Introduction
Longitudinal melanonychia encompasses several distinct conditions, including lentigo, nail matrix nevus, and subungual melanoma. Lentigo and nail matrix nevus represent benign lesions, while subungual melanoma is a malignant entity.[1] Subungual melanoma arises from melanocytes within the nail apparatus and represents a specific subtype of cutaneous malignant melanoma. Most commonly, this form corresponds to acral lentiginous melanoma, a variant that typically develops on the palms and soles of the feet. First recognized by Boyer in 1834, subungual melanoma was later described in more clinical detail by Hutchinson in 1886. Hutchinson identified a hallmark feature known as the "Hutchinson sign," characterized by pigmentation extending from the nail onto the adjacent skin, a finding strongly associated with subungual melanoma.[2][3][4]
Also referred to as nail unit melanoma, subungual melanoma accounts for up to 3% of all melanoma cases in individuals with lightly pigmented skin. Among populations with darkly pigmented skin, the percentage rises significantly, accounting for up to 30% of melanoma diagnoses.[14] In approximately 65% of cases, nail melanoma initially presents as a dark, vertical pigmented band affecting a single nail.[15] These bands often exceed 3 mm in width and display proximal widening along with irregular lateral borders. Nail plate dystrophy frequently accompanies the pigmentation, serving as an additional clinical clue to the diagnosis.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Malignant melanoma of the nail matrix originates from melanocytes, as with cutaneous melanoma. Unlike cutaneous malignant melanoma, subungual melanoma does not appear to be related to sun exposure.[5]
Epidemiology
Subungual melanomas represent a rare subset of malignant melanomas, accounting for approximately 0.7% to 3.5% of cases worldwide. Melanoma of the nail apparatus occurs with equal frequency across all racial groups; however, it stands as the most common subtype among African-American, Asian, and Hispanic populations. Among individuals of African descent, subungual melanoma accounts for 75% of melanoma cases, compared to 25% in Chinese populations and 10% in Japanese populations. These higher proportions reflect the lower incidence of cutaneous malignant melanoma in these groups, largely due to increased melanin pigmentation, which provides a protective barrier against ultraviolet (UV) radiation.
The condition tends to present most frequently in the seventh decade of life in men and the sixth decade in women. The digits most commonly affected include the great toe and the thumb, which together account for 75% to 90% of subungual melanoma cases. These digits likely face greater risk due to their higher exposure to trauma, which may contribute to the development of malignancy within the nail unit.[6][7]
Pathophysiology
Subungual melanoma develops due to increased melanin production by melanocytes. Once activated, these melanocytes differentiate into matrix cells and migrate distally within the nail unit. Compared to normal skin, the nail unit contains fewer melanocytes, and melanonychia most often involves the distal nail matrix. Melanocyte dormancy in the proximal matrix contributes to its lower likelihood of involvement in melanonychia. In contrast, nearly half of the melanocytes in the distal matrix retain the potential for activation. As a result, longitudinal melanonychia arises through 1 of 2 mechanisms: activation of dormant proximal melanocytes or proliferation of melanocytes in the distal nail matrix.[8][9]
Melanocytic activation may occur under several conditions. In individuals with darkly pigmented skin, physiological activation often occurs. Hormonal stimulation during pregnancy can also trigger this process. Additional causes include inflammatory or infectious nail disorders, eg, nail psoriasis or lichen planus, adverse effects from certain medications, repetitive frictional trauma, and systemic disorders like Laugier-Hunziker syndrome or Peutz-Jeghers syndrome. This type of activation—referred to as functional melanonychia, melanocytic stimulation, or hypermelanosis—leads to increased melanin production within the nail matrix epithelium without an accompanying rise in melanocyte numbers.
