Introduction
Osteoporosis (translating to 'porous bones' from the Greek words Osteon and Poros) is characterized by low bone mineral density (BMD) with a propensity for fractures.[1] This condition occurs from microarchitectural alterations of the bone, and apart from fractures, can be associated with impairment in quality of life, disability, morbidity, and mortality. Colloquially referred to as 'the silent disease' or 'silent epidemic', osteoporosis is often not diagnosed until after a fracture has occurred.[1] Osteoporosis is not a new disease, and as historians suggest, it was present thousands of years ago, as evidenced by the collapsed vertebrae of Egyptian mummies during excavations.[2] The groundwork leading to our current understanding of osteoporosis arose from the curiosity of British surgeon Sir Astley Cooper, noting the association between abnormal bones and fractures in 1822.[3]
Around a decade later, the work of French pathologist Jean Lobstein (who coined the term osteoporosis) confirmed the findings of Sir Astley Cooper, noting porous bones.[3] In 1941, the American endocrinologist Fuller Albright noted the association between the weakening of vertebral bodies and fracture risk in patients with loss of ovarian function; he subsequently reported the reversal with the introduction of estrogen, laying the foundation for our understanding of the pathophysiology of osteoporosis in postmenopausal females.[3] Osteoporosis can affect both sexes, but it disproportionately affects more postmenopausal females. The disease is more common in the advanced age group and is expected to increase exponentially with the aging population. Screening and treatment guidelines are established; however, education and awareness are substandard, with frequent underdiagnosis and missed opportunities to implement screening and treatment.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
In 1983, Drs Riggs and Melton posed a classification distinguishing 2 types of primary osteoporosis. The classification helped further our understanding of osteoporosis, guiding targeted prevention and treatment strategies for these distinct types. Type 1 was informally noted as ‘postmenopausal’ and occurred from hormonal changes of estrogen imbalance.[4] Type 1 occurs approximately 10 to 15 years following menopause, in the typical age group of 50 to 70 years; however, it can affect up to one-quarter of women in early menopause.[5][6] Due to the loss of estrogen in Type 1, there is a shift towards dramatic bone loss (relative to bone formation) with the acceleration of trabecular bone loss (rather than cortical), involving regions with trabecular bone predominance, such as the vertebral body which has a trabecular-to-cortical ratio of 75:25, in comparison to the femoral neck at ratio of 30:70.[7]
Type 2 osteoporosis, also known as ‘senile’, occurs from low turnover and is noted to be a result of gradual trabecular and cortical bone loss (with mostly cortical predominance); it may be present in both sexes, typically in those over age 70.[5][7] Subsequently, as osteoporosis was studied in further detail, it was apparent that it could also occur secondary to medication or underlying pathophysiological processes, for which the classification ‘secondary osteoporosis’ was created.[8] Men are more likely to have a secondary cause for bone loss compared to women, estimated to be 50% to 80% compared to about 30%.[8] Secondary osteoporosis risk factors are often classified as ‘modifiable’ or ‘non-modifiable’ (see Table. Risk Factors for Secondary Osteoporosis).[9][10]
Table. Risk Factors for Secondary Osteoporosis
| Lifestyle Factors | Endocrine | Gastrointestinal | Genetic | Rheumatologic/Connective Tissue | Medications | Hematologic | Miscellaneous |
| Smoking | Hyperparathyroidism | Celiac disease | Hypophosphatasia | Osteogenesis imperfecta | Heparin | Multiple myeloma | Chronic kidney disease |
| Anorexia nervosa | Hyperthyroidism | Inflammatory bowel disease | Parental history of hip fracture | Ehlers-Danlos syndrome | Lithium | Chronic hemolytic anemia | Chronic obstructive pulmonary disease |
| Vitamin D deficiency | Diabetes | Cirrhosis | Homocystinuria | Marfan syndrome | Glucocorticoids | Systemic mastocytosis | Congestive heart failure |
| Prior fracture | Delayed puberty | Malabsorption | Cystic fibrosis | Rheumatoid arthritis | Opiates | Haemophilia | Multiple sclerosis |
| Vitamin A toxicity | Hypogonadism (primary or secondary) | Pancreatic disease | Glycogen storage disease | Systemic lupus erythematosus | Aromatase inhibitors | Lymphoma | Chronic metabolic acidosis |
| Low calcium intake | Hyperprolactinemia | Primary biliary cholangitis | Menkes kinky hair syndrome | Muscular dystrophy | Cyclosporine | Sickle cell disease | HIV/AIDS |
| High salt intake | Panhypopituitarism | Gastrointestinal surgery | Gaucher disease | Idiopathic scoliosis | Gonadotrophin-releasing hormone agonists | Thalassemia | Post-transplantation |
| Aluminum (antacids) | Amenorrhea | Gastric bypass | Riley-Day syndrome | Sarcoidosis | Anticonvulsants (Phenytoin) | Leukaemia | |
| Inactivity/immobilization | Cushing syndrome | Parenteral nutrition | Androgen insensitivity | Barbiturates | Amyloidosis | ||
| High caffeine intake | Adrenal insufficiency | Porphyria | Tacrolimus | ||||
| Excessive alcohol | Hypercalciuria | Hemochromatosis | Thyroxine | ||||
| Falls | Growth hormone deficiency | Klinefelter syndrome | HIV medication (Tenofovir) | ||||
| Low body mass index | Turner syndrome | Canagliflozin | |||||
| Thiazolidinediones |
Table Obtained from Osteoporosis in Males StatPearls [10]
Epidemiology
Osteoporosis is a crisis of global proportions, with between 200 and 500 million people affected internationally, with 6.3% of men and 21.2% of women older than age 50 diosed from this osseous disease.[11] There are notable regional variations of osteoporosis, with a greater prevalence in developing (compared to developed) countries.[12] Similarly, Asia has the highest reported prevalence of osteoporosis in the world, which is correlated with Asian patients often having below-average BMD.[12]
Worldwide, up to 37 million fragility fractures occur annually in patients older than age 55, which equates to 70 fractures per minute.[13] The European Union has designated fragility fracture as the fourth most burdensome noncommunicable disease (behind ischemic heart disease, dementia, and lung cancer).[14] Moreover, there is a large economic burden, which will continue to increase alongside the aging population. The economic burden in the United Kingdom is around £4 billion per annum and €56 billion in the European Union. In the United States, the Bone Health and Osteoporosis Foundation estimates a cost of approximately $19 billion annually.[15][16] Apart from cost, fragility fractures are a significant health burden, with the European Union noting 1,180,000 quality-adjusted life-years lost, with twice as many in females compared to males.[17] Furthermore, 26,300 life-years were lost in the European Union due to incident fractures in the year 2010.[14]
In the United States, around 1.9 million fragility fractures occur annually, with the dominant fracture types including 700,000 clinical vertebral fractures and 300,000 hip fractures, and they are associated with around 500,000 hospital admissions, 2.5 million office visits, and 180,000 nursing home admissions.[18][19][20] Currently, Medicare pays for about 80% of these fractures, with hip fractures accounting for 72% of the cost.[20] Estimates suggest that by 2040, the number of fractures will increase to 3.2 million annually, and the cost of care is expected to increase to $95 billion.[21]
As stated, women are disproportionately affected by osteoporosis and fragility fractures. In the European Union in 2010, approximately 43,000 deaths were estimated to have occurred following a fracture, with 50% being from hip fractures, 28% from vertebral fractures, and 22% from other fracture types in women, compared to 37%, 29%, and 14% for men, respectively.[17] Although women are at a higher risk for osteoporosis and subsequent fractures, men experience greater mortality following a fracture.[22] When comparing osteoporosis to other female health crises, the risk of a lifetime hip fracture in a White woman is around 1 in 6, compared to a breast cancer diagnosis of 1 in 9. Moreover, the risk of death from a hip fracture in a 50-year-old White woman in the United States is 2.8% for the remainder of her life, which is equivalent to the risk of death from breast cancer, and 4 times greater than the risk from endometrial cancer [International Osteoporosis Foundation-Epidemiology of osteoporosis and fragility fractures. 2024. https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures#ref_bottom_68].
In the European Union, around 22 million women between the ages of 50 and 84 years old are believed to have had a diagnosis of osteoporosis in 2010, with estimates suggesting an increase of 23% by 2025 (up to 33.9 million).[17][23] Globally, osteoporosis is estimated to affect one-tenth of females aged 60, one-fifth aged 70, two-fifths aged 80, and two-thirds older than 90.[24] The prevalence of osteoporosis in women in 2010 was 9% in the United Kingdom, 15% in France, 15% in Germany, and 38% in Japan.[25]
The Global Longitudinal Study of Osteoporosis in Women (GLOW) analyzed 60,000 postmenopausal women in the United States, Australia, Canada, and several European countries, noting 4,122 fractures over 3 years; 86% were non-hip/nonvertebral, 8% were clinical vertebral, and 6% were hip fractures. Findings from GLOW further noted seasonal variation with hip fractures (with more likely to occur in the spring) compared to other fractures, also noting 65% of non-hip/nonvertebral fractures occurring outside and 61% of vertebral fractures occurring indoors, with an equivalent risk indoors versus outdoors for hip fractures. The data from GLOW reinforced the importance of considering a preceding fall about an osteoporotic fracture, occurring in 68% to 86% of non hip/nonvertebral fractures and 68-83% of hip fractures, as well as around 45% of vertebral fractures associated with falls in this age group.[26]
In 2010, the prevalence of osteoporosis in the United States in the population older than 50 was 10.3%.[25] Of the roughly 10.3 million Americans with a diagnosis of osteoporosis, around 80% (nearly 8 million) of these patients were women.[27][28] In the United States, the lifetime risk for a low-trauma fracture is around 40% for a woman older than the age of 50 (17.5% being hip fractures, 16.0% forearm fractures, and 15.6% symptomatic/clinical vertebral fractures) with nearly 1 in 2 White females experiencing an osteoporotic fracture in their life.[15][19] Moreover, within the United States, the highest annual hip fracture rates occur among White women (140.7 per 100,000), followed by Asian (85.4 per 100,000), Black (57.3 per 100,000), and Hispanic (49.7 per 100,000) women.[12]
Although African American women on average have higher BMD, when diagnosed with osteoporosis, there is an equivocal risk for fragility fractures compared to that of their White counterparts.[29] The United States Preventive Services Task Force (USPSTF) discovered that African American females are 40% less likely than White females to undergo BMD screening.[12] Similarly, following the repair of hip fractures, African American females are less likely to undergo a bone densitometry assessment and are less likely to receive treatment for osteoporosis compared to White women, for either prevention or following a fragility fracture.[12] Likewise, referral rates for densitometry screening amongst Hispanic women are lower compared to those of White women.[12]
More than 14 million hip fractures occur globally in people older than 65, with the number expected to double from 2018 to 2050, ultimately leading to a 240% increase in women compared to 1990s rates.[30][31] Of these hip fractures, around 75% occur in women.[32] The Nordic countries are noted to be among the highest reported incidences of hip fractures worldwide.[33] In the United States, the annual incidence of hip fractures per 100,000 individuals ranges from 511 to 553 for women, with most occurring at an average age of 82, with an annual incidence of a second hip fracture being 2% to 10%, occurring on average 2 years after the first.[12][19] A 50-year-old female has a 17.5% lifetime risk of a hip fracture.[34]
Wrist fractures demonstrate an increase in age-adjusted incidence in women between the ages of 45 and 60, followed by relative stability. Wrist fractures occur earlier in life than other fractures [International Osteoporosis Foundation- Epidemiology. https://www.osteoporosis.foundation/health-professionals/fragility-fractures/epidemiology]. This differs from men, who account for 15% of wrist fractures and do not demonstrate an increased incidence with age.[35] Around 326,838 wrist fractures occur annually, with a 16% lifetime risk for a Colle’s fracture in a 50-year-old woman in the United States.[36][37] In the United Kingdom, the incidence of distal forearm fractures is 39.7 per 10,000 person-years in women, compared to 8.9 per 10,000 person-years in men, for individuals older than 50.[15] Contrary to hip and vertebral fractures, however, there does not appear to be an associated increased risk for mortality following distal forearm fractures.[15]
Vertebral compression fractures are the most common fractures, but are typically asymptomatic and are often incidentally found on imaging.[38] Existing vertebral fractures increase the risk of a subsequent fracture by 5-fold.[39] Only about one-third of vertebral fractures come to clinical attention when they can be diagnosed radiographically.[19] Nearly one-quarter of postmenopausal women have a vertebral fracture, and around 55% of patients with a hip fracture have evidence of a prior vertebral fracture.[39][40] Vertebral fractures from osteoporosis occur around once every 22 seconds in both men and women in those aged 50 and older.[41] A 65-year-old woman with 1 vertebral fracture appears to have a 1 in 4 chance of another fracture within the next 5 years, which is reduced to 1 in 8 with treatment.[42] A White female older than 50 in the United States has around a 16% lifetime risk of a vertebral fracture.[43] The European Vertebral Osteoporosis Study noted that men have a greater incidence of vertebral deformities before age 65, after which women demonstrate a greater incidence.[44] Moreover, the European Prospective Osteoporosis Study noted an age-standardized incidence of vertebral fractures of 10.7 per 1000 person-years in women compared to 5.7 per 1000 person-years in men.[44] Similarly, the Norwegian Tromso Study noted 3% of vertebral fractures in females younger than 60, compared to 19% in females older than 70.[45]
The osteoporosis ‘treatment gap’ is the number of patients who meet the indication for therapy minus the number of patients who receive treatment. The treatment gap is a global concern, with the International Osteoporosis Foundation recording a treatment gap of 73% among women from France, Germany, Italy, Spain, and the United Kingdom.[14][21] In 2019, approximately 15 million women in Europe who were eligible did not receive treatment for osteoporosis.[16] Up to 95% of patients who are discharged following the repair of a hip fracture do not receive treatment or a management plan, with men far less likely to receive treatment compared to women.[21][46] When treatment is commenced, however, it is also estimated that around 70% of patients will not continue treatment past the first year.[47]
In addition to the absence of treatment, screening for osteoporosis is not prioritized. Less than one-third of patients with a fragility fracture have either a bone density test or have received treatment for underlying osteoporosis.[48] An estimated 91% of women older than 65 with a previous fragility fracture are not screened for osteoporosis.[28] Estimates suggest around 21% of females aged 60 to 64 receive screening for osteoporosis, 27% aged 65 to 79, and 13% older than 80.[49] Similarly, after a new osteoporotic fracture, about 9% of female Medicare fee-for-service beneficiaries undergo BMD testing within 6 months.[28] In 2012, among an estimated 2 million Americans with 2.3 million osteoporotic fractures, less than 10% underwent BMD testing within 6 months of their fracture, with a second fracture occurring in more than 300,000 individuals within 3 years.[15]
A survey performed in the United States among females with postmenopausal osteoporosis noted that only 31% of females stated that after seeing a healthcare professional following a recent osteoporotic fracture, they received follow-up or a referral to the appropriate consultant. Around 35% of respondents were unaware that osteoporosis was the cause of their fracture, with almost half of these respondents blaming the fracture on ‘clumsiness’ rather than poor bone health. Moreover, more than half of the respondents were unaware that 1 osteoporotic fracture may increase the risk for another fracture. In the same survey, for patients who did follow up after an osteoporotic fracture but did not commence therapy, half of all cases were due to patient refusal. For those who did commence therapy, an estimated 27% were noted to have taken a drug holiday or stopped medication, with 47% of respondents admitting that this was done of their own accord without a medical recommendation.[49] Similarly, up to 30% of patients who receive a prescription do not commence the medication, and up to 70% do not continue treatment past 1 year.[50][51]
Pathophysiology
Two predominant cells are in a constant interplay between bone formation and resorption, notably the osteoblasts forming osteoid matrix and the osteoclasts digesting calcified bone matrix.[52] A third type of cell, the osteocytes, functions in overall regulation.[52] Osteoblasts release receptor activator of nuclear factor Kappa-B ligand (RANKL), which acts upon RANK receptors on osteoclast precursors, stimulating active resorption.[53] Osteoprotegerin (also known as osteoclastogenesis inhibitory factor) is released from osteocytes and binds to RANKL to inhibit its function, leading to an inhibition of bone resorption (see Image. Role of RANKL/RANK/OPG Axis on Bone Homeostasis and Immune System).[53]
BMD depends upon 2 principal factors: the peak bone mass and the rate of decline. Peak bone mass is defined as the maximal skeletal mass and strength attained.[54] Around 50% of peak bone mass is obtained in the adolescent years in females ('peaks'), followed by slow building continuing until the third decade, where peak bone mass is reached.[28] Estimates suggest that around 60% to 80% of peak bone mass is genetically predetermined.[19] Compared to men, however, peak bone mass is around 10% lower in females, meaning women start resorption from a lower bone density.[55] An increase in peak bone mass in children and adolescents can reduce the risk of an osteoporotic fracture during adulthood by nearly 50%.[28] Compared to males, females have less periosteal bone formation, but more endocortical apposition, largely due to estrogen inhibition of periosteal apposition. In contrast, androgens stimulate apposition, which culminates in wider bones in men.[56]
The onset of bone loss is more rapid in women and occurs at a younger age.[57] Men and women both start to lose bone in the middle of the third decade, with women losing up to 50% of trabecular bone and 35% of cortical bone (see Image. Normal Versus Osteoporotic Bone).[7] The Dubbo Osteoporosis Epidemiological Study noted an annual bone loss of 0.96% per year for women at the femoral neck, with more rapid decline occurring in ages 65 to 69 years. Similarly, the Framingham Osteoporosis Study noted an average 4-year bone loss of up to 4.8% for women at the hip/lumbar spine/radius.[57][58] With aging, trabecular perforations and loss occur in women, compared to trabecular thinning, but not loss, in men.[59]
Menopause is defined as the complete cessation of menstruation in a woman for at least 1 year or more; in the United States, the average age of menopause is 51 years.[12] With the increase in life expectancy, women now spend more than one-third of their lives beyond menopause, correlating with an increased prevalence of osteoporosis.[12] Estrogen decline leads to rapid acceleration of bone loss starting the year before menopause and continuing for another 3 years, followed by deceleration to a moderate rate of bone loss over the following 4 to 8 years.[60] The most significant decrease in BMD occurs approximately 5% per year in the first year following menopause and reaches 1% to 1.5% per year in the subsequent years.[25] The 'accelerated' phase of trabecular bone loss occurs largely from loss of connectivity from perforation, followed by the slower phase of cortical site involvement; this latter phase occurs in both sexes due to a decreased osteoblast number and rate of bone formation.[61]
The average decrease in BMD over the menopausal transition is between 10% and 12% in the spine and hip (1 T-score unit), meaning half of the women are losing bone even faster, with up to 20% loss in the 7 years around menopause.[19][60] Moreover, around one-quarter of postmenopausal females are classified as "fast bone losers." [60] One caveat to consider is that thin women appear to lose bone mass faster than overweight individuals.[19] By age 80, around 30% of peak bone mass is lost.[19] There is an estimation that a 10% loss of bone mass at the hip is associated with a 2.5 times greater risk for a hip fracture, and a 10% loss of bone mass in the vertebrae is associated with a 2-fold greater risk of vertebral fractures.[62]
While trabecular bone loss is related to estrogen decline, a notable proportion of trabecular bone loss is due to aging rather than estrogen deficiency. As an example, between menopause and age 75, around 22% of total body bone mineral is lost, with estimates suggesting 7.75% is due to estrogen deprivation, and 13.3% secondary to aging. In the femoral neck, 5.3% of the bone loss is related to estrogen deficiency, and 14% is from aging.[63]
Another under-recognized category is known as 'lactation-associated osteoporosis'.[64] During lactation, rapid asymptomatic decreases in BMD can be associated with recovery during/after weaning. The skeletal resorption has been linked to excess parathyroid hormone-related protein released from the mammary glands during breastfeeding, a phenomenon which can lead to hypercalcemia in breastfeeding females.[65] Limited case reports have noted fractures during this time; however, other data support the notion of a lasting benefit to BMD and reduction in fracture risk in those with a history of breastfeeding. Preliminary studies have also suggested a positive association between pregnancy and fracture risk at the onset of menopause, noting a significant reduction in the risk of fracture after an average of 3 pregnancies.[64]
Histopathology
A bone biopsy is not routinely indicated for the diagnosis of osteoporosis and is typically restricted to when an atypical, unclear presentation occurs. Obtaining a biopsy will alter the course of treatment and can assist with excluding other pertinent pathological causes of osteoporosis, such as mastocytosis or multiple myeloma.[66] A bone biopsy is typically performed as a trans-iliac bone biopsy, with double tetracycline labelling to help provide data on bone turnover. Patients are administered tetracycline, which binds to the newly formed bone and will appear as a linear fluorescence.
A second tetracycline dose is administered around 2 weeks later, appearing as a second linear fluorescence on the biopsy. The distance between the 2 labels is measured, and the amount of bone formed during that interval is calculated.[67] A bone biopsy may also demonstrate a response (or lack of) to treatment.[67] Histologic findings of osteoporosis include thinning of the trabecular bone, which is usually generalized, and perforations of the trabeculae, along with decreased wall width.[68]
History and Physical
Clinical History
A comprehensive history is paramount to arriving at the correct diagnosis, stratifying risk, and allowing consideration for the correct treatment regimen. The following components are essential to a thorough clinical history.
- Age: Apart from sex, age is one of the strongest risk factors for osteoporosis and subsequent morbidity and mortality. An estimated 85% of female nursing home residents older than 80 have a diagnosis of osteoporosis.[69] Age is associated with decreased bone mass, with increased cortical porosity by 176% to 259% from ages 20 to 90.[70] Additionally, age increases the risk of fractures independent of bone mass. For example, with the same BMD, a 20-year increase in age can lead to a 4-fold increase in fracture risk.[71] Moreover, every 20-year increase in age is associated with a 10-fold increase in the risk of femoral neck fractures.[59]
- Fracture history: A prior fracture after menopause is correlated with an increased risk for future fractures. While the risk gradually decreases, it persists for up to 10 years, with the highest risk being in the first 2 years, and the average risk over time being about 2-fold greater than expected for a female with her stated age and BMD.[19][72] Considering the mechanism of action of the fracture and identify if it was low-impact (fragility) related or a consequence of trauma is important. Furthermore, it is also vital to consider the bone involved in the fracture.
- Family history: Nearly 20% of females in the United States with a diagnosis of osteoporosis report a positive family history. The presence of osteoporosis in the family is a significant risk factor for offspring and is greatest when 2 or more relatives are affected.[73] Moreover, maternal hip fractures are associated with an increased risk of hip fractures in women. The American Association of Clinical Endocrinologists (AACE) recommends always inquiring about a family history of osteoporosis, in particular parental hip fractures.[74]
- Strength: Sarcopenia is a risk factor for falls. Starting in the fourth decade, 3% to 5% of muscle mass is lost per decade, generally worsening by 1% to 2% annually after age 50.[75] Epidemiological studies note that women have a greater fall risk than men and are more likely to experience a fracture after a fall compared to men.[76] Patients should be screened for a history of falls at each medical visit, and the fall risks in their living spaces, such as unstable floors and carpets, should be considered. Counseling on fall prevention is an essential part of the management of osteoporosis.
- Comorbid conditions: Comorbid conditions may be a causative factor for secondary osteoporosis, involving multiple different bodily systems, including gastrointestinal, endocrine, hematologic, rheumatologic, pulmonary, renal, and hepatic, among others (see Table. Risk Factors for Secondary Osteoporosis).
- List of medications: Reviewing the list of medications at each medical professional contact is imperative. Medications may directly lead to weakened BMD, predispose to vitamin and mineral deficiencies, lead to poor healing after a fracture, cause muscle weakening, increase the risk of a fall and fracture, or increase the risk of falls through impaired consciousness (see Table. Risk Factors for Secondary Osteoporosis). The Beers Criteria should be reviewed in all geriatric patients.[77]
- Social history: Smoking cigarettes is both directly, through weakened BMD, and indirectly linked to osteoporosis and fractures, with reduced levels of estrogen and earlier menopause.[19] Similarly, consumption of more than 2 units of alcohol daily in women (more than 3 in men) is associated with weakened BMD and can indirectly increase the risk of falls through depression of the central nervous system.[19] Illicit substance use that includes exogenous anabolic steroids or opioids should be screened for, which can lead to hypogonadism and increased risk for fractures. Physical activity should be assessed to determine if patients are active or sedentary. Occupation should additionally be asked, which may be linked to physical activity. Furthermore, the amount of outdoor time and sunlight exposure should be investigated, as this is usually related to overall vitamin D levels.
- Dietary history: The patient’s diet should be reviewed, any dietary restrictions assessed, and the amount of daily calcium and vitamin D intake evaluated. Patients should also be asked if they are taking over-the-counter supplements, which may include vitamin D and calcium.
Physical Examination
Although history is the most important tool in assessing a patient, the physical examination can furthermore be used to estimate risk, guide decision-making with treatment, and allow for diagnosis of secondary conditions.
- Height and weight: Routine height measurements should be obtained at each medical encounter; a decrease in height can be a subtle sign of a vertebral fracture. A height loss of 1.5 inches or more is an indication to obtain vertebral imaging.[19] With progressive vertebral collapse, patients may often show a kyphotic posture, colloquially known as the ‘Dowager’s Hump’ with upper spine involvement.[78] Patients who are underweight (weight <127 lbs or body mass index <21kg/m2) are considered the lower quartile of weight for women older than 65 in the United States and are at risk for low BMD and subsequent fractures.[19]
- Mobility: Gait, balance, strength, and mobility should be assessed at each clinical encounter to assess the likelihood of an impending fall and subsequent fracture.
- Malnutrition: Malnutrition may be readily noted on an exam, evidenced by nail changes, hair changes, loss of muscle mass, changes in menstruation, altered libido, dry skin, and a low body mass index. Such patients are likely to have decreased BMD and are at a heightened risk for fractures.
- Hormonal excess: Signs of hormonal excess may suggest an underlying cause of osteoporosis (or be identified as a risk for osteoporosis). Examples include the presence of hyperthyroid symptomatology like a goiter, tremors, exophthalmos, or tachycardia, and a cushingoid appearance like moon facies, a dorsocervical fat pad, and abdominal striae.
- Hormonal deficiency: Signs of hypogonadism may be present, suggestive of either premature ovarian failure or menopause (depending upon the age and clinical condition). Examples may include poor libido, infertility, vaginal dryness, breast changes, and cessation of the menses.
- Oral exam: While an oral exam does not identify osteoporosis, nor provide insight into prognosis, long-term treatment with bisphosphonates or denosumab can lead to osteonecrosis of the jaw, and it is therefore recommended by the AACE to perform an oral examination assessing baseline hygiene and bone health if considering treatment with such agents.[74]
Evaluation
Evaluating osteoporosis in females involves a comprehensive assessment of risk factors, clinical history, and diagnostic testing. Early and accurate identification is essential for timely intervention and fracture prevention.
Diagnosis
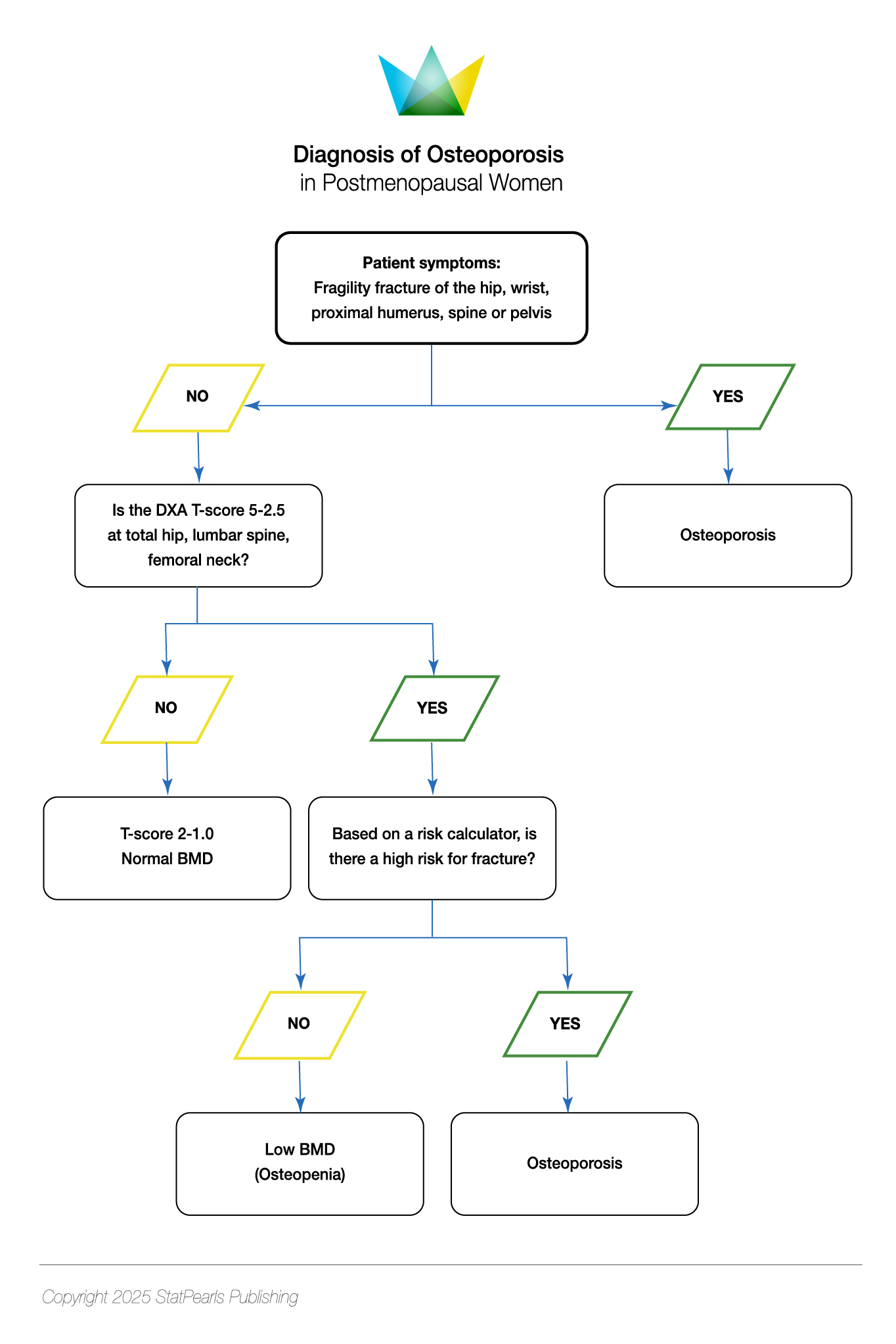
Osteoporosis was defined in 1993 by the World Health Organization (WHO) as a systemic disease involving skeletal tissue with low bone mass and distorted microarchitecture, causing fragility and an increased susceptibility to fractures.[79] In 1994, the WHO provided a radiological definition of osteoporosis, with a BMD of -2.5 standard deviations (SD) or lower than the mean value for young adult women (T-score of ≤ - 2.5 SD) on dual-energy x-ray absorptiometry (DEXA) of the lumbar spine, femoral neck, total proximal femur or distal one-third (33%) of the radius.[32] The definition was further expanded to include those with a history of fragility fractures of the spine or hip (spontaneous or occurring with minimal trauma), irrespective of BMD; it should be noted that the majority of fractures in postmenopausal women occur without a densitometric diagnosis of osteoporosis [International Osteoporosis Foundation - Epidemiology of osteoporosis and fragility fractures. 2024. https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures#ref_bottom_68]. Osteoporosis is classified by the WHO based on T-scores as follows:
- Normal: ≥-1.0 SD
- Osteopenia: -1.0 to -2.5 SD
- Osteoporosis: ≤-2.5 SD [80]
The WHO provides a further category, known as ‘severe’ (established) osteoporosis, which includes a T-score of ≤-2.5 SD and 1 or more fragility fractures.[81] While a T-score of ≤-2.5 SD confers the greatest risk for fracture, overall, there are more fractures with a T-score of –1.0 to –2.5 SD due to more patients in this category.[20] Therefore, osteoporosis can be diagnosed with a T-score between -1.0 and -2.5 SD if there is an increased fracture risk using risk-assessment tools (with country-specific thresholds).[74] A T-score between -1.0 and -2.5 SD and a fragility fracture of the proximal humerus, pelvis, or distal forearm may furthermore be classified as osteoporosis (see Table. Diagnosis of Osteoporosis in Postmenopausal Women).[74] The Study of Osteoporotic Fractures (SOF) was a landmark trial performed over 31 years (1986-2017), which provided insight into risk factors and fracture risk prediction in women; the SOF accurately predicted, via DEXA, the risk for osteoporosis-related fractures in asymptomatic women.[82]
While the T-score compares the patient in question to a young adult reference population, the Z-score compares the bone density of a woman to an age-matched population; a Z-score of -2.0 SD or lower is below the expected range for that age and should prompt assessment for secondary causes.[20] The WHO thresholds are based on data demonstrating the fracture risk in postmenopausal women; as a result, the International Society for Clinical Densitometry recommends using the WHO classification with postmenopausal women, and also with men, 50 years of age and older, but advises against using this criterion in those patients younger than 50.[83]
BMD is one of the strongest predictors of fracture, particularly of the hip.[74] The decrease in BMD at the site measured does not solely confer fracture risk at that site but can also be used to predict fracture risk at other sites. A 1 SD decrease at the lumbar spine, femoral neck, and distal radius, and corresponding fractures are as follows:
- Lumbar Spine: All Fractures: 1.7-fold increase
- Vertebral Fracture: 2.3-fold increase
- Hip Fracture: 1.6-fold increase
- Forearm Fracture: 1.5-fold increase
- Femoral Neck: All Fractures: 1.6-fold increase
- Hip Fracture: 2.6-fold increase
- Vertebral Fracture: 1.8-fold increase
- Forearm Fracture: 1.4-fold increase
- Distal Radius: All Fractures: 1.4-fold increase
- Forearm Fracture: 1.7-fold increase
- Vertebral Fracture: 1.7-fold increase
- Hip Fracture: 1.8-fold increase [84]
While the lumbar spine and femoral neck can be used, the North American Menopause Society (NAMS) notes that the strongest correlation between BMD and fracture risk is at the hip. The spine is prone to interference from surrounding vasculature, calcification, and osteophytes. However, the spine demonstrates less variability and can detect earlier responses to treatment. When neither the hip nor the spine can be used, the distal one-third (33% radius) can be used and is also recommended at the site to measure patients with primary hyperparathyroidism.[19] Although some guidelines recommend measuring BMD concurrently at 2 spots (most commonly used being the proximal femur and lumbar spine), the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis notes that this recommendation does not improve overall fracture prediction and can lead to T-score discordances.[85] A T-score discordance can lead to an approximate 10% change in fracture risk for each unit of T-score discordance.[17]
Screening
The Bone Health and Osteoporosis Foundation, alongside the AACE and the USPTF, recommend obtaining a DEXA scan for all women 65 years and older, with a frequency no greater than once every 1 to 2 years.[28][74] NAMS additionally recommends bone density screening in patients with a history of fractures since menopause, those with a known medical cause of bone loss (or fracture), and in females 50 years or older with 1 additional risk factor, such as weight less than 127 lbs, BMI less than 21 kg/m2, parental history of hip fracture, current tobacco user, or if discontinuing estrogen with additional risk factors for fractures.[19] In 2025, the USPTF updated its guidelines to provide a Grade B recommendation to screen for osteoporosis in females younger than 65 with 1 or more risk factors.[86]
NAMS recommends using the Osteoporosis Self-Assessment Tool to select young postmenopausal women to undergo BMD testing. The Osteoporosis Self-Assessment Tool requires 2 inputs, age and body weight, and identifies those likely to have low BMD.[19] The best-known fracture risk assessment is the Fracture Risk Assessment Tool (FRAX), which was developed in 2008 and is recommended by multiple societal guidelines, including (but not limited to) the AACE, Endocrine Society, and the American Society for Bone and Mineral Research.[87] Other tools include the Garvan Fracture Risk, the QFracture, the POL-RISK, the Canadian Association of Radiologists and Osteoporosis Canada Calculator (CAROC), the Simplified Calculated Osteoporosis Risk Estimation (SCORE), Osteoporosis Index of Risk (OSIRIS), and the Osteoporosis Risk Assessment Instrument (ORAI). The AACE and the National Osteoporosis Foundation recommend using the FRAX score in postmenopausal women older than 50.[20][74]
The FRAX tool allows for input of age, sex, height, weight, prior fracture, parental hip fracture, tobacco use, corticosteroid use, rheumatoid arthritis, alcohol, secondary osteoporosis, and femoral neck BMD (if known); notable drawbacks of FRAX include failing to include falls as a risk factor, not allowing for certain medications linked to low BMD, failure to include diabetes mellitus as a risk factor, not allowing for the quantification of corticosteroid dose, alcohol and cigarette use, nor duration, and type of fracture or recency of fracture. Moreover, FRAX may underestimate the risk for those with discordant bone mineral densities (such as low spine but normal femoral neck). Moreover, FRAX has not been studied in those with current (or prior) pharmacotherapy.[19]
In a 65-year-old White female with a BMI of 25 kg/m2 and no risk factors, who does not have a documented BMD at the femoral neck, the 10-year risk of a major osteoporotic fracture is 9.3%, and the risk of hip fracture is 1.3%. At the same time, the USPTF does not recommend using these thresholds for decision making; they state that the results of the risk assessment can assist with informing decisions about further screening with DEXA.[86] FRAX furthermore can help with deciding upon commencing pharmacological therapy when the 10-year risk for hip and major osteoporotic fractures is elevated; the latest limits used for recommending pharmacotherapy are a 10-year risk for hip fracture of 3% or more or greater or over and for major osteoporotic fracture, a risk of 20% or greater.
Laboratory Tests
The AACE recommends obtaining a baseline complete blood cell count, comprehensive metabolic panel (with calcium, phosphate, total protein, albumin, alkaline phosphatase, liver enzymes, creatinine and electrolytes), 25-hydroxy-vitamin D and 24-hour urine calcium/sodium/creatinine excretion in all patients at baseline, with the latter test aiming to assess for calcium malabsorption or hypercalciuria.[74] Additional tests that could be considered based on the clinical context to evaluate for secondary causes include the following:
- Thyroid-stimulating hormone (hyperthyroidism)
- Intact parathyroid hormone (hyperparathyroidism, primary and/or secondary)
- Serum protein electrophoresis and free kappa/lambda light chains (multiple myeloma)
- Intestinal biopsy (celiac disease)
- 24-hour urinary free cortisol (Cushing syndrome)
- Serum tryptase or urine N-methylhistidine (mastocytosis)
- Rheumatoid factor (rheumatoid arthritis)
- Prolactin, follicle-stimulating hormone, luteinizing hormone (hypogonadism)
- Skin biopsy (connective tissue diseases)
- Genetic markers (such as COL1A for osteogenesis imperfecta) [20]
Additionally, the AACE recommends obtaining baseline bone turnover markers at initial evaluation and follow-up; elevated levels may predict a rapid loss of bone and heightened fracture risk.[74] The International Federation of Clinical Chemistry recommends serum bone formation marker P1NP (aminoterminal propeptide of type 1 collagen) and bone resorption marker CTX-1 (C-telopeptide of type 1 collagen cross-links) to be used as the reference markers for bone turnover markers.[88]
Other Studies
Although DEXA is the gold standard for assessing BMD, limitations to DEXA include the inability to separate trabecular from cortical bone. DEXA does not provide information on the changes in size or geometry of bones with age.[89] This point is relevant as most fractures in those older than age 65 occur at cortical sites, and postmortem studies in females demonstrate that most bone loss is related to intracortical porosity.[90] Other modalities that are used instead of (or in addition to) DEXA include the following:
- Trabecular bone score: Performed in addition to DEXA as add-on software, analyzing variation in the grey level (trabecular structure) at the lumbar spine, and assessing homogeneity of bone texture. The score is not used to diagnose osteoporosis, nor can it guide treatment decisions. Still, it can improve the accuracy of fracture prediction, especially when combined with FRAX.[74]
- Vertebral fracture assessment: Incorporated with DEXA technology (often done simultaneously) to image the spine and identify vertebral fractures with a lower cost and less radiation exposure than traditional plain radiography. The European Society for Clinical and Economic Aspects of Osteoporosis recommends obtaining vertebral fracture assessment as either lateral lumbar and thoracic spine radiography or lateral spine DEXA imaging.[17] The International Society for Clinical Densitometry recommends obtaining vertebral fracture assessment in patients with a T-score ≤-1.0 and at least 1 of the following: female age 70 and older, height loss ≥4 cm, self-reported history (but most of the times undocumented) of prior vertebral fracture, or corticosteroid therapy ≥5mg of prednisone daily for ≥3 months.[91]
- Peripheral DEXA: A portable device incorporating the same technology used in central DEXA measurements, which can be used at the calcaneus, finger, and forearm. This carries a risk of both technical error and misinterpretation of fracture risk. Moreover, there are no standardized references for T-scores at peripheral sites.[92]
- Quantitative heel ultrasound: Calculates stiffness rather than BMD. Although more convenient and without radiation exposure, this ultrasound cannot be used to classify or diagnose osteoporosis as it does not measure BMD. Moreover, it cannot be used for monitoring therapy and has not been shown to reduce fracture risk.[92]
- Quantitative computed tomography: This imaging technique assesses the volumetric density of bone (g/cm3) and is therefore not affected by the size of bones. This imaging analyzes separate cortical and trabecular bone structures, can identify fractures and surrounding areas (even assessing healing), and investigates metastatic disease.[92] Although the cost is similar to DEXA, it involves more radiation exposure and cannot be used to diagnose osteoporosis.[20] The International Society of Clinical Densitometry notes that vertebral fractures can be predicted in postmenopausal women, and a peripheral quantitative computed tomography at the distal radius can also predict hip fracture risk (but not vertebral) in this population. The International Society of Clinical Densitometry notes that if a central DEXA cannot be obtained, quantitative computed tomography of the spine (or peripheral quantitative computed tomography of the distal radius) can be used to assess patients who would benefit from pharmacological therapy. Similarly, quantitative computed tomography can be used to monitor treatment at the lumbar spine [The International Society For Clinical Densitometry, 2019. https://iscd.org/learn/official-positions/adult-positions/].
- Hip Structural Analysis: Structural properties of the femur can be predicted based on the DEXA scan, considering multiple parameters and characteristics that help predict an index of strength, which could subsequently estimate the likelihood of the femur withstanding impact to the greater trochanter.[93]
- Finite element analysis: Also referred to as a structural engineering model, this is a 3-dimensional image of bone created using computed tomography or DEXA images that undergoes electronically simulated forces until a fracture occurs. This can be used for assessing bone strength but is limited to the research setting, and cannot be used for diagnosis, decision-making regarding treatment, or monitoring treatment response.[94]
- Radiofrequency echographic multispectrometry: This non-ionizing (ultrasound) technique assesses bone strength at axial sites of the skeleton.[95]
- Pulse-echo ultrasonography: This sonographic technique measures cortical bone thickness at peripheral sites, providing a density index.[96]
Treatment / Management
Regardless of the improvement in the T-score with treatment, the diagnosis of osteoporosis persists.[74] Treatment can involve both nonpharmacological and pharmacological options.(A1)
Nonpharmacological Treatments
- Alcohol: The AACE advises postmenopausal women to limit alcohol intake to no more than 2 units of alcohol daily, as it has been shown that consuming more than 3 units per day is associated with a 38% increase in major osteoporotic fractures and a 68% increase in hip fractures.[19] Conversely, numerous studies, such as Epidémiologie de l'Osteoporose (EPIDOS), noted that moderate drinking was associated with a protective relationship and a higher trochanteric BMD in older women.[97]
- Smoking cessation: Women who smoke are noted on average to have lower BMD and an estimated 30% increase in risk of fractures, which is independent of BMD.[19] On average, smokers are thinner, experience menopause at a younger age, and have lower estradiol levels.[19] Studies have furthermore noted that smoking cessation can lead to an increase in BMD and reduce fracture risk; similarly, smoking cessation appears to be associated with increased bone formation marker levels, such as osteocalcin.[98][99]
- Physical activity: Moderate exercise is associated with an improvement in BMD. Moreover, physical activity during young adulthood maximizes BMD, limits the associated loss of bone with aging, and can improve both stability and strength, overall minimizing the risk of falls and fractures in older individuals.[38] Skeletal adaptations are slow, taking a minimum of approximately 8 months of exercise to achieve a significant change in bone mass (as it takes around 4 months to complete bone remodelling), and benefits do not persist (but are shown to be lost) when physical activity is stopped.[38] Therefore, the Bone Health and Osteoporosis Foundation recommends lifelong physical activity, including weight-bearing exercise, balance training, and muscle strengthening for 30 minutes, 5 days per week (or 75 minutes twice weekly).[21] Numerous programs, such as tai chi, target strength and balance, and many others, protect against falls in older individuals.[100] Patients should also be encouraged to maintain a straight spine and avoid arching (and twisting) when possible, ultimately protecting the spine.[38] The AACE advises healthcare professionals to provide counseling on reducing fall risk and consider a referral to physical therapy to prevent falls.[74] Numerous studies have demonstrated the benefits of physical activity in postmenopausal females, including the following:
- Erlangen Fitness and Osteoporosis Prevention Study (EFOPS): Long-term exercise reduced fracture risk and slowed BMD decline.[101]
- European Prospective Osteoporosis Study (EPOS): Regular physical activity (resistance and weight-bearing) increased BMD and reduced fracture risk.[102]
- Lifting Intervention For Training Muscle and Osteoporosis Rehabilitation (LIFTMOR): High-intensity and resistance training improved BMD.[103]
(A1)
- Diet: Adequate dairy intake is required to attain regular calcium intake. Additionally, especially in patients with sarcopenia, high protein intake may lead to a reduction in falls. In patients hospitalized with a fracture, limited data support the role of a high-protein diet in shortening recovery time and improving functional recovery.[104] (A1)
- Calcium intake: Numerous societies, including but not limited to the AACE, advise a dietary intake of 1200 mg of calcium per day for women older than 50.[74] The average dietary calcium intake in the United States is around 600 to 800 mg, with nearly 33% coming from dairy products.[74][105] The European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis advises calcium supplementation only when dietary intake is less than 800 mg daily.[17] There is conflicting data on whether excess calcium supplementation (with or without vitamin D) can increase cardiovascular risk and mortality; the National Osteoporosis Foundation suggests there is no link and states a daily intake of up to 2000 to 2500 mg of calcium is safe from a cardiovascular standpoint.[106] (A1)
- Vitamin D supplementation: The AACE advises maintaining serum 25-hydroxy-vitamin D above 30 ng/mL in osteoporotic patients, stating a daily dose of 1000 to 2000 IU of vitamin D3 is typically sufficient, with higher dosages likely needed in obesity and malabsorptive states.[74] The USPTF concluded that there was insufficient evidence regarding daily vitamin D and calcium supplementation to prevent postmenopausal fractures.[107] Moreover, there is conflicting evidence of reducing fall risk in older individuals with vitamin D; however, the USPTF recommends against prescribing vitamin D solely for this purpose.[108] Similarly, there is conflicting evidence regarding reducing fracture risk and increasing BMD in patients taking vitamin D supplementation. However, most trials were done with concurrent calcium, not vitamin D alone. The European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis advises calcium (500 mg to 1200 mg) and vitamin D supplementation (400 IU to 800 IU) in daily doses in patients who receive bone protective pharmacologic therapy, as they note most trials assessing osteoporosis therapy administered concurrent calcium and vitamin D.[17] (A1)
- Other supplements: Probiotics, magnesium, vitamin K1, and phytoestrogens are not recommended to prevent or manage postmenopausal osteoporosis and fractures due to limited and insufficient data.[19] Moreover, vitamin A is not recommended, as excessive dosages have shown a detrimental effect on bones.[109] While data regarding caffeine and BMD is inconclusive, the AACE recommends limiting caffeine intake to <1-2 servings (8-12 ounces) of caffeinated drinks per day, citing observational studies demonstrating reduced calcium absorption and increased rates of fractures.[74] (A1)
- Equipment: Devices to assist with mobilization, such as a walker or roller, are recommended for patients with impaired mobility. Moreover, a hip protector can be considered for those at recurrent risk of falls.
Pharmacological Treatments
Pharmacological management is indicated for the 'prevention' and 'treatment' of osteoporosis and associated fractures; the distinction is essential as the Food and Drug Administration's (FDA) designated approval and dosage of medication differ based on the primary reason for administration. Numerous societies, including the Endocrine Society, recommend calcium, vitamin D, and adjunctive measures for osteoporosis therapy in postmenopausal women.[110] The AACE recommends treatment for female patients with the following:(A1)
- Low BMD and a history of fragility fractures at the hip or spine
- T-score ≤-2.5 SD in the total hip, femoral neck, or distal one-third radius
- T-score between –1.0 and–2.5 SD with a FRAX score of 10-year probability for a major osteoporotic fracture ≥20%, or ≥ 3% 10-year probability for a hip fracture [74] (A1)
Furthermore, the AACE advises consideration for treatment in other females with the following:
- Recent fracture within the past 12 months
- Fracture while on approved osteoporosis therapy
- Multiple fractures
- Fractures while on any medications known to cause harm to the skeleton [74] (A1)
Current medications approved for prevention of bone loss in postmenopausal females without a diagnosis of osteoporosis include estrogen preparations (with and without progestogen), bazedoxifene (with estrogen preparation), raloxifene, alendronate, risedronate, ibandronate, zoledronate, and tibolone (the latter is not available in the United States).[19] Regarding treatment, classes are categorized as 'antiresorptive' or 'anabolic', with certain medications functioning as a combination of both classes. Additionally, medications can be classified according to which site (hip, vertebral, and nonvertebral) reduces the risk of fractures (see Table. Approved Medications for Prevention and/or Treatment of Bone Loss).
Table. Approved Medications for Prevention and/or Treatment of Bone Loss
|
Medication |
Hip Fracture Reduction |
Vertebral Fracture Reduction |
Nonvertebral Fracture Reduction |
Prevention |
Treatment |
|
Alendronate |
Y |
Y |
Y |
Y |
Y |
|
Ibandronate |
N |
Y |
N |
Y |
Y |
|
Risedronate |
Y |
Y |
Y |
Y |
Y |
|
Zoledronate |
Y |
Y |
Y |
Y |
Y |
|
Denosumab |
Y |
Y |
Y |
N |
Y |
|
Teriparatide |
N |
Y |
Y |
N |
Y |
|
Abaloparatide |
N |
Y |
Y |
N |
Y |
|
Romosozumab |
Y |
Y |
Y |
N |
Y |
|
Calcitonin |
N |
Y |
N |
N |
Y |
|
Raloxifene |
N |
Y |
N |
Y |
Y |
|
Bazedoxifene |
N |
Y |
N |
Y |
N |
|
Estrogen +/- Progestogen |
Y |
Y |
Y |
Y |
N |
|
Tibolone |
N |
Y |
Y |
Y |
N |
|
Strontium Ranelate |
Y |
Y |
Y |
N |
Y |
Antiresorptive Therapy
Bisphosphonates
Bisphosphonates are the most commonly used therapeutic agents prescribed for osteoporosis, with an estimated 150 million prescriptions between 2005 and 2009 in the United States, with 5.1 million patients older than 55 receiving a prescription for a bisphosphonate in 2008.[111] Currently, bisphosphonates are within the third generation, and the FDA approves 4 for both prevention and treatment of osteoporosis (alendronate, ibandronate, risedronate, and zoledronate).[38][112](B2)
- Alendronate is a second-generation bisphosphonate approved in 1995, which is administered orally.[113] Prevention dosages include 5 mg oral daily or 35 mg oral weekly.[74] Treatment dosages include 10 mg oral daily or 70 mg oral weekly; special formulations exist, including combination with vitamin D, 2800 IU (or 5600 IU) of vitamin D as weekly administration, and an effervescent tablet.[74] Alendronate was shown to reduce all types of fractures and increase BMD in the Fracture Intervention Trials (FIT), Fracture Intervention Trial Long-Term Extension (FLEX), and Fosamax International Trial (FOSIT).[114][115][116] Alendronate is also used in the prevention and management of steroid-induced osteoporosis.[117] (A1)
- Ibandronate is a third-generation bisphosphonate approved in 2003, which is administered orally.[118] Prevention dosages include 2.5 mg oral daily or 150 mg oral monthly; treatment dosages are the same as prevention.[74] Moreover, ibandronate treatment dosage is available as a 3 mg intravenous infusion administered every 3 months.[74] Ibandronate differs from the 3 other approved bisphosphonates, as it has only been shown to reduce vertebral fractures (but not the hip or nonvertebral fractures).[19] Ibandronate was studied in the Oral Ibandronate Osteoporosis Vertebral fracture Trial in North America and Europe (BONE), Monthly Oral Ibandronate in Ladies (MOBILE), and Dosing IntraVenous Administration (DIVA) trials.[119][120][121] (A1)
- Risedronate is a third-generation bisphosphonate approved in 2000, which is administered orally.[122] Prevention dosages include 5 mg oral daily, 35 mg oral weekly, or 150 mg oral monthly; similar to ibandronate, the treatment dosages for risedronate are the same as prevention doses.[74] Risedronate does have a special formulation that can be taken with or without food and is delayed-release (past the stomach), which is beneficial in patients with preexisting upper gastrointestinal disease. However, the rates of adverse gastrointestinal events (including upper) are not lowered.[74] Risedronate was studied in the Vertebral Efficacy with Risedronate Therapy North America (VERT NA), Vertebral Efficacy with Risedronate Therapy Multinational (VERT MN), Hip Intervention Program (HIP), and the Risedronate and Alendronate (REAL) studies.[123][124][125] (A1)
- Zoledronate is a third-generation bisphosphonate approved in 2007, which is administered intravenously.[126] The prevention dosage is 5 mg every 2 years, whereas the treatment dosage is 5 mg yearly.[74] Zoledronic acid was studied in the Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly (HORIZON; both Pivotal Fracture and Recurrent Fracture Trials), and the ZOledroNate Treatment in Efficacy to Osteoporosis (ZONE) trials.[127][128][129] (A1)
Bisphosphonates function to bind to hydroxyapatite in bone (at sites of active remodeling) to reduce bone formation; this is achieved through inhibition of proton vacuolar adenosine triphosphatase (ATPase), and alteration of the ruffled border and cytoskeleton.[74] Moreover, aminobisphosphonates inhibit the farnesyl pyrophyophoate synthase (involved in the mevalonate pathway), inhibiting isoprenylation of guanosine triphosphate binding proteins.[38] The bone affinity of the 'broad-spectrum' bisphosphonates ranks as follows: zoledronate, alendronate, and risedronate.[74](A1)
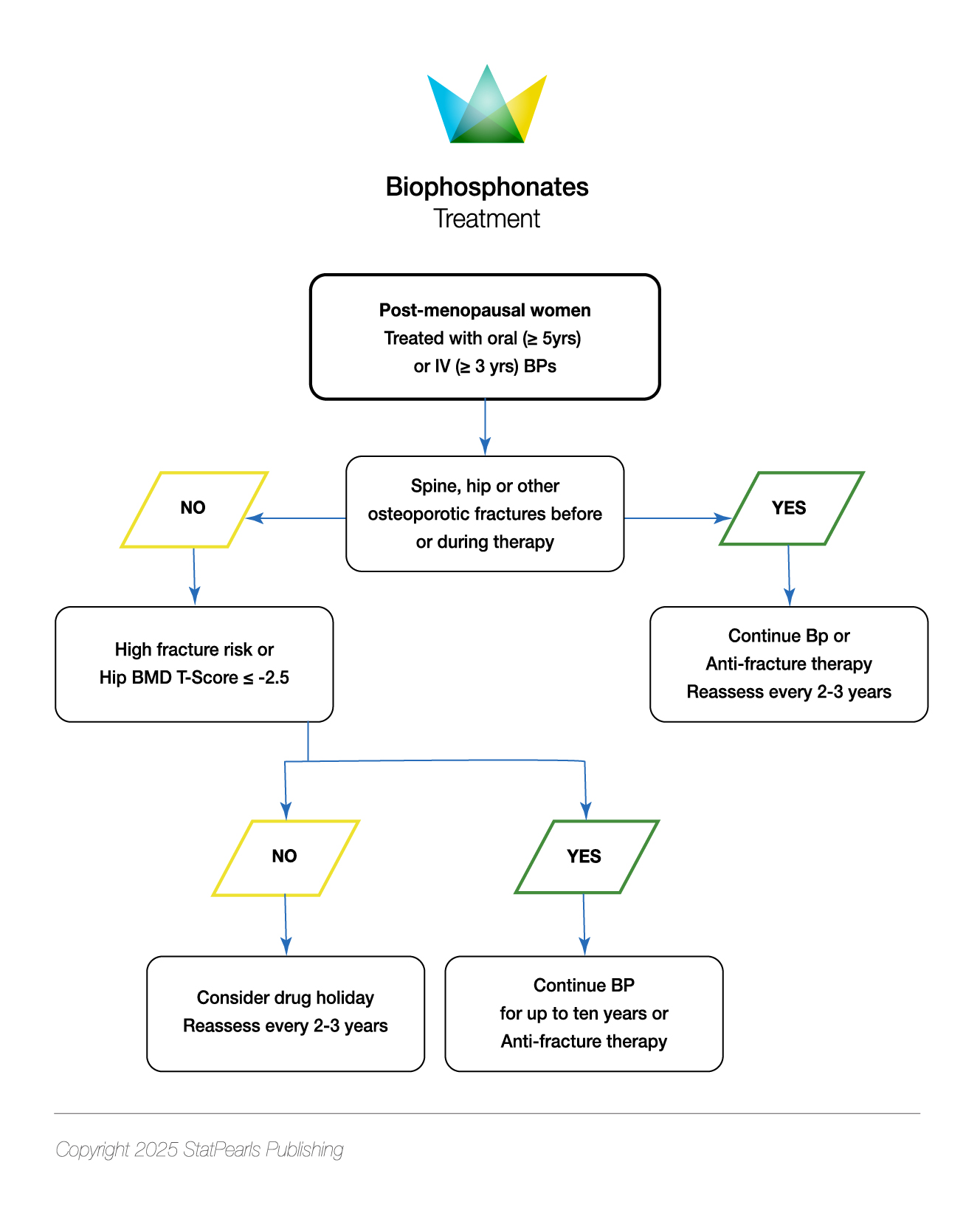
Oral bisphosphonates are inadequately absorbed (<3% in a fasting state) and rapidly cleared from the plasma, with about half deposited in the bone and the rest renally excreted.[38] The half-life of bisphosphonates is prolonged, in the manner of years, and differs from the other classes of medications, in that the effects may persist for years following discontinuation.[130] Oral bisphosphonates are typically the first-line treatment for patients not at very high risk for fractures. Oral bisphosphonates must be taken after prolonged fasting (typically taken in the morning), with a full glass of water, followed by sitting upright for 30 to 60 minutes and waiting this period before ingesting food or administering other medications; despite this, however, patients may experience dyspepsia.[74](A1)
In patients who are unable to follow the administration regimen noted previously, or who have active esophageal disease (such as strictures, achalasia, varices, or scleroderma), or who have malabsorptive states (such as celiac disease, Crohn disease, infiltrative disease, or prior gastric bypass), intravenous formulation is recommended.[74][110] All patients must have their vitamin D levels measured and treated before starting therapy due to the risk of hypocalcemia.[110] Risedronate and ibandronate cannot be used for a glomerular filtration rate less than 30 mL/min, and alendronate and zoledronic acid cannot be used with a glomerular filtration rate less than 35 mL/min (rapid administration of intravenous nitrogen-containing bisphosphonates can lead to both transient and permanent decreases in renal function).[74](A1)
Administration of intravenous zoledronic acid has been associated with an 'acute phase reaction', which occurs after the first time of medication administration in about 30% of subjects and is characterized by muscle aches, flu-like symptoms, and fever, which can last for several days; pretreatment with acetaminophen may reduce symptoms.[74] Moreover, there is inconsistent evidence regarding an increase in atrial fibrillation with intravenous zoledronic acid, which was noted in the HORIZON study.[131] Uveitis has rarely been linked to bisphosphonate therapy, and conflicting evidence has suggested an increased risk of esophageal cancer; however, the FDA has concluded there is no definitive association.[74][132] Bisphosphonates have been associated with osteonecrosis of the jaw and atypical femur fractures.[17] Despite the beneficial effects of bisphosphonates, due to the long list of adverse effects, approximately 50% of patients will discontinue bisphosphonate therapy within 1 year of use.[38](A1)
Denosumab
Denosumab is a fully human monoclonal antibody, approved in 2010 for the treatment of osteoporosis.[133] This drug's mechanism of action involves targeting the receptor activator of the nuclear factor Kappa B receptor ligand (RANKL), which prevents its attachment to the RANK.[133] Denosumab is administered as a 60 mg subcutaneous injection every 6 months and does not have a limit of duration. However, the Endocrine Society recommends reassessing fracture risk after 5 to 10 years of use and considering continuing or transitioning to other therapy if fracture risk remains high.[133][134] Similar to bisphosphonates, long-term risks include osteonecrosis of the jaw and atypical femur fractures (albeit a reduced risk compared to bisphosphonates) and skin rash, cellulitis, and injection site reactions.[74] Denosumab, unlike bisphosphonates, is safe to use in patients with renal disease.[135] Denosumab is approved for the treatment of glucocorticoid-induced osteoporosis in both men and women at high risk for fracture, and is recommended for women on aromatase inhibitor therapy for breast cancer.[133](A1)
Data suggest that switching from bisphosphonates to denosumab can lead to additional gains in BMD.[136] Denosumab is noted to reduce vertebral, hip, and nonvertebral fractures, as noted in the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) and Denosumab and Calcium Osteoporosis Study (DANCE) studies.[133][137] Upon discontinuation of denosumab therapy, there is a risk for rapid loss of bone and rebound fractures (particularly vertebral), as noted in the extension of the FREEDOM trial; the risk appears to be related to the duration of treatment, and is more commonly seen in those with prior vertebral fractures.[133] Additionally, this phenomenon appears to be more common in women.[138] After discontinuation, therapy must be provided with bisphosphonates (alendronate or zoledronic acid); bisphosphonates are preferred over anabolic agents, which can lead to a transient decrease in BMD.[139](A1)
Bazedoxifene
Unlike other countries, both the United States and Canada have only approved bazedoxifene in combination with a conjugated estrogen (20 mg bazedoxifene with 0.45 mg conjugated estrogen); the FDA approved bazedoxifene in combination with a conjugated estrogen in 2013 for the treatment of vasomotor symptoms and prevention of postmenopausal osteoporosis.[19][140] The rationale for such a combination was to increase BMD further and reduce hot flushes, alongside a reduced risk of breast tenderness and adverse effects upon the breast and uterus compared to monotherapy with estrogen.[110] Compared to estrogen/progestogen hormonal therapy, however, there is less of an increase in BMD.[110](A1)
The Selective Estrogens, Menopause, and Response to Therapy (SMART) trial assessed bazedoxifene combined with conjugated estrogens, demonstrating increased BMD at the lumbar spine and total hip and reduced bone turnover markers. However, bazedoxifene (with and without conjugated estrogen) has only been shown to reduce vertebral fractures.[141] Adverse events of bazedoxifene include leg cramps and deep venous thromboses; unlike raloxifene, the impact on breast cancer prevention is unknown.[110] Caution is furthermore advised in initiating hormone therapy in women older than 60.[19] Candidates for bazedoxifene with conjugated estrogen are typically younger postmenopausal women with a uterus who require symptomatic vasomotor relief and prevention of bone loss.[110](A1)
Raloxifene
Like bazedoxifene, raloxifene also belongs to the class of selective estrogen receptor modulators.[74] Unlike bazedoxifene, however, raloxifene was approved as monotherapy in 1997 for prevention and in 1999 for treatment of postmenopausal osteoporosis, administered as 60 mg orally once daily.[142] Raloxifene has only been shown to reduce vertebral fractures, as noted in the Multiple Outcomes of Raloxifene Evaluation Study (MORE), the Continuing Outcomes Relevant to Evista (CORE), and Selective Treatment of Osteoporosis with Raloxifene (STAR) trials.[143][144][145] Once raloxifene has been discontinued, the skeletal benefit is lost within the subsequent 1 to 2 years; furthermore, raloxifene may increase bone loss when administered to premenopausal women.[74][146] Raloxifene may cause hot flashes, leg cramps, deep venous thromboses, and fatal stroke.[110] A particular benefit to raloxifene, however, is the approval for the prevention of invasive breast cancer for those at high risk.[110] Raloxifene may be appropriate for postmenopausal women at high risk of invasive breast cancer, or those with low BMD in the spine but not the hip (discordance).[74][110](A1)
Hormonal Therapy
Hormonal therapy (estrogen with or without progestin) has been approved for the prevention of osteoporosis in postmenopausal females since 1986.[147] Estrogen appears to have both antiresorptive and anabolic effects on bone.[60] Numerous different formulations are available, as well as both oral and transdermal options. However, there are no significant differences between the two routes of administration; transdermal has a lower risk of venous thrombosis and stroke.[38] Data from the Women’s Health Initiative (WHI) noted health concerns with estrogen-progestogen therapy, with an increased risk of breast cancer, stroke, and thromboembolic events. In contrast, those with a hysterectomy receiving conjugated estrogen had an increase in stroke and deep venous thromboses, without breast cancer or coronary heart disease.[38]
Subgroup analysis noted the timing of hormonal therapy influences risks and benefits, with more favorable effects in those under age 60 or within 10 years of menopause.[148] Both the Kronos Early Estrogen Prevention Study (KEEPS) and the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial noted therapy increased BMD at both the hip and spine. The WHI noted a reduction in vertebral, hip, and nonvertebral fractures, with meta-analyses suggesting 5 to 7 years of treatment is required to reduce the fracture risk significantly.[149][150][151][152] The Endocrine Society solely recommends estrogen therapy in postmenopausal women at high risk of fractures with a prior hysterectomy, who are under 60, 10 years past menopause, low risk for deep venous thromboses, no prior myocardial infarction or stroke, no diagnosis of breast cancer, those with bothersome vasomotor symptoms, those willing to take menopausal hormone therapy, and for whom bisphosphonates or denosumab are contraindicated.[110](A1)
The AACE recommends cyclic or daily progestin in addition to estrogen in patients with a uterus to prevent endometrial hyperplasia; however, their advice is for treatment to be used at the lowest dose and for the shortest time necessary.[74] When hormonal therapy is discontinued, skeletal benefits are lost after a few months, with a decrease in BMD of around 6% within the first year (and by 2 years returns to the level of a woman who has never taken estrogen), and it has been noted that bone turnover markers return to levels seen pretreatment within a few months; the WHI noted discontinuation returned the fracture risk to baseline.[19](A1)
Strontium Ranelate
Strontium ranelate was previously approved in Europe for the treatment of osteoporosis in postmenopausal women but was never approved in the United States or Canada; it is no longer available due to the heightened risk for cardiovascular events, venous thromboses, and cutaneous adverse reactions, and so its production was discontinued by the manufacturer in 2017.[19][153] Strontium ranelate is a divalent cation with a molecular weight greater than calcium, and is deposited in the skeleton, increasing BMD, although some of this effect is likely to be an artifact.[19] Strontium was noted to reduce vertebral, nonvertebral, and hip fractures in postmenopausal women in the Spinal Osteoporosis Therapeutic Intervention (SOTI) and Treatment Of Postmenopausal Osteoporosis (TROPOS) trials.[154](B3)
Tibolone
Tibolone is a synthetic steroid with estrogenic, progestogenic, and androgenic properties, derived from Mexican yam.[19] This drug is available in Mexico but was never approved in the United States or Canada, and is approved for the prevention of postmenopausal fractures and is administered at 2.5 mg orally daily.[19] The Long-Term Intervention on Fractures with Tibolone (LIFT) study showed a reduction in both vertebral and nonvertebral fractures, without significantly affecting hip fractures.[155] The LIFT study also demonstrated beneficial effects in managing hot flashes and reducing the risk of falls and colon cancer.[155] Although tibolone has shown a decreased risk of breast cancer in the LIFT study, the Livial Intervention Following Breast Cancer: Efficacy, Recurrence and Tolerability Endpoints (LIBERATE) study noted an increased recurrence in those with prior breast cancer.[156] Moreover, tibolone is noted to increase the risk of vaginal discharge, bleeding and stroke.[110](A1)
Calcitonin
Calcitonin is a 32-amino acid peptide produced by parafollicular cells (also known as C-cells) within the thyroid gland.[38] Calcitonin's main function is to inhibit bone resorption by directly affecting osteoclasts.[157] Calcitonin was initially approved by the FDA for osteoporosis in 1984 as an injection; however, alternative (and more efficient) routes of administration were developed, and nasal spray was approved in 1995.[38][158] Calcitonin is approved for the treatment of osteoporosis for women who are at least 5 years past menopause.[38] A particular benefit of calcitonin is its ability to lead to rapid reductions in serum calcium and acute pain, especially in vertebral crush fractures.[159] Human and salmon calcitonin are similar, differing by 1 amino acid; therefore, therapeutic calcitonin is derived from salmon.[38](A1)
Injectable calcitonin is available at 100 IU daily (either subcutaneous or intramuscular), and nasal spray is available at 200 IU (1 spray) daily.[74] Limited results from m.studies, such as the Prevent Recurrence of Osteoporotic (PROOF) study, have demonstrated nasal calcitonin to reduce the risk of vertebral fractures in postmenopausal women, but not hip or nonvertebral fractures; no published data suggest a reduction in fracture risk with the injectable solution.[74][160] The main contraindication to calcitonin therapy is hypersensitivity, for which a skin test is recommended before treatment in those with clinical suspicion.[74](A1)
With parenteral administration, adverse effects include nausea, local inflammation, and vasomotor symptoms like sweating and flushing; with nasal administration, patients may experience nasal discomfort, irritation of the mucosa, and epistaxis.[74] When calcitonin is discontinued, skeletal benefits are rapidly lost over 1 to 2 years.[74] Calcitonin has been withdrawn from the European Medicines Agency and by Health Canada due to concerns regarding an association with various malignancies (including prostate and liver). However, the FDA did not find sufficient evidence to suggest a causal relationship but advised to evaluate the benefit-to-risk ratio with each patient.[74][110](A1)
Anabolic Therapy
Teriparatide
Contrary to persistent parathyroid hormone stimulation on the bone, intermittent administration leads to bone formation.[161] Teriparatide is a synthetic parathyroid hormone peptide containing the first 34 amino acids (out of 84) approved by the FDA in 2002.[19][162] Teriparatide has been shown to stimulate both endocortical and trabecular bone surfaces, increase skeletal mass and volume, improve microarchitecture, increase width, and improve bone strength.[19] Teriparatide is approved for the treatment of osteoporosis in those at high risk and has also been approved for glucocorticoid-related osteoporosis.[163] Teriparatide is administered as a 20 μg subcutaneous injection daily via a disposable pen device into the thigh or abdomen; the pen requires refrigeration between uses.[38] Furthermore, biosimilars are now available, as the patent of the initial preparation expired in 2019.[164](B3)
Teriparatide has been shown to increase BMD and reduce the risk of both vertebral and nonvertebral fractures; however, currently, there is no data to support a reduction in hip fractures.[74] The NAMS cites 1 meta-analysis showing a reduction in hip fractures, and the European Society for Clinical and Economic Aspects of Osteoporosis suggests this finding is likely due to an absence of evidence rather than evidence of absence when relating hip fracture reduction to teriparatide.[17][19] Teriparatide was studied in the Fracture Prevention Trial (FPT), Vertebral Efficacy with Risedronate and Teriparatide (VERO), Teriparatide Once-Weekly Efficacy Research (TOWER), EUROpean Study of FORSteo (EUROFORS), and Direct analysis of Nonvertebral Fracture in the Community Experience (DANCE) studies.[165][166][167][168][169] Adverse effects include injection site reactions, nausea, orthostatic hypotension, leg cramps, hypercalcemia (that is typically transient), increased urinary calcium excretion, and increased serum uric acid.[38](A1)
Previously, the FDA had a black-box warning for osteosarcoma and a limit of 2 years of usage; in November 2020, the black-box warning for osteosarcoma was removed as human data collected since approval in 2002 failed to show an increased risk of this type of tumor. Also, teriparatide may be used beyond 2 years in those patients who remain at a very high risk for fractures.[162] Baseline calcium, vitamin D, and alkaline phosphatase are required before initiation, and teriparatide should not be administered in individuals with hypercalcemia or those with suspected Paget’s disease, skeletal metastases, or radiation exposure that increases the risk for osteosarcoma.
At the same time, hypercalciuria incidence is not greater on this medication compared to placebo; caution is advised in using this medication in patients with a history of recent (or active) urolithiasis.[74] Similarly, while calcium monitoring is not required during treatment, it is important to note that serum calcium will reach its maximum value by 6 hours and return to baseline around 16 to 24 hours after every dosage.[74] Following discontinuation of teriparatide, the anabolic effects of bone are lost, and it is therefore recommended for the medication to be followed by a bisphosphonate or denosumab; the Endocrine Society furthermore recommends considering raloxifene or menopausal hormone therapy.[74][110](A1)
Abaloparatide
Abaloparatide is a synthetic analog created from a modified parathyroid hormone-related peptide using the first 34 amino acids and functions similarly to teriparatide, approved by the FDA in 2017.[74][170] Abaloparatide is a daily injection of 80 μg via a pen device; however, unlike teriparatide, it does not require refrigeration after use and can be stored at room temperature for up to 30 days.[38] The adverse event profile is similar for abaloparatide; however, hypercalcemia is less common (and less severe) compared to teriparatide.[171] While the black-box warning for osteosarcoma was dropped for both teriparatide and abaloparatide in November 2020, abaloparatide is still limited to 2 a total duration.[172](A1)
As with teriparatide, abaloparatide is contraindicated in those at high risk for osteosarcoma. BMD gains appear to be greater with abaloparatide than teriparatide, with a slightly greater significant fracture reduction with abaloparatide for major osteoporosis-related fractures. Similar to teriparatide, abaloparatide has been shown to reduce only vertebral and nonvertebral fractures.[19] Abaloparatide was studied in the Abaloparatide Comparator Trial in Vertebral Endpoints (ACTIVE), ACTIVExtend, and ACTIVE-J (in Japan) trials.[173][174][175] Again, bone loss occurs upon discontinuation, and an antiresorptive medication must follow treatment.[74](A1)
Romosozumab
Romosozumab is a novel medication that functions as a dual antiresorptive and anabolic agent and was approved in 2019.[74][176] This drug is an anti-sclerostin monoclonal antibody, activating the 'wnt' (wingless/integrated) signaling pathway.[176] In the United States, romosozumab is only approved for treatment in females [134] and is administered as a 210 mg monthly injection (2 injections of 105 mg), with treatment for up to 1 year.[177] Numerous trials, including the Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk (ARCH), Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) and the Study evaluating the effects of Romosozumab Compared to Teriparatide in postmenopausal women with osteoporosis transitioning from bisphosphonate therapy (STRUCTURE) all noted a reduction in vertebral, hip and nonvertebral fractures.[178][179][180] Romosozumab has a black-box warning of an increased risk for stroke and myocardial infarction, as the ARCH study noted a higher incidence in the romosozumab-treated group compared to alendronate; as a result, the medication is not recommended in those with a prior history of such.[74] With the completion of treatment, transition to an antiresorptive agent is advised to maintain the skeletal benefits obtained from the anabolic treatment.[74](A1)
Surgical Treatment
Occasionally, patients such as those at risk for vertebral fractures and those with a fracture requiring stabilization and pain relief may undergo surgical procedures. The 2 most common procedures performed include kyphoplasty and vertebroplasty. Kyphoplasty involves balloon inflation introduced surgically after approximating fractured bone pieces, with material introduced to harden and stabilize bones. In contrast, vertebroplasty directly inserts low-viscosity cement material into the collapsed vertebral body.[17] A vertebroplasty is used for unstable fractures to prevent further deformities or loss of height, and it differs from kyphoplasty in that it does not involve manipulation of fractured bone pieces.[74](A1)
Notable risks from these procedures include bone cement leak, persistent pain, allergic reaction to bone cement, infection, paralysis, and pulmonary embolism.[181] An additional concern is the potential for increased risk of vertebral fractures in adjacent vertebrae. However, evidence is mixed.[74] A further treatment is known as a local osteo-enhancement procedure, which involves the injection of a resorbable synthetic bone graft substitute into the femoral neck to increase BMD; on follow-up imaging, it appears to be completely resorbed and replaced with bone.[17](A1)
The AACE advises against either a kyphoplasty or vertebroplasty, citing unclear benefits and concern for increased risk of adjacent vertebral fractures.[74] The European Society for Clinical and Economic Aspects of Osteoporosis notes that these procedures can be considered after persistent pain for 2 to 3 weeks after a vertebral fracture despite adequate analgesia; however, it again cites the concern for new fractures (albeit evidence is inconsistent).[17](A1)
Initiation and Sequence of Therapy
The AACE advises stratifying patients based on risk of fracture to allow for deciding optimal therapy, typically to distinguish between 'high risk' and 'very high risk'.[74] Numerous societies provide differing definitions of 'very high risk'; however, the AACE defines as 'very high risk' those with a recent fracture (within the prior 12 months), fracture while on osteoporosis therapy, a history of multiple fractures, fractures while exposed on medications known to impair BMD (such as corticosteroids), a very low T-score (≤-3.0 SD), a high risk for falls (or history of falls leading to injury), or a FRAX-score of more than 30% for major osteoporotic fracture or more than 4.5% risk for hip fracture.[74] Moreover, the AACE states that those without the characteristics as mentioned above but with a diagnosis of osteoporosis are classified as 'high risk'.[74](A1)
The Endocrine Society, however, stratifies patients as 'low risk' when they have no history of spine or hip fractures, have BMD T-scores at hip and spine above –1.0 SD, 10-year hip fracture risk less than 3% and 10 year major osteoporotic fracture risk less than 20%; 'moderate risk' those with no history of spine or hip fractures, a BMD T-score at the hip and spine above –2.5 SD, a 10-year hip fracture risk less than 3% or 10-year major osteoporotic fracture risk less than 20%; 'high risk' those with history of spine or hip fracture, BMD T-score at hip or spine less than or equal to 2.5 SD, 10-year risk of hip fracture greater than or equal to 3% or major osteoporotic fracture greater than or equal to 20% and 'very high risk' those with multiple spine fractures and BMD T-score at the hip or spine less than or equal to 2.5 SD.[110] Neither the NAMS nor the European Society for Clinical and Economic Aspects of Osteoporosis provides cut-off levels (categories) for the risk of fractures. However, note that certain patients are at higher risk than others, and advise on assessing an individualized risk stratification.[17][19](A1)
Most guidelines, however, recommend using an antiresorptive agent in patients who are not at 'very high risk' for fracture; this typically entails an oral bisphosphonate (intravenous if there are contraindications to the oral route) or denosumab if bisphosphonates are contraindicated (such as in patients with renal failure). The National Osteoporosis Guideline Group, however, recommends intravenous zoledronic acid as first-line treatment following a hip fracture.[182] An individualized approach is recommended; for example, in a patient at risk for spinal fracture (and not other sites) as well as at risk for invasive breast cancer, raloxifene can be considered. Similarly, for females younger than 60 and within 10 years of menopause who have bothersome vasomotor symptoms and without contraindications (and are not at a 'very high risk' for fractures), bazedoxifene (with conjugated estrogen) or hormonal therapy can be considered.
For those who are at a heightened risk for fractures ('very high risk'), osteoanabolic agents (teriparatide, abaloparatide, and romosozumab) are recommended, followed by a transition to an antiresorptive agent to maintain the skeletal benefits which would otherwise be rapidly lost; this is typically a bisphosphonate or denosumab.[74] Treatment requires an individualized approach to consider contraindications to each medication and assess the site where the patient is at risk for fracture; for example, if a female patient is at very high risk for a hip fracture, neither teriparatide nor abaloparatide has demonstrated clinical risk reduction, and it may be more appropriate to commence romosozumab (see Table. Lifestyle and Nutritional Optimization for Bone Health for All Postmenopausal Women).(A1)
Neither the AACE nor the European Society for Clinical and Economic Aspects of Osteoporosis recommends combination therapy, citing the lack of adequately powered studies.[17][74] One exception, however, is noted by the AACE, for patients who are already on raloxifene for breast cancer prevention as the main indication, a bisphosphonate or denosumab can be added to reduce the risk of a hip fracture.[74] Limited data, however, suggest that administering zoledronate at the same time as teriparatide results in the most rapid increase in BMD at both the hip (achieved by the zoledronate) and lumbar spine (achieved by the teriparatide).[183] Similarly, the AACE noted that the greatest increase in BMD is seen with combined teriparatide and denosumab as demonstrated in the Denosumab and Teriparatide Administration (DATA) study; however, they cited no data to demonstrate fracture risk reduction and also noted that the bone formation markers were reduced.[74][184] Further limitations include excess cost and increased likelihood of adverse effects with combination therapy.[74](A1)
For patients on corticosteroid therapy, the American College of Rheumatology advises assessing clinical fracture risk in all patients continuing (or initiating) glucocorticoid therapy of at least 2.5 mg equivalent of prednisone for at least 3 months.[185] The International Osteoporosis Foundation and the European Calcified Tissue Society recommend therapy in postmenopausal women with an equivalent of at least 7.5 mg daily of prednisolone for at least 3 months, further advising treatment at the onset of commencing corticosteroid therapy.[186] Bisphosphonates are typically the initial therapy; denosumab is also approved for this indication (as is teriparatide), but is more expensive. One caveat concerns further infection risk with combined denosumab and corticosteroid therapy.
Duration of Treatment
The AACE defines successful treatment as stable or increasing BMD, without new fractures (or progression of vertebral fractures).[74] In those with recurrent fractures or who continue to demonstrate bone loss while on therapy, assessment for medication adherence and consideration for alternative causes should be pursued. The AACE notes that a single fracture does not suggest treatment failure and recommends considering 2 or more fractures as treatment failure.[74] Moreover, osteoporosis therapy does not eliminate the risk of fracture in its entirety, and differing societies debate the strength of the association between fracture and treatment failure. The Endocrine Society suggests that failure of therapy is defined as loss of BMD, which is greater than the least significant change (5% in the femoral neck, 4% in total hip, and 5% in the lumbar spine) over 2 years, with a decrease in bone turnover markers on antiresorptive therapy.[110](A1)
As noted, certain medications have a limited duration, such as romosozumab, which is approved for 1 year of use, and abaloparatide, which is approved for 2 years; teriparatide is no longer limited to 2 years and can be used in certain situations beyond this time. Following discontinuation of an osteoanabolic agent, an antiresorptive medication must be started. Non-bisphosphonate antiresorptive therapy (particularly denosumab) does not have a treatment limit and can be continued for as long as clinically indicated; however, upon discontinuation, there is a rapid loss of BMD and risk of vertebral fracture. This effect was particularly notable during the SARS-CoV-2 pandemic, whereby patients often saw delays in visiting their physician; upon discontinuing denosumab, a bisphosphonate must be started within 1 month to prevent rebound (especially vertebral) fractures.[74](A1)
Bisphosphonates differ from the other medications in that the treatment effects persist, even after discontinuation of the medication for a few years. Due to the increased risk for both atypical femur fractures and osteonecrosis of the jaw (which are strongly associated with the duration of treatment), patients are advised to consider a drug holiday after 5 years of oral treatment if fracture risk is no longer elevated (T-score >–2.5 SD or patient remaining fracture-free) and to continue for another 5 years if fracture risk persists.[111] With intravenous zoledronic acid, a drug holiday can be considered after 3 years, extended up to 6 years in those with a very high risk.[111] Once a drug holiday has started, BMD should be reassessed every 2 to 4 years. For those with increased bone turnover markers or a decrease in BMD, reinitiating therapy may be required (see Table. Bisphosphonates Treatment).[110](A1)
The AACE advises obtaining a DEXA scan every 1 to 2 years until findings are stable, after which the frequency can be decreased.[74] As noted above, for those on bisphosphonate therapy, a repeat DEXA should be considered at 5 years to assess if a drug holiday is possible. Additionally, the AACE recommends using bone turnover markers to assess patient compliance and therapy efficacy.[74] With antiresorptive therapy, reductions are seen (at or below the median value for premenopausal women), demonstrating a fracture risk reduction; increases suggest a favorable response to anabolic therapy.[74] The American Association of Clinical Chemistry recommends C-terminal telopeptide type-1 collagen (CTX) as the preferred resorption marker, and serum carboxy-terminal propeptide of type-1 collagen (PINP) as the preferred marker for bone formation.[187](A1)
Bone turnover markers are advantageous in detecting changes earlier (in 3 to 6 months) compared to waiting 1 to 2 years to see improvement on DEXA scans.[74] Bone turnover marker changes are compared with the least significant change (such as with BMD), with 56% for serum CTX and 38% for serum P1NP; if changes in bone turnover markers are greater than the least significant change, this suggests that the treatment goal has been reached.[110] The Endocrine Society states that up to 90% of women are expected to respond by 12 weeks on oral bisphosphonate therapy.[110] Bone turnover markers can help identify treatment failure or poor medication adherence. They may also guide decisions regarding drug holidays. If patients are compliant with their medication but show no biochemical improvement, this could indicate improper administration or issues with absorption.[110] However, one drawback to bone turnover markers is that after an acute fracture, bone turnover marker levels may transiently rise and can be misleading.[74] (A1)
Differential Diagnosis
When evaluating osteoporosis in females, it is essential to consider other conditions that can mimic or contribute to decreased bone density. The following is a list of differential diagnoses that should be ruled out during assessment.
The differential diagnosis for osteoporosis is as follows:
- Osteopenia
- Osteogenesis imperfecta
- Osteomalacia
- Vitamin D deficiency or insufficiency
- Hyperparathyroidism
- Hyperthyroidism
- Paget disease of the bone
- Mineral and bone disorders of chronic kidney disease
- Primary bone malignancies
- Metastases to the skeleton
- Avascular necrosis
- Hematologic malignancies
- Mimickers of vertebral osteoporosis (such as osteoarthritis, scoliosis, or Scheuermann disease) [188][189]
Prognosis
In the European Union, 43,000 deaths related to fractures were reported in 2010, with around 50% due to hip fractures, 28% to vertebral fractures, and 22% to other types of fractures.[17] An estimated 50% of postmenopausal women will experience a fragility fracture.[190] Once a fracture has been sustained, 10% of patients will experience a further fracture within 1 year, 18% within 2 years, and 31% within 5 years.[191] Additional complications include mortality, a decrease in quality of life, pain, and loss of independence.
Vertebral fractures are the most common osteoporotic fractures; however, less than 10% will result in hospitalization, despite pain and decreased quality of life [International Osteoporosis Foundation - Epidemiology. https://www.osteoporosis.foundation/health-professionals/fragility-fractures/epidemiology]. Vertebral fractures are associated with a nearly 5-fold risk of future vertebral fractures, as well as a 2-fold risk for hip fractures, and a 1.5-fold risk for distal forearm fractures.[20] There is an 8-fold increased hazard ratio in age-adjusted mortality from vertebral fractures; survival rates are estimated to be 53.9% at 3 years, 30.9% at 5 years, and 10.5% at 7 years in the United States.[192][193] Data from the United Kingdom General Practice Research Database (UK GPRD) demonstrated a survival of 86.5% in women at 12 months, and 56.5% at 5 years.[15]
Hip fractures are the greatest cause of morbidity and mortality, particularly within 1 year.[20] Following a hip fracture, 60% of women will lose their ability to walk independently and never regain prior function, 25% will die within 1 year (compared to 33% in men), and another 25% will require care in a nursing home [International Osteoporosis Foundation - Epidemiology of osteoporosis and fragility fractures. 2024. https://www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures#ref_bottom_68][38]. Six months following a hip fracture, only 15% of the patients are noted to be able to walk unaided across a room.[194] There is an estimation that approximately 31,000 deaths occur annually within 6 months of a hip fracture in the United States.[15] As noted previously, distal forearm fractures do not appear to increase mortality risk.[15]
Complications
Osteoporosis and subsequent fractures are associated with a heightened risk for future fractures, as well as mortality. For those with a fragility fracture, other complications noted may include loss of independence, immobility, and persistent pain. Complications may be fracture-site specific; as an example, vertebral fractures may lead to height loss and kyphosis, as well as restrictive lung disease, and altered abdominal anatomy with distension, constipation, anorexia, or premature satiety.[20] Hip fractures may lead to inadvertent nerve or artery damage and a retroperitoneal hematoma.
A distal forearm fracture may lead to avascular necrosis of the bone or inadvertent nerve injury. Complications can also occur from surgical treatment; following hip fracture repair, there is a risk for surgical site infections, prosthetic dislocation, and venous thromboembolism, among other complications. Two prominent complications can occur from antiresorptive therapy (mostly the bisphosphonates and, to a lesser extent, denosumab), which require awareness and monitoring: atypical femur fractures and osteonecrosis of the jaw. Moreover, preliminary data suggest romosozumab (from its action upon suppression of bone resorption) has also been linked to both complications.[134]
Atypical femur fractures were first reported in 2005 and are seen with prolonged therapy of antiresorptive agents.[195] Atypical femur fractures are defined by the Second Task Force of the American Society for Bone and Mineral Research as a fracture located along the femoral diaphysis (distal to lesser trochanter, but proximal to the supracondylar flare) in addition to 4 out of the 5 following major criteria: (1) minimal, or no trauma associated with the fracture; (2) non-comminuted; (3) line of fracture originates at lateral cortex (transverse in orientation); (4) complex fractures extend through both cortices (may have medial spike; incomplete fractures involve lateral cortex); (5) flaring is noted at the site of fracture (periosteal/endosteal thickening of the lateral cortex).[38][196]
Other minor features may include delayed fracture healing, increased thickness of the diaphysis, bilateral femoral diaphyseal fractures (complete or incomplete), or a prodrome of aching and dullness in the thigh or groin area.[38] The pathophysiology is incompletely understood but believed to occur from suppressed bone turnover, allowing for microdamage accumulation.[195] The risk of an atypical femur fracture is between 3.2 and 50 per 100,000 person-years on bisphosphonate therapy, but increases to around 100 per 100,000 person-years with long-term therapy; notable risk factors include glucocorticoid use, hypovitaminosis D, and duration of treatment longer than 3 to 5 years total.[38]
Epidemiological data suggest Asian women are more affected by this complication than White women.[12] Imaging should encompass both lower extremities, as one-third of atypical femur fractures affect the contralateral leg.[38] Management requires surgical fixation if there is a complete fracture (or incomplete with severe pain) with intramedullary nailing or plating; if incomplete and without pain, conservative methods such as 3 months on crutches could be followed.[38] All patients should discontinue their medication and commence vitamin D and calcium supplementation. There is limited data to support the benefit of teriparatide if fractures fail to heal.[195]
Osteonecrosis of the jaw is an additional complication of long-term antiresorptive therapy, which was first reported in 2003.[197] This phenomenon is defined by the International Task Force on Osteonecrosis of the Jaw as bone exposed in the maxillofacial region, which does not heal after 8 weeks since the initial identification.[38][198] Furthermore, there must be a preceding exposure to antiresorptive therapy, and no history of radiation exposure to the craniofacial region.[198]
The pathophysiology of osteonecrosis of the jaw is not entirely understood. Still, it is believed to occur because of abnormal bone remodeling and an insult such as an infection or other dental interventions.[199] The incidence of osteonecrosis of the jaw, as well as the incidence of atypical femur fractures, is lower in denosumab-treated patients.[200] Moreover, limited evidence also supports teriparatide therapy for treating osteonecrosis of the jaw.[201]
Consultations
Patients are best treated in a multidisciplinary approach to allow for a comprehensive evaluation with risk stratification and management. Multiple healthcare professionals are essential in preventing, screening, identifying, and managing osteoporosis. Patients may encounter a primary care physician, nutritionist/dietitian, endocrinologist, rheumatologist, geriatrician, or orthopedic surgeon (at the onset of a fragility fracture), and care is best when coordinated between the different specialties. A fracture liaison service is a program of coordinated transition management that has demonstrated improved quality of life and is recommended by the AACE.[74]
Deterrence and Patient Education
Prevention of postmenopausal osteoporosis involves maintaining adequate bone health through vitamin D and calcium intake (primarily dietary, with supplementation if not at goal), regular weight-bearing and resistance exercises, fall-risk modification, alcohol moderation, and smoking cessation. Routine BMD screening with DEXA is advised for women older than age 65 (or younger with risk factors), to allow for earlier detection and intervention before fragility fractures and decreases in quality of life. Once diagnosed, treatment involves maintaining bone health and commencing pharmacologic therapy, usually stratified based on risk. Patient education is equally vital regarding treatment and should emphasize the silent nature of the disease and the importance of medication adherence. Implementing a fracture liaison service may improve osteoporosis management by allowing timely evaluation, initiation of treatment, and long-term follow-up for those with fragility fractures.
Enhancing Healthcare Team Outcomes
Effective management of osteoporosis involves a coordinated team of healthcare professionals. This allows for timely diagnosis, provides evidence-based management, and improves patient outcomes. A clear definition of responsibilities among team members is essential. The care coordination may involve primary care clinicians, nursing professionals, subspecialists (including but not limited to endocrinologists, rheumatologists, and geriatricians), radiologists, physical therapists, and surgeons (particularly orthopedic surgeons involved in patient care after a fracture).
Physicians may lead screening, diagnosis, and treatment plans, with nurses providing optimal patient education, ensuring medication adherence, and providing fall prevention counseling. Pharmacists are essential in guiding the safety and effectiveness of osteoporotic pharmaceutical agents, while physical therapists focus on balance exercises, strength training, and assessment of mobility, especially to reduce fall risk. Utilizing strategic approaches with integration of screening protocols, using country-specific fracture risk assessment tools (such as FRAX), and providing education to ensure timely commencement of treatment is the gold standard in care.
Ethical considerations include emphasizing informed consent, shared decision-making, and addressing racial disparities in the screening and treatment access of osteoporosis, particularly among high-risk populations. A fracture liaison service effectively ensures follow-up and timely evaluation and medication initiation for patients with osteoporotic fractures, improves postfracture care, and reduces the risk of subsequent fractures. Patient care and safety are enhanced when interprofessional collaboration is prioritized, reducing morbidity, mortality, and overall healthcare costs.
Media
(Click Image to Enlarge)
Normal Versus Osteoporotic Bone. Normal composition of bone with trabeculation pattern compared to loss of trabeculae and excessive pores in bone affected with osteoporosis.
BruceBlaus, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
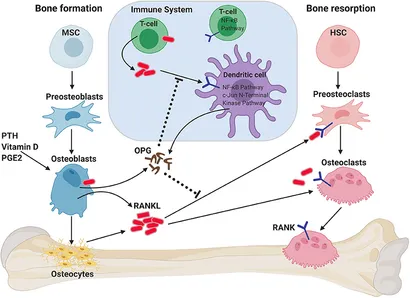
Role of RANKL/RANK/OPG Axis on Bone Homeostasis and Immune System. RANKL is secreted by osteoblasts and osteocytes when stimulated by parathyroid hormone (PTH), vitamin D, and/or prostaglandin 2 (PGE2). RANKL binds to RANK on the membrane of osteoclast progenitors (preosteoclasts), which results in bone resorption by mature osteoclasts. Osteoprotegerin (OPG) binds to RANKL, thus inhibiting RANK signaling and bone resorption. RANK/RANKL also plays a role in immune cell regulation and the crosstalk between both systems (termed osteoimmunology).
(Click Image to Enlarge)
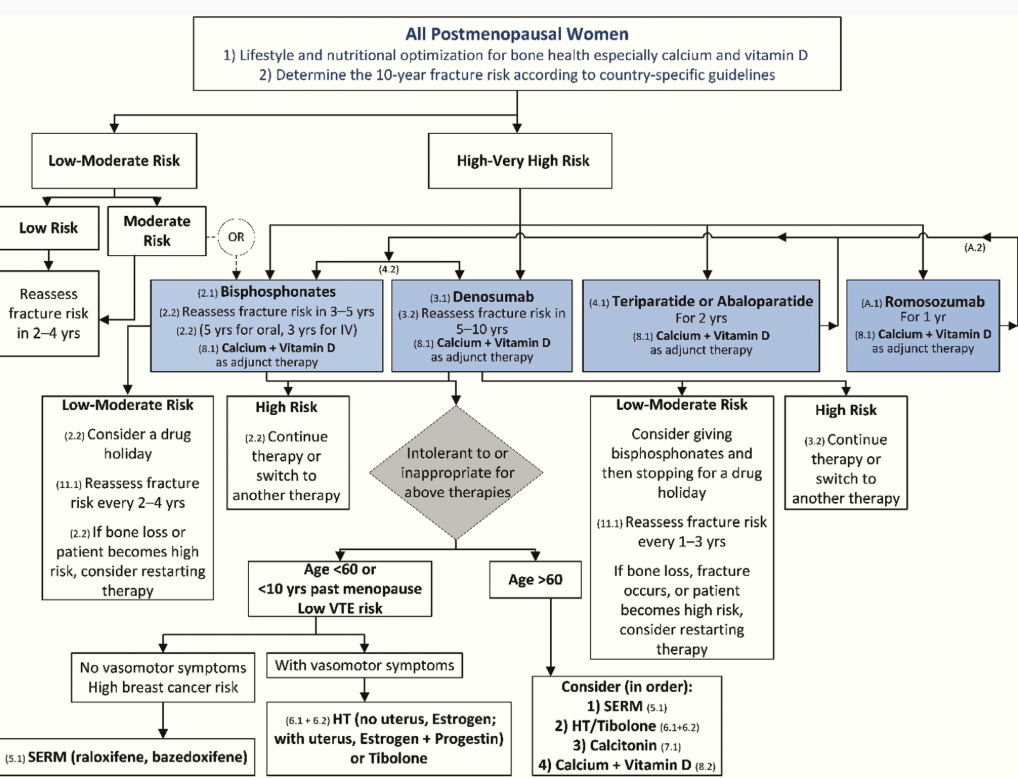
Lifestyle and Nutritional Optimization for Bone Health for All Postmenopausal Women. This chart guides women with osteoporosis with nutrition and lifestyle choice information.
Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society guideline update. J Clin Endocrinol Metab. 2020;105(3):587–594. doi: 10.1210/clinem/dgaa048.
References
Varo N. Bone remodeling markers: monitoring the changing bone. Advances in laboratory medicine. 2024 Mar:5(1):1-3. doi: 10.1515/almed-2024-0009. Epub 2024 Feb 27 [PubMed PMID: 38634082]
Level 3 (low-level) evidenceStride PJ, Patel N, Kingston D. The history of osteoporosis: why do Egyptian mummies have porotic bones? The journal of the Royal College of Physicians of Edinburgh. 2013:43(3):254-61. doi: 10.4997/JRCPE.2013.314. Epub [PubMed PMID: 24087808]
Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis. Journal of internal medicine. 2015 Jun:277(6):650-61. doi: 10.1111/joim.12369. Epub [PubMed PMID: 25832448]
Riggs BL, Melton LJ 3rd. Evidence for two distinct syndromes of involutional osteoporosis. The American journal of medicine. 1983 Dec:75(6):899-901 [PubMed PMID: 6650542]
Poduval M, Vishwanathan K. Definition and Evolution of the Term Osteoporosis. Indian journal of orthopaedics. 2023 Dec:57(Suppl 1):42-44. doi: 10.1007/s43465-023-01013-2. Epub 2023 Oct 31 [PubMed PMID: 38107798]
Ji MX, Yu Q. Primary osteoporosis in postmenopausal women. Chronic diseases and translational medicine. 2015 Mar:1(1):9-13. doi: 10.1016/j.cdtm.2015.02.006. Epub 2015 Mar 21 [PubMed PMID: 29062981]
Hunter DJ, Sambrook PN. Bone loss. Epidemiology of bone loss. Arthritis research. 2000:2(6):441-5 [PubMed PMID: 11094456]
Sheu A, Diamond T. Secondary osteoporosis. Australian prescriber. 2016 Jun:39(3):85-7. doi: 10.18773/austprescr.2016.038. Epub 2016 Jun 1 [PubMed PMID: 27346916]
Thulkar J, Singh S, Sharma S, Thulkar T. Preventable risk factors for osteoporosis in postmenopausal women: Systematic review and meta-analysis. Journal of mid-life health. 2016 Jul-Sep:7(3):108-113 [PubMed PMID: 27721637]
Level 1 (high-level) evidenceAnastasopoulou C, Barnett MJ, Rodrigues Silva Sombra L, Garla VV. Osteoporosis in Males. StatPearls. 2025 Jan:(): [PubMed PMID: 30860766]
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008 Mar:42(3):467-75. doi: 10.1016/j.bone.2007.11.001. Epub 2007 Nov 17 [PubMed PMID: 18180210]
Onizuka N, Onizuka T. Disparities in Osteoporosis Prevention and Care: Understanding Gender, Racial, and Ethnic Dynamics. Current reviews in musculoskeletal medicine. 2024 Sep:17(9):365-372. doi: 10.1007/s12178-024-09909-8. Epub 2024 Jun 25 [PubMed PMID: 38916641]
Level 3 (low-level) evidenceHassan AB, Almarabheh A, Almekhyal A, Karashi AR, Saleh J, Shaikh M, Alawadhi A, Jahrami H. Frequency of Osteoporosis-Related Fractures in the Kingdom of Bahrain. Healthcare (Basel, Switzerland). 2024 Dec 12:12(24):. doi: 10.3390/healthcare12242515. Epub 2024 Dec 12 [PubMed PMID: 39765942]
Borgström F, Karlsson L, Ortsäter G, Norton N, Halbout P, Cooper C, Lorentzon M, McCloskey EV, Harvey NC, Javaid MK, Kanis JA, International Osteoporosis Foundation. Fragility fractures in Europe: burden, management and opportunities. Archives of osteoporosis. 2020 Apr 19:15(1):59. doi: 10.1007/s11657-020-0706-y. Epub 2020 Apr 19 [PubMed PMID: 32306163]
Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C. The epidemiology of osteoporosis. British medical bulletin. 2020 May 15:133(1):105-117. doi: 10.1093/bmb/ldaa005. Epub [PubMed PMID: 32282039]
Kanis JA, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, McCloskey EV, Willers C, Borgström F. SCOPE 2021: a new scorecard for osteoporosis in Europe. Archives of osteoporosis. 2021 Jun 2:16(1):82. doi: 10.1007/s11657-020-00871-9. Epub 2021 Jun 2 [PubMed PMID: 34080059]
Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2019 Jan:30(1):3-44. doi: 10.1007/s00198-018-4704-5. Epub 2018 Oct 15 [PubMed PMID: 30324412]
Lewiecki EM, Ortendahl JD, Vanderpuye-Orgle J, Grauer A, Arellano J, Lemay J, Harmon AL, Broder MS, Singer AJ. Healthcare Policy Changes in Osteoporosis Can Improve Outcomes and Reduce Costs in the United States. JBMR plus. 2019 Sep:3(9):e10192. doi: 10.1002/jbm4.10192. Epub 2019 May 13 [PubMed PMID: 31667450]
. Management of osteoporosis in postmenopausal women: the 2021 position statement of The North American Menopause Society. Menopause (New York, N.Y.). 2021 Sep 1:28(9):973-997. doi: 10.1097/GME.0000000000001831. Epub [PubMed PMID: 34448749]
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R, National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014 Oct:25(10):2359-81. doi: 10.1007/s00198-014-2794-2. Epub 2014 Aug 15 [PubMed PMID: 25182228]
Level 1 (high-level) evidenceLeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, Siris ES. The clinician's guide to prevention and treatment of osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2022 Oct:33(10):2049-2102. doi: 10.1007/s00198-021-05900-y. Epub 2022 Apr 28 [PubMed PMID: 35478046]
Kannegaard PN, van der Mark S, Eiken P, Abrahamsen B. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age and ageing. 2010 Mar:39(2):203-9. doi: 10.1093/ageing/afp221. Epub 2010 Jan 14 [PubMed PMID: 20075035]
Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Archives of osteoporosis. 2013:8(1):136. doi: 10.1007/s11657-013-0136-1. Epub 2013 Oct 11 [PubMed PMID: 24113837]
Salari N, Darvishi N, Bartina Y, Larti M, Kiaei A, Hemmati M, Shohaimi S, Mohammadi M. Global prevalence of osteoporosis among the world older adults: a comprehensive systematic review and meta-analysis. Journal of orthopaedic surgery and research. 2021 Nov 13:16(1):669. doi: 10.1186/s13018-021-02821-8. Epub 2021 Nov 13 [PubMed PMID: 34774085]
Level 1 (high-level) evidenceHemmati E, Mirghafourvand M, Mobasseri M, Shakouri SK, Mikaeli P, Farshbaf-Khalili A. Prevalence of primary osteoporosis and low bone mass in postmenopausal women and related risk factors. Journal of education and health promotion. 2021:10():204. doi: 10.4103/jehp.jehp_945_20. Epub 2021 Jun 30 [PubMed PMID: 34395641]
Costa AG, Wyman A, Siris ES, Watts NB, Silverman S, Saag KG, Roux C, Rossini M, Pfeilschifter J, Nieves JW, Netelenbos JC, March L, LaCroix AZ, Hooven FH, Greenspan SL, Gehlbach SH, Díez-Pérez A, Cooper C, Compston JE, Chapurlat RD, Boonen S, Anderson FA Jr, Adachi JD, Adami S. When, where and how osteoporosis-associated fractures occur: an analysis from the Global Longitudinal Study of Osteoporosis in Women (GLOW). PloS one. 2013:8(12):e83306. doi: 10.1371/journal.pone.0083306. Epub 2013 Dec 11 [PubMed PMID: 24349484]
Carlson BC, Robinson WA, Wanderman NR, Sebastian AS, Nassr A, Freedman BA, Anderson PA. A Review and Clinical Perspective of the Impact of Osteoporosis on the Spine. Geriatric orthopaedic surgery & rehabilitation. 2019:10():2151459319861591. doi: 10.1177/2151459319861591. Epub 2019 Jul 17 [PubMed PMID: 31360592]
Level 3 (low-level) evidenceMcPhee C, Aninye IO, Horan L. Recommendations for Improving Women's Bone Health Throughout the Lifespan. Journal of women's health (2002). 2022 Dec:31(12):1671-1676. doi: 10.1089/jwh.2022.0361. Epub 2022 Nov 7 [PubMed PMID: 36346282]
Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clinical orthopaedics and related research. 2011 Jul:469(7):1891-9. doi: 10.1007/s11999-011-1863-5. Epub [PubMed PMID: 21431462]
Dong Y, Zhang Y, Song K, Kang H, Ye D, Li F. What was the Epidemiology and Global Burden of Disease of Hip Fractures From 1990 to 2019? Results From and Additional Analysis of the Global Burden of Disease Study 2019. Clinical orthopaedics and related research. 2023 Jun 1:481(6):1209-1220. doi: 10.1097/CORR.0000000000002465. Epub 2022 Nov 2 [PubMed PMID: 36374576]
Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1997:7(5):407-13 [PubMed PMID: 9425497]
Lorentzon M, Johansson H, Harvey NC, Liu E, Vandenput L, McCloskey EV, Kanis JA. Osteoporosis and fractures in women: the burden of disease. Climacteric : the journal of the International Menopause Society. 2022 Feb:25(1):4-10. doi: 10.1080/13697137.2021.1951206. Epub 2021 Jul 28 [PubMed PMID: 34319208]
Meyer AC, Ek S, Drefahl S, Ahlbom A, Hedström M, Modig K. Trends in Hip Fracture Incidence, Recurrence, and Survival by Education and Comorbidity: A Swedish Register-based Study. Epidemiology (Cambridge, Mass.). 2021 May 1:32(3):425-433. doi: 10.1097/EDE.0000000000001321. Epub [PubMed PMID: 33512961]
Lips P. Epidemiology and predictors of fractures associated with osteoporosis. The American journal of medicine. 1997 Aug 18:103(2A):3S-8S; discussion 8S-11S [PubMed PMID: 9302892]
Owen RA, Melton LJ 3rd, Johnson KA, Ilstrup DM, Riggs BL. Incidence of Colles' fracture in a North American community. American journal of public health. 1982 Jun:72(6):605-7 [PubMed PMID: 7072880]
Edwards BJ, Song J, Dunlop DD, Fink HA, Cauley JA. Functional decline after incident wrist fractures--Study of Osteoporotic Fractures: prospective cohort study. BMJ (Clinical research ed.). 2010 Jul 8:341():c3324. doi: 10.1136/bmj.c3324. Epub 2010 Jul 8 [PubMed PMID: 20616099]
Cummings SR, Black DM, Rubin SM. Lifetime risks of hip, Colles', or vertebral fracture and coronary heart disease among white postmenopausal women. Archives of internal medicine. 1989 Nov:149(11):2445-8 [PubMed PMID: 2818106]
Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Zaheer S, LeBoff MS. Osteoporosis: Prevention and Treatment. Endotext. 2000:(): [PubMed PMID: 25905299]
Alexandru D, So W. Evaluation and management of vertebral compression fractures. The Permanente journal. 2012 Fall:16(4):46-51 [PubMed PMID: 23251117]
Gonnelli S, Caffarelli C, Maggi S, Rossi S, Siviero P, Gandolini G, Cisari C, Rossini M, Iolascon G, Letizia Mauro G, Crepaldi G, Nuti R, BREAK Study Group. The assessment of vertebral fractures in elderly women with recent hip fractures: the BREAK Study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013 Apr:24(4):1151-9. doi: 10.1007/s00198-012-2119-2. Epub 2012 Aug 22 [PubMed PMID: 23011681]
Nicolaes J, Liu Y, Zhao Y, Huang P, Wang L, Yu A, Dunkel J, Libanati C, Cheng X. External validation of a convolutional neural network algorithm for opportunistically detecting vertebral fractures in routine CT scans. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2024 Jan:35(1):143-152. doi: 10.1007/s00198-023-06903-7. Epub 2023 Sep 7 [PubMed PMID: 37674097]
Level 1 (high-level) evidenceKaptoge S, Armbrecht G, Felsenberg D, Lunt M, O'Neill TW, Silman AJ, Reeve J, EPOS Study Group. When should the doctor order a spine X-ray? Identifying vertebral fractures for osteoporosis care: results from the European Prospective Osteoporosis Study (EPOS). Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004 Dec:19(12):1982-93 [PubMed PMID: 15537441]
Litwic A, Edwards M, Cooper C, Dennison E. Geographic differences in fractures among women. Women's health (London, England). 2012 Nov:8(6):673-84. doi: 10.2217/whe.12.54. Epub [PubMed PMID: 23181532]
Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocrine reviews. 2008 Jun:29(4):441-64. doi: 10.1210/er.2008-0002. Epub 2008 May 1 [PubMed PMID: 18451258]
Waterloo S, Ahmed LA, Center JR, Eisman JA, Morseth B, Nguyen ND, Nguyen T, Sogaard AJ, Emaus N. Prevalence of vertebral fractures in women and men in the population-based Tromsø Study. BMC musculoskeletal disorders. 2012 Jan 17:13():3. doi: 10.1186/1471-2474-13-3. Epub 2012 Jan 17 [PubMed PMID: 22251875]
Cawthon PM. Gender differences in osteoporosis and fractures. Clinical orthopaedics and related research. 2011 Jul:469(7):1900-5. doi: 10.1007/s11999-011-1780-7. Epub [PubMed PMID: 21264553]
Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Rosen CJ. The Epidemiology and Pathogenesis of Osteoporosis. Endotext. 2000:(): [PubMed PMID: 25905357]
Ross BJ, Lee OC, Harris MB, Dowd TC, Savoie FH 3rd, Sherman WF. Rates of Osteoporosis Management and Secondary Preventative Treatment After Primary Fragility Fractures. JB & JS open access. 2021 Apr-Jun:6(2):. doi: 10.2106/JBJS.OA.20.00142. Epub 2021 Jun 14 [PubMed PMID: 34136740]
Lewiecki EM, Leader D, Weiss R, Williams SA. Challenges in osteoporosis awareness and management: results from a survey of US postmenopausal women. Journal of drug assessment. 2019:8(1):25-31. doi: 10.1080/21556660.2019.1579728. Epub 2019 Feb 6 [PubMed PMID: 30834163]
Level 3 (low-level) evidenceHall SF, Edmonds SW, Lou Y, Cram P, Roblin DW, Saag KG, Wright NC, Jones MP, Wolinsky FD. Patient-reported reasons for nonadherence to recommended osteoporosis pharmacotherapy. Journal of the American Pharmacists Association : JAPhA. 2017 Jul-Aug:57(4):503-509. doi: 10.1016/j.japh.2017.05.003. Epub 2017 Jun 8 [PubMed PMID: 28602783]
Tai TW, Chen HY, Shih CA, Huang CF, McCloskey E, Lee JK, Yeap SS, Cheung CL, Charatcharoenwitthaya N, Jaisamrarn U, Kuptniratsaikul V, Yang RS, Lin SY, Taguchi A, Mori S, Li-Yu J, Ang SB, Chan DC, Chan WS, Ng H, Chen JF, Tu ST, Chuang HH, Chang YF, Chen FP, Tsai KS, Ebeling PR, Marin F, Nistal Rodríguez FJ, Shi H, Hwang KR, Kim KK, Chung YS, Reid IR, Chandran M, Ferrari S, Lewiecki EM, Hew FL, Ho-Pham LT, Nguyen TV, Nguyen VH, Lekamwasam S, Pandey D, Bhadada S, Chen CH, Hwang JS, Wu CH. Asia-Pacific consensus on long-term and sequential therapy for osteoporosis. Osteoporosis and sarcopenia. 2024 Mar:10(1):3-10. doi: 10.1016/j.afos.2024.02.001. Epub 2024 Mar 16 [PubMed PMID: 38690538]
Level 3 (low-level) evidenceFlorencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed research international. 2015:2015():421746. doi: 10.1155/2015/421746. Epub 2015 Jul 13 [PubMed PMID: 26247020]
Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed research international. 2020:2020():6910312. doi: 10.1155/2020/6910312. Epub 2020 Feb 19 [PubMed PMID: 32149122]
Bonjour JP, Theintz G, Law F, Slosman D, Rizzoli R. Peak bone mass. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1994:4 Suppl 1():7-13 [PubMed PMID: 8081064]
Rył A, Szylińska A, Skonieczna-Żydecka K, Miazgowski T, Rotter I. The Impact of Metabolic Syndrome on Bone Mass in Men: Systematic Review and Meta-Analysis. Biomedicines. 2023 Jul 6:11(7):. doi: 10.3390/biomedicines11071915. Epub 2023 Jul 6 [PubMed PMID: 37509553]
Level 1 (high-level) evidenceSeeman E. Pathogenesis of bone fragility in women and men. Lancet (London, England). 2002 May 25:359(9320):1841-50 [PubMed PMID: 12044392]
Alswat KA. Gender Disparities in Osteoporosis. Journal of clinical medicine research. 2017 May:9(5):382-387. doi: 10.14740/jocmr2970w. Epub 2017 Apr 1 [PubMed PMID: 28392857]
Jones G, Nguyen T, Sambrook P, Kelly PJ, Eisman JA. Progressive loss of bone in the femoral neck in elderly people: longitudinal findings from the Dubbo osteoporosis epidemiology study. BMJ (Clinical research ed.). 1994 Sep 17:309(6956):691-5 [PubMed PMID: 7950520]
Chen H, Zhou X, Fujita H, Onozuka M, Kubo KY. Age-related changes in trabecular and cortical bone microstructure. International journal of endocrinology. 2013:2013():213234. doi: 10.1155/2013/213234. Epub 2013 Mar 18 [PubMed PMID: 23573086]
Yong EL, Logan S. Menopausal osteoporosis: screening, prevention and treatment. Singapore medical journal. 2021 Apr:62(4):159-166. doi: 10.11622/smedj.2021036. Epub [PubMed PMID: 33948669]
Feng X, McDonald JM. Disorders of bone remodeling. Annual review of pathology. 2011:6():121-45. doi: 10.1146/annurev-pathol-011110-130203. Epub [PubMed PMID: 20936937]
Level 3 (low-level) evidenceKlotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000 Apr:15(4):721-39 [PubMed PMID: 10780864]
Almeida M, Laurent MR, Dubois V, Claessens F, O'Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiological reviews. 2017 Jan:97(1):135-187 [PubMed PMID: 27807202]
Herath M, Cohen A, Ebeling PR, Milat F. Dilemmas in the Management of Osteoporosis in Younger Adults. JBMR plus. 2022 Jan:6(1):e10594. doi: 10.1002/jbm4.10594. Epub 2022 Jan 19 [PubMed PMID: 35079682]
John Levy Barnett M. A Scoping Review of the Apparent Phenomenon of the Improvement in Hypoparathyroidism in Pregnant and Postpartum Females. Cureus. 2023 Sep:15(9):e46123. doi: 10.7759/cureus.46123. Epub 2023 Sep 28 [PubMed PMID: 37790033]
Level 2 (mid-level) evidenceKann PH, Pfützner A, Delling G, Schulz G, Meyer S. Transiliac bone biopsy in osteoporosis: frequency, indications, consequences and complications. An evaluation of 99 consecutive cases over a period of 14 years. Clinical rheumatology. 2006 Feb:25(1):30-4 [PubMed PMID: 15915321]
Level 3 (low-level) evidenceColazo JM, Quirion J, Judice AD, Halpern J, Schwartz HS, Tanner SB, Lawrenz JM, Dahir KM, Holt GE. Utility of iliac crest tetracycline-labelled bone biopsy in osteoporosis and metabolic bone disease: An evaluation of 95 cases over a period of 25 years. Bone reports. 2023 Dec:19():101715. doi: 10.1016/j.bonr.2023.101715. Epub 2023 Sep 24 [PubMed PMID: 37811524]
Level 3 (low-level) evidenceMarcu F, Bogdan F, Muţiu G, Lazăr L. The histopathological study of osteoporosis. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2011:52(1 Suppl):321-5 [PubMed PMID: 21424070]
Duque G, Lord SR, Mak J, Ganda K, Close JJ, Ebeling P, Papaioannou A, Inderjeeth CA. Treatment of Osteoporosis in Australian Residential Aged Care Facilities: Update on Consensus Recommendations for Fracture Prevention. Journal of the American Medical Directors Association. 2016 Sep 1:17(9):852-9. doi: 10.1016/j.jamda.2016.05.011. Epub 2016 Jun 24 [PubMed PMID: 27349626]
Level 3 (low-level) evidenceMacdonald HM, Nishiyama KK, Kang J, Hanley DA, Boyd SK. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCT study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011 Jan:26(1):50-62. doi: 10.1002/jbmr.171. Epub [PubMed PMID: 20593413]
Naot D, Watson M, Choi AJ, Musson DS, Callon KE, Zhu M, Gao R, Caughey W, Pitto RP, Munro JT, Horne A, Gamble GD, Dalbeth N, Reid IR, Cornish J. The effect of age on the microarchitecture and profile of gene expression in femoral head and neck bone from patients with osteoarthritis. Bone reports. 2020 Dec:13():100287. doi: 10.1016/j.bonr.2020.100287. Epub 2020 Jun 5 [PubMed PMID: 32551338]
Söreskog E, Ström O, Spångéus A, Åkesson KE, Borgström F, Banefelt J, Toth E, Libanati C, Charokopou M. Risk of major osteoporotic fracture after first, second and third fracture in Swedish women aged 50 years and older. Bone. 2020 May:134():115286. doi: 10.1016/j.bone.2020.115286. Epub 2020 Feb 15 [PubMed PMID: 32070789]
Robitaille J, Yoon PW, Moore CA, Liu T, Irizarry-Delacruz M, Looker AC, Khoury MJ. Prevalence, family history, and prevention of reported osteoporosis in U.S. women. American journal of preventive medicine. 2008 Jul:35(1):47-54. doi: 10.1016/j.amepre.2008.03.027. Epub [PubMed PMID: 18541176]
Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS/AMERICAN COLLEGE OF ENDOCRINOLOGY CLINICAL PRACTICE GUIDELINES FOR THE DIAGNOSIS AND TREATMENT OF POSTMENOPAUSAL OSTEOPOROSIS-2020 UPDATE. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2020 May:26(Suppl 1):1-46. doi: 10.4158/GL-2020-0524SUPPL. Epub [PubMed PMID: 32427503]
Level 1 (high-level) evidenceZacker RJ. Health-related implications and management of sarcopenia. JAAPA : official journal of the American Academy of Physician Assistants. 2006 Oct:19(10):24-9 [PubMed PMID: 17066677]
Gale CR, Cooper C, Aihie Sayer A. Prevalence and risk factors for falls in older men and women: The English Longitudinal Study of Ageing. Age and ageing. 2016 Nov:45(6):789-794 [PubMed PMID: 27496938]
By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society. 2023 Jul:71(7):2052-2081. doi: 10.1111/jgs.18372. Epub 2023 May 4 [PubMed PMID: 37139824]
Katzman WB, Wanek L, Shepherd JA, Sellmeyer DE. Age-related hyperkyphosis: its causes, consequences, and management. The Journal of orthopaedic and sports physical therapy. 2010 Jun:40(6):352-60. doi: 10.2519/jospt.2010.3099. Epub [PubMed PMID: 20511692]
Aibar-Almazán A, Voltes-Martínez A, Castellote-Caballero Y, Afanador-Restrepo DF, Carcelén-Fraile MDC, López-Ruiz E. Current Status of the Diagnosis and Management of Osteoporosis. International journal of molecular sciences. 2022 Aug 21:23(16):. doi: 10.3390/ijms23169465. Epub 2022 Aug 21 [PubMed PMID: 36012730]
Akkawi I, Zmerly H. Osteoporosis: Current Concepts. Joints. 2018 Jun:6(2):122-127. doi: 10.1055/s-0038-1660790. Epub 2018 Jun 14 [PubMed PMID: 30051110]
Maclaughlin EJ, Sleeper RB, McNatty D, Raehl CL. Management of age-related osteoporosis and prevention of associated fractures. Therapeutics and clinical risk management. 2006 Sep:2(3):281-95 [PubMed PMID: 18360603]
Black DM, Cauley JA, Wagman R, Ensrud K, Fink HA, Hillier TA, Lui LY, Cummings SR, Schousboe JT, Napoli N. The Ability of a Single BMD and Fracture History Assessment to Predict Fracture Over 25 Years in Postmenopausal Women: The Study of Osteoporotic Fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018 Mar:33(3):389-395. doi: 10.1002/jbmr.3194. Epub 2017 Jul 18 [PubMed PMID: 28719727]
Leslie WD, Adler RA, El-Hajj Fuleihan G, Hodsman AB, Kendler DL, McClung M, Miller PD, Watts NB, International Society for Clinical Densitometry. Application of the 1994 WHO classification to populations other than postmenopausal Caucasian women: the 2005 ISCD Official Positions. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2006 Jan-Mar:9(1):22-30 [PubMed PMID: 16731428]
Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ (Clinical research ed.). 1996 May 18:312(7041):1254-9 [PubMed PMID: 8634613]
Level 1 (high-level) evidenceKanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013 Jan:24(1):23-57. doi: 10.1007/s00198-012-2074-y. Epub 2012 Oct 19 [PubMed PMID: 23079689]
US Preventive Services Task Force, Nicholson WK, Silverstein M, Wong JB, Chelmow D, Coker TR, Davis EM, Jaén CR, Krousel-Wood M, Lee S, Li L, Mangione CM, Ogedegbe G, Rao G, Ruiz JM, Stevermer J, Tsevat J, Underwood SM, Wiehe S. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2025 Feb 11:333(6):498-508. doi: 10.1001/jama.2024.27154. Epub [PubMed PMID: 39808425]
Kanis JA, Johansson H, Harvey NC, McCloskey EV. A brief history of FRAX. Archives of osteoporosis. 2018 Oct 31:13(1):118. doi: 10.1007/s11657-018-0510-0. Epub 2018 Oct 31 [PubMed PMID: 30382424]
Vasikaran S, Cooper C, Eastell R, Griesmacher A, Morris HA, Trenti T, Kanis JA. International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clinical chemistry and laboratory medicine. 2011 Aug:49(8):1271-1274. doi: 10.1515/CCLM.2011.602. Epub 2011 May 24 [PubMed PMID: 21605012]
Dhainaut A, Hoff M, Syversen U, Haugeberg G. Technologies for assessment of bone reflecting bone strength and bone mineral density in elderly women: an update. Women's health (London, England). 2016:12(2):209-16. doi: 10.2217/whe.15.94. Epub 2016 Feb 22 [PubMed PMID: 26900798]
Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet (London, England). 2010 May 15:375(9727):1729-36. doi: 10.1016/S0140-6736(10)60320-0. Epub [PubMed PMID: 20472174]
Level 2 (mid-level) evidenceChou SH, Vokes TJ, Ma SL, Costello M, Rosen HR, Schousboe JT. Simplified criteria for selecting patients for vertebral fracture assessment. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2014 Jul-Sep:17(3):386-91. doi: 10.1016/j.jocd.2013.11.003. Epub 2014 Feb 25 [PubMed PMID: 24582084]
Lewiecki EM, Lane NE. Common mistakes in the clinical use of bone mineral density testing. Nature clinical practice. Rheumatology. 2008 Dec:4(12):667-74. doi: 10.1038/ncprheum0928. Epub 2008 Oct 21 [PubMed PMID: 18936788]
Beck TJ. Extending DXA beyond bone mineral density: understanding hip structure analysis. Current osteoporosis reports. 2007 Jun:5(2):49-55 [PubMed PMID: 17521505]
Level 3 (low-level) evidenceDanielson ME, Beck TJ, Karlamangla AS, Greendale GA, Atkinson EJ, Lian Y, Khaled AS, Keaveny TM, Kopperdahl D, Ruppert K, Greenspan S, Vuga M, Cauley JA. A comparison of DXA and CT based methods for estimating the strength of the femoral neck in post-menopausal women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013 Apr:24(4):1379-88. doi: 10.1007/s00198-012-2066-y. Epub 2012 Jul 19 [PubMed PMID: 22810918]
Al Refaie A, Baldassini L, Mondillo C, Giglio E, De Vita M, Tomai Pitinca MD, Gonnelli S, Caffarelli C. Radiofrequency Echographic Multi Spectrometry (R.E.M.S.): New Frontiers for Ultrasound Use in the Assessment of Bone Status-A Current Picture. Diagnostics (Basel, Switzerland). 2023 May 9:13(10):. doi: 10.3390/diagnostics13101666. Epub 2023 May 9 [PubMed PMID: 37238151]
Dovjak P, Iglseder B, Rainer A, Dovjak G, Weber M, Pietschmann P. Pulse-echo ultrasound measurement in osteoporosis screening: a pilot study in older patients. Aging clinical and experimental research. 2023 Jun:35(6):1221-1230. doi: 10.1007/s40520-023-02404-z. Epub 2023 Apr 24 [PubMed PMID: 37093523]
Level 3 (low-level) evidenceGanry O, Baudoin C, Fardellone P, Dubreuil A, EPIDOS Group. Alcohol consumption by non-institutionalised elderly women: the EPIDOS Study. Public health. 2001 May:115(3):186-91 [PubMed PMID: 11429713]
Park JS, Kang KC, Park SJ, Kim JK, Han K, Hong JY. The positive impact of smoking cessation on fracture risk in a nationwide cohort study. Scientific reports. 2024 Apr 30:14(1):9892. doi: 10.1038/s41598-024-60301-5. Epub 2024 Apr 30 [PubMed PMID: 38688971]
Kiyota Y, Muramatsu H, Sato Y, Kobayashi T, Miyamoto K, Iwamoto T, Matsumoto M, Nakamura M, Tateno H, Sato K, Miyamoto T. Smoking cessation increases levels of osteocalcin and uncarboxylated osteocalcin in human sera. Scientific reports. 2020 Oct 8:10(1):16845. doi: 10.1038/s41598-020-73789-4. Epub 2020 Oct 8 [PubMed PMID: 33033284]
Zhang Y, Chen H. Effect of Tai Chi exercise on bone health and fall prevention in postmenopausal women: a meta-analysis. Journal of orthopaedic surgery and research. 2024 Aug 10:19(1):471. doi: 10.1186/s13018-024-04962-y. Epub 2024 Aug 10 [PubMed PMID: 39127644]
Level 1 (high-level) evidenceKemmler W, Bebenek M, Kohl M, von Stengel S. Exercise and fractures in postmenopausal women. Final results of the controlled Erlangen Fitness and Osteoporosis Prevention Study (EFOPS). Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015 Oct:26(10):2491-9. doi: 10.1007/s00198-015-3165-3. Epub 2015 May 12 [PubMed PMID: 25963237]
Silman AJ, O'Neill TW, Cooper C, Kanis J, Felsenberg D. Influence of physical activity on vertebral deformity in men and women: results from the European Vertebral Osteoporosis Study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1997 May:12(5):813-9 [PubMed PMID: 9144348]
Watson SL, Weeks BK, Weis LJ, Harding AT, Horan SA, Beck BR. High-Intensity Resistance and Impact Training Improves Bone Mineral Density and Physical Function in Postmenopausal Women With Osteopenia and Osteoporosis: The LIFTMOR Randomized Controlled Trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018 Feb:33(2):211-220. doi: 10.1002/jbmr.3284. Epub 2017 Oct 4 [PubMed PMID: 28975661]
Level 1 (high-level) evidenceSchürch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Annals of internal medicine. 1998 May 15:128(10):801-9 [PubMed PMID: 9599191]
Level 1 (high-level) evidenceDrewnowski A. The contribution of milk and milk products to micronutrient density and affordability of the U.S. diet. Journal of the American College of Nutrition. 2011 Oct:30(5 Suppl 1):422S-8S [PubMed PMID: 22081688]
Kopecky SL, Bauer DC, Gulati M, Nieves JW, Singer AJ, Toth PP, Underberg JA, Wallace TC, Weaver CM. Lack of Evidence Linking Calcium With or Without Vitamin D Supplementation to Cardiovascular Disease in Generally Healthy Adults: A Clinical Guideline From the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Annals of internal medicine. 2016 Dec 20:165(12):867-868. doi: 10.7326/M16-1743. Epub 2016 Oct 25 [PubMed PMID: 27776362]
US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist AH, Kubik M, Landefeld S, Mangione CM, Silverstein M, Simon MA, Tseng CW. Vitamin D, Calcium, or Combined Supplementation for the Primary Prevention of Fractures in Community-Dwelling Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 Apr 17:319(15):1592-1599. doi: 10.1001/jama.2018.3185. Epub [PubMed PMID: 29677309]
Grigorie D, Sucaliuc A. PREVENTION OF FALLS AND FRACTURES - TO "D" OR NOT TO "D"? Acta endocrinologica (Bucharest, Romania : 2005). 2018 Apr-Jun:14(2):235-237. doi: 10.4183/aeb.2018.235. Epub [PubMed PMID: 31149263]
Yee MMF, Chin KY, Ima-Nirwana S, Wong SK. Vitamin A and Bone Health: A Review on Current Evidence. Molecules (Basel, Switzerland). 2021 Mar 21:26(6):. doi: 10.3390/molecules26061757. Epub 2021 Mar 21 [PubMed PMID: 33801011]
Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2019 May 1:104(5):1595-1622. doi: 10.1210/jc.2019-00221. Epub [PubMed PMID: 30907953]
Level 1 (high-level) evidenceAdler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, Compston JE, Drake MT, Edwards BJ, Favus MJ, Greenspan SL, McKinney R Jr, Pignolo RJ, Sellmeyer DE. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016 Jan:31(1):16-35. doi: 10.1002/jbmr.2708. Epub [PubMed PMID: 26350171]
Quesnel AM, Seton M, Merchant SN, Halpin C, McKenna MJ. Third-generation bisphosphonates for treatment of sensorineural hearing loss in otosclerosis. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012 Oct:33(8):1308-14. doi: 10.1097/MAO.0b013e318268d1b3. Epub [PubMed PMID: 22935809]
Level 2 (mid-level) evidenceCummings SR, Santora AC, Black DM, Russell RGG. History of alendronate. Bone. 2020 Aug:137():115411. doi: 10.1016/j.bone.2020.115411. Epub 2020 May 11 [PubMed PMID: 32437874]
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet (London, England). 1996 Dec 7:348(9041):1535-41 [PubMed PMID: 8950879]
Level 1 (high-level) evidenceBlack DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006 Dec 27:296(24):2927-38 [PubMed PMID: 17190893]
Level 1 (high-level) evidencePols HA, Felsenberg D, Hanley DA, Stepán J, Muñoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1999:9(5):461-8 [PubMed PMID: 10550467]
Level 1 (high-level) evidenceWang YK, Zhang YM, Qin SQ, Wang X, Ma T, Guo JB, Zhu C, Luo ZJ. Effects of alendronate for treatment of glucocorticoid-induced osteoporosis: A meta-analysis of randomized controlled trials. Medicine. 2018 Oct:97(42):e12691. doi: 10.1097/MD.0000000000012691. Epub [PubMed PMID: 30334952]
Level 1 (high-level) evidencePyon EY. Once-monthly ibandronate for postmenopausal osteoporosis: review of a new dosing regimen. Clinical therapeutics. 2006 Apr:28(4):475-90 [PubMed PMID: 16750461]
Felsenberg D, Miller P, Armbrecht G, Wilson K, Schimmer RC, Papapoulos SE. Oral ibandronate significantly reduces the risk of vertebral fractures of greater severity after 1, 2, and 3 years in postmenopausal women with osteoporosis. Bone. 2005 Nov:37(5):651-4 [PubMed PMID: 16126016]
Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, Christiansen C, Rowell L, Mairon N, Bonvoisin B, Drezner MK, Emkey R, Felsenberg D, Cooper C, Delmas PD, Miller PD. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Annals of the rheumatic diseases. 2006 May:65(5):654-61 [PubMed PMID: 16339289]
Eisman JA, Civitelli R, Adami S, Czerwinski E, Recknor C, Prince R, Reginster JY, Zaidi M, Felsenberg D, Hughes C, Mairon N, Masanauskaite D, Reid DM, Delmas PD, Recker RR. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. The Journal of rheumatology. 2008 Mar:35(3):488-97 [PubMed PMID: 18260172]
Wells GA, Hsieh SC, Zheng C, Peterson J, Tugwell P, Liu W. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. The Cochrane database of systematic reviews. 2022 May 3:5(5):CD004523. doi: 10.1002/14651858.CD004523.pub4. Epub 2022 May 3 [PubMed PMID: 35502787]
Level 1 (high-level) evidenceIolascon G, Sirico F, Ferrante A, Gimigliano R, Gimigliano F. Risedronate's efficacy: from randomized clinical trials to real clinical practice. Clinical cases in mineral and bone metabolism : the official journal of the Italian Society of Osteoporosis, Mineral Metabolism, and Skeletal Diseases. 2010 Jan:7(1):19-22 [PubMed PMID: 22461286]
Level 1 (high-level) evidenceDeal CL. Risedronate prevents hip fractures, but who should get therapy? Cleveland Clinic journal of medicine. 2002 Dec:69(12):964, 968-70, 973-6 [PubMed PMID: 12546269]
Silverman SL, Watts NB, Delmas PD, Lange JL, Lindsay R. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007 Jan:18(1):25-34 [PubMed PMID: 17106785]
Grey A. Intravenous zoledronate for osteoporosis: less might be more. Therapeutic advances in musculoskeletal disease. 2016 Aug:8(4):119-23. doi: 10.1177/1759720X16650866. Epub 2016 May 19 [PubMed PMID: 27493690]
Level 3 (low-level) evidenceD'Andrea E, Schneeweiss S, Franklin JM, Kim SC, Glynn RJ, Lee SB, Wang SV. Efficacy Versus Effectiveness: The HORIZON-Pivotal Fracture Trial and Its Emulation in Claims Data. Arthritis & rheumatology (Hoboken, N.J.). 2025 Jan:77(1):12-21. doi: 10.1002/art.42968. Epub 2024 Sep 18 [PubMed PMID: 39129266]
Adachi JD, Lyles KW, Colón-Emeric CS, Boonen S, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Bucci-Rechtweg C, Su G, Eriksen EF, Magaziner JS. Zoledronic acid results in better health-related quality of life following hip fracture: the HORIZON-Recurrent Fracture Trial. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011 Sep:22(9):2539-49. doi: 10.1007/s00198-010-1514-9. Epub 2011 Jan 20 [PubMed PMID: 21249332]
Level 2 (mid-level) evidenceNakamura T, Fukunaga M, Nakano T, Kishimoto H, Ito M, Hagino H, Sone T, Taguchi A, Tanaka S, Ohashi M, Ota Y, Shiraki M. Efficacy and safety of once-yearly zoledronic acid in Japanese patients with primary osteoporosis: two-year results from a randomized placebo-controlled double-blind study (ZOledroNate treatment in Efficacy to osteoporosis; ZONE study). Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2017 Jan:28(1):389-398. doi: 10.1007/s00198-016-3736-y. Epub 2016 Sep 8 [PubMed PMID: 27631091]
Level 1 (high-level) evidenceWatts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. The Journal of clinical endocrinology and metabolism. 2010 Apr:95(4):1555-65. doi: 10.1210/jc.2009-1947. Epub 2010 Feb 19 [PubMed PMID: 20173017]
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR, HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. The New England journal of medicine. 2007 May 3:356(18):1809-22 [PubMed PMID: 17476007]
Level 1 (high-level) evidenceChartrand NA, Lau CK, Parsons MT, Handlon JJ, Ronquillo YC, Hoopes PC, Moshirfar M. Ocular Side Effects of Bisphosphonates: A Review of Literature. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2023 Jan-Feb:39(1):3-16. doi: 10.1089/jop.2022.0094. Epub 2022 Nov 21 [PubMed PMID: 36409537]
Kendler DL, Cosman F, Stad RK, Ferrari S. Denosumab in the Treatment of Osteoporosis: 10 Years Later: A Narrative Review. Advances in therapy. 2022 Jan:39(1):58-74. doi: 10.1007/s12325-021-01936-y. Epub 2021 Nov 11 [PubMed PMID: 34762286]
Level 3 (low-level) evidenceShoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. The Journal of clinical endocrinology and metabolism. 2020 Mar 1:105(3):. pii: dgaa048. doi: 10.1210/clinem/dgaa048. Epub [PubMed PMID: 32068863]
Abduelkarem AR, Guella A, Hamrouni AM, Hassanein MM, Nasr A, Rana O. Denosumab Use in Chronic Kidney Disease Associated Osteoporosis: A Narrative Review. Risk management and healthcare policy. 2023:16():1809-1813. doi: 10.2147/RMHP.S426869. Epub 2023 Sep 11 [PubMed PMID: 37719685]
Level 3 (low-level) evidenceMok CC, Ho LY, Ma KM. Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: a 12-month randomized controlled trial. Bone. 2015 Jun:75():222-8. doi: 10.1016/j.bone.2015.03.002. Epub 2015 Mar 8 [PubMed PMID: 25761434]
Level 1 (high-level) evidenceSilverman SL, Siris E, Belazi D, Recknor C, Papaioannou A, Brown JP, Gold DT, Lewiecki EM, Quinn G, Balasubramanian A, Yue S, Stolshek B, Kendler DL. Persistence at 24 months with denosumab among postmenopausal women with osteoporosis: results of a prospective cohort study. Archives of osteoporosis. 2018 Aug 7:13(1):85. doi: 10.1007/s11657-018-0491-z. Epub 2018 Aug 7 [PubMed PMID: 30088189]
Walker MD, Shane E. Postmenopausal Osteoporosis. The New England journal of medicine. 2023 Nov 23:389(21):1979-1991. doi: 10.1056/NEJMcp2307353. Epub [PubMed PMID: 37991856]
Yu EW, Tsourdi E, Clarke BL, Bauer DC, Drake MT. Osteoporosis Management in the Era of COVID-19. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2020 Jun:35(6):1009-1013. doi: 10.1002/jbmr.4049. Epub 2020 May 26 [PubMed PMID: 32406536]
Umland EM, Karel L, Santoro N. Bazedoxifene and Conjugated Equine Estrogen: A Combination Product for the Management of Vasomotor Symptoms and Osteoporosis Prevention Associated with Menopause. Pharmacotherapy. 2016 May:36(5):548-61. doi: 10.1002/phar.1749. Epub 2016 May 11 [PubMed PMID: 27027527]
Pinkerton JV, Harvey JA, Lindsay R, Pan K, Chines AA, Mirkin S, Archer DF, SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. The Journal of clinical endocrinology and metabolism. 2014 Feb:99(2):E189-98. doi: 10.1210/jc.2013-1707. Epub 2014 Jan 17 [PubMed PMID: 24438370]
Level 1 (high-level) evidenceMessalli EM, Scaffa C. Long-term safety and efficacy of raloxifene in the prevention and treatment of postmenopausal osteoporosis: an update. International journal of women's health. 2010 Aug 9:1():11-20 [PubMed PMID: 21072271]
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Glüer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999 Aug 18:282(7):637-45 [PubMed PMID: 10517716]
Level 1 (high-level) evidenceSiris ES, Harris ST, Eastell R, Zanchetta JR, Goemaere S, Diez-Perez A, Stock JL, Song J, Qu Y, Kulkarni PM, Siddhanti SR, Wong M, Cummings SR, Continuing Outcomes Relevant to Evista (CORE) Investigators. Skeletal effects of raloxifene after 8 years: results from the continuing outcomes relevant to Evista (CORE) study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005 Sep:20(9):1514-24 [PubMed PMID: 16059623]
Vogel VG. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert review of anticancer therapy. 2009 Jan:9(1):51-60. doi: 10.1586/14737140.9.1.51. Epub [PubMed PMID: 19105706]
Eng-Wong J, Reynolds JC, Venzon D, Liewehr D, Gantz S, Danforth D, Liu ET, Chow C, Zujewski J. Effect of raloxifene on bone mineral density in premenopausal women at increased risk of breast cancer. The Journal of clinical endocrinology and metabolism. 2006 Oct:91(10):3941-6 [PubMed PMID: 16868059]
Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. The American journal of medicine. 2005 Dec 19:118 Suppl 12B():64-73 [PubMed PMID: 16414329]
Manson JE, Bassuk SS, Kaunitz AM, Pinkerton JV. The Women's Health Initiative trials of menopausal hormone therapy: lessons learned. Menopause (New York, N.Y.). 2020 Aug:27(8):918-928. doi: 10.1097/GME.0000000000001553. Epub [PubMed PMID: 32345788]
Miller VM, Naftolin F, Asthana S, Black DM, Brinton EA, Budoff MJ, Cedars MI, Dowling NM, Gleason CE, Hodis HN, Jayachandran M, Kantarci K, Lobo RA, Manson JE, Pal L, Santoro NF, Taylor HS, Harman SM. The Kronos Early Estrogen Prevention Study (KEEPS): what have we learned? Menopause (New York, N.Y.). 2019 Sep:26(9):1071-1084. doi: 10.1097/GME.0000000000001326. Epub [PubMed PMID: 31453973]
. Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. The Writing Group for the PEPI. JAMA. 1996 Nov 6:276(17):1389-96 [PubMed PMID: 8892713]
Lorentzon M, Johansson H, Harvey NC, Liu E, Vandenput L, Crandall CJ, Cauley JA, LeBoff MS, McCloskey EV, Kanis JA. Menopausal hormone therapy reduces the risk of fracture regardless of falls risk or baseline FRAX probability-results from the Women's Health Initiative hormone therapy trials. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2022 Nov:33(11):2297-2305. doi: 10.1007/s00198-022-06483-y. Epub 2022 Jul 14 [PubMed PMID: 35833956]
Fink HA, MacDonald R, Forte ML, Rosebush CE, Ensrud KE, Schousboe JT, Nelson VA, Ullman K, Butler M, Olson CM, Taylor BC, Brasure M, Wilt TJ. Long-Term Drug Therapy and Drug Discontinuations and Holidays for Osteoporosis Fracture Prevention: A Systematic Review. Annals of internal medicine. 2019 Jul 2:171(1):37-50. doi: 10.7326/M19-0533. Epub 2019 Apr 23 [PubMed PMID: 31009947]
Level 1 (high-level) evidenceKolitz E, McKesey J, Kwan E, Gill JG, Mauskar M. Strontium citrate associated drug reaction with eosinophilia and systemic symptoms syndrome with granulomatous dermatitis. JAAD case reports. 2021 Apr:10():85-88. doi: 10.1016/j.jdcr.2021.02.002. Epub 2021 Feb 17 [PubMed PMID: 33778142]
Level 3 (low-level) evidenceBlake GM, Fogelman I. Strontium ranelate: a novel treatment for postmenopausal osteoporosis: a review of safety and efficacy. Clinical interventions in aging. 2006:1(4):367-75 [PubMed PMID: 18046914]
Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, Mol-Arts M, Kloosterboer L, Mosca L, Christiansen C, Bilezikian J, Kerzberg EM, Johnson S, Zanchetta J, Grobbee DE, Seifert W, Eastell R, LIFT Trial Investigators. The effects of tibolone in older postmenopausal women. The New England journal of medicine. 2008 Aug 14:359(7):697-708. doi: 10.1056/NEJMoa0800743. Epub [PubMed PMID: 18703472]
Bundred NJ, Kenemans P, Yip CH, Beckmann MW, Foidart JM, Sismondi P, Schoultz Bv, Vassilopoulou-Sellin R, Galta RE, Lieshout EV, Mol-Arts M, Planellas J, Kubista E. Tibolone increases bone mineral density but also relapse in breast cancer survivors: LIBERATE trial bone substudy. Breast cancer research : BCR. 2012 Jan 17:14(1):R13 [PubMed PMID: 22251615]
Keller J, Catala-Lehnen P, Huebner AK, Jeschke A, Heckt T, Lueth A, Krause M, Koehne T, Albers J, Schulze J, Schilling S, Haberland M, Denninger H, Neven M, Hermans-Borgmeyer I, Streichert T, Breer S, Barvencik F, Levkau B, Rathkolb B, Wolf E, Calzada-Wack J, Neff F, Gailus-Durner V, Fuchs H, de Angelis MH, Klutmann S, Tsourdi E, Hofbauer LC, Kleuser B, Chun J, Schinke T, Amling M. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nature communications. 2014 Oct 21:5():5215. doi: 10.1038/ncomms6215. Epub 2014 Oct 21 [PubMed PMID: 25333900]
Srinivasan A, Wong FK, Karponis D. Calcitonin: A useful old friend. Journal of musculoskeletal & neuronal interactions. 2020 Dec 1:20(4):600-609 [PubMed PMID: 33265089]
Boucher E, Rosgen B, Lang E. Efficacy of calcitonin for treating acute pain associated with osteoporotic vertebral compression fracture: an updated systematic review. CJEM. 2020 May:22(3):359-367. doi: 10.1017/cem.2019.490. Epub [PubMed PMID: 32188529]
Level 1 (high-level) evidenceChesnut CH 3rd, Silverman S, Andriano K, Genant H, Gimona A, Harris S, Kiel D, LeBoff M, Maricic M, Miller P, Moniz C, Peacock M, Richardson P, Watts N, Baylink D. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. The American journal of medicine. 2000 Sep:109(4):267-76 [PubMed PMID: 10996576]
Level 1 (high-level) evidenceJilka RL, O'Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone. 2009 Feb:44(2):275-86. doi: 10.1016/j.bone.2008.10.037. Epub 2008 Oct 22 [PubMed PMID: 19010455]
Krege JH, Gilsenan AW, Komacko JL, Kellier-Steele N. Teriparatide and Osteosarcoma Risk: History, Science, Elimination of Boxed Warning, and Other Label Updates. JBMR plus. 2022 Sep:6(9):e10665. doi: 10.1002/jbm4.10665. Epub 2022 Aug 14 [PubMed PMID: 36111201]
Eastell R, Walsh JS. Anabolic treatment for osteoporosis: teriparatide. Clinical cases in mineral and bone metabolism : the official journal of the Italian Society of Osteoporosis, Mineral Metabolism, and Skeletal Diseases. 2017 May-Aug:14(2):173-178. doi: 10.11138/ccmbm/2017.14.1.173. Epub 2017 Oct 25 [PubMed PMID: 29263728]
Level 3 (low-level) evidenceFernández-Carneado J, Vallès-Miret M, Arrastia-Casado S, Almazán-Moga A, Macias MJ, Martin-Malpartida P, Vilaseca M, Díