Introduction
Tay-Sachs disease is a progressive, lethal neurodegenerative disorder caused by a deficiency of the enzyme hexosaminidase-A that results in the accumulation of GM2 gangliosides. GM2 gangliosidoses comprise 3 different diseases: Tay-Sachs disease, Sandhoff disease, and the AB variant. GM2 gangliosidoses manifest mainly with central nervous system dysfunction. Sandhoff disease is different from the other 2 gangliosidoses with its systemic involvement, including hepatosplenomegaly, cardiomegaly, macroglossia, and skeletal abnormalities.
The disease is classified into infantile, juvenile, and adult forms based on the age at presentation. Early diagnosis of Tay-Sachs is clinically challenging because of subtle clinical features and nonspecific biochemical findings. Accurate diagnosis is essential for proper management and reducing complications associated with the disease.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Tay-Sachs disease belongs to a group of autosomal recessively inherited lysosomal storage disorders called GM2 gangliosidoses. GM2 gangliosides accumulate inside lysosomes, primarily affecting the nervous system and resulting in neuronal dysfunction and neurodegeneration. The other GM2 gangliosidoses are Sandhoff disease and the AB variant.[1]
Tay-Sachs disease is an autosomal recessive disorder caused by a mutation in HEXA, which encodes the enzymes beta-hexosaminidase A. HEXA is located at 15q23. More than 130 mutations have been identified, including single gene deletions, substitution, insertion splicing alteration, duplication, and complex gene rearrangements.[2] Very rare founder variants of Tay-Sachs disease have been identified recently in India.[3]
Epidemiology
Tay-Sachs disease is rare in the general population, and the incidence is about 1 in 100,000 live births in the United States (US), whereas the carrier frequency is about 1 in 250.[4][5] The disease is more frequent in the people of Ashkenazi Jewish heritage. Epidemiological studies on carrier states in the Jewish community in the US showed a prevalence of about 1 in 29 and 1 in 3500 live births affected by Tay-Sachs disease.[6] A Cajun community in Lousiana, an old-order Amish community in Pennsylvania, and non-Jewish French Canadians living near St Lawrence also have a high incidence of Tay-Sachs disease.[1]
Pathophysiology
Tay-Sachs disease is caused by beta-hexosaminidase A (Hex A) deficiency, responsible for GM2 ganglioside degradation. Alpha and beta subunits of Hex A are synthesized at the endoplasmic reticulum. The enzyme is transported to the Golgi network following glycosylation, intramolecular disulfide bond formation, and dimerization in the endoplasmic reticulum. The most important step is posttranslational modification of the enzyme with mannose-6-phosphate, which helps the lysosome recognize the enzyme. The presentation of GM2 ganglioside to the active site of Hex A requires an activator protein GM2A, which makes the enzyme lipophilic.[7][8]
Gangliosides are the major glycolipid of the neuronal cell membrane, ensuring normal cellular activity.[9] Ganglioside expression in the brain is highly region-specific, highly regulated, and correlated with neurodevelopmental milestones, including neural tube formation, neuritogenesis, axonogenesis, synaptogenesis, and myelination. Ganglioside accretion occurs as early as the 10th week of gestation and continues through 5 years of life. Ganglioside expression also plays a significant role in modulating ion channel and receptor signaling, ensuring optimum function and adaption of neuronal circuits involved in neurotransmission, memory, and learning.
However, deficiency of Hex A causes an accumulation of the gangliosides up to toxic levels, especially in the neurons. While it is clear that the accumulation of ganglioside is the cause, the exact mechanism that translates the primary insult into neuronal death is unclear.[10] Progressive neurodegeneration, microglia proliferation, and accumulation of the complex lipids in neuronal macrophages occur.[10] Other pathological processes in the disease include abnormal endosomal transport, impaired autophagy, progressive accumulation of alpha-synuclein, and anti-ganglioside antibodies.[11]
Histopathology
Hexaminidase levels are virtually absent from all the tissues, including plasma and leucocytes. Ganglioside accumulation is seen in all tissues but predominately in the central and autonomic nervous systems and the retina. Patients with acute infantile Tay-Sachs have an excessive accumulation of GM2 ganglioside (at least 12% of dry weight). In contrast, adult forms have less accumulation and may even be restricted to specific regions. For example, the cortex is almost unaffected in adult forms, whereas brainstem nuclei, spinal cord, and hippocampus are markedly affected.[12][13]
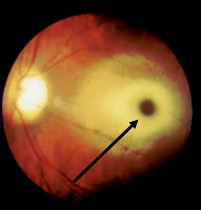
Histopathological examination reveals neurons are ballooned with cytoplasmic vacuoles, constituting lysosomes distended with ganglioside. Sudan Black B and Oil Red O staining are typically positive.[14] Under electron microscopy, a whorled configuration within lysosomes resembling onion-skin layers is the most prominent type seen. GM2 gangliosides accumulate in the retinal ganglion cells, particularly in the margins of the macula, causing cherry-red spots.Ganglion cells are densely packed around the fovea, and swelling in this area leads to a grey-white appearance. In contrast, the center of the fovea lacks ganglion cells, which allows it to maintain a reddish appearance. Toxic accumulation of intralysosomal gangliosides causes early visual symptoms.[14]
History and Physical
Tay-Sachs disease manifests a broad spectrum of clinical features. This disease process has infantile, juvenile, and adult-onset types.
Infantile Tay-Sachs Disease
Infantile Tay-Sachs disease is a prototype of degenerative diseases of grey matter in infancy. The infant is usually normal at birth, and the symptoms start between 3 to 6 months but can manifest as early as 1 week of life.[9] Nonhydrops fetalis can be the initial presentation representing prenatal involvement. The classical initial clinical features are mild motor weakness, irritability, and hypersensitivity to auditory and other sensory stimuli. An exaggerated startle (auditory myoclonus) response is considered an initial and helpful sign in the diagnosis.
A cherry-red spot in the retina visualized during fundoscopic examination is almost a near-specific finding in patients with Tay-Sachs disease (see Image. Tay-Sachs Disease).[15][16] This finding has been noted as early as 2 days of life. The cherry-red spot is due to attenuation of the normal color of the macula and choroid contrasted with pallor produced by the swollen ganglion cells in the remainder of the retina. By 6 months of age, all the patients develop this finding; by 12 to 18 months, vision is reduced; and by 30 months, most patients become blind. Patients also develop narrowing of retinal vessels, nystagmus, and optic atrophy.[17] The child can also have doll-like facies.
Neurological symptoms are the hallmark of Tay-Sachs disease. Infants are usually hypotonic from birth and present with developmental delays or regression by 4 to 6 months of age. Symptoms rapidly progress by 8 to 10 months, spontaneous and voluntary movements diminish, and the infant becomes progressively less responsive.[18][19] The patient also develops seizures by 12 months, typically of the tonic-myoclonic type. Spasticity and seizures mark the final phase of the illness. Myoclonic seizures can be massive and multiple and often refractory to medications, with more than two-thirds of patients needing more than 2 anticonvulsants.[20] Generalized, focal, and gelastic seizures can also occur. Around the same age, the patient also develops ataxia, dyskinesia, sleep disturbances, episodes of screaming, and irritability. By 18 months of age, patients usually develop macrocephaly.[21] Increasing head circumference is due to reactive cerebral gliosis and not hydrocephalus. By 2 years, patients deteriorate and develop decerebrate posturing, dysphagia, and progress to an unresponsive and vegetative state.
Cardiovascular complications are rare but can occur due to the accumulation of the substrate. Prolonged QT intervals and nonspecific T-wave changes are reported.[22] Hepatosplenomegaly is typically absent in Tay-Sachs disease. Patients often develop infections, and respiratory infections are reported frequently as the cause of death. Interestingly, Tay-Sachs carriers are more resistant to mycobacterial infection, which typically lack alpha units and alpha-beta heterodimer of beta-hexosaminidase, and have increased production of HexB (beta subunit) by as much as 200%. The beta subunit of HexB confers resistance against mycobacterial infection.[23]
Juvenile Tay-Sachs Disease
Juvenile Tay-Sachs disease manifests in early childhood between ages 2 to 10, caused by the reduced activity level of Hex A. The earlier the onset of the symptoms, the more quickly the disease progresses. Early symptoms include incoordination, clumsiness, and muscle weakness. Other common symptoms include ataxia, dysarthria, dysphagia, and the progression of spasticity.[24] A cherry-red spot is not consistently observed. Optic atrophy and retinitis pigmentosa can be observed later in life.[25] A vegetative state with decerebrate posturing occurs by age 10 to 15 and is followed within a few years by death, usually from a respiratory infection.
Adult Tay-Sachs Disease
Adult Tay-Sachs disease is a very rare disorder, and diagnosis is often delayed at least by 8 years.[26] The adult form is less aggressive, resulting from a small mutation and high residual activity of Hex A, which demonstrates at least 5% to 20% of normal activity. Typical symptoms start in adolescence or early adulthood but can appear later,in those aged between 20 and 30.[27] Affected individuals have several different phenotypes, including progressive lower motor neuron disease, cognitive impairment, psychiatric symptoms or depression, dystonia, and cerebellar symptoms of dysarthria, ataxia, and tremor.[28] Mentation and verbal skills are affected later in the course of illness. Psychiatric features are also common in adult-onset Tay-Sachs disease. About 40% of individuals develop psychiatric manifestations without dementia, including recurrent psychotic depression, hebephrenic schizophrenia with disorganization of thoughts, delusion and hallucination, paranoia, and bipolar symptoms.[29][30] In any patient with psychiatric illness, the presence of cognitive decline or neurological symptoms should raise suspicion of storage disorders.
