Introduction
Cerebrospinal fluid (CSF) is contained within the brain ventricles and the subarachnoid spaces of the cranium and spine (see Image. Cerebrospinal Fluid Distribution). Essential physiological functions include nutrient delivery, waste clearance, and mechanical protection of the brain.[1] In adults, total CSF volume measures approximately 150 ml—about 125 ml within the subarachnoid spaces and 25 ml within the ventricles. Older individuals may have volumes approaching 350 ml due to cerebral atrophy.[2]
The choroid plexus accounts for most CSF production, with minor contributions from less well-defined sources. Daily secretion ranges from 400 to 600 ml in adults, supporting complete CSF renewal 4 to 5 times every 24 hours in young adults. Decreased turnover may promote metabolite accumulation observed in aging and neurodegenerative disease. The composition of CSF is tightly regulated. Any deviation may carry diagnostic significance.[3]
Cellular Level
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Cellular Level
Around 80% of CSF production occurs via a network of modified ependymal cells known as the choroid plexus. This structure consists of a highly specialized, simple cuboidal epithelium continuous with the ependymal lining of the cerebral ventricles. The epithelium encloses clusters of fenestrated capillaries, enabling plasma filtration.[4] Apical surfaces of choroid plexus cells contain dense microvilli. Tight junctions between these cells form the blood-CSF barrier, which regulates CSF composition (see Image. Blood-CSF Barrier at the Choroid Plexus).
Lack of a functional barrier between CSF and the extracellular space of the brain places further importance on the blood-CSF barrier in maintaining brain homeostasis. Larger molecules, including cells, proteins, and glucose, are excluded, whereas ions and small solutes such as vitamins and nutrients pass more readily into CSF.[5] Water crosses the choroid plexus epithelium through apical aquaporin 1 channels. Substances unable to cross passively but essential to brain function may be actively synthesized or transported by epithelial cells into CSF. A lumen-positive voltage potential of approximately 5 mV exists across the choroid plexus epithelium. This electrochemical gradient draws sodium, chloride, and bicarbonate ions from plasma into CSF, generating an osmotic gradient that promotes water movement into the ventricular system.
Compared to plasma, CSF contains higher concentrations of sodium, chloride, and magnesium but lower concentrations of potassium and calcium. Only trace amounts of cells, protein, and immunoglobulins are present in CSF, in contrast to plasma. No cellular elements pass directly through the blood-CSF barrier, although small numbers of white blood cells may enter CSF via indirect mechanisms. Normal CSF cell counts are typically below 5 cells/ml. Despite fluctuations in blood composition and cerebral perfusion, CSF composition remains stable, preserving a consistent intraventricular environment essential for normal neuronal function (see Image. Brain Ventricular System and Surface Anatomy).
Function
CSF supports the brain by providing protection, metabolic exchange, and waste clearance. Hydromechanical protection of the neuroaxis occurs through 2 primary mechanisms. First, CSF functions as a shock absorber, cushioning the brain against the rigid confines of the skull. Second, CSF permits buoyancy of the brain and spinal cord, reducing the effective weight of the brain from approximately 1,500 grams to 50 grams. This reduction in weight minimizes mechanical stress on cerebral parenchyma and intracranial vessels during traumatic impact. Additional physiological roles include maintaining homeostasis of the interstitial fluid surrounding brain tissue. Stable extracellular conditions are essential for preserving normal neuronal activity.
Glymphatic System
The brain contains a fluid clearance network resembling lymphatic vessels, termed the "glymphatic system," first described in 2012.[6] This system comprises perivascular spaces surrounding cerebral blood vessels. Specialized astrocytic processes, known as endfeet, envelop these vessels (see Image. Glymphatic System).
CSF enters the brain along periarterial spaces, propelled by arterial pulsations. Astrocyte endfeet express high levels of aquaporin 4 water channels, which facilitate fluid transfer from periarterial spaces into the extracellular compartment of brain parenchyma. Fluid containing metabolic waste products exits along perivenous pathways, draining toward meningeal lymphatic vessels and cervical lymph nodes.
Glymphatic transport peaks during nonrapid eye movement sleep and declines during wakefulness. Reduced activity during wakefulness limits neurotransmitter diffusion and preserves synaptic precision. Primary functions of the glymphatic system include clearance of metabolic byproducts and regulation of extracellular fluid volume.[7]
Convective glymphatic flow transports soluble proteins and metabolic waste from the interstitial compartment to meningeal lymphatics and cervical nodes. This transport system complements capillary nutrient delivery by distributing solutes that lack specific blood-brain barrier (BBB) transporters. Additional proposed roles include redistribution of neurotransmitters and neurohormones beyond their release sites, as well as brain-wide dissemination of peptides and hormones introduced into CSF.
Mechanism
CSF is continuously secreted with a stable and tightly regulated composition, preserving homeostasis within the intracranial environment. Propulsion of CSF along the neuroaxis, from sites of secretion to sites of absorption, occurs primarily through the rhythmic systolic pulse wave transmitted via the choroidal arteries. Lesser determinants of CSF flow include respiratory frequency, body posture, jugular venous pressure, physical exertion, and circadian variation.
Primary secretion sites are the choroid plexuses located within the cerebral ventricles, with the lateral ventricles serving as the dominant contributors. The classical model attributes 80% of CSF production to the choroid plexus and 20% to the BBB endothelium. Revised models describe additional extrachoroidal sources. These mechanisms include transendothelial influx of fluid across the BBB driven by ion and solute cotransporters that create osmotic gradients. The extensive capillary surface area within brain parenchyma supports significant extracellular fluid entry, some of which drains into the ventricular system. Arterioles within the subarachnoid space express aquaporin water channels and sodium-potassium-chloride cotransporter 1, suggesting a role for localized CSF secretion beyond classical ventricular sources.
CSF flows through the ventricular system in a unidirectional, rostral-to-caudal manner. Flow begins in the lateral ventricles, proceeds through the interventricular foramina into the 3rd ventricle, continues through the cerebral aqueduct into the 4th ventricle, and exits via the median aperture (foramen of Magendie) and the paired lateral foramina of Luschka into the subarachnoid space at the base of the brain.
The subarachnoid space, located between the arachnoid membrane and pia mater, contains CSF external to the ventricular system. The arachnoid membrane adheres to the dura mater, forming the outer boundary of the subarachnoid space. This membrane consists of fibroblasts connected by tight junctions composed of claudin 11, which function to seal the CSF compartment. Arachnoid trabeculae extend into the subarachnoid space and merge with the underlying pia mater. The pia mater is a single-cell layer closely applied to the surfaces of brain parenchyma and astrocytic processes.
Once within the subarachnoid space, CSF exhibits gentle multidirectional flow that promotes equilibration of composition throughout the compartment. While circulating over the cerebral convexities and along the spinal cord, CSF maintains continuous exchange across interstitial and perivascular interfaces.
The classical theory holds that CSF absorption occurs via arachnoid villi and granulations—protrusions of arachnoid mater that extend through the dura mater into the lumen of a venous sinus or lacuna (see Image. Cerebrospinal Fluid Circulation). Arachnoid villi represent smaller structures, while granulations are larger invaginations. A pressure gradient of 3 to 5 mm Hg between the subarachnoid space and venous sinuses drives CSF into the venous system through these structures, facilitating absorption.
However, contemporary models no longer recognize this route as the principal mechanism of CSF egress. Dorsal and basal dural lymphatic vessels now appear to serve as major outflow pathways, draining CSF to cervical lymph nodes. Additional clearance routes include perineural pathways along cranial nerves, particularly through the nasal cribriform plate, and spinal nerves. CSF also traverses the adventitia of basal cerebral arteries to reach perivascular lymphatic vessels. Further elimination occurs through spinal nerve root sheaths, draining into paravertebral lymphatics and the epidural venous plexus.[8] CSF clearance is influenced by individual posture, local pressure differentials, and underlying pathophysiology.
Related Testing
Lumbar puncture, also referred to as spinal tap, is a commonly performed invasive procedure in which CSF is withdrawn from the subarachnoid space. This procedure is used to measure intracranial pressure and to obtain CSF samples for diagnostic evaluation. Frequent indications include the assessment of acute headache and suspected infections of the central nervous system.
Patients are typically positioned either in the lateral recumbent or upright sitting posture. A sterile spinal needle is inserted slowly between the lumbar vertebrae, most often between the L3-L4 or L4-L5 interspaces, until the subarachnoid space is entered. Needle placement may be guided by fluoroscopy or ultrasound to improve procedural success and reduce the risk of trauma.
