 Anatomy, Head and Neck, Temporomandibular Joint
Anatomy, Head and Neck, Temporomandibular Joint
Introduction
The stomatognathic system comprises various anatomical structures responsible for mouth opening, swallowing, breathing, phonation, sucking, and multiple facial expressions. These structures include the temporomandibular joint (TMJ), maxilla and mandible, associated muscles and tendons, dental arches, salivary glands, and the hyoid bone, as well as muscles connecting this bone to the scapula, sternum, and neck.
The TMJ performs complex movements across multiple orthogonal planes and axes of rotation, functioning synergistically with the other components (see Image. Temporomandibular Joint). Coordination with the contralateral TMJ ensures synchronized movement. Dysfunction or pathology of this structure can lead to pain, limited jaw movement, and impaired quality of life. Medical management focuses on diagnosing disorders such as TMJ dysfunction, arthritis, and trauma. Surgical intervention may be necessary for severe joint degeneration, ankylosis, tumors, or congenital anomalies.
A thorough understanding of the TMJ’s functional anatomy, embryological development, and age-related anatomical differences enhances clinicians’ ability to assess and manage joint function effectively. Awareness of surgical, clinical, and physiological factors influencing the TMJ supports informed decision-making. Integrating manual therapeutic approaches with interprofessional collaboration can optimize joint rehabilitation outcomes.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The TMJ is a diarthrosis, more specifically classified as a ginglymoarthrodial joint. The joint is enclosed by a capsule and contains a synovial cavity and articular cartilage. Synovial fluid and several ligaments are present within the joint. The TMJ is the articulation between the temporal bone cavity and the mandibular condyle.
Anatomy
The cranial surface of the TMJ comprises the squamous part of the temporal bone, known as the glenoid fossa, which accommodates the mandibular condyle. The posterior region of the fossa forms the posterior articular ridge, adjacent laterally to the postglenoid process. The postglenoid process contributes to the upper wall of the external acoustic meatus.
The anterior boundary of the glenoid fossa is the articular eminence, a medial bony prominence at the posterior border of the zygomatic bone. The preglenoid plane, which is slightly inclined, leads into the articular eminence, which lies anterior to the fossa along the base of the skull. This region facilitates the movements of the articular disc and condyle. On the lateral surface of the articular eminence, near the root of the zygomatic process, lies a bony ridge known as the articular tubercle.
The glenoid fossa is wider mediolaterally than anteroposteriorly. The inferior articular surface of the glenoid fossa corresponds to the superior aspect of the mandible.[1] This surface includes the mandibular condyle, which measures approximately 15 to 20 mm transversely and 8 to 10 mm anteroposteriorly.
The articular disc covering the condyle and interposed beneath the glenoid fossa has a biconcave or oval shape. The cartilaginous disc features an anterior portion measuring about 2 mm and a posterior portion approximately 3 mm thick, with a thinner middle region. The anterior portion consists of an upper fibroelastic fascia layer and a lower fibrous layer. The upper part contacts the postglenoid process, preventing the disc from slipping during mouth opening. The lower part restricts excessive rotational movement of the disc relative to the mandibular condyle.
The anterior portion of the articular disc contacts the joint capsule, articular eminence, condyle, and the upper area of the lateral pterygoid muscle. The posterior segment of the articular disc relates to the bilateral retrodiscal tissue located behind the condyle, the glenoid fossa, the condyle itself, and the temporal bone. The medial and lateral aspects of the cartilaginous disc attach to the mandibular condyle. The disc’s edges partially fuse with the fibrous capsule surrounding the joint.
Several ligaments regulate TMJ forces and transmit multiple proprioceptive afferents. Proprioception of the joint arises from components including the capsule, masticatory muscles, skin receptors, and receptors within the periodontal ligaments. Tension sensed by the articular ligaments plays a critical role in TMJ function.[2]
The sphenomandibular ligament (SML) is a remnant of the Meckel cartilage. This fibrous structure originates from the sphenoid spine, which also gives rise to the pterygospinous ligament, and inserts into the medial wall of the TMJ capsule along its course toward the mandible. The SML interacts with the malleus and contributes fibers to the anterior ligament of the malleus through the petrotympanic fissure. The ligament continues inferiorly to attach to the lingula of the mandible.
