Definition/Introduction
Magnetic resonance imaging (MRI) is a noninvasive imaging technique known for its exceptional soft tissue contrast and expanding range of physiological and functional applications. Unlike imaging modalities that rely on ionizing radiation, MRI has been a cornerstone of diagnostic radiology since the 1980s. The technique utilizes a strong static magnetic field, rapidly changing gradient magnetic fields, radiofrequency waves, and computer processing to produce detailed images. Despite its many advantages, MRI carries certain risks. As clinical demand continues to increase, healthcare professionals must receive proper training in MRI safety to minimize potential hazards to patients.[1]
MRI scanners utilize 3 main types of magnetic fields, each associated with distinct safety concerns. First, the strong static magnetic field can attract and accelerate ferromagnetic objects toward the scanner’s core, potentially turning them into hazardous projectiles. This field can also displace implants or interfere with the functionality of electronic medical devices, such as pacemakers and infusion pumps. Second, the radiofrequency field, generated by radiofrequency coils, can cause tissue heating, especially when implants are present. Non-ferromagnetic implants may generate heat buildup due to eddy currents circulating within metal components exposed to oscillating magnetic fields.[2][3]
Third, the time-varying, fast-switching gradient magnetic fields are responsible for the spatial encoding of MRI signals. However, they can stimulate muscles or peripheral nerves and increase the temperature of implants. These fields also produce significant acoustic noise, often exceeding 100 dB, which can potentially damage hearing. As a result, headphones or earplugs are essential for patients and all individuals present in the scanner room during imaging.[4]
Magnetic fields in MRI scanners can trigger 5 critical interactions in patients with metallic foreign bodies, including projectile motion, implant displacement or twisting, tissue burns, imaging artifacts, and device malfunction (eg, pacemaker interference).[5] Comprehensive screening for metallic foreign bodies should be conducted before every MRI scan to ensure patient safety.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Radiologists, referring physicians, and MRI technologists must assess magnetic resonance (MR) safety, evaluate patient conditions, and confirm the compatibility of medical devices to prevent harm. MRI contraindications are classified into absolute and relative categories.
Absolute Contraindications
Cardiac implantable electronic devices (CIEDs)—including pacemakers, implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices—pose significant risks during MRI. These include inappropriate therapy delivery, device heating or displacement, and the potential for inducing arrhythmias. Patients with CIEDs must be scheduled during dedicated MRI time slots with support from an electrophysiology nurse or technician. MRI-conditional CIEDs are now widely available and increasingly utilized.[6]
Metallic intraocular foreign bodies can migrate or cause significant injury when exposed to the magnetic field. Patients with a history of facial trauma involving metal fragments or unprotected welding must undergo an orbital x-ray, which should be reviewed by a radiologist before proceeding with the MRI scan.
Other absolute contraindications include implantable neurostimulation systems, cochlear or ear implants, and drug infusion pumps used to deliver insulin, analgesics, or chemotherapy. For example, patients with bone-anchored hearing aids (BAHAs) may undergo MRI at 1.5 Tesla only after removing the battery, and scheduling must accommodate specific preparations for cochlear implants. Infusion pumps should be removed when possible. Additional contraindicated items include catheters with metallic components (eg, Swan-Ganz catheters), metallic fragments such as bullets, shrapnel, or pellets, cerebral artery aneurysm clips, magnetic dental implants, tissue expanders, prosthetic limbs, hearing aids, and body piercings.
MRI safety depends not only on the object itself but also on the scanner’s magnetic field strength. Some items may be safe at 1.5 Tesla but pose risks at 3 Tesla. Each implant or device must be verified through a certified MRI safety database or the manufacturer's documentation. Radiologists and technologists should be trained to access and interpret this information accurately. Most modern implants and medical materials are made from non-ferromagnetic substances and are labeled as " MR-safe" or "MR-conditional." In the absence of validated safety data, any implant or device should be assumed to be "MR-unsafe."
Relative Contraindications
Several relative contraindications require case-by-case evaluation before proceeding with an MRI. Patients with coronary or peripheral artery stents, programmable shunts, or airway stents may undergo scanning, but specific precautions must be taken. For patients with programmable shunts, it is essential to ensure reprogramming with their healthcare provider after the procedure. Plastic tracheostomy tubes are MRI-compatible; however, if the material is unknown or metallic, the tracheostomy tube must be replaced with a plastic one before the patient enters the scanner.