CSF is collected sequentially once it begins to flow into 4 sterile tubes. Laboratory analysis of CSF can identify abnormal constituents that aid in diagnosis. For example, xanthochromia, a yellow-orange discoloration caused by red blood cell breakdown, suggests subarachnoid hemorrhage (SAH). Elevated concentrations of immunoglobulins, referred to as oligoclonal bands, may indicate systemic infection or autoimmune disease.
Normal CSF parameters are as follows:
- Color: Clear
- Opening pressure: 8 to 15 mm Hg
- Glucose concentration: 50 to 80 mg/dL, or approximately 2/3 of the concurrent blood glucose level
- Protein concentration: 15 to 45 mg/dL
- Mononuclear cell count: 0 to 5 cells/mm3
Absolute contraindications to lumbar puncture include the presence of an intracranial space-occupying lesion, coagulopathy or other bleeding disorders, and local infection at the intended puncture site. Lumbar puncture is generally safe when performed with appropriate precautions. Although serious complications are rare, potential risks include infection, hemorrhage, radicular pain, and cerebral herniation. The most common complication is postlumbar puncture headache, which typically begins within 24 hours and resolves spontaneously by the 10th day in most cases.[9][10]
Clinical Significance
Hydrocephalus
Hydrocephalus is a pathological condition characterized by abnormal accumulation of CSF due to increased production, impaired flow, or decreased absorption. Ventricular enlargement occurs in response to elevated CSF volume and may result in structural damage to brain parenchyma. This condition may be congenital or acquired.
Obstruction within the ventricular system is known as noncommunicating, or obstructive, hydrocephalus. The most common cause is a space-occupying lesion, such as a tumor or abscess, blocking a foramen. Because CSF secretion is continuous, obstruction results in accumulation proximal to the blockage.
Stenosis of the cerebral aqueduct, one of the most frequent causes of obstructive hydrocephalus, leads to enlargement of both lateral ventricles and the 3rd ventricle. The condition is classified as communicating, or nonobstructive, hydrocephalus when CSF flow is obstructed beyond the ventricular system, either within the subarachnoid space or at the site of absorption.
Hydrocephalus may result from genetic abnormalities, intracranial infection, intraventricular hemorrhage, traumatic injury, or central nervous system neoplasm. Clinical manifestations include headache, seizure, nausea, vomiting, visual disturbances, and progressive cognitive decline.
Diagnosis is established through imaging modalities such as ultrasound, computed tomographic (CT) scan, or magnetic resonance imaging (MRI). The most frequently employed intervention is surgical shunt placement to divert CSF from the ventricles to another region of the body for reabsorption into the systemic circulation.
Alternative treatments include endoscopic 3rd ventriculostomy, a procedure that involves creating an opening in the floor of the 3rd ventricle to permit CSF flow past an obstruction, and coagulation of selected sections of the choroid plexus to reduce CSF production. Hydrocephalus carries a significant risk of irreversible neurologic deterioration, coma, or death when left untreated.[11][12]
CSF Leak
CSF leak is a condition in which CSF escapes from the subarachnoid space through a defect in the surrounding dura mater. The volume of fluid lost varies widely, ranging from minimal to substantial. Significant CSF loss may result in spontaneous intracranial hypotension (SIH).
SIH typically presents with a positional headache caused by downward displacement of the brain due to reduced buoyant support. Other common symptoms include posterior neck stiffness, nausea, and vomiting. The estimated annual incidence is 4 per 100,000. Women are affected approximately twice as often, with a peak incidence at around 40 years of age.
Underlying causes include spinal CSF leakage from dural tears, meningeal diverticula, and CSF venous fistulas. Early imaging is critical. Clinicians should obtain brain and spine MRI at symptom onset to detect extradural fluid collections and guide further evaluation. Contrast-enhanced brain imaging typically reveals diffuse meningeal enhancement, venous sinus engorgement, and downward displacement of brain structures. Spine imaging may demonstrate longitudinal epidural fluid collections. These collections are frequently associated with discogenic microspurs or dural tears.
High-yield leak detection methods include CT myelography, digital subtraction myelography, and ultrafast CT myelography. A CSF venous fistula should be suspected in the absence of epidural fluid on MRI. Lateral decubitus CT myelography and digital subtraction myelography are preferred for identifying such fistulas.
