged as active members of the healthcare team throughout the continuum of care. Families should receive training during their child's therapy to ensure they can provide effective care as the child grows. Essential considerations include patient mobilization, proper use of orthotics and assistive devices, and safe body mechanics. Additionally, families should be educated on recognizing signs and symptoms of severe conditions that may develop, including urinary tract infections, hydrocephalus, or seizures.
Once children are developmentally capable of understanding their condition and needs, they should be included in the decision-making process and provided with education on self-care. Patients with spina bifida occulta may have minimal comorbidities, while those with open dysraphisms typically require more intensive monitoring. These patients may need to learn self-catheterization techniques or how to manage a bowel program independently.[50] As they age, many patients express concerns about ambulation. Fortunately, most achieve functional ambulation, with only about 7% requiring a wheelchair for mobility. Most patients will obtain functional ambulation, with only 7% requiring wheelchair-level ambulation. Psychosocial factors should also be addressed to enhance patient participation in healthcare and improve outcomes. A randomized controlled trial showed that cognitive behavioral therapy, when integrated into a high-intensity rehabilitation program, resulted in improvements in self-care, cognition, mood, and independence.[51] Spina bifida can significantly impact activities of daily living and reduce the quality of life for survivors.[52]
Enhancing Healthcare Team Outcomes
Improving health literacy and implementing a surveillance system to monitor and prevent spinal birth defects are essential for better patient outcomes.[53] Mandatory folic acid food fortification has been shown to reduce the prevalence of NTDs.[54] Additionally, transparent, interdisciplinary counseling is critical when considering fetal surgery for spina bifida.[31]
An interprofessional team approach, both medically and psychosocially, is crucial for achieving the best outcomes for patients with spina bifida.[55] A collaborative team approach involving several specialists and therapy staff can holistically address the full spectrum of disease.