Melanocytic hyperplasia, or matrix melanocyte proliferation, represents a less common cause of longitudinal melanonychia and typically occurs in 3 specific contexts: nevus, lentigo, and melanoma. Benign melanocytic hyperplasia gives rise to nail matrix lentigines and nevi, while malignant forms include both in situ and invasive melanoma of the nail apparatus.[10]
Histopathology
Histopathological Features of Subungual Melanoma
The histopathological features of subungual melanoma differ from other melanocytic lesions due to the distinct anatomy of the nail unit. Pathologic specimens typically show an increased number of melanocytes within the basal layer, where pleomorphic melanocytes with irregular nuclei predominate. Invasive nail melanoma typically exhibits a melanocyte count 2 to 3 times higher than that observed in benign melanonychia. Additional distinguishing features include multinucleation, cellular atypia, and extensive pagetoid spread of melanocytes throughout the epidermis. Inflammatory infiltrates frequently appear in advanced melanoma, whereas benign melanonychia lacks this inflammatory response.
Most histologic specimens of subungual melanoma align with the pattern of acral lentiginous melanoma, which is found in approximately 67% of cases. This melanoma subtype also commonly affects the palms and soles of the feet. Approximately 50% of subungual melanoma cases exhibit features of superficial spreading, and some display characteristics of both acral lentiginous and superficial spreading patterns. Acral lentiginous melanoma is marked by acanthosis, elongation of the rete ridges, and proliferation of atypical melanocytes within the epidermis. Malignant melanocytes often replace the entire basal keratinocyte layer.
Histopathologic examination remains the gold standard for diagnosing longitudinal melanonychia. However, differentiating early-stage melanoma involving the nail matrix from benign melanocytic lesions can present considerable challenges. Cellular atypia, pagetoid spread, and melanocytic nest formation frequently occur not only in melanoma in situ but also in benign conditions, eg, lentigines and nevi.[11] To reach an accurate diagnosis of melanoma and distinguish it from these differentials, careful evaluation of key histologic indices becomes essential.
Melanocytic activation
Nonspecific melanin pigmentation of the matrix epithelium with identical melanocyte numbers represents the activation of melanocytes. Early-stage melanocytic activation might be diagnosed with Fontana-Masson staining. The main advantage of Fontana-Masson staining is in cases with limited visible pigmentation. That said, pigmented dendritic keratinocytes and sparse melanophages, without any evidence of atypia, are expected in the superficial dermis.[12]
Lentigo is characterized by a mild to moderate increase in the number of melanocytes in the matrix (approximately 30 per mm). The increased melanocytes are individually located and aligned sparsely in the basal layer.[13] Cytologic atypia could be mildly present. The pagetoid spread is not diffuse. The pigmentation is observed only in the lower third of the nail epithelium. However, full-thickness pigmentation has been reported in some circumstances. Fontana-Masson staining reveals the fine granularity of melanin.[12]
Nevus
A melanocytic nevus of the nail matrix is identified by melanocytic hyperplasia and nest formation.[13] Further histopathologic indexes of a melanocytic nevus include: a lentiginous pattern at the center of the nevus, suprabasal pagetoid spread tendency, mild nuclear pleomorphism, and periungual pigmentation.[14]
Melanoma in-situ
Characterized by an increased number of melanocytes in the basal cell layer (on average 90 per mm), with a single melanocyte, a few nests, nuclear atypia, and pagetoid spreading. Atypical melanocytes, which are melanoma markers in situ, have large, hyperchromatic, and pleomorphic nuclei, as well as branching dendrites.[6]
History and Physical
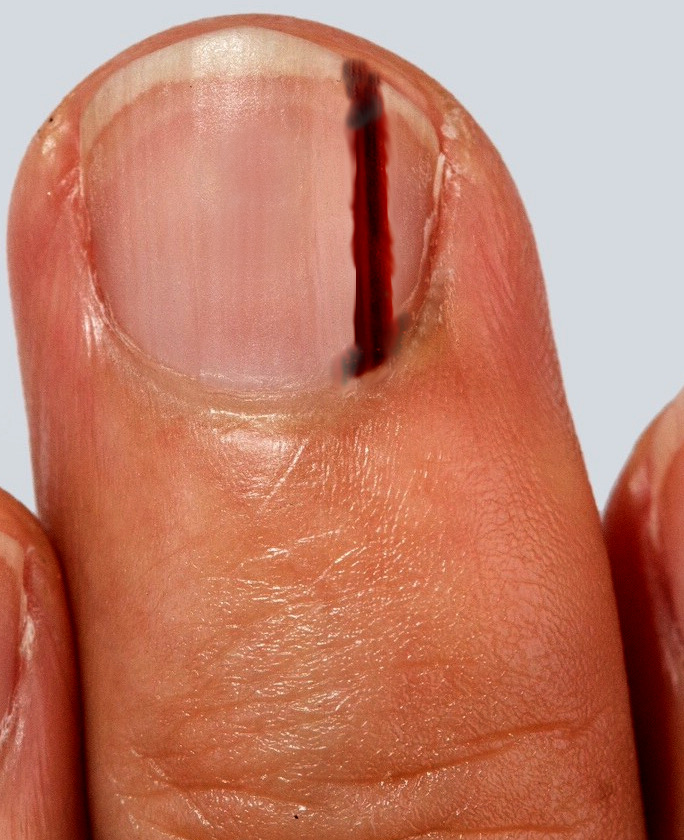