Evaluation
The classical clinical findings of progressive weakness with developmental delay or regression, inattentiveness, and exaggerated startle response with physical findings of a cherry-red spot, generalized hypotonia with sustained clonus, or hyperreflexia should warrant further evaluation for gangliosidoses. The first step in the evaluation involves the demonstration of Hex A and total Hexosaminidase levels in the serum. Individuals with the infantile form of Tay-Sachs disease have no or extremely low enzyme activity (0% to 5%) in addition to normal or elevated Beta hexosaminidase levels (HEX B isoenzyme). Individuals with juvenile or adult forms have low enzyme activity (10% to 15%). If the initial testing shows reduced enzyme activity and the patient is from an Azheknazi or French Canadian background, consider targeted gene testing.[31]
Genetic Studies
The first step in carrier testing for Ashkenazi Jewish ancestry depends on whether comprehensive Ashkenazi Jewish carrier screening is desired or only Tay-Sachs screening is required.[32] A comprehensive Ashkenazi Jewish carrier panel includes Tay-Sachs, Canavan disease, familial dysautonomia, Gauchers, Bloom disease, Niemann Pick type A and B, Mucolipidosis IV, and Fanconi anemia type C.
Molecular genetic testing includes sequencing, targeted analysis for pathogenic variants, and deletion/duplication analysis. The targeted analysis is performed if the enzyme activity is absent or low on the initial assay. The panel consists of 6 common pathogenic variants. The panel includes 3 null alleles p.Tyr427IlefsTer5, c.1421+1G>C, and c.1073+G>A, which in the homozygous or compound heterozygous state is associated with Tay-Sachs disease. Allele p.Gly269Ser is associated with an adult-onset form of Hex A deficiency. Two pseudodeficiency alleles (p.Arg247Trp and p.Arg249Trp) are not associated with neurological disease but with reduced degradation of the synthetic substrate when Hex A activity is determined in vitro.[33] The test results must be interpreted carefully because the pseudodeficiency allele does not reduce the enzyme activity with the natural substrate in vivo. About 35 % of non-Jewish individuals identified as heterozygotes by Hex A enzyme testing are carriers for the pseudodeficiency allele, whereas it is 2% in the Jewish population.
Prenatal testing on fetal cells can be performed by chorionic villus sampling at 10 to 12 weeks of gestation or by amniocentesis at 15 to 18 weeks of gestation in families where the Hex A enzyme assay shows parents are heterozygous and molecular genetic testing has ruled out a pseudodeficiency allele in either parent. In families with identified pathogenic variants, preimplantation genetic testing is an option.
Imaging Studies
Neuroradiological findings in Tay-Sachs disease are described in 3 phases of the clinical course.[34][35] In the initial phase, cerebral white matter and basal ganglia show hypodensity in computed tomography (CT) and T2 hyperintense signal changes on magnetic resonance imaging (MRI) (see Image. Brain MRI of Tay-Sachs Disease). Caudate nuclei are enlarged and extend into the lateral ventricle in the first and second phases. The brain becomes atrophic in the final phase. The combination of hyperdensity on CT images and T2-weighted hypointense/T1-weighted hyperintense MRI signal involving bilateral thalami suggests Tay-Sachs disease.[36]
Magnetic resonance (MR) spectroscopy demonstrated an increase in myoinositol/creatinine and choline/creatinine ratio with a decrease in N-acetyl aspartate/creatinine ratio. MR spectroscopy is sensitive and specific to neuroaxonal damage in late-onset Tay-Sachs disease.[37] MR spectroscopy might be a good tool for detecting and quantifying neuronal damage and monitoring the treatment response in patients with late-onset Tay-Sachs disease using pharmacological agents to reduce ganglioside accumulation. Pontocerebellar atrophy is an imaging hallmark of late- or adult-onset Tay-Sachs disease.[38]
Treatment / Management
Treatment of Tay-Sachs disease is largely supportive with the goals of providing adequate nutrition, controlling seizures, managing the infectious sequelae, protecting the airway, and early aggressive physical and occupational therapy. Seizure control usually requires multiple antiepileptics. However, seizures become progressive and change in pattern, so frequent dose changes and initiating new medications are essential. As the child with Tay-Sachs disease becomes more disabled and debilitated, good bowel management becomes essential. Therapeutic modalities for Tay-Sachs disease include enzyme replacement therapy, cell transplantation, substrate reduction therapy, enzyme enhancing therapy, and gene therapy.[4]
Enzyme Replacement Therapy
Enzyme replacement therapy is a promising option for Tay-Sachs disease. Currently, enzyme replacement therapy is less effective in Tay-Sachs disease due to the inability to cross the blood-brain barrier and prevent neurological complications. Results in a recent encouraging report showed that using a recombinant chimeric protein composed of Hex A linked to 2 blood-brain barrier entry elements (dual trojan horse protein), a transferrin receptor binding sequence, and a granulocyte-colony stimulating factor yielded good outcomes.[39] Another major challenge in enzyme replacement therapy is synthesizing both subunits. The synthetic Hex A is treated with alpha-mannosidase to expose mannose-6 residues on the N-glycans.[40]
Enzyme Enhancing Therapy
Most mutations causing Tay-Sachs disease are not localized to the active site but often cause instability of native folded protein. Strategies to reduce the substrate include using molecules called chaperones to stabilize the enzyme. Interestingly, the pharmacological chaperones used are enzyme-specific competitive inhibitors. A trial of the Hex A inhibitor pyrimethamine demonstrated 4-fold increases in Hex A levels, but clinical benefits are not reported.