[11] In an article by Dicianno, the "specialty medical home" is proposed as the ideal approach for managing spina bifida, offering a variation of the commonly referenced "medical home model." In the medical home model, the primary care physician serves as the central hub, compiling all medical information and acting as the referring clinician to manage the coordination among specialists. However, it has been suggested that the specialty physician should serve as the medical home in certain conditions, as most comorbidities are directly related to the underlying condition. Dicianno recommends this "specialty medical home" model for spina bifida to foster a collaborative approach led by a subspecialist who is well-versed in the condition and its sequelae.[56]
This interprofessional team-based medical care should continue throughout adulthood; however, many patients face challenges transitioning seamlessly into adult care. Involving parents, caretakers, the transitioning youth, and both pediatric and adult clinicians in this process is essential. Early preparation is crucial for a smooth transition and should begin well before the actual shift in care. Transitioning to adult care can be difficult for patients, caregivers, and clinicians, as patients often spend many years with a specific physician team. The transition timing should consider the patient’s age as well as their cognitive readiness to ensure the patient is ready to transition from pediatric to adult care. This transition requires significant collaboration among the pediatric clinician, the new specialty clinician, and other healthcare team consultants.[50] This team-based approach involves clinicians, physical, occupational, and speech therapists, and neuropsychologists to help navigate the disease and its sequelae. Results from a randomized controlled trial by Kahn et al showed that high-intensity, interprofessional rehabilitation with incorporated cognitive behavioral therapy significantly improved cognitive function, mood, independence, and outcomes related to bowel and bladder dysfunctions.[51]
Media
(Click Image to Enlarge)
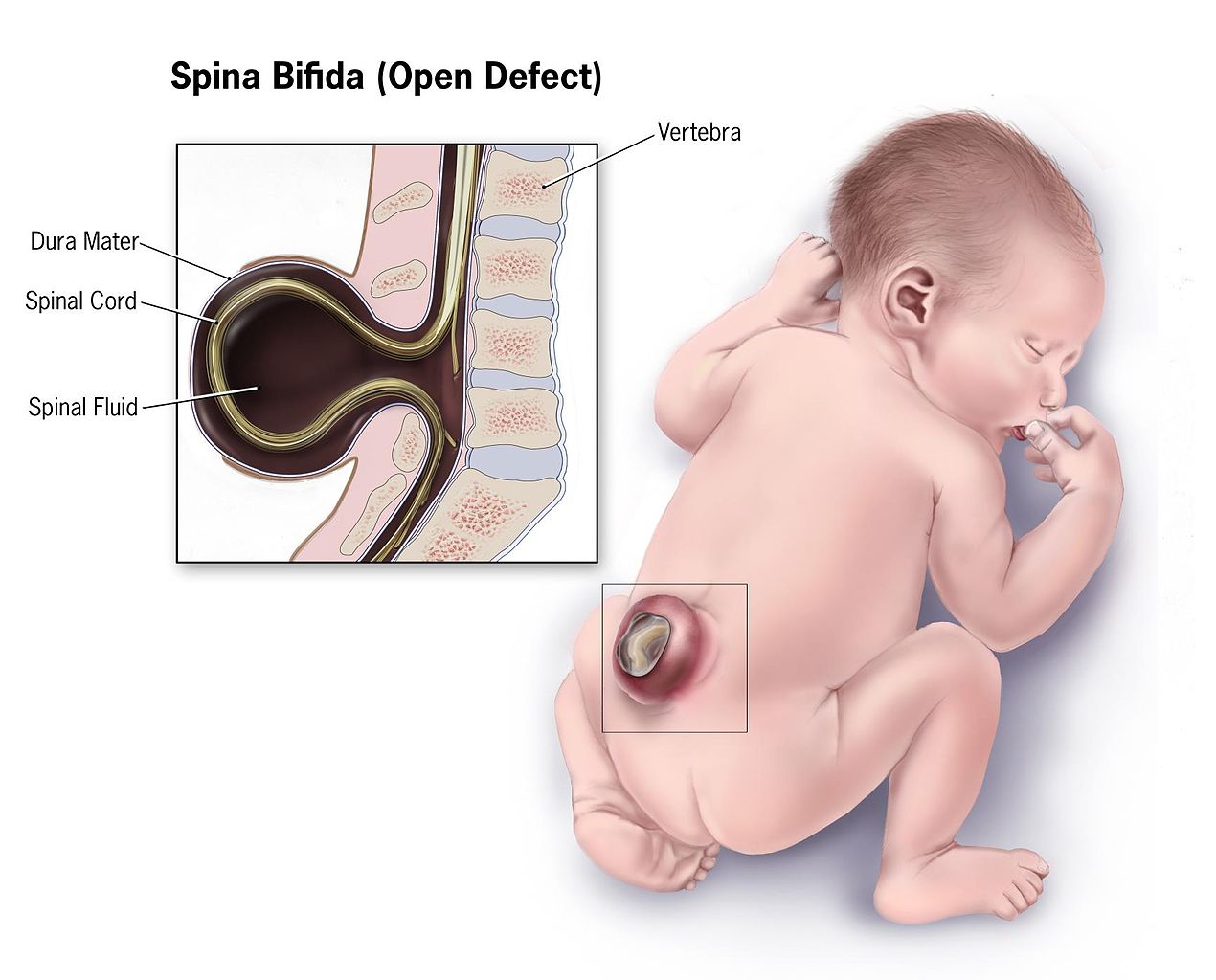
Infant With Spina Bifida. This illustration depicts the characteristic features of an infant with spina bifida.
Centers for Disease Control and Prevention, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
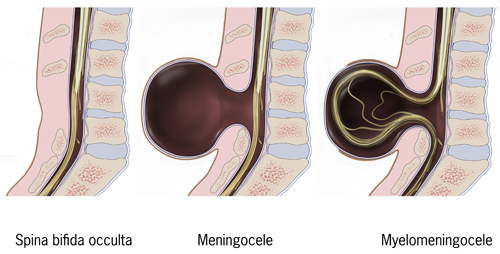
Types of Spina Bifida. Meningocele is a type of neural tube defect where the meninges, the protective membranes surrounding the spinal cord, protrude through a spinal defect, forming a sac filled with cerebrospinal fluid. Meningoceles are typically covered by skin and generally present without neurological symptoms.