Intrauterine devices (IUDs) must be evaluated by make and model. Unknown IUDs may only be scanned at 1.5 Tesla. Other devices requiring caution include ocular prostheses, stapes implants, surgical clips, wire sutures, penile prostheses, joint replacements, and inferior vena cava filters. If the make and model of an inferior vena cava filter are unknown, scanning at 1.5 Tesla may proceed if at least 6 weeks have passed since implantation. Patients with Harrington rods may safely undergo MRI at 1.5 Tesla.
Medication patches must be removed before the scan. Tattoos in the imaging area that are less than 6 weeks old require rescheduling. For older tattoos, ice packs or padding should be placed between the tattoo and the scanner bore or MRI coil. Patients must be instructed to use the communication ball if they experience warmth in tattooed areas.
A recent colonoscopy—especially within the past 8 weeks—requires confirmation of any retained endoscopic devices, such as clips or capsule endoscopes. If the presence of such devices is confirmed or cannot be ruled out, consultation with a radiologist or referring physician is necessary before proceeding.
Additional devices or implants may also present safety concerns. Radiologists and technologists must assess each device type before scanning. Claustrophobia can hinder the completion of the scan; these patients benefit from a thorough explanation of the procedure in advance and may require sedation. Open or wide-bore MRI systems can offer relief in such cases.[7][8]
Patients who are unable to remain still or follow breathing instructions require special attention. Pain, discomfort, or anxiety can lead to motion during scanning, which degrades image quality and reduces diagnostic accuracy. Certain sequences require patients to hold their breath and remain motionless. Individuals with a high body mass index may not fit into standard scanners, but wider-bore systems are available and can accommodate these patients.
Precautions During Gadolinium-Based Contrast Administration
Gadolinium-based contrast agents used in MRI are chelates that vary in stability, viscosity, and osmolality. While gadolinium is generally considered safe, rare allergic or anaphylactic reactions may occur. Careful evaluation is essential for specific patient groups before contrast administration. Patients on dialysis or with a history of renal disease, such as renal transplantation, a single kidney, or renal malignancy, require special attention. Individuals who received gadolinium-based contrast within the previous 24 hours should also be assessed. A history of allergic or anaphylactic reactions to gadolinium warrants caution and may require premedication or consideration of alternative imaging strategies.
Patients at risk for nephrogenic systemic fibrosis, particularly those with impaired renal function, must be screened before contrast injection. Estimating the glomerular filtration rate (GFR) is essential for patients with diabetes or hypertension receiving pharmacological treatment. If the GFR is below 35 mL/min/1.73 m², consultation with a radiologist is required. Gadolinium administration should generally be avoided in patients with an estimated GFR below 30 mL/min/1.73 m2, unless deemed essential by the radiologist.
Pregnant patients require an individualized risk-benefit assessment before using gadolinium contrast. The decision to proceed should be based on clinical necessity, considering the uncertain long-term fetal effects of gadolinium exposure.
Pregnancy and Breastfeeding
MRI is a valuable imaging tool for evaluating both obstetric and non-obstetric conditions during pregnancy. No known adverse effects have been associated with non-contrast MRI exposure to fetuses. However, gadolinium is classified as a class C agent by the US Food and Drug Administration (FDA), and definitive safety data for MRI during pregnancy is lacking. A patient who is pregnant or may be pregnant should inform the radiology team and physician before the procedure. MRI during pregnancy should only be performed when necessary to evaluate suspected abnormalities, and a 1.5-Tesla scanner should be used.[9]
Gadolinium contrast is excreted into breast milk in amounts less than 0.04% of the dose administered to the mother. Of this small amount, only 0.8% is absorbed by the infant. Therefore, breastfeeding can generally continue without concern following an MRI.[10]
Clinical Significance
MRI has become an increasingly important component of diagnostic strategies, playing a key role in guiding therapeutic decision-making. This modality offers high-resolution imaging of soft tissues without the use of ionizing radiation, making it a safe and preferred option for diagnosing musculoskeletal, neurological, and cardiovascular conditions. However, certain restrictions and contraindications exist due to the equipment's magnetic fields, machine structure, and gadolinium contrast agents. Healthcare professionals must be adequately trained in MRI safety to mitigate potential risks to both patients and staff. Close coordination between the radiology team and physicians is essential to ensure appropriate imaging, patient safety, and optimal diagnostic outcomes.[11]
Nursing, Allied Health, and Interprofessional Team Interventions
All patients must complete a thorough safety screening before undergoing an MRI scan due to potential safety risks. The screening process typically includes a verbal interview to identify any contraindications. Patients should be asked about the presence of any foreign substances or materials that could interfere with the MRI. If a patient has a device or implant, its make and model must be verified using appropriate databases and MRI safety resources. Consultation with a radiologist or the referring physician may be necessary to determine whether MRI is the most appropriate imaging modality. Patients should wear gowns to eliminate the risk of metallic components in clothing, and they must be informed in advance about the procedure details and expected duration.