Subungual melanoma typically presents as brown to black discoloration of the nail bed, which may appear as a linear streak or an area of irregular pigmentation (see Image. Subungual Melanoma). As the condition progresses, the affected nail may undergo thickening, splitting, or destruction, often accompanied by pain and inflammation. The presence of periungual pigmentation—known as the Hutchinson sign—provides strong support for the clinical diagnosis of melanoma.
The Hutchinson sign refers to pigment extension onto the proximal or lateral nail folds.[9][15] Historically regarded as pathognomonic for subungual melanoma, this sign also appears in other conditions. Approximately 30% of patients with nail lentigines, nevi, or melanonychia associated with darkly pigmented skin, Laugier-Hunziker syndrome, or Peutz-Jeghers syndrome may exhibit similar periungual discoloration.[16][17] In such cases, the pigmentation is termed a "pseudo-Hutchinson sign" to distinguish it from the true indicator of melanoma.[18][19]
A thorough patient history should address recent trauma to rule out subungual hematoma and assess for infections that could suggest pigmented fungal nail disease. Levit and colleagues introduced the ABCDEF guidelines to assist in identifying nail lesions that may indicate melanoma. These criteria include:
- Age and ancestry: 50 to 70 years of age and African, Japanese, Chinese, and Native American heritage
- Brown-black band >3 mm with an irregular border
- Change in size and growth rate
- High-risk digits: thumb, big toe, or index finger
- Extension of discoloration into the skin surrounding the nail (Hutchinson sign)
- Family history of melanoma
Evaluation
The primary method for making a diagnosis is through a full-thickness biopsy of the nail bed. A recent study by Reilly et al, published in December 2017, demonstrated a significant disparity between the tumor thickness on initial biopsy and the final tumor thickness. This point emphasizes the importance of obtaining a high-quality biopsy to facilitate the initial histological assessment, followed by a management plan.
A nail bed biopsy should be performed using a digital nerve block. A tourniquet is useful for hemostasis, but the digit should not be exsanguinated in malignancy. The nail plate should be carefully removed using a periosteal elevator. To visualize the germinal matrix, 2 radial incisions can be made at a 45-degree angle on either side of the eponychial fold. A full-thickness biopsy should then be taken of the pigmented area. Ideally, this should be an excisional biopsy if possible. The defect is then repaired using an absorbable suture, and the nail is replaced in the eponychial fold.
Following the excisional biopsy of the pigmented lesion in the nail bed, a further management plan can be made based on the histological report. The report will detail the subtype of malignant melanoma, the Breslow thickness, and other histological characteristics.[15][16]
Treatment / Management
The interprofessional melanoma team will use this information to determine what further investigations are necessary and to determine the appropriate treatment. The mainstay of management of melanoma of any kind is excision. The initial excision is a biopsy, which provides a histological report, and guidelines determine the wide local excision margin based on the depth of the tumor. This is the same for subungual melanoma; however, to achieve the recommended clinical margins, an amputation is often necessary. A conservative digit amputation tailored to tumor depth (eg, distal phalanx versus ray amputation) to achieve clear margins while preserving function is the standard of care.[4][17][20]
The lymph node basins should be clinically assessed, and those with clinically palpable nodes should undergo further evaluation with imaging, possibly accompanied by a biopsy. Subsequently, a lymph node dissection should be performed if the disease is detected. Based on guidelines, lesions with a thickness greater than 0.8 mm and no lymphadenopathy should be considered for sentinel lymph node biopsy.