Centers for Disease Control and Prevention, Public Domain, via Wikimedia Commons
References
Badejo OA, Shokunbi MT, Adeolu AA, Oderinde IO, Akinmoladun JA, Ogbole GI. Atypical Variants of Spinal Dysraphism: A Case Series. Journal of the West African College of Surgeons. 2025 Jan-Mar:15(1):118-126. doi: 10.4103/jwas.jwas_186_23. Epub 2024 Aug 2 [PubMed PMID: 39735810]
Level 2 (mid-level) evidenceOkon II, Temitope AE, Ogundele IO, Akpan U, Mbong EO, Kasimieh O, Chaurasia B, James E, Gbadebo E, Precious FK, Jader A, Okesanya OJ, Karmani V, Erhayanmen M, Lucero-Prisno Iii DE. The current state of Spina Bifida in low- and middle-income countries: where does Africa stand? Neuro-Chirurgie. 2025 Jan:71(1):101616. doi: 10.1016/j.neuchi.2024.101616. Epub 2024 Nov 6 [PubMed PMID: 39515639]
Parajuli YG, Sinclair M. Cognitive, Behavioral and Educational Outcomes in Children Aged 5-11 Years With Spina Bifida in Northern Ireland. Birth defects research. 2025 Jan:117(1):e2434. doi: 10.1002/bdr2.2434. Epub [PubMed PMID: 39825676]
Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN, Shaw GM. Spina bifida. Nature reviews. Disease primers. 2015 Apr 30:1():15007. doi: 10.1038/nrdp.2015.7. Epub 2015 Apr 30 [PubMed PMID: 27189655]
Wolujewicz P, Aguiar-Pulido V, Thareja G, Suhre K, Elemento O, Finnell RH, Ross ME. Integrative computational analyses implicate regulatory genomic elements contributing to spina bifida. Genetics in medicine open. 2024:2():101894. doi: 10.1016/j.gimo.2024.101894. Epub 2024 Sep 14 [PubMed PMID: 39669613]
Aydin S, Jenkins A, Detchou D, Barrie U. Folate fortification for spina bifida: preventing neural tube defects. Neurosurgical review. 2024 Oct 4:47(1):724. doi: 10.1007/s10143-024-02959-z. Epub 2024 Oct 4 [PubMed PMID: 39365348]
Goel P, Sharma M, Kaushik H, Kumar S, Singh H, Jain V, Dhua AK, Yadav DK, Kumar N, Agarwala S. Genetic Markers of Spina Bifida in an Indian Cohort. Journal of Indian Association of Pediatric Surgeons. 2024 Sep-Oct:29(5):529-535. doi: 10.4103/jiaps.jiaps_64_24. Epub 2024 Sep 9 [PubMed PMID: 39479418]
Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, Frohnert B, Kirby RS, Centers for Disease Control and Prevention. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification - United States, 1995-2011. MMWR. Morbidity and mortality weekly report. 2015 Jan 16:64(1):1-5 [PubMed PMID: 25590678]
Abdelmageed S, Votoupal M, Lam SK, Garcia RM. Epidemiology and morbidity of spina bifida in Hispanic Americans: a systematic review. BMJ public health. 2024 Jun:2(1):e000746. doi: 10.1136/bmjph-2023-000746. Epub 2024 Jun 22 [PubMed PMID: 40018258]
Level 1 (high-level) evidenceFeng M, Chen PC, Lin GR, Lin TY, Hsieh TT, Shaw SW. The clinical experience of fetoscopic repair of myelomeningocele in Taiwan: The dilemma in prenatal decision-making and first successful case. Taiwanese journal of obstetrics & gynecology. 2024 Nov:63(6):904-908. doi: 10.1016/j.tjog.2024.07.018. Epub [PubMed PMID: 39482001]
Level 3 (low-level) evidenceSchmitt N, Schubert AK, Wulf H, Keil C, Sutton CD, Bedei I, Kalmus G. Initial experience with the anaesthetic management of fetoscopic spina bifida repair at a German University Hospital: A case series of 15 patients. European journal of anaesthesiology and intensive care. 2024 Apr:3(2):e0047. doi: 10.1097/EA9.0000000000000047. Epub 2024 Feb 9 [PubMed PMID: 39917608]