The mylohyoid nerve and several vessels cross the SML, maintaining contact with the pterygomandibular fascia. The SML lies superiorly and laterally in relation to the lateral pterygoid muscle, the internal maxillary artery, the auriculotemporal nerve, the inferior alveolar nerve, and the middle meningeal artery. The ligament primarily protects the TMJ from excessive condylar translation after approximately 10° of mouth opening.
The stylomandibular ligament (STML) extends from the styloid process of the temporal bone to the posterior margin of the mandible, including the jaw angle. The STML is a thickening of the deep cervical fascia, specifically the parotid fascia, and functions to limit excessive mandibular protrusion. Embryologically, this fibrous structure derives from the 1st and 2nd branchial arches, the latter giving rise to the middle ear stapes through the Reichert cartilage. Along its course, the STML covers the medial surface of the medial pterygoid muscle.
The pterygomandibular ligament (PTML) or raphe is a thickening of the buccopharyngeal fascia. This ligament extends from the apex of the hamulus of the medial pterygoid plate to the posterior region of the retromolar trigone of the mandible. The PTML is in contact with the buccinator muscle anteriorly and the pharyngeal constrictor muscle posteriorly. Embryologically, this structure derives from the mesenchymal connection of the 1st and 2nd branchial arches. The PTML functions to limit excessive mandibular movements.
The Pinto ligament, also known as the malleolomandibular or discomalleolar ligament, originates from the tympanic portion during embryological development. The ligament has 2 parts. The middle ear portion encompasses the malleus and the anterior ligament of the malleus. The extratympanic segment includes the posterosuperior part of the TMJ capsule, in contact with retrodiscal tissues and passing through the petrotympanic fissure. The Pinto ligament serves 2 main functions. In the TMJ, this ligament protects the synovial membrane from tension generated by surrounding structures. In the middle ear, this structure appears to regulate or influence adequate pressure within this region.[3][4]
The collateral ligament consists of 2 bundles of symmetrical fibers originating from the intermediate fascia of the articular disc and inserting at the medial and lateral poles of the mandibular condyle. This ligament anchors the disc to the condyle.[5][6]
The TMJ is associated with several muscles responsible for its movement and protection. Muscles that close the jaw include the masseter, temporalis, and medial pterygoid. Muscles that open the jaw include the lateral pterygoid, geniohyoid, mylohyoid, and digastric.
Function
During mouth opening, the TMJ exhibits a combination of rotational movement within the discomandibular space and translational movement within the discotemporal space, with rotation occurring before translation. The condyle can move laterally through rotation, followed by anterior sliding of the same condylar structure, while the contralateral condyle undergoes anterior translation and rotation medially. Posterior movement of one condyle occurs simultaneously with anterior sliding of the opposite condyle. Bilateral or ipsilateral TMJ protrusion results from anterior sliding of the condyles.
The complex movements of the TMJ enable multiple essential functions, including chewing, sucking, swallowing, phonation, facial expression, breathing, protrusion, retrusion, lateralization of the jaw, and mouth opening. The TMJ also plays a role in maintaining the appropriate middle ear pressure.
Embryology
The TMJ develops from the 1st pharyngeal arch, with a mesodermal component giving rise to muscles and vessels, and mesenchyme derived from neural crest cells forming the bones and cartilage. TMJ development occurs in 3 stages: blastemic, cavitation, and maturation.
The blastemic stage begins during the 7th to 8th week of gestation, with the formation of the glenoid fossa and the condylar blastema. The blastema consists of undifferentiated cells that proliferate to establish the foundational structure of the joint.
The lower joint space begins to form during the cavitation stage. The blastemas differentiate into multiple layers, giving rise to the lower synovial layer and the future articular disc. This stage occurs between the 9th and 10th weeks of gestation.
The maturation stage begins around the 11th week, with the formation of the upper joint space. TMJ development continues throughout gestation. The joint capsule is established by approximately 17 weeks. Cartilage development within the capsule becomes identifiable between 19 and 20 weeks.
The morphology of the glenoid fossa and the condyle is influenced by the mechanical forces exerted by surrounding vessels and muscles. At birth, the TMJ is not fully developed compared with other synovial joints. Mandibular formation begins during the 4th week of gestation, with the TMJ developing concurrently with the ear.
The mandibular arch is more obtuse in children than in adults, whose arches exhibit a more angular shape. The glenoid fossa in infants is looser, and cartilage has not yet formed, being replaced by fibrous connective tissue. The condyles grow posteriorly, laterally, and superiorly between 5 and 10 years of age, while mechanical forces from the teeth and masticatory muscles continue to shape the joint.
Blood Supply and Lymphatics
Arterial blood supply to the TMJ is provided primarily by the superficial temporal, maxillary, and masseteric arteries. Additional contributions arise from the posterior auricular, ascending pharyngeal (branches of the external carotid), and ascending palatine arteries.[7]
Venous drainage occurs via the pterygoid venous plexus in the retrodiscal region, which communicates with the internal maxillary, sphenopalatine, middle meningeal, deep temporal, masseteric, and inferior alveolar veins. Lymphatic drainage of the TMJ is variable, particularly in pathological conditions when lymph nodes may increase in number. Under normal circumstances, the lymphatic vessels associated with the TMJ primarily drain toward the submandibular triangle.
Nerves
The TMJ contains multiple proprioceptive receptors, particularly within the parenchyma of the articular disc, including Golgi-Mazzoni and Ruffini endings, as well as myelinated and unmyelinated nerve fibers. Innervation of the articular capsule varies by region. The anterolateral portion receives fibers from the masseteric nerve, a branch of the mandibular division of the trigeminal nerve. The lateral region of the capsule is innervated by the auriculotemporal nerve, also a branch of the mandibular division of cranial nerve V.[8][9]
Muscles
Four muscles make direct contact with the TMJ. These muscles include the masseter, temporalis, and the medial and lateral pterygoids.
The masseter, including its perimysium, contacts the anterior edge of the articular disc. Originating from the zygomatic arch with multiple muscular layers, the masseter inserts on the lateral surface of the mandibular ramus and the lateral surface of the coronoid process. This muscle primarily elevates the mandible. The masseteric branch of the trigeminal nerve provides innervation.
The temporalis originates from the temporal fossa of the skull and the medial surface of the zygomatic process, inserting on the coronoid process of the mandible. Anterior contact with the articular disc can occur in some individuals. Primary action is elevation of the mandible. Innervation is via the deep temporal branches of the mandibular division of the trigeminal nerve.
The lateral (external) pterygoid consists of an upper head and a lower head. The upper head originates from the extracranial surface of the greater wing of the sphenoid and inserts anteromedially on the joint capsule, as well as on the anteromedial surface of the condylar neck within the superior portion of the pterygoid fovea. This head contacts the articular disc at its anteromedial aspect. The inferior head arises from the lateral surface of the lateral lamina of the pterygoid process of the sphenoid and inserts on the pterygoid fovea.
Bilateral activation of the lateral pterygoid protrudes the mandible, whereas unilateral activation causes contralateral lateral deviation of the mandible. During mouth opening, the lateral pterygoid pulls the condyle forward. During mouth closing, the muscle pulls the disc anteromedially. Both heads are active during the early stages of mouth opening and the initial phases of closing.
The medial (internal) pterygoid originates from the pterygoid fossa, the pyramidal process of the palatine, and the maxillary tuberosity, inserting on the medial surface of the mandibular ramus and angle. Like the lateral pterygoid, the medial pterygoid receives innervation from the mandibular branch of the trigeminal nerve. The muscle's primary actions include elevation and protrusion of the mandible.
Physiologic Variants
Pneumatization of the articular tubercle represents an anatomical variant of the TMJ. Bone cavities or air cells are present in the root of the zygomatic arch and the tubercle or eminence of the temporal bone. These cavities may occur unilaterally or bilaterally. Normal development includes the reabsorption of the cavities during puberty. However, reabsorption may not occur in some cases. This anatomical variation does not appear to affect TMJ function or symptomatology negatively.[10]
Surgical Considerations
Surgical procedures for the TMJ are indicated for various medical reasons, including local or systemic disease, trauma, alteration of joint morphology, and loss of function. The surgical approach of choice depends on the specific problem, anatomical and physiological considerations, the patient's psychoemotional needs, the surgeon’s experience, and presurgical objectives.
Orthognathic surgery performed for aesthetic or functional purposes, such as mandibular advancement, improving mouth opening, or preventing joint dislocation, does not provide definitive conclusions regarding the balance of benefits and side effects. Postoperative functional outcomes are likely more favorable when the TMJ is free of concomitant pathologies, including arthritis and arthrosis.[11][12]
Knowledge regarding the effects of surgery in the presence of synovial plicae is limited. Functional alterations caused by the plica may remain asymptomatic and are often detected only through instrumental examination.[13]
Total joint reconstruction or alloplastic joint replacement demonstrates favorable postsurgical outcomes in cases of idiopathic condylar resorption.[14][15] Arthroscopic approaches also achieve good results for the repair or removal of the articular disc.[16]
Clinical Significance
Multiple pathologies can affect the TMJ and result in varying degrees of clinical dysfunction. Rheumatoid arthritis may present with crepitus, limited range of motion (ROM), increased stiffness, pain, and joint sounds. Psoriatic arthropathy often manifests as joint sounds, morning stiffness, and pain. Ankylosing spondylitis can cause joint sounds, pain in the lateral pterygoid muscles, hypertrophy of the masseter, and ROM restriction. Pain originating from the joint may be associated with articular sounds. Fibromyalgia syndrome can produce myalgia and generalized TMJ pain. Muscular pain may also contribute to dysfunction. Multiple sclerosis may be associated with myalgia of the TMJ and pain during mandibular movement.[17]
Clinical Evaluation
Clinical evaluation of the TMJ begins with observation of natural mandibular movements. Assessment includes facial symmetry, the range and quality of TMJ movements, the onset and severity of pain during opening, closing, and accessory movements, and the presence of joint sounds.
Palpation of the masticatory muscles, both internally and externally, is performed, along with evaluation for enlarged submandibular lymph nodes. Muscle strength is tested in isolation, as well as in combination during complex mandibular movements. Passive manipulation of the jaw, using a gloved hand, allows evaluation of the TMJ ligament apparatus. Intraoral examination is conducted to identify anatomical anomalies.
Palpation of the joint during mandibular opening enables the detection of additional abnormalities. With fingers supporting the TMJ, the patient is asked to perform tongue protrusion, rotation, neck flexion and extension, and lateral inclinations during mouth opening. These maneuvers help reveal accentuation of TMJ dysfunction in association with other musculoskeletal structures.
Several tools can enhance the measurement of mandibular ROM, including the Boley gauge and the TheraBite ROM scale. These evaluations are based on the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD), updated in 2016.[18]
Children with a class II malocclusion exhibit a higher prevalence of TMJ dysfunction. Occurrence of dysfunction during the growth phase may result in asymmetry of the mandibular ramus.
Instrumental Assessments
Instrumental assessments are essential for accurate diagnosis and should be performed before initiating any therapy for TMJ dysfunction.[19] Panoramic radiography allows evaluation of the relationship between the mandibular condyles and the corresponding glenoid fossae. Multislice or cone-beam computed tomography enables detailed assessment of the morphology of TMJ components, bone position, and potential pathologies. Magnetic resonance imaging provides visualization of the articular disc, as well as joint anatomy, function, and form.
Other Issues
Approximately 85% to 90% of TMJ disorder symptoms are managed with noninvasive, nonsurgical approaches. Noninvasive management strategies include manual physiotherapy and osteopathy, patient education, pharmacological treatment, and splint therapy.[20][21]
The relationship between TMJ function, anatomy, and posture remains a topic of considerable debate. Difficulty in establishing a definitive link arises from the anatomical complexity of the TMJ, which includes the capsule, muscles, ligaments, nerves, and articular disc, and from challenges in comparing its function with other regions of the body. Research has not yet identified a standardized unit of measurement or an ideal tool to assess the relationship between TMJ and posture. Consideration of the patient’s clinical history and the duration of joint dysfunction is essential for proper evaluation.
A relationship exists between TMJ function and the cervical-hyoid-scapula tract.[22] Patients with TMJ dysfunction exhibit abnormal plantar support pressure compared with individuals without TMJ alterations.[23][24]
Degenerative diseases of the TMJ represent the 2nd most common musculoskeletal problem, causing pain and reduced movement. Aging constitutes a major factor in TMJ-related disorders. The geroscience hypothesis suggests that delaying age-related changes, such as cellular senescence of muscle-joint tissues and cytokine secretion, may help prevent or postpone the onset of TMJ dysfunction. Some authors propose that substances such as quercetin, an antioxidant, and dasatinib, an anticancer agent, may slow TMJ degeneration.[25] Recent studies indicate that patients with TMJ dysfunction display greater electrical activity in their masticatory muscles compared with subjects without TMJ disorders.[26]
Excessive driving represents a potential cause of impaired TMJ function. Longer driving durations increase the risk of developing temporomandibular disorders, although the underlying mechanisms remain unclear.[27] Neck pain and shoulder pain also constitute possible risk factors for joint dysfunction.[28]
Alterations in the arthrokinematics of the TMJ can contribute to vertigo and dizziness, potentially through trigeminal neural connections.[29] Joint dysfunctions may also be associated with low back pain, although the specific mechanisms have not been fully elucidated.[30]
TMJ dysfunction has been observed in patients with breast cancer-related lymphedema. The condition likely develops in the postoperative period, when patients adopt positions aimed at relieving pain.[31]
Media
(Click Image to Enlarge)
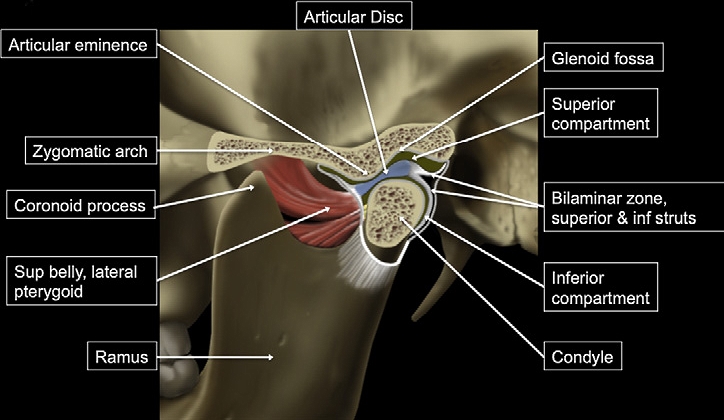
Temporomandibular Joint. This illustration shows the temporomandibular joint, highlighting key structures including the articular disc, glenoid fossa, superior and inferior compartments, condyle, articular eminence, bilaminar zone (superior and inferior struts), zygomatic arch, coronoid process, lateral pterygoid superior belly, and the mandibular ramus.
Contributed by Bruno Bordoni, PhD.
References
Bender ME, Lipin RB, Goudy SL. Development of the Pediatric Temporomandibular Joint. Oral and maxillofacial surgery clinics of North America. 2018 Feb:30(1):1-9. doi: 10.1016/j.coms.2017.09.002. Epub [PubMed PMID: 29153232]
Cuccia AM, Caradonna C, Caradonna D. Manual therapy of the mandibular accessory ligaments for the management of temporomandibular joint disorders. The Journal of the American Osteopathic Association. 2011 Feb:111(2):102-12 [PubMed PMID: 21357496]
Sencimen M, Yalçin B, Doğan N, Varol A, Okçu KM, Ozan H, Aydintuğ YS. Anatomical and functional aspects of ligaments between the malleus and the temporomandibular joint. International journal of oral and maxillofacial surgery. 2008 Oct:37(10):943-7. doi: 10.1016/j.ijom.2008.07.003. Epub 2008 Sep 2 [PubMed PMID: 18768297]
Mérida-Velasco JR, de la Cuadra-Blanco C, Pozo Kreilinger JJ, Mérida-Velasco JA. Histological study of the extratympanic portion of the discomallear ligament in adult humans: a functional hypothesis. Journal of anatomy. 2012 Jan:220(1):86-91. doi: 10.1111/j.1469-7580.2011.01447.x. Epub 2011 Nov 4 [PubMed PMID: 22050648]
Bravetti P, Membre H, El Haddioui A, Gérard H, Fyard JP, Mahler P, Gaudy JF. Histological study of the human temporo-mandibular joint and its surrounding muscles. Surgical and radiologic anatomy : SRA. 2004 Oct:26(5):371-8 [PubMed PMID: 15290103]
Sato I, Shindo K, Ezure H, Shimada K. Morphology of the lateral ligament in the human temporomandibular joint. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 1996 Feb:81(2):151-6 [PubMed PMID: 8665306]
Cuccia AM, Caradonna C, Caradonna D, Anastasi G, Milardi D, Favaloro A, De Pietro A, Angileri TM, Caradonna L, Cutroneo G. The arterial blood supply of the temporomandibular joint: an anatomical study and clinical implications. Imaging science in dentistry. 2013 Mar:43(1):37-44. doi: 10.5624/isd.2013.43.1.37. Epub 2013 Mar 11 [PubMed PMID: 23525363]
Asaki S, Sekikawa M, Kim YT. Sensory innervation of temporomandibular joint disk. Journal of orthopaedic surgery (Hong Kong). 2006 Apr:14(1):3-8 [PubMed PMID: 16598078]
Davidson JA, Metzinger SE, Tufaro AP, Dellon AL. Clinical implications of the innervation of the temporomandibular joint. The Journal of craniofacial surgery. 2003 Mar:14(2):235-9 [PubMed PMID: 12621296]
Bichir C, Rusu MC, Vrapciu AD, Măru N. The temporomandibular joint: pneumatic temporal cells open into the articular and extradural spaces. Folia morphologica. 2019:78(3):630-636. doi: 10.5603/FM.a2018.0111. Epub 2018 Dec 11 [PubMed PMID: 30536358]
Nadershah M, Mehra P. Orthognathic surgery in the presence of temporomandibular dysfunction: what happens next? Oral and maxillofacial surgery clinics of North America. 2015 Feb:27(1):11-26. doi: 10.1016/j.coms.2014.09.002. Epub [PubMed PMID: 25483441]
Valladares-Neto J, Cevidanes LH, Rocha WC, Almeida Gde A, Paiva JB, Rino-Neto J. TMJ response to mandibular advancement surgery: an overview of risk factors. Journal of applied oral science : revista FOB. 2014 Jan-Feb:22(1):2-14. doi: 10.1590/1678-775720130056. Epub [PubMed PMID: 24626243]
Level 3 (low-level) evidenceMurakami K, Hori S, Yamaguchi Y, Mercuri LG, Harayama N, Maruo S, Takahashi T. Synovial plicae and temporomandibular joint disorders: surgical findings. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2015 May:73(5):827-33. doi: 10.1016/j.joms.2014.12.018. Epub 2014 Dec 24 [PubMed PMID: 25795190]
Mehra P, Nadershah M, Chigurupati R. Is Alloplastic Temporomandibular Joint Reconstruction a Viable Option in the Surgical Management of Adult Patients With Idiopathic Condylar Resorption? Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2016 Oct:74(10):2044-54. doi: 10.1016/j.joms.2016.04.012. Epub 2016 Apr 23 [PubMed PMID: 27186870]
Chigurupati R, Mehra P. Surgical Management of Idiopathic Condylar Resorption: Orthognathic Surgery Versus Temporomandibular Total Joint Replacement. Oral and maxillofacial surgery clinics of North America. 2018 Aug:30(3):355-367. doi: 10.1016/j.coms.2018.05.004. Epub 2018 Jul 5 [PubMed PMID: 30008344]
Liu X, Zheng J, Cai X, Abdelrehem A, Yang C. Techniques of Yang's arthroscopic discopexy for temporomandibular joint rotational anterior disc displacement. International journal of oral and maxillofacial surgery. 2019 Jun:48(6):769-778. doi: 10.1016/j.ijom.2018.12.003. Epub 2018 Dec 26 [PubMed PMID: 30594477]
O'Connor RC, Fawthrop F, Salha R, Sidebottom AJ. Management of the temporomandibular joint in inflammatory arthritis: Involvement of surgical procedures. European journal of rheumatology. 2017 Jun:4(2):151-156. doi: 10.5152/eurjrheum.2016.035. Epub 2017 Feb 23 [PubMed PMID: 28638693]
Shaffer SM, Brismée JM, Sizer PS, Courtney CA. Temporomandibular disorders. Part 1: anatomy and examination/diagnosis. The Journal of manual & manipulative therapy. 2014 Feb:22(1):2-12. doi: 10.1179/2042618613Y.0000000060. Epub [PubMed PMID: 24976743]
Jung HD, Kim SY, Park HS, Jung YS. Orthognathic surgery and temporomandibular joint symptoms. Maxillofacial plastic and reconstructive surgery. 2015 Dec:37(1):14. doi: 10.1186/s40902-015-0014-4. Epub 2015 May 28 [PubMed PMID: 26029683]
Cuccia AM, Caradonna C, Annunziata V, Caradonna D. Osteopathic manual therapy versus conventional conservative therapy in the treatment of temporomandibular disorders: a randomized controlled trial. Journal of bodywork and movement therapies. 2010 Apr:14(2):179-84. doi: 10.1016/j.jbmt.2009.08.002. Epub 2009 Sep 20 [PubMed PMID: 20226365]
Level 1 (high-level) evidenceShaffer SM, Brismée JM, Sizer PS, Courtney CA. Temporomandibular disorders. Part 2: conservative management. The Journal of manual & manipulative therapy. 2014 Feb:22(1):13-23. doi: 10.1179/2042618613Y.0000000061. Epub [PubMed PMID: 24976744]
An JS, Jeon DM, Jung WS, Yang IH, Lim WH, Ahn SJ. Influence of temporomandibular joint disc displacement on craniocervical posture and hyoid bone position. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics. 2015 Jan:147(1):72-9. doi: 10.1016/j.ajodo.2014.09.015. Epub [PubMed PMID: 25533074]
Souza JA, Pasinato F, Corrêa EC, da Silva AM. Global body posture and plantar pressure distribution in individuals with and without temporomandibular disorder: a preliminary study. Journal of manipulative and physiological therapeutics. 2014 Jul-Aug:37(6):407-14. doi: 10.1016/j.jmpt.2014.04.003. Epub 2014 Aug 6 [PubMed PMID: 25108750]
Cuccia AM. Interrelationships between dental occlusion and plantar arch. Journal of bodywork and movement therapies. 2011 Apr:15(2):242-50. doi: 10.1016/j.jbmt.2010.10.007. Epub 2010 Dec 9 [PubMed PMID: 21419367]
Zhou Y, Xu M, Yadav S. Temporomandibular joint aging and potential therapies. Aging. 2021 Jul 15:13(14):17955-17956. doi: 10.18632/aging.203332. Epub 2021 Jul 15 [PubMed PMID: 34264857]
Xu L, Zhang L, Lu J, Fan S, Cai B, Dai K. Head and neck posture influences masticatory muscle electromyographic amplitude in healthy subjects and patients with temporomandibular disorder: a preliminary study. Annals of palliative medicine. 2021 Mar:10(3):2880-2888. doi: 10.21037/apm-20-1850. Epub 2021 Mar 3 [PubMed PMID: 33691457]
Zhu J, Yuan X, Zhang Y. The potential association between sedentary behaviors and risk of temporomandibular disorders: evidence from Mendelian randomization analysis. Journal of oral & facial pain and headache. 2024 Dec:38(4):91-100. doi: 10.22514/jofph.2024.042. Epub 2024 Dec 12 [PubMed PMID: 39800960]
Song L, Zhao M, Wang Y. Exploring the causal relationship between chronic pain and temporomandibular disorders: A two-sample Mendelian randomization study. Archives of oral biology. 2025 May:173():106191. doi: 10.1016/j.archoralbio.2025.106191. Epub 2025 Feb 14 [PubMed PMID: 39965291]
Alves IS, Gebrim EMS, Passos UL. Imaging of Vertigo and Dizziness: A Site-based Approach, Part 1 (Middle Ear, Bony Labyrinth, and Temporomandibular Joint). Seminars in ultrasound, CT, and MR. 2024 Oct:45(5):360-371. doi: 10.1053/j.sult.2024.09.003. Epub 2024 Oct 5 [PubMed PMID: 39374861]
Piva SR, Smith C, Anderst W, Bell KM, Darwin J, Delitto A, Flynn C, Greco CM, McKernan GP, Schneider MJ, Sowa GA, Sundaram M, Vo NV, Zhou L, Patterson CG. Demographic and Biomedical Characteristics of an Observational Cohort With Chronic Low Back Pain: A Descriptive Analysis. JOR spine. 2025 Sep:8(3):e70094. doi: 10.1002/jsp2.70094. Epub 2025 Jul 14 [PubMed PMID: 40662112]
Akbulut Bayrak A, Tekbudak MY, Gultekin S, Keser I. Investigation of temporomandibular dysfunction in patients with breast cancer-related lymphedema. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2025 May 7:33(6):455. doi: 10.1007/s00520-025-09500-y. Epub 2025 May 7 [PubMed PMID: 40332621]