Differential Diagnosis
The 2 most common misdiagnoses of subungual melanoma are striate melanonychia and onychomycosis. Striate melanonychia is the deposition of melanin in the nail plate and is typically not a solitary pigmented streak. Onychomycosis is a fungal nail infection producing a painless, dystrophic pigment change in the nail. Other differential diagnoses include a subungual hematoma, which is usually a painful lesion that presents following trauma and migrates distally as the nail grows, and a junctional naevus, a single benign lesion not in a streaked pattern. Subungual hematoma and nail bed infections should not be ignored while evaluating a patient with suspicious subungual melanoma.
Surgical Oncology
Surgical management of subungual melanoma has remained a subject of controversy over the past decade. For many years, digital amputation represented the standard approach. In 1965, Das Gupta and colleagues conducted a study involving 34 patients to evaluate outcomes following amputation at the interphalangeal joint compared to more proximal levels. The study reported that none of the patients who underwent amputation at the interphalangeal joint survived beyond 5 years.
Based on these findings, the prevailing surgical philosophy shifted toward more aggressive interventions, favoring proximal amputation at the level of the carpometacarpal or tarsometatarsal joint. This approach, however, can lead to substantial functional impairment, particularly when the thumb or great toe is involved, as these digits play critical roles in hand and foot mechanics. Although the study highlighted important survival outcomes, it lacked details regarding tumor depth, and half of the patients presented with either metastatic or recurrent disease. These limitations suggest that the conclusions may not fully reflect the spectrum of disease severity or the potential effectiveness of less radical surgical approaches.[21][22][23]
More recently, this aggressive treatment has become less acceptable, leading to the investigation of less radical surgical options to preserve length and obtain surgical clearance. Cochran et al published a literature review in 2014, specifically examining the management options for subungual melanoma. This review concluded that in situ excision surgery for lower-grade melanomas is a reasonable option. This translates as excision down to the periosteum or paratenon. Other deeper, higher-grade melanomas could undergo amputation at a more distal level (interphalangeal joint) than advised by Das Gupta. However, despite the encouraging evidence that surgeons are considering less aggressive options, the data quality is weak, with no randomized controlled trials and mostly retrospective data collection. The surgical excision method remains a topic of controversy and should be evaluated on a case-by-case basis.
Recent years have seen a shift in clinical thinking, with an increasing interest in function-preserving surgeries that aim to achieve oncologic control while minimizing unnecessary morbidity. In 2014, Cochran et al published a literature review analyzing surgical options for subungual melanoma. The review supported in situ excision for lower-grade melanomas, recommending resection down to the periosteum or paratenon. For deeper or higher-grade tumors, amputation at a more distal level, eg, the interphalangeal joint, may provide adequate surgical margins while preserving more function than the previously favored proximal amputations. Although this more conservative trend in treatment appears promising, the evidence remains limited by low-quality data, predominantly from retrospective studies. The absence of randomized controlled trials continues to hinder the formulation of definitive recommendations, and surgical decisions must be made on an individual basis, taking into account tumor characteristics, functional considerations, and patient preferences.
The following guidelines should help to determine the excision of subungual melanoma, but may need to be adjusted to allow the most functional outcome for the patient:
- In situ: excision depth of 0.5 cm to 1 cm
- Invasive melanoma <1 mm Breslow thickness: excision depth of 1 cm
- Depth of 1 to 2 mm: excision depth of 1 cm to 2 cm
- Greater than 2 mm: excision depth of 2 cm
Those with clinically palpable nodes should be offered a lymph node dissection. A sentinel lymph node biopsy should be offered to all patients with melanoma with depth ≥0.8 mm. For with a node-positive for micrometastasis should be offered a completion lymph node dissection.
Radiation Oncology
Radiotherapy plays a limited role in the treatment of melanoma and should be reserved for carefully selected clinical situations. The use of radiotherapy may follow lymphadenectomy when bulky lymphadenopathy or extracapsular disease involves the lymph node basin, thereby helping to reduce the risk of local recurrence. In such cases, radiotherapy serves as an adjuvant therapy to manage residual microscopic disease.
Cerebral metastases also represent a key indication for radiotherapy in melanoma. Treatment of brain metastases often includes stereotactic radiosurgery or whole-brain radiotherapy, depending on the number, size, and location of lesions. In these settings, radiotherapy aims to improve neurological outcomes, reduce symptoms, and enhance quality of life.
Medical Oncology
Subungual melanoma, a rare variant of acral lentiginous melanoma, has been underrepresented in prospective immunotherapy trials due to its low incidence. However, retrospective studies and subgroup analyses have included patients with subungual disease, particularly within broader cohorts of acral melanoma. These analyses suggest that subungual melanoma generally demonstrates a lower response rate to checkpoint inhibitors compared to cutaneous melanoma, likely due to a lower tumor mutational burden and distinct molecular profiles.
Single-agent anti–PD-1 therapies, eg, nivolumab and pembrolizumab, have shown limited efficacy, whereas combination regimens, like nivolumab plus ipilimumab, have demonstrated higher response rates in acral melanoma overall; however, subungual-specific outcomes remain poorly defined.[24][25] Continued inclusion of subungual melanoma patients in prospective trials, along with the development of dedicated registries, is essential to clarify the role of immunotherapy in this population.
Staging
Subungual melanoma is staged using the American Joint Committee on Cancer (AJCC) staging and classification system. The Tumour Node Metastasis (TNM) staging system is used in a similar manner to other malignant melanomas.
Prognosis
Several studies have reported a poorer prognosis for subungual melanoma compared to cutaneous melanoma. This difference likely reflects delays in diagnosis, as subungual lesions often present later due to their concealed location and resemblance to benign conditions. When diagnosed at comparable stages, the prognosis for subungual melanoma aligns closely with that of other cutaneous malignant melanomas.
The American Cancer Society has published the following updated prognostic statistics based on disease staging:
- Stage IA: The 5-year survival rate reaches approximately 97%, with a 10-year survival rate of nearly 95%.
- Stage IB: This stage carries a 5-year survival rate of about 92% and a 10-year rate of roughly 86%.
- Stage IIA: Survival drops to 81% at 5 years and 67% at 10 years.
- Stage IIB: This stage shows further decline, with survival rates of 70% at 5 years and 57% at 10 years.
- Stage IIC: Survival rates fall to 53% at 5 years and 40% at 10 years.
- Stage IIIA: The 5-year survival rate is approximately 78%, with a 10-year survival rate of around 68%.
- Stage IIIB: The 5-year survival rate is around 59%; the 10-year survival is approximately 43%.
- Stage IIIC: The 5-year survival rate is around 40%. The 10-year survival is approximately 24%.
- Stage IV: Melanoma at this stage carries the lowest survival rates, with 5-year survival rates ranging from 15% to 20% and 10-year survival rates between 10% and 15%. Outcomes improve when metastasis is limited to distant skin or lymph nodes, and when lactate dehydrogenase (LDH) levels remain within normal limits.
Complications
Subungual melanoma can lead to significant complications, largely due to delays in diagnosis and treatment. Often mistaken for benign nail conditions, this rare melanoma subtype frequently presents at a more advanced stage, increasing the risk of local tissue invasion, nodal involvement, and distant metastasis. Complications may include functional impairment of the affected digit, chronic pain, cosmetic nail deformity (universally seen in all patients), and, in severe cases, amputation.
Advanced disease may result in lymphatic spread or systemic involvement, contributing to lower survival rates. Prognosis tends to mirror that of cutaneous melanoma when detected early, but delayed recognition commonly leads to poorer outcomes and more aggressive disease progression. Collaboration with plastic or hand surgeons may further optimize cosmetic outcomes, particularly when complex nail bed reconstruction or flap coverage is needed.