Level 2 (mid-level) evidenceCanfield MA, Mai CT, Wang Y, O'Halloran A, Marengo LK, Olney RS, Borger CL, Rutkowski R, Fornoff J, Irwin N, Copeland G, Flood TJ, Meyer RE, Rickard R, Alverson CJ, Sweatlock J, Kirby RS, National Birth Defects Prevention Network. The association between race/ethnicity and major birth defects in the United States, 1999-2007. American journal of public health. 2014 Sep:104(9):e14-23. doi: 10.2105/AJPH.2014.302098. Epub 2014 Jul 17 [PubMed PMID: 25033129]
Trudell AS, Odibo AO. Diagnosis of spina bifida on ultrasound: always termination? Best practice & research. Clinical obstetrics & gynaecology. 2014 Apr:28(3):367-77. doi: 10.1016/j.bpobgyn.2013.10.006. Epub 2013 Dec 3 [PubMed PMID: 24373566]
Martínez-Lage JF, Moltó MA, Pagán JA. [Latex allergy in patients with spina bifida: prevention and treatment]. Neurocirugia (Asturias, Spain). 2001:12(1):36-42 [PubMed PMID: 11706433]
Zhu Z, Li H. First-trimester diagnosis of open spina bifida using three-dimensional ultrasound. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2023 Jun:161(3):1095-1097. doi: 10.1002/ijgo.14708. Epub 2023 Feb 15 [PubMed PMID: 36728582]
Paschereit F, Schindelmann KH, Hummel M, Schneider J, Stoltenburg-Didinger G, Kaindl AM. Cerebral Abnormalities in Spina Bifida: A Neuropathological Study. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2022 Mar-Apr:25(2):107-123. doi: 10.1177/10935266211040500. Epub 2021 Oct 6 [PubMed PMID: 34614376]
Biggio JR Jr, Owen J, Wenstrom KD, Oakes WJ. Can prenatal ultrasound findings predict ambulatory status in fetuses with open spina bifida? American journal of obstetrics and gynecology. 2001 Nov:185(5):1016-20 [PubMed PMID: 11717624]
Appasamy M, Roberts D, Pilling D, Buxton N. Antenatal ultrasound and magnetic resonance imaging in localizing the level of lesion in spina bifida and correlation with postnatal outcome. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2006 May:27(5):530-6 [PubMed PMID: 16619377]
Level 2 (mid-level) evidenceGarg SP, Shah KV, Lentskevich M, Yau A, Gosain AK. Prenatal Spina Bifida Repair: A Survey of Current Practice in the United States. Plastic and reconstructive surgery. Global open. 2024 Dec:12(12):e6377. doi: 10.1097/GOX.0000000000006377. Epub 2024 Dec 19 [PubMed PMID: 39703379]
Level 3 (low-level) evidenceZargarzadeh N, Sambatur E, Abiad M, Rojhani E, Javinani A, Northam W, Chmait RH, Krispin E, Aagaard K, Shamshirsaz AA. Gestational age at birth varies by surgical technique in prenatal open spina bifida repair: a systematic review and meta-analysis. American journal of obstetrics and gynecology. 2025 Feb 19:():. pii: S0002-9378(25)00094-8. doi: 10.1016/j.ajog.2025.02.014. Epub 2025 Feb 19 [PubMed PMID: 39983885]
Level 1 (high-level) evidenceAthiel Y, Cariot L, Jouannic JM, Maillet C, Mauffré V, Adam C, Huet H, Larghero J, Nasone J, Guilbaud L. Safety and efficacy of human umbilical cord-derived mesenchymal stromal cells in fetal ovine myelomeningocele repair. Stem cell research & therapy. 2024 Nov 20:15(1):444. doi: 10.1186/s13287-024-03991-y. Epub 2024 Nov 20 [PubMed PMID: 39568021]
Kwasnicki A, Stevenson CB, Forde B, Habli M, McKinney D, Goetz E, Lim FY, Peiro JL. Cryopreserved decellularized human umbilical cord matrix allograft as duraplasty for fetoscopic prenatal spina bifida repair. Journal of neurosurgery. Pediatrics. 2025 Feb 1:35(2):149-157. doi: 10.3171/2024.8.PEDS2488. Epub 2024 Nov 8 [PubMed PMID: 39514855]
Krause M, Leibnitz F, Knüpfer MM, Merkenschlager A, Griessenauer CJ, Gburek-Augustat J. The potential impact of intraoperative neurophysiological monitoring on neurological function outcomes after postnatal spina bifida repair. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2025 Feb 24:41(1):119. doi: 10.1007/s00381-025-06778-5. Epub 2025 Feb 24 [PubMed PMID: 39992435]