Although gadolinium chelates are generally well-tolerated, MRI technologists and physicians must remain vigilant for potential adverse reactions. MRI departments should be equipped with appropriate medications and facilities to manage any reactions to contrast agents. The MRI scanner generates loud knocking noises that can potentially damage hearing. These noises can be minimized by providing patients with headphones or earplugs during the scan. Throughout the procedure, staff must continuously monitor the patient. An intercommunication system enables the patient to speak with the MRI technologist or radiology nurse, use a squeeze ball for signaling, and receive instructions as needed.
Nursing, Allied Health, and Interprofessional Team Monitoring
The 2024 edition of the American College of Radiology Manual on MR Safety offers an updated and comprehensive guide for managing MRI facilities. This document addresses MRI-specific hazards, such as projectile risks, radiofrequency burns, and device interactions, while providing best practices for safety screening, staffing, equipment usage, and emergency protocols.
Key points include:
- A classification of safety zones (Zone I–IV) and MR-controlled access areas (see Image. MRI Safety Zones (I–IV) and MR-Controlled Access Areas).
- Defined roles for MR safety personnel, such as the MR Medical Director, MR Safety Officer, and MR Safety Expert.
- Updated screening protocols for patients, staff, and devices, with special attention to implants and ferromagnetic materials.
- Guidance on managing special patient populations, including pregnant, pediatric, and detained individuals.
- Coverage of alternative MR environments, such as positron emission tomography/MR, intraoperative MR, and 7-Tesla systems.
- Clear emergency procedures for incidents such as magnet quench or fire.
The manual is a practical, evidence-based educational resource and a procedural blueprint, emphasizing risk mitigation, regular training, and ongoing safety assessments.
Media
(Click Image to Enlarge)
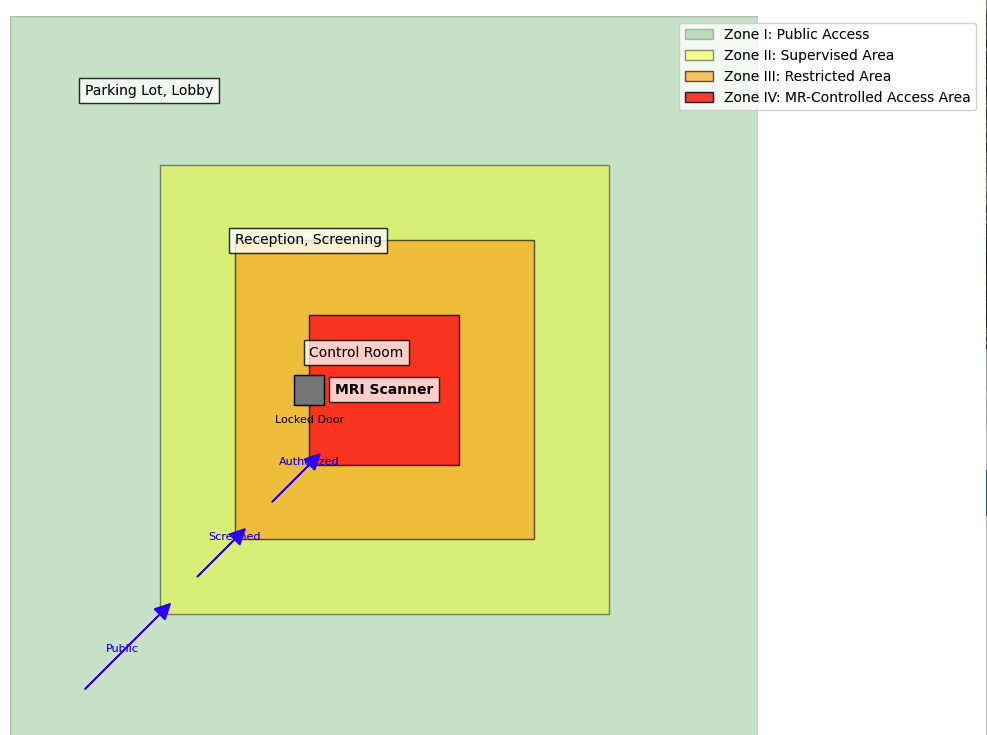
MRI Safety Zones (I–IV) and MR-Controlled Access Areas. This diagram illustrates the various magnetic resonance imaging (MRI) safety zones (I-IV) and magnetic resonance (MR)-controlled access areas, highlighting the varying levels of restriction and control required for each zone to ensure the safety of patients and staff within MRI environments. Zone IV is the MR-controlled access area with strict safety protocols.
Contributed by A Thomas, MD
References
Feychting M. Health effects of static magnetic fields--a review of the epidemiological evidence. Progress in biophysics and molecular biology. 2005 Feb-Apr:87(2-3):241-6 [PubMed PMID: 15556662]
Level 2 (mid-level) evidenceShellock FG, Crues JV. Corneal temperature changes induced by high-field-strength MR imaging with a head coil. Radiology. 1988 Jun:167(3):809-11 [PubMed PMID: 3363146]
Shellock FG, Crues JV. Temperature changes caused by MR imaging of the brain with a head coil. AJNR. American journal of neuroradiology. 1988 Mar-Apr:9(2):287-91 [PubMed PMID: 3128077]
Sammet S. Magnetic resonance safety. Abdominal radiology (New York). 2016 Mar:41(3):444-51. doi: 10.1007/s00261-016-0680-4. Epub [PubMed PMID: 26940331]
Stecco A, Saponaro A, Carriero A. Patient safety issues in magnetic resonance imaging: state of the art. La Radiologia medica. 2007 Jun:112(4):491-508 [PubMed PMID: 17563855]
Korutz AW, Obajuluwa A, Lester MS, McComb EN, Hijaz TA, Collins JD, Dandamudi S, Knight BP, Nemeth AJ. Pacemakers in MRI for the Neuroradiologist. AJNR. American journal of neuroradiology. 2017 Dec:38(12):2222-2230. doi: 10.3174/ajnr.A5314. Epub 2017 Jul 13 [PubMed PMID: 28705821]
Murphy KJ, Brunberg JA. Adult claustrophobia, anxiety and sedation in MRI. Magnetic resonance imaging. 1997:15(1):51-4 [PubMed PMID: 9084025]
Level 2 (mid-level) evidenceThorpe S, Salkovskis PM, Dittner A. Claustrophobia in MRI: the role of cognitions. Magnetic resonance imaging. 2008 Oct:26(8):1081-8. doi: 10.1016/j.mri.2008.01.022. Epub 2008 Jun 3 [PubMed PMID: 18524527]
Mervak BM, Altun E, McGinty KA, Hyslop WB, Semelka RC, Burke LM. MRI in pregnancy: Indications and practical considerations. Journal of magnetic resonance imaging : JMRI. 2019 Mar:49(3):621-631. doi: 10.1002/jmri.26317. Epub 2019 Jan 31 [PubMed PMID: 30701610]
Newman J. Breastfeeding and radiologic procedures. Canadian family physician Medecin de famille canadien. 2007 Apr:53(4):630-1 [PubMed PMID: 17872711]
Sammet S, Sammet CL. Implementation of a comprehensive MR safety course for medical students. Journal of magnetic resonance imaging : JMRI. 2015 Dec:42(6):1478-86. doi: 10.1002/jmri.24993. Epub 2015 Jul 14 [PubMed PMID: 26172156]