Initial treatment includes bed rest, fluid resuscitation, and caffeine administration. Nontargeted epidural blood patches are typically administered within 2 weeks of diagnosis. Persistent symptoms warrant myelographic evaluation to localize the leak and guide targeted blood patching, transvenous embolization for CSF venous fistulas, or surgical intervention.[13][14] Surgical repair using suture placement or aneurysm clip application is considered safe and effective in resolving CSF leak symptoms.[15] Complications of SIH include subdural hematoma, cerebral venous thrombosis, and superficial siderosis.
Meningitis
Meningitis refers to inflammation of the meninges, the protective coverings of the brain. This condition is classified as either aseptic or bacterial. Aseptic meningitis may result from fungal infection, adverse drug reactions, or metastatic malignancy, although viral infection accounts for the majority of cases. Classic symptoms include fever, nuchal rigidity, and photophobia.
Diagnosis is established through CSF analysis obtained by lumbar puncture. Polymerase chain reaction testing of CSF improves diagnostic sensitivity for viral causes. Treatment for aseptic meningitis is typically supportive, focusing on antipyretic and analgesic management.
Bacterial meningitis occurs less frequently than aseptic meningitis but carries greater morbidity and mortality. Widespread vaccination has led to a substantial decline in the incidence of this condition. Symptomatology overlaps with aseptic meningitis, but the clinical course is typically more severe. Additional findings may include altered mental status, seizure, and focal neurologic deficits.
Diagnosis is likewise confirmed by lumbar puncture. CSF often appears cloudy and typically demonstrates hypoglycorrhachia along with positive Gram stain or culture. Empiric administration of broad-spectrum antibiotics is required immediately in all suspected cases. Antimicrobial therapy should be adjusted accordingly once microbiologic results become available. Patients require admission to the intensive care unit for continuous monitoring. Most individuals with bacterial meningitis recover without long-term complications when treated appropriately and without delay.[16]
Subarachnoid Hemorrhage
SAH refers to bleeding into the subarachnoid space, where blood mixes with CSF. Traumatic injury is the leading cause of SAH. Among nontraumatic cases, approximately 80% result from rupture of a cerebral aneurysm. Other nontraumatic etiologies include arteriovenous malformations and vasculitic disorders.
Clinical presentation typically involves the sudden onset of severe headache, often described as a "thunderclap headache" or "the worst headache" of the patient's life. Associated symptoms may include vomiting, seizure, loss of consciousness, and in some cases, death.
Noncontrast CT imaging of the head serves as the initial diagnostic modality. Sensitivity is highest within the first few hours after hemorrhage but declines over time. Lumbar puncture is indicated in patients with an unremarkable head scan but continued suspicion for SAH. A positive result includes persistent red blood cells in both the 1st and 4th collection tubes or the presence of xanthochromia. Management focuses on preventing rebleeding and minimizing secondary brain injury through careful hemodynamic monitoring, neurosurgical intervention, and supportive care.[17]
Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension, also known as pseudotumor cerebri syndrome, is characterized by elevated intracranial pressure in the absence of hydrocephalus, mass lesion, or abnormal CSF composition. This condition primarily affects women of reproductive age with obesity and has an estimated annual incidence of 2.4 per 100,000. The underlying pathogenesis is uncertain. The most widely accepted hypothesis suggests impaired CSF absorption at the level of the arachnoid granulations or olfactory lymphatics.
Headache is the most frequently reported symptom, although no specific clinical features reliably distinguish it from other headache types. In some cases, asymptomatic individuals are identified by the presence of papilledema during routine ophthalmologic examination. Other manifestations include pulsatile tinnitus, transient visual obscurations, visual field deficits, and progressive visual loss.
Neuroimaging with MRI and venography should be performed within 24 hours to exclude intracranial mass lesions and cerebral venous sinus thrombosis. Spinal fluid analysis is essential for diagnosis. CSF opening pressure typically exceeds 250 mm H2O in adults.