McDevitt WM, Afshari FT, Gallo P, Quinn L, Martin-Lamb D, Pepper J, Lo WB, Rodrigues D, Solanki GA. Intraoperative neuromonitoring and mapping during spinal cord untethering surgery; a single-centre paediatric neurosurgery unit experience. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2024 Nov 30:41(1):20. doi: 10.1007/s00381-024-06665-5. Epub 2024 Nov 30 [PubMed PMID: 39614997]
Farmer DL, Thom EA, Brock JW 3rd, Burrows PK, Johnson MP, Howell LJ, Farrell JA, Gupta N, Adzick NS, Management of Myelomeningocele Study Investigators. The Management of Myelomeningocele Study: full cohort 30-month pediatric outcomes. American journal of obstetrics and gynecology. 2018 Feb:218(2):256.e1-256.e13. doi: 10.1016/j.ajog.2017.12.001. Epub 2017 Dec 12 [PubMed PMID: 29246577]
Levin-Decanini T, Houtrow A, Katz A. The Evolution of Spina Bifida Treatment Through a Biomedical Ethics Lens. HEC forum : an interdisciplinary journal on hospitals' ethical and legal issues. 2017 Sep:29(3):197-211. doi: 10.1007/s10730-017-9327-2. Epub [PubMed PMID: 28555303]
Houtrow AJ, MacPherson C, Jackson-Coty J, Rivera M, Flynn L, Burrows PK, Adzick NS, Fletcher J, Gupta N, Howell LJ, Brock JW 3rd, Lee H, Walker WO, Thom EA. Prenatal Repair and Physical Functioning Among Children With Myelomeningocele: A Secondary Analysis of a Randomized Clinical Trial. JAMA pediatrics. 2021 Apr 1:175(4):e205674. doi: 10.1001/jamapediatrics.2020.5674. Epub 2021 Apr 5 [PubMed PMID: 33555337]
Level 1 (high-level) evidenceMcKernan G, Mesoros M, Dicianno BE. Machine Learning Algorithms for Prediction of Ambulation and Wheelchair Transfer Ability in Spina Bifida. Archives of physical medicine and rehabilitation. 2025 May:106(5):682-687. doi: 10.1016/j.apmr.2024.11.013. Epub 2024 Dec 2 [PubMed PMID: 39631515]
Choi EK, Ji Y, Jung E, Bae E. Factors associated with transition readiness among adolescents and young adults with spina bifida in South Korea. Journal of child health care : for professionals working with children in the hospital and community. 2024 Dec 7:():13674935241302438. doi: 10.1177/13674935241302438. Epub 2024 Dec 7 [PubMed PMID: 39644213]
Naus CA, Mann DG, Andropoulos DB, Belfort MA, Sanz-Cortes M, Whitehead WE, Sutton CD. "This is how we do it" Maternal and fetal anesthetic management for fetoscopic myelomeningocele repairs: the Texas Children's Fetal Center protocol. International journal of obstetric anesthesia. 2025 Feb:61():104316. doi: 10.1016/j.ijoa.2024.104316. Epub 2024 Dec 16 [PubMed PMID: 39721283]
Keil C, Sass B, Schulze M, Köhler S, Axt-Fliedner R, Bedei I. The Intrauterine Treatment of Open Spinal Dysraphism. Deutsches Arzteblatt international. 2025 Jan 24:122(2):33-37. doi: 10.3238/arztebl.m2024.0239. Epub [PubMed PMID: 39654393]
Kulesz PA, Juranek JJ, Fletcher JM, Houtrow AJ, Bilaniuk L, Pruthi S, Glenn OA, MacPherson C. Relations of hippocampal and ventricle volumes to Memory Outcomes in the Management of Myelomeningocele Study (MOMS) prenatal surgery clinical trial. Neuropsychology. 2025 Feb:39(2):162-171. doi: 10.1037/neu0000977. Epub [PubMed PMID: 39946636]
Corroenne R, Rangwani S, Whitehead WE, Johnson RM, Nassr AA, Buskmiller C, Munoz JL, Castillo J, Castillo H, Donepudi RV, Belfort MA, Sanz Cortes M. Neurodevelopmental Outcomes after Fetoscopic Myelomeningocele Repair. The Journal of pediatrics. 2025 Apr:279():114472. doi: 10.1016/j.jpeds.2025.114472. Epub 2025 Jan 17 [PubMed PMID: 39828055]