Substrate Reduction Therapy
The rationale behind substrate reduction therapy is balancing substrate synthesis with diminished enzyme degradative power. The substrate-reduction drug miglustat (N-butyldeoxynojirimycin) has succeeded in mouse models but not humans. Currently, the US Food and Drug Administration has not approved the use of miglustat in treating Tay-Sachs disease.
Gene Therapy
Since a single disorder causes Tay-Sachs disease, gene therapy is an excellent therapeutic option. Significant progress has been made in developing adeno-associated virus-based vectors.[41][42][43] The major limitation of adeno-based vectors is their capacity to carry constructs. For clinically significant therapy, a vector should carry Hex A isoenzyme, which should carry alpha and beta subunits. Genome editing therapies using zinc-finger nucleases are being explored. There are a few scattered reports of tumor development in mice models kept alive for a prolonged time following gene therapy, so a cautious approach is warranted. Massive efforts are being invested in developing viral vectors to deliver isoenzymes effectively.[40]
Bone Marrow Transplantation
Another breakthrough approach involves transplanting ex vivo modified multipotent neural cells with human HEXA expression produced by retroviral transduction. Overall, adequate production and distribution of Hex A are required for a better therapeutic effect in Tay-Sachs disease. So far, substrate reduction therapy, bone marrow transplantation, and enzyme replacement therapy have shown low efficacy in preventing neurodegeneration. Hence, a combination of multiple therapies at an early age is essential since myelination defects appear early and worsen with time.
Differential Diagnosis
Activator Deficient Tay-Sachs Disease
Activator deficient Tay-Sachs disease (AB variant) is a variant of Tay-Sachs disease with the classical findings of neuroregression, exaggerated startle response, and a macular cherry-red spot without hepatosplenomegaly. This condition should be considered in patients with classical findings of Tay-Sachs disease but with normal Hex A and Hex B levels. Ganglioside accumulation occurs due to the deficiency of the intralysosomal GM2 glycoprotein required for GM2 ganglioside degradation.[44]
Sandhoff Disease and Other Lysosomal Storage Disorders
Sandhoff disease is characterized by progressive neurodegeneration starting at 6 months of life and is associated with hyperacusis, macular cherry-red spots, and blindness. Throughout the clinical course, diagnosis, and management, Sandhoff disease is indistinguishable from Tay-Sachs disease except for the visceral and bone involvement. Hepatomegaly is common in Sandhoff disease. Sandhoff disease is a severe Tay-Sachs disease caused by a HEXB mutation and is usually not limited to any specific ethnic group. Progressive neurodegeneration with cherry-red spots is common in other lysosomal storage disorders, including GM1 gangliosidoses, infantile Gaucher disease, Niemann-Pick disease type A, and galactosialidosis.
Late-Onset Tay-Sachs Disease Differential Diagnoses
The differential diagnosis for late-onset Tay-Sachs disease with neurological findings includes adolescent-onset spinal muscular atrophy, Friedreich ataxia, amyotrophic lateral sclerosis, Kufs disease (adult-onset neuronal cerebral lipofuscinosis), and late-onset forms of lysosomal storage disorders. The differential diagnosis of adult-onset Tay-Sachs with neuropsychiatric manifestation includes hepatolenticular degeneration, Niemann-Pick disease Type C, cerebrotendinous xanthomatosis, ceroid neuronal lipofuscinosis, metachromatic leucodystrophy, and X-linked adrenoleukodystrophy. All the above diagnoses should be considered in young patients with refractory or unusual neuropsychiatric symptom clusters.
Prognosis
Tay-Sachs disease is a progressive neurodegenerative disease. Progressive neurological deterioration occurs, and the resulting seizures often remain refractory to treatment. Even with the best care, patients with infantile Tay-Sachs disease usually die by the age of 4 to 5. Death usually results from recurrent infections. In late-onset disease, there are progressive gait difficulties and motor impairment, which often requires the use of adaptive equipment and mobility assistance. Concomitant psychiatric symptoms often remain resistant to treatment. Progressive neurological deterioration often leads to a vegetative state, and death usually occurs by 10 to 15 years of age.
Complications
The complications include progressive neurological deterioration, spasticity, refractory seizures, and progressive visual impairment, finally leading to a vegetative state. Patients with late-onset disease develop progressive motor impairment and balance issues and are at risk of falls and developing psychiatric symptoms that
may remain resistant to treatment.