Weight reduction remains the only proven disease-modifying intervention and should be initiated as early as possible. Acetazolamide may improve visual field function and reduce the frequency or severity of headaches. Surgical interventions, including CSF diversion procedures or optic nerve sheath fenestration, may be necessary in cases with fulminant onset or progressive visual decline despite medical therapy. Most individuals diagnosed with idiopathic intracranial hypertension achieve favorable outcomes. However, a minority of patients experience chronic headache or irreversible visual impairment.[18][19]
Dementia
Newer theories implicate glymphatic system dysfunction as a final convergent mechanism in the development of dementia. Advancing age reduces glymphatic clearance and accelerates extracellular protein accumulation. Impaired perivascular CSF flow leads to protein stagnation and promotes the misfolding and aggregation of amyloid-β, τ, and α-synuclein. These protein aggregates propagate along perivascular and interstitial pathways in a prion-like manner. Local inflammation and neuronal loss subsequently occur in regions of aggregate deposition.
Media
(Click Image to Enlarge)
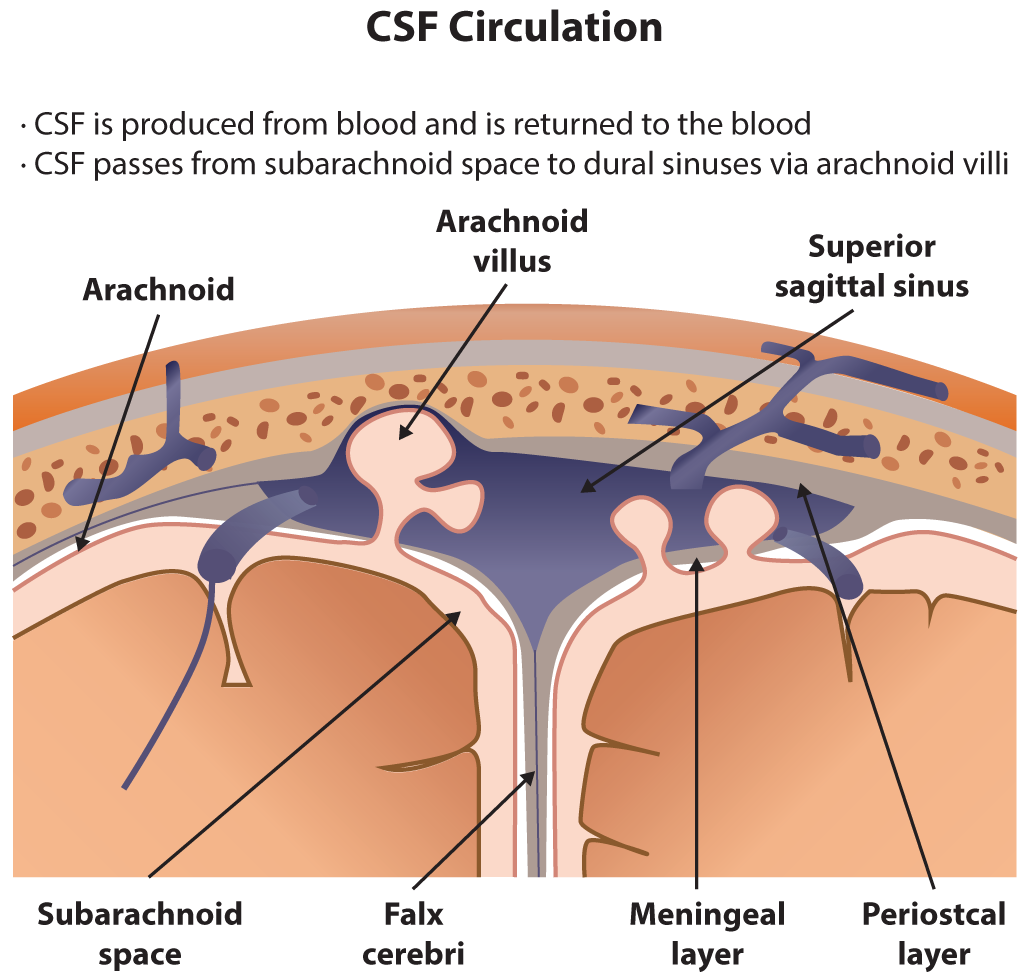
Cerebrospinal Fluid Circulation. The illustration depicts cerebrospinal fluid circulation through the subarachnoid space, arachnoid villi, and superior sagittal sinus. Key anatomical structures include the falx cerebri, meningeal and periosteal layers of the dura mater, and the arachnoid membrane.
Contributed by B Parker
(Click Image to Enlarge)
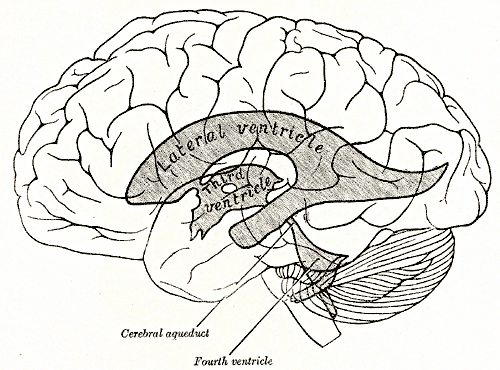
Brain Ventricular System and Surface Anatomy. This diagram illustrates the relationship of the brain's ventricular system to its outer surface. The lateral ventricle, 3rd ventricle, cerebral aqueduct, and 4th ventricle are highlighted to show the flow of cerebrospinal fluid through the central nervous system.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
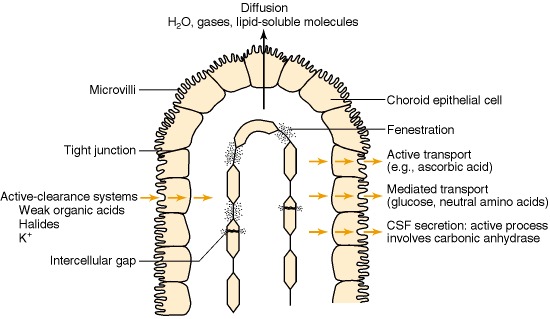
Blood-CSF Barrier at the Choroid Plexus. The image illustrates the brain's ventricular system, where the blood-CSF barrier is located at the choroid plexus epithelium. Unlike brain capillaries, those in the choroid plexus are fenestrated, allowing free molecular movement. However, tight junctions between epithelial cells regulate CSF composition. Microvilli on the apical surface enhance secretion, with transport mechanisms including diffusion, facilitated diffusion, and active transport.
Laterra J, Keep R, Betz LA, et al. Blood—Cerebrospinal Fluid Barrier. In: Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27998/
(Click Image to Enlarge)
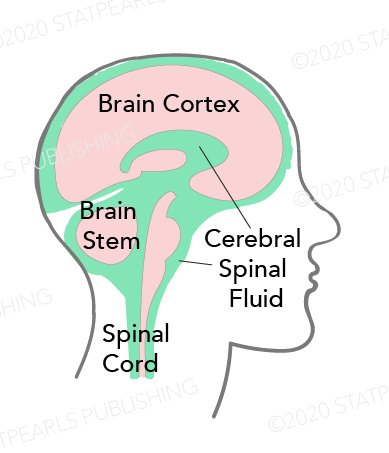
Cerebrospinal Fluid Distribution. This image highlights the circulation of cerebrospinal fluid (CSF) within the central nervous system. CSF surrounds the brain cortex, brain stem, and spinal cord, providing mechanical protection and facilitating nutrient exchange. The diagram shows how CSF occupies the subarachnoid space and ventricular system, cushioning neural structures and maintaining intracranial pressure.
Contributed by Katherine Humphreys
(Click Image to Enlarge)
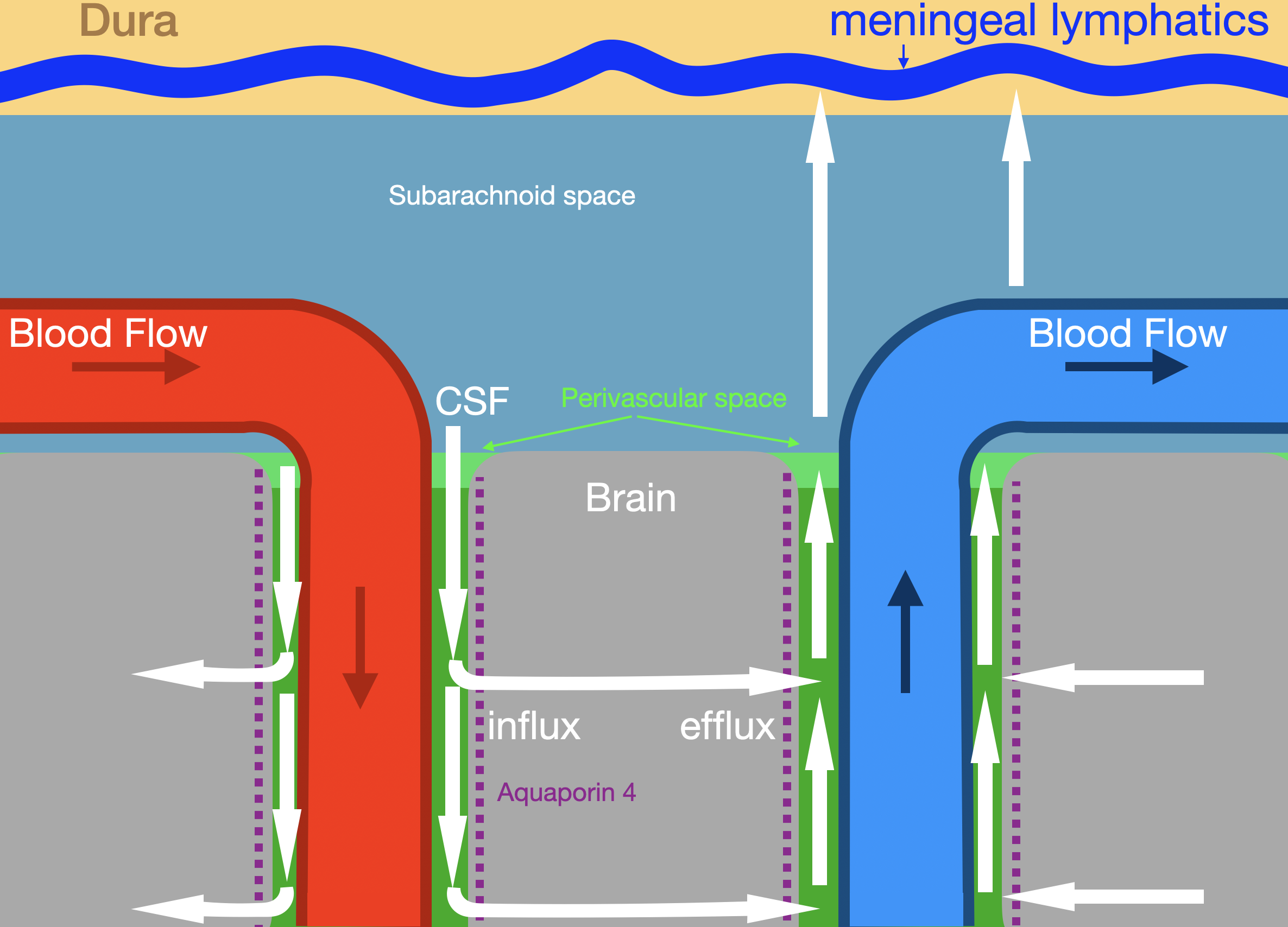
Glymphatic System. The illustration shows glymphatic fluid flow (white arrows) along periarterial and perivenous pathways. Labeled structures include the dura, meningeal lymphatics, subarachnoid space, brain parenchyma, arterial and venous circulation, and aquaporin 4 channels mediating influx and efflux.
Contributed by Konstantinos Margetis MD, PhD
References
Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Experimental neurology. 2015 Nov:273():57-68. doi: 10.1016/j.expneurol.2015.07.027. Epub 2015 Aug 4 [PubMed PMID: 26247808]