Showalter VC, Salazar AC, Wilson JM, Ming JM. Pediatric urology patient transition to adulthood: Brief report on the barriers and shortcomings in a resource poor state. Health care transitions. 2024:2():100062. doi: 10.1016/j.hctj.2024.100062. Epub 2024 Jul 9 [PubMed PMID: 39712595]
Tennant PW, Pearce MS, Bythell M, Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet (London, England). 2010 Feb 20:375(9715):649-56. doi: 10.1016/S0140-6736(09)61922-X. Epub 2010 Jan 19 [PubMed PMID: 20092884]
Houtrow AJ, Thom EA, Fletcher JM, Burrows PK, Adzick NS, Thomas NH, Brock JW 3rd, Cooper T, Lee H, Bilaniuk L, Glenn OA, Pruthi S, MacPherson C, Farmer DL, Johnson MP, Howell LJ, Gupta N, Walker WO. Prenatal Repair of Myelomeningocele and School-age Functional Outcomes. Pediatrics. 2020 Feb:145(2):. doi: 10.1542/peds.2019-1544. Epub [PubMed PMID: 31980545]
Mannino JE, Reens H, Smith K, Kysh L, Nelson SR, Wang Y, Raam M, Roland M, Speybroeck AV, Betz CL. Psychosocial needs and outcomes of adults with spina bifida: A scoping review, 1974-2023. Health care transitions. 2024:2():100041. doi: 10.1016/j.hctj.2024.100041. Epub 2024 Feb 14 [PubMed PMID: 39712619]
Level 2 (mid-level) evidenceYun H, Yang SH, Lee H, Kim SW, Lee YS, Ji Y, Park J, Ji JE, Choi EK. Clinical profile of Korean children with spina bifida: a single-center prospective cohort study. BMC pediatrics. 2024 Dec 3:24(1):791. doi: 10.1186/s12887-024-05229-5. Epub 2024 Dec 3 [PubMed PMID: 39623314]
Hensel DJ, Young AI, Szymanski KM. Daily Experiences of Urinary and Fecal Incontinence in Young Adults with Spina Bifida: Preliminary Results from an Ecological Momentary Assessment Study. medRxiv : the preprint server for health sciences. 2025 Jan 12:():. pii: 2025.01.10.24313751. doi: 10.1101/2025.01.10.24313751. Epub 2025 Jan 12 [PubMed PMID: 39830248]
Level 2 (mid-level) evidenceMay JM, DeMaio EL, Larson JE. Long-term Clinical and Radiographic Results of Posteromedial Lateral Release for Neuromuscular Clubfoot Deformity. Journal of pediatric orthopedics. 2025 Feb 1:45(2):87-92. doi: 10.1097/BPO.0000000000002848. Epub 2024 Nov 6 [PubMed PMID: 39501684]
Mariani A, Leclair MD, Faraj S, Péré M, Perrouin-Verbe MA, Loubersac T. Long-term outcomes of "clam" bladder augmentation in a pediatric population with neurogenic refractory bladder dysfunction: A 20-year follow-up experience at single center. The French journal of urology. 2025 Feb 7:35(5):102863. doi: 10.1016/j.fjurol.2025.102863. Epub 2025 Feb 7 [PubMed PMID: 39922313]
Etter MM, Greuter L, Guzman R, Soleman J, Licci M. Management of a large pseudomeningocele and cerebrospinal fluid fistula after microsurgical resection of recurrent lipomyelomeningocele in children. Neurosurgical focus. 2025 Feb 1:58(2):E17. doi: 10.3171/2024.11.FOCUS24730. Epub [PubMed PMID: 39891945]
Franco CL, de Oliveira Júnior JP, Morais BA, Pereira NM, Junior VPP, Germano JRG, de Melo ACT, Ribeiro PRJ. Do routine antibiotics change the myelomeningocele infection rate? A case series. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2025 Jan 28:41(1):95. doi: 10.1007/s00381-024-06748-3. Epub 2025 Jan 28 [PubMed PMID: 39873857]
Level 2 (mid-level) evidenceDias LS, Swaroop VT, de Angeli LRA, Larson JE, Rojas AM, Karakostas T. Myelomeningocele: a new functional classification. Journal of children's orthopaedics. 2021 Feb 1:15(1):1-5. doi: 10.1302/1863-2548.15.200248. Epub [PubMed PMID: 33643452]
Graham HK, Harvey A, Rodda J, Nattrass GR, Pirpiris M. The Functional Mobility Scale (FMS). Journal of pediatric orthopedics. 2004 Sep-Oct:24(5):514-20 [PubMed PMID: 15308901]
Swaroop VT, Dias L. Orthopedic management of spina bifida. Part I: hip, knee, and rotational deformities. Journal of children's orthopaedics. 2009 Dec:3(6):441-9. doi: 10.1007/s11832-009-0214-5. Epub 2009 Oct 25 [PubMed PMID: 19856195]
Betancourt D, Shumate C, Yantz C, Gandhi H, Drummond-Borg M, Kubenka C, Singletary C, Riconda D, Agopian AJ. Self-Reported Access to Specialized Genetics Providers Among Families of Young Children With Birth Defects in Texas. American journal of medical genetics. Part A. 2025 Jun:197(6):e64022. doi: 10.1002/ajmg.a.64022. Epub 2025 Feb 17 [PubMed PMID: 39957499]
Wald NJ, Vale SH, Bestwick JP, Morris JK. Blood folate level needed for fully effective fortification in the prevention of neural tube defects. Archives of disease in childhood. 2025 Feb 12:():. pii: archdischild-2024-328115. doi: 10.1136/archdischild-2024-328115. Epub 2025 Feb 12 [PubMed PMID: 39939144]
Lapa DA, Rangwala S, Zebian B, Brown M, Chu J, Chmait RH. Late Prenatal Development of Hindbrain Herniation in Open Spina Bifida. Fetal diagnosis and therapy. 2025 Feb 13:():1-9. doi: 10.1159/000543850. Epub 2025 Feb 13 [PubMed PMID: 39947152]
Binks JA, Barden WS, Burke TA, Young NL. What do we really know about the transition to adult-centered health care? A focus on cerebral palsy and spina bifida. Archives of physical medicine and rehabilitation. 2007 Aug:88(8):1064-73 [PubMed PMID: 17678671]
Khan F, Amatya B, Ng L, Galea M. Rehabilitation outcomes in persons with spina bifida: A randomised controlled trial. Journal of rehabilitation medicine. 2015 Sep:47(8):734-40. doi: 10.2340/16501977-1999. Epub [PubMed PMID: 26181910]
Level 1 (high-level) evidenceYounsi N, Stein R, Szymanski KM. Adaptation of the German language version of the QUAlity of life assessment of spina bifida for adults (QUALAS-A-G). The journal of spinal cord medicine. 2024 Dec 11:():1-6. doi: 10.1080/10790268.2024.2420141. Epub 2024 Dec 11 [PubMed PMID: 39660977]
Level 2 (mid-level) evidenceBerihu BA, Mulugeta A, Magana T, Tessema M, Gebreegziabher T, Berhe Y, Welderufael AL, Mekonen HK. Neural tube defects in a war-torn Tigray regional state of Ethiopia: a retrospective study of 54,626 deliveries. BMC pregnancy and childbirth. 2025 Feb 3:25(1):108. doi: 10.1186/s12884-025-07254-3. Epub 2025 Feb 3 [PubMed PMID: 39901097]
Level 2 (mid-level) evidenceArynchyna-Smith A, Arynchyn AN, Kancherla V, Anselmi K, Aban I, Hoogeveen RC, Steffen LM, Becker DJ, Kulczycki A, Carlo WA, Blount JP. Improvement of serum folate status in the US women of reproductive age with fortified iodised salt with folic acid (FISFA study). Public health nutrition. 2024 Oct 24:27(1):e218. doi: 10.1017/S1368980024001903. Epub 2024 Oct 24 [PubMed PMID: 39445493]
Moodley N, Weidler EM, Ochoa B, Eldredge RS, Rakkar M, Boles K, van Leeuwen K. Satisfaction With Multidisciplinary Team Structure and Function in a Pediatric Outpatient Clinic. Journal of pediatric surgery. 2025 Mar:60(3):162103. doi: 10.1016/j.jpedsurg.2024.162103. Epub 2024 Dec 14 [PubMed PMID: 39733606]
Dicianno BE. 21st century challenges to the provision of health care to adults with spina bifida: a rehabilitation approach. Archives of physical medicine and rehabilitation. 2014 Sep:95(9):1601-2. doi: 10.1016/j.apmr.2014.01.011. Epub 2014 Jan 29 [PubMed PMID: 24486240]