Deterrence and Patient Education
Patients should be counseled that subungual melanoma is not associated with UV exposure, unlike cutaneous melanomas. Instead, its pathogenesis is thought to be related to mechanical trauma, genetic mutations, or other unknown factors. While specific preventive strategies remain limited, maintaining good hand and foot hygiene may offer general benefits. Patients should be advised to perform regular self-examinations of the nails and nail beds to monitor for signs of recurrence or new pigmented lesions, and promptly report any concerning changes to a healthcare practitioner.
Pearls and Other Issues
For clinicians, always removing nail polish in applicable patients before dermatological examination is essential to perform a thorough evaluation.
Enhancing Healthcare Team Outcomes
Effective management of subungual melanoma relies on a cohesive interprofessional approach that prioritizes early detection, accurate diagnosis, and timely treatment. Physicians, advanced practitioners, and nurses must remain vigilant during routine physical exams, particularly in populations at higher risk, including individuals of Black, Hispanic, and Asian descent. Because subungual melanoma typically arises in non-sun-exposed areas, such as the nail beds, and lacks the UV-related associations of other melanomas, clinical suspicion must be guided by subtle signs, including longitudinal melanonychia, nail dystrophy, and periungual pigmentation. Nurses and primary care clinicians serve on the front lines of screening, ensuring nail assessments become a standard part of routine care and initiating prompt referrals to dermatology when necessary. Dermatologists play a key role in confirming the diagnosis through biopsy, while providing guidance on subsequent staging and treatment plans.
Following diagnosis, care coordination involves seamless communication between dermatologists, surgical specialists, and oncologists to determine the appropriate extent of excision or amputation, assess lymph node involvement, and plan long-term follow-up. Pharmacists support safe medication use when systemic therapy is indicated and can reinforce patient education to ensure effective treatment outcomes. Across all phases of care, nurses help patients understand their condition, manage anxiety, and recognize early signs of recurrence. Interprofessional collaboration—centered on clear communication, mutual accountability, and coordinated roles—ensures that patients receive timely, equitable, and patient-centered care. This collaborative effort ultimately strengthens diagnostic accuracy, reduces delays, improves survival rates, and enhances the overall quality of life for patients with subungual melanoma.[26][27][28][29]
Media
(Click Image to Enlarge)
References
Haneke E. Important malignant and new nail tumors. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2017 Apr:15(4):367-386. doi: 10.1111/ddg.13223. Epub [PubMed PMID: 28378483]
Nunes LF, Quintella Mendes GL, Koifman RJ. Acral melanoma: a retrospective cohort from the Brazilian National Cancer Institute (INCA). Melanoma research. 2018 Oct:28(5):458-464. doi: 10.1097/CMR.0000000000000476. Epub [PubMed PMID: 30020197]
Level 2 (mid-level) evidenceOzdemir F, Errico MA, Yaman B, Karaarslan I. Acral lentiginous melanoma in the Turkish population and a new dermoscopic clue for the diagnosis. Dermatology practical & conceptual. 2018 Apr:8(2):140-148. doi: 10.5826/dpc.0802a14. Epub 2018 Apr 30 [PubMed PMID: 29785333]
Nakamura Y. [II. Diagnosis and Treatment for Nail Apparatus(Subungual)Melanoma]. Gan to kagaku ryoho. Cancer & chemotherapy. 2018 Apr:45(4):619-621 [PubMed PMID: 29650816]
Boespflug A, Debarbieux S, Depaepe L, Chouvet B, Maucort-Boulch D, Dalle S, Balme B, Thomas L. Association of subungual melanoma and subungual squamous cell carcinoma: A case series. Journal of the American Academy of Dermatology. 2018 Apr:78(4):760-768. doi: 10.1016/j.jaad.2017.09.038. Epub 2017 Sep 22 [PubMed PMID: 28947295]
Level 2 (mid-level) evidenceDecker A, Connolly KL, Lee EH, Busam KJ, Nehal KS. Frequency of Subungual Melanoma in Longitudinal Melanonychia: A Single-Center Experience. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2017 Jun:43(6):798-804. doi: 10.1097/DSS.0000000000001112. Epub [PubMed PMID: 28296790]
Ingkaninanda P, Visessiri Y, Rutnin S. Clinicopathological Features and Prognostic Factors of Malignant Melanoma: A Retrospective Analysis of Thai Patients in Ramathibodi Hospital. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2015 Aug:98(8):820-7 [PubMed PMID: 26437541]
Level 2 (mid-level) evidenceLee DK, Lipner SR. Optimal diagnosis and management of common nail disorders. Annals of medicine. 2022 Dec:54(1):694-712. doi: 10.1080/07853890.2022.2044511. Epub [PubMed PMID: 35238267]
Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatologic clinics. 2015 Apr:33(2):185-95. doi: 10.1016/j.det.2014.12.002. Epub 2015 Feb 7 [PubMed PMID: 25828711]
Varas-Meis E, Delgado-Vicente S, Fernández-Canga P, Rodríguez Prieto MA. Blaschkoid hypermelanosis in a patient with ring 18 chromosome. Indian journal of dermatology, venereology and leprology. 2020 May-Jun:86(3):316-318. doi: 10.4103/ijdvl.IJDVL_282_18. Epub [PubMed PMID: 32209754]
Alessandrini A, Dika E, Starace M, Chessa MA, Piraccini BM. Diagnosis of Melanonychia. Dermatologic clinics. 2021 Apr:39(2):255-267. doi: 10.1016/j.det.2020.12.004. Epub 2021 Feb 10 [PubMed PMID: 33745638]
André J, Sass U, Richert B, Theunis A. Nail pathology. Clinics in dermatology. 2013 Sep-Oct:31(5):526-39. doi: 10.1016/j.clindermatol.2013.06.005. Epub [PubMed PMID: 24079581]
Göktay F, Güldiken G, Altan Ferhatoğlu Z, Güneş P, Atış G, Haneke E. The role of dermoscopy in the diagnosis of subungual glomus tumors. International journal of dermatology. 2022 Jul:61(7):826-832. doi: 10.1111/ijd.16042. Epub 2022 Jan 24 [PubMed PMID: 35073425]
Güneş P, Göktay F, Haneke E. A case of adult-onset longitudinal melanonychia due to nail matrix compound nevus. Journal of cutaneous pathology. 2020 Dec:47(12):1159-1163. doi: 10.1111/cup.13800. Epub 2020 Sep 10 [PubMed PMID: 32640104]
Level 3 (low-level) evidenceTalavera-Belmonte A, Bonfill-Ortí M, Martínez-Molina L, Fornons-Servent R, Bauer-Alonso A, Ferreres-Riera JR, Marcoval J. Subungual Melanoma: A Descriptive Study of 34 Patients. Actas dermo-sifiliograficas. 2018 Nov:109(9):801-806. doi: 10.1016/j.ad.2018.06.010. Epub 2018 Aug 3 [PubMed PMID: 30082026]
Ohn J, Jo G, Cho Y, Sheu SL, Cho KH, Mun JH. Assessment of a Predictive Scoring Model for Dermoscopy of Subungual Melanoma In Situ. JAMA dermatology. 2018 Aug 1:154(8):890-896. doi: 10.1001/jamadermatol.2018.1372. Epub [PubMed PMID: 29926108]
Wollina U, Nenoff P, Haroske G, Haenssle HA. The Diagnosis and Treatment of Nail Disorders. Deutsches Arzteblatt international. 2016 Jul 25:113(29-30):509-18. doi: 10.3238/arztebl.2016.0509. Epub [PubMed PMID: 27545710]