Yamada S, Mase M. Cerebrospinal Fluid Production and Absorption and Ventricular Enlargement Mechanisms in Hydrocephalus. Neurologia medico-chirurgica. 2023 Apr 15:63(4):141-151. doi: 10.2176/jns-nmc.2022-0331. Epub 2023 Mar 1 [PubMed PMID: 36858632]
Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. European annals of otorhinolaryngology, head and neck diseases. 2011 Dec:128(6):309-16. doi: 10.1016/j.anorl.2011.03.002. Epub 2011 Nov 18 [PubMed PMID: 22100360]
Damkier HH, Brown PD, Praetorius J. Epithelial pathways in choroid plexus electrolyte transport. Physiology (Bethesda, Md.). 2010 Aug:25(4):239-49. doi: 10.1152/physiol.00011.2010. Epub [PubMed PMID: 20699470]
Level 3 (low-level) evidenceDamkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiological reviews. 2013 Oct:93(4):1847-92. doi: 10.1152/physrev.00004.2013. Epub [PubMed PMID: 24137023]
Level 3 (low-level) evidenceIliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Science translational medicine. 2012 Aug 15:4(147):147ra111. doi: 10.1126/scitranslmed.3003748. Epub [PubMed PMID: 22896675]
Level 3 (low-level) evidenceNedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science (New York, N.Y.). 2020 Oct 2:370(6512):50-56. doi: 10.1126/science.abb8739. Epub [PubMed PMID: 33004510]
Rasmussen MK, Mestre H, Nedergaard M. Fluid transport in the brain. Physiological reviews. 2022 Apr 1:102(2):1025-1151. doi: 10.1152/physrev.00031.2020. Epub 2021 May 5 [PubMed PMID: 33949874]
Doherty CM, Forbes RB. Diagnostic Lumbar Puncture. The Ulster medical journal. 2014 May:83(2):93-102 [PubMed PMID: 25075138]
Wright BL, Lai JT, Sinclair AJ. Cerebrospinal fluid and lumbar puncture: a practical review. Journal of neurology. 2012 Aug:259(8):1530-45. doi: 10.1007/s00415-012-6413-x. Epub 2012 Jan 26 [PubMed PMID: 22278331]
Level 3 (low-level) evidenceOrešković D, Klarica M. Development of hydrocephalus and classical hypothesis of cerebrospinal fluid hydrodynamics: facts and illusions. Progress in neurobiology. 2011 Aug:94(3):238-58. doi: 10.1016/j.pneurobio.2011.05.005. Epub 2011 May 27 [PubMed PMID: 21641963]