Consultations
Following the initial diagnosis, patients with either infantile-onset or late-onset Tay-Sachs disease require consultations with multiple professionals. Evaluation by a neurologist is essential to assess and manage the neurologic symptoms. This includes brain MRI, electroencephalography, and assessing the need for antiepileptic drug treatment and monitoring. Ophthalmologic evaluation is required to assess visual impairment and its progression. A speech therapy referral is necessary to evaluate the swallowing dysfunction and risk of aspiration. In case of risk of aspiration referral for gastrostomy and involvement by feeding, the team is required. Respiratory team involvement is essential to assess the airway.
Physiotherapy and occupational therapy referrals are required to manage neuromuscular impairment. Referral to clinical genetics services is required for genetic counseling, screening of at-risk family members, and prenatal or preimplantation genetic diagnosis. Patients with late-onset Tay-Sach disease might require referral to a psychiatrist or psychological medicine team for the assessment of psychiatric symptoms and the need for treatment. Additionally, providing appropriate social support to the family through the involvement of the social work team is vital.
Deterrence and Patient Education
Parents and caregivers should be appropriately counseled regarding the diagnosis, progression, and anticipated complications of Tay-Sachs disease. Families should be informed about the expected outcome, such as progressive neurological deterioration, the refractory nature of the seizures, and the risk of aspiration and recurrent infections. Patients with late-onset Tay-Sachs disease (juvenile- or adult-onset) should be informed about the risk of falls due to ataxia, and appropriate measures such as assistive devices should be advised. In addition, treatment of psychiatric symptoms is essential.
Appropriate genetic counseling should be offered to those who are carriers and at risk of being carriers. Autosomal recessive disorders result when 1 copy of the abnormal gene for the same trait is inherited from each parent. Thus, parents of an affected child with Tay-Sach disease are obligate heterozygote carriers. Therefore, 2 carriers, the father and mother, have a 25% chance that the offspring is affected and a 25% chance that their offspring is healthy, while half of their offspring (50%) may be carriers like their parents. Heterozygotes or carriers are usually asymptomatic.
Enhancing Healthcare Team Outcomes
Managing Tay-Sachs disease requires a coordinated, interprofessional approach due to its progressive and incurable nature. Clinicians, including neonatologists and pediatric neurologists, play a pivotal role in early detection, especially in infants showing developmental delays or regression while being vigilant for juvenile and adult-onset forms. Advanced clinicians, such as nurse practitioners and physician assistants, support ongoing monitoring and management, ensuring patients receive timely care. Genetic counselors are crucial in providing prenatal risk counseling and guiding families through genetic testing.
Nurses contribute to patient-centered care by offering emotional support, coordinating care, and educating families. Pharmacists help manage symptom relief, particularly for seizures, ensuring medication safety and efficacy. Physiotherapists and occupational therapists collaborate to optimize physical function, enhancing quality of life. Effective interprofessional communication ensures seamless care transitions and minimizes errors, which improves outcomes—while coordinated efforts provide comprehensive support to families. This team-based approach improves patient-centered care, safety, and long-term outcomes for those affected by Tay-Sachs disease.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
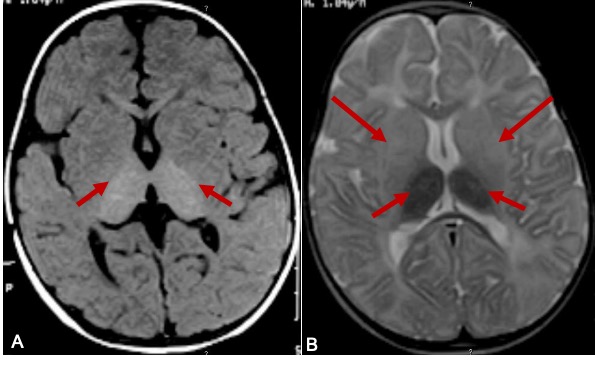
Brain MRI of Tay-Sachs Disease. This image is a brain MRI of a 9-month-old boy with Tay-Sachs disease. The T1 weighted axial image (A) showing hyperintensity of the thalami (arrows). T2W axial image (B) shows hypomyelination, hyperintense signal changes in basal ganglia (arrows), and hypointense signal changes in thalami (small arrows).
Contributed by B Parayil Sankaran MD, DM, FRACP, PhD
References
Solovyeva VV, Shaimardanova AA, Chulpanova DS, Kitaeva KV, Chakrabarti L, Rizvanov AA. New Approaches to Tay-Sachs Disease Therapy. Frontiers in physiology. 2018:9():1663. doi: 10.3389/fphys.2018.01663. Epub 2018 Nov 20 [PubMed PMID: 30524313]
Gravel RA, Triggs-Raine BL, Mahuran DJ. Biochemistry and genetics of Tay-Sachs disease. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1991 Aug:18(3 Suppl):419-23 [PubMed PMID: 1834320]
Sheth J, Nair A, Sheth F, Ajagekar M, Dhondekar T, Panigrahi I, Bavdekar A, Nampoothiri S, Datar C, Gandhi A, Muranjan M, Kaur A, Desai M, Mistri M, Patel C, Naik P, Shah M, Godbole K, Kapoor S, Gupta N, Bijarnia-Mahay S, Kadam S, Solanki D, Desai S, Iyer A, Patel K, Patel H, Shah RC, Mehta S, Shah R, Bhavsar R, Shah J, Pandya M, Patel B, Shah S, Shah H, Shah S, Bajaj S, Shah S, Thaker N, Kalane U, Kamate M, Kn VR, Tayade N, Jagadeesan S, Jain D, Chandarana M, Singh J, Mehta S, Suresh B, Sheth H. Burden of rare genetic disorders in India: twenty-two years' experience of a tertiary centre. Orphanet journal of rare diseases. 2024 Aug 13:19(1):295. doi: 10.1186/s13023-024-03300-z. Epub 2024 Aug 13 [PubMed PMID: 39138584]
Picache JA, Zheng W, Chen CZ. Therapeutic Strategies For Tay-Sachs Disease. Frontiers in pharmacology. 2022:13():906647. doi: 10.3389/fphar.2022.906647. Epub 2022 Jul 5 [PubMed PMID: 35865957]
Lew RM, Burnett L, Proos AL, Delatycki MB. Tay-Sachs disease: current perspectives from Australia. The application of clinical genetics. 2015:8():19-25. doi: 10.2147/TACG.S49628. Epub 2015 Jan 21 [PubMed PMID: 25653550]
Level 3 (low-level) evidenceRozenberg R, Pereira Lda V. The frequency of Tay-Sachs disease causing mutations in the Brazilian Jewish population justifies a carrier screening program. Sao Paulo medical journal = Revista paulista de medicina. 2001 Jul 5:119(4):146-9 [PubMed PMID: 11500789]
Dersh D, Iwamoto Y, Argon Y. Tay-Sachs disease mutations in HEXA target the α chain of hexosaminidase A to endoplasmic reticulum-associated degradation. Molecular biology of the cell. 2016 Dec 1:27(24):3813-3827 [PubMed PMID: 27682588]
Fernandes Filho JA, Shapiro BE. Tay-Sachs disease. Archives of neurology. 2004 Sep:61(9):1466-8 [PubMed PMID: 15364698]
Level 3 (low-level) evidenceKolter T. Ganglioside biochemistry. ISRN biochemistry. 2012:2012():506160. doi: 10.5402/2012/506160. Epub 2012 Dec 19 [PubMed PMID: 25969757]
Cachón-González MB, Wang SZ, Ziegler R, Cheng SH, Cox TM. Reversibility of neuropathology in Tay-Sachs-related diseases. Human molecular genetics. 2014 Feb 1:23(3):730-48. doi: 10.1093/hmg/ddt459. Epub 2013 Sep 20 [PubMed PMID: 24057669]
Level 3 (low-level) evidenceKodama T, Togawa T, Tsukimura T, Kawashima I, Matsuoka K, Kitakaze K, Tsuji D, Itoh K, Ishida Y, Suzuki M, Suzuki T, Sakuraba H. Lyso-GM2 ganglioside: a possible biomarker of Tay-Sachs disease and Sandhoff disease. PloS one. 2011:6(12):e29074. doi: 10.1371/journal.pone.0029074. Epub 2011 Dec 20 [PubMed PMID: 22205997]
Level 3 (low-level) evidenceNestrasil I, Ahmed A, Utz JM, Rudser K, Whitley CB, Jarnes-Utz JR. Distinct progression patterns of brain disease in infantile and juvenile gangliosidoses: Volumetric quantitative MRI study. Molecular genetics and metabolism. 2018 Feb:123(2):97-104. doi: 10.1016/j.ymgme.2017.12.432. Epub 2017 Dec 20 [PubMed PMID: 29352662]
Moriwaki S, Takashima S, Yoshida H, Kawano N, Goto M. Histological observation of the brain of Tay-Sachs disease with seizure and chronic DPH intoxication--report of an autopsy case. Acta pathologica japonica. 1977 May:27(3):387-407 [PubMed PMID: 200060]
Level 3 (low-level) evidenceFerreira CR, Gahl WA. Lysosomal storage diseases. Translational science of rare diseases. 2017 May 25:2(1-2):1-71. doi: 10.3233/TRD-160005. Epub 2017 May 25 [PubMed PMID: 29152458]
Nakamura S, Saito Y, Ishiyama A, Sugai K, Iso T, Inagaki M, Sasaki M. Correlation of augmented startle reflex with brainstem electrophysiological responses in Tay-Sachs disease. Brain & development. 2015 Jan:37(1):101-6. doi: 10.1016/j.braindev.2014.01.011. Epub 2014 Feb 15 [PubMed PMID: 24534057]
Chen H, Chan AY, Stone DU, Mandal NA. Beyond the cherry-red spot: Ocular manifestations of sphingolipid-mediated neurodegenerative and inflammatory disorders. Survey of ophthalmology. 2014 Jan-Feb:59(1):64-76. doi: 10.1016/j.survophthal.2013.02.005. Epub 2013 Sep 5 [PubMed PMID: 24011710]
Level 3 (low-level) evidenceStrupp M, Kremmyda O, Adamczyk C, Böttcher N, Muth C, Yip CW, Bremova T. Central ocular motor disorders, including gaze palsy and nystagmus. Journal of neurology. 2014 Sep:261 Suppl 2(Suppl 2):S542-58. doi: 10.1007/s00415-014-7385-9. Epub [PubMed PMID: 25145891]
Karimzadeh P, Jafari N, Nejad Biglari H, Jabbeh Dari S, Ahmad Abadi F, Alaee MR, Nemati H, Saket S, Tonekaboni SH, Taghdiri MM, Ghofrani M. GM2-Gangliosidosis (Sandhoff and Tay Sachs disease): Diagnosis and Neuroimaging Findings (An Iranian Pediatric Case Series). Iranian journal of child neurology. 2014 Summer:8(3):55-60 [PubMed PMID: 25143775]
Level 2 (mid-level) evidenceBley AE, Giannikopoulos OA, Hayden D, Kubilus K, Tifft CJ, Eichler FS. Natural history of infantile G(M2) gangliosidosis. Pediatrics. 2011 Nov:128(5):e1233-41. doi: 10.1542/peds.2011-0078. Epub 2011 Oct 24 [PubMed PMID: 22025593]
Level 2 (mid-level) evidenceKarimzadeh P. Approach to neurometabolic diseases from a pediatric neurological point of view. Iranian journal of child neurology. 2015 Winter:9(1):1-16 [PubMed PMID: 25767534]
Pavone P, Praticò AD, Rizzo R, Corsello G, Ruggieri M, Parano E, Falsaperla R. A clinical review on megalencephaly: A large brain as a possible sign of cerebral impairment. Medicine. 2017 Jun:96(26):e6814. doi: 10.1097/MD.0000000000006814. Epub [PubMed PMID: 28658095]
Lin AE, Basson CT, Goldmuntz E, Magoulas PL, McDermott DA, McDonald-McGinn DM, McPherson E, Morris CA, Noonan J, Nowak C, Pierpont ME, Pyeritz RE, Rope AF, Zackai E, Pober BR. Adults with genetic syndromes and cardiovascular abnormalities: clinical history and management. Genetics in medicine : official journal of the American College of Medical Genetics. 2008 Jul:10(7):469-94 [PubMed PMID: 18580689]
Spyropoulos B, Moens PB, Davidson J, Lowden JA. Heterozygote advantage in Tay-Sachs carriers? American journal of human genetics. 1981 May:33(3):375-80 [PubMed PMID: 7246543]
Lawson CA, Martin DR. Animal models of GM2 gangliosidosis: utility and limitations. The application of clinical genetics. 2016:9():111-20. doi: 10.2147/TACG.S85354. Epub 2016 Jul 20 [PubMed PMID: 27499644]
Level 3 (low-level) evidenceUdwadia-Hegde A, Hajirnis O. Temporary Efficacy of Pyrimethamine in Juvenile-Onset Tay-Sachs Disease Caused by 2 Unreported HEXA Mutations in the Indian Population. Child neurology open. 2017 Jan-Dec:4():2329048X16687887. doi: 10.1177/2329048X16687887. Epub 2017 Jan 17 [PubMed PMID: 28503624]
Xiao C, Koziura M, Cope H, Spillman R, Tan K, Hisama FM, Tifft CJ, Toro C. Adults with lysosomal storage diseases in the undiagnosed diseases network. Molecular genetics & genomic medicine. 2022 Sep:10(9):e2013. doi: 10.1002/mgg3.2013. Epub 2022 Jul 18 [PubMed PMID: 35848209]
Barritt AW, Anderson SJ, Leigh PN, Ridha BH. Late-onset Tay-Sachs disease. Practical neurology. 2017 Oct:17(5):396-399. doi: 10.1136/practneurol-2017-001665. Epub 2017 Jul 24 [PubMed PMID: 28739864]
Fullam S, Togher Z, Power A, Kennelly L, McHugh JC, O'Dowd S, Tubridy N, Hardiman O, Costigan D, Ryan A, Lefter S, Connolly S, Murphy SM. Late-onset Tay-Sachs disease presenting with a neuromuscular phenotype-a case series. European journal of neurology. 2024 Jan:31(1):e16069. doi: 10.1111/ene.16069. Epub 2023 Sep 27 [PubMed PMID: 37754769]
Level 2 (mid-level) evidenceMacQueen GM, Rosebush PI, Mazurek MF. Neuropsychiatric aspects of the adult variant of Tay-Sachs disease. The Journal of neuropsychiatry and clinical neurosciences. 1998 Winter:10(1):10-9 [PubMed PMID: 9547461]
Zelnik N, Khazanov V, Sheinkman A, Karpati AM, Peleg L. Clinical manifestations of psychiatric patients who are carriers of tay-sachs disease. Possible role of psychotropic drugs. Neuropsychobiology. 2000:41(3):127-31 [PubMed PMID: 10754426]
Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, Toro C, Shirvan L, Tifft C. HEXA Disorders. GeneReviews(®). 1993:(): [PubMed PMID: 20301397]
Kaplan F. Tay-Sachs disease carrier screening: a model for prevention of genetic disease. Genetic testing. 1998:2(4):271-92 [PubMed PMID: 10464605]
Triggs-Raine BL, Mules EH, Kaback MM, Lim-Steele JS, Dowling CE, Akerman BR, Natowicz MR, Grebner EE, Navon R, Welch JP. A pseudodeficiency allele common in non-Jewish Tay-Sachs carriers: implications for carrier screening. American journal of human genetics. 1992 Oct:51(4):793-801 [PubMed PMID: 1384323]
Level 3 (low-level) evidenceFukumizu M, Yoshikawa H, Takashima S, Sakuragawa N, Kurokawa T. Tay-Sachs disease: progression of changes on neuroimaging in four cases. Neuroradiology. 1992:34(6):483-6 [PubMed PMID: 1436455]
Level 3 (low-level) evidenceBano S, Prasad A, Yadav SN, Chaudhary V, Garga UC. Neuroradiological findings in GM2 gangliosidosis variant B1. Journal of pediatric neurosciences. 2011 Jul:6(2):110-3. doi: 10.4103/1817-1745.92824. Epub [PubMed PMID: 22408656]
Level 3 (low-level) evidenceMugikura S, Takahashi S, Higano S, Kurihara N, Kon K, Sakamoto K. MR findings in Tay-Sachs disease. Journal of computer assisted tomography. 1996 Jul-Aug:20(4):551-5 [PubMed PMID: 8708054]
Level 3 (low-level) evidenceAydin K, Bakir B, Tatli B, Terzibasioglu E, Ozmen M. Proton MR spectroscopy in three children with Tay-Sachs disease. Pediatric radiology. 2005 Nov:35(11):1081-5 [PubMed PMID: 16079982]
Level 3 (low-level) evidenceMájovská J, Hennig A, Nestrasil I, Schneider SA, Jahnová H, Vaněčková M, Magner M, Dušek P. Pontocerebellar atrophy is the hallmark neuroradiological finding in late-onset Tay-Sachs disease. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2022 May:43(5):3273-3281. doi: 10.1007/s10072-021-05757-3. Epub 2021 Nov 20 [PubMed PMID: 34800199]
Osher E, Anis Y, Singer-Shapiro R, Urshanski N, Unger T, Albeck S, Bogin O, Weisinger G, Kohen F, Valevski A, Fattal-Valevski A, Sagi L, Weitman M, Shenberger Y, Sagiv N, Navon R, Wilchek M, Stern N. Treating late-onset Tay Sachs disease: Brain delivery with a dual trojan horse protein. Molecular therapy. Methods & clinical development. 2024 Sep 12:32(3):101300. doi: 10.1016/j.omtm.2024.101300. Epub 2024 Jul 17 [PubMed PMID: 39211733]
Cachon-Gonzalez MB, Zaccariotto E, Cox TM. Genetics and Therapies for GM2 Gangliosidosis. Current gene therapy. 2018:18(2):68-89. doi: 10.2174/1566523218666180404162622. Epub [PubMed PMID: 29618308]
Harkins AL, Ambegaokar PP, Keeler AM. Immune responses to central nervous system directed adeno-associated virus gene therapy: Does direct CNS delivery make a difference? Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2024 Jul:21(4):e00435. doi: 10.1016/j.neurot.2024.e00435. Epub 2024 Aug 23 [PubMed PMID: 39180957]
Flotte TR, Cataltepe O, Puri A, Batista AR, Moser R, McKenna-Yasek D, Douthwright C, Gernoux G, Blackwood M, Mueller C, Tai PWL, Jiang X, Bateman S, Spanakis SG, Parzych J, Keeler AM, Abayazeed A, Rohatgi S, Gibson L, Finberg R, Barton BA, Vardar Z, Shazeeb MS, Gounis M, Tifft CJ, Eichler FS, Brown RH Jr, Martin DR, Gray-Edwards HL, Sena-Esteves M. AAV gene therapy for Tay-Sachs disease. Nature medicine. 2022 Feb:28(2):251-259. doi: 10.1038/s41591-021-01664-4. Epub 2022 Feb 10 [PubMed PMID: 35145305]
Yu TW, Bodamer O. A solid start for gene therapy in Tay-Sachs disease. Nature medicine. 2022 Feb:28(2):236-237. doi: 10.1038/s41591-022-01687-5. Epub [PubMed PMID: 35145310]
Hall PL, Laine R, Alexander JJ, Ankala A, Teot LA, Lidov HGW, Anselm I. GM2 Activator Deficiency Caused by a Homozygous Exon 2 Deletion in GM2A. JIMD reports. 2018:38():61-65. doi: 10.1007/8904_2017_31. Epub 2017 May 25 [PubMed PMID: 28540636]