Daneshbod Y, Akrami M, Fanaee S, Baboli KM, Mirfazaelian H. Pigmented lesion on nail bed: Pseudo-Hutchinson sign. Cleveland Clinic journal of medicine. 2022 Jun 1:89(6):303-304. doi: 10.3949/ccjm.89a.21070. Epub 2022 Jun 1 [PubMed PMID: 35649562]
Baran LR, Ruben BS, Kechijian P, Thomas L. Non-melanoma Hutchinson's sign: a reappraisal of this important, remarkable melanoma simulant. Journal of the European Academy of Dermatology and Venereology : JEADV. 2018 Mar:32(3):495-501. doi: 10.1111/jdv.14715. Epub 2018 Jan 29 [PubMed PMID: 29178539]
Sinno S, Wilson S, Billig J, Shapiro R, Choi M. Primary melanoma of the hand: An algorithmic approach to surgical management. Journal of plastic surgery and hand surgery. 2015:49(6):339-45. doi: 10.3109/2000656X.2015.1053396. Epub 2015 Jun 7 [PubMed PMID: 26051472]
Cochran AM, Buchanan PJ, Bueno RA Jr, Neumeister MW. Subungual melanoma: a review of current treatment. Plastic and reconstructive surgery. 2014 Aug:134(2):259-273. doi: 10.1097/PRS.0000000000000529. Epub [PubMed PMID: 25068326]
Tan KB, Moncrieff M, Thompson JF, McCarthy SW, Shaw HM, Quinn MJ, Li LX, Crotty KA, Stretch JR, Scolyer RA. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. The American journal of surgical pathology. 2007 Dec:31(12):1902-12 [PubMed PMID: 18043047]
Level 3 (low-level) evidenceLevit EK, Kagen MH, Scher RK, Grossman M, Altman E. The ABC rule for clinical detection of subungual melanoma. Journal of the American Academy of Dermatology. 2000 Feb:42(2 Pt 1):269-74. doi: 10.1016/S0190-9622(00)90137-3. Epub [PubMed PMID: 10642684]
Level 3 (low-level) evidenceJohnson DB, Peng C, Abramson RG, Ye F, Zhao S, Wolchok JD, Sosman JA, Carvajal RD, Ariyan CE. Clinical Activity of Ipilimumab in Acral Melanoma: A Retrospective Review. The oncologist. 2015 Jun:20(6):648-52. doi: 10.1634/theoncologist.2014-0468. Epub 2015 May 11 [PubMed PMID: 25964307]
Level 2 (mid-level) evidenceNakamura Y, Namikawa K, Kiniwa Y, Kato H, Yamasaki O, Yoshikawa S, Maekawa T, Matsushita S, Takenouchi T, Inozume T, Nakai Y, Fukushima S, Saito S, Otsuka A, Fujimoto N, Isei T, Baba N, Matsuya T, Tanaka R, Kaneko T, Onishi M, Kuwatsuka Y, Nagase K, Onuma T, Nomura M, Umeda Y, Yamazaki N. Efficacy comparison between anti-PD-1 antibody monotherapy and anti-PD-1 plus anti-CTLA-4 combination therapy as first-line immunotherapy for advanced acral melanoma: A retrospective, multicenter study of 254 Japanese patients. European journal of cancer (Oxford, England : 1990). 2022 Nov:176():78-87. doi: 10.1016/j.ejca.2022.08.030. Epub 2022 Oct 1 [PubMed PMID: 36194906]
Level 2 (mid-level) evidenceChamberlain A, Ng J. Cutaneous melanoma--atypical variants and presentations. Australian family physician. 2009 Jul:38(7):476-82 [PubMed PMID: 19575065]
Pavri SN, Clune J, Ariyan S, Narayan D. Malignant Melanoma: Beyond the Basics. Plastic and reconstructive surgery. 2016 Aug:138(2):330e-340e. doi: 10.1097/PRS.0000000000002367. Epub [PubMed PMID: 27465194]
Möhrle M, Lichte V, Breuninger H. [Operative therapy of acral melanomas]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. 2011 May:62(5):362-7. doi: 10.1007/s00105-010-2084-7. Epub [PubMed PMID: 21468730]
Sureda N, Phan A, Poulalhon N, Balme B, Dalle S, Thomas L. Conservative surgical management of subungual (matrix derived) melanoma: report of seven cases and literature review. The British journal of dermatology. 2011 Oct:165(4):852-8. doi: 10.1111/j.1365-2133.2011.10477.x. Epub 2011 Aug 4 [PubMed PMID: 21812768]
Level 3 (low-level) evidence