Level 3 (low-level) evidenceKahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC. Hydrocephalus in children. Lancet (London, England). 2016 Feb 20:387(10020):788-99. doi: 10.1016/S0140-6736(15)60694-8. Epub 2015 Aug 6 [PubMed PMID: 26256071]
Cheema S, Anderson J, Angus-Leppan H, Armstrong P, Butteriss D, Carlton Jones L, Choi D, Chotai A, D'Antona L, Davagnanam I, Davies B, Dorman PJ, Duncan C, Ellis S, Iodice V, Joy C, Lagrata S, Mead S, Morland D, Nissen J, Pople J, Redfern N, Sayal PP, Scoffings D, Secker R, Toma AK, Trevarthen T, Walkden J, Beck J, Kranz PG, Schievink W, Wang SJ, Matharu MS. Multidisciplinary consensus guideline for the diagnosis and management of spontaneous intracranial hypotension. Journal of neurology, neurosurgery, and psychiatry. 2023 Oct:94(10):835-843. doi: 10.1136/jnnp-2023-331166. Epub 2023 May 5 [PubMed PMID: 37147116]
Level 3 (low-level) evidenceCheema S, Mehta D, Qureshi A, Sayal P, Kamourieh S, Davagnanam I, Matharu M. Spontaneous intracranial hypotension. Practical neurology. 2024 Mar 19:24(2):98-105. doi: 10.1136/pn-2023-003986. Epub 2024 Mar 19 [PubMed PMID: 38135500]
Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006 May 17:295(19):2286-96 [PubMed PMID: 16705110]
Putz K, Hayani K, Zar FA. Meningitis. Primary care. 2013 Sep:40(3):707-26. doi: 10.1016/j.pop.2013.06.001. Epub 2013 Jul 25 [PubMed PMID: 23958365]
Abraham MK, Chang WW. Subarachnoid Hemorrhage. Emergency medicine clinics of North America. 2016 Nov:34(4):901-916. doi: 10.1016/j.emc.2016.06.011. Epub [PubMed PMID: 27741994]
Friedman DI. The pseudotumor cerebri syndrome. Neurologic clinics. 2014 May:32(2):363-96. doi: 10.1016/j.ncl.2014.01.001. Epub 2014 Feb 28 [PubMed PMID: 24703535]
Level 3 (low-level) evidenceMollan SP, Davies B, Silver NC, Shaw S, Mallucci CL, Wakerley BR, Krishnan A, Chavda SV, Ramalingam S, Edwards J, Hemmings K, Williamson M, Burdon MA, Hassan-Smith G, Digre K, Liu GT, Jensen RH, Sinclair AJ. Idiopathic intracranial hypertension: consensus guidelines on management. Journal of neurology, neurosurgery, and psychiatry. 2018 Oct:89(10):1088-1100. doi: 10.1136/jnnp-2017-317440. Epub 2018 Jun 14 [PubMed PMID: 29903905]
Level 3 (low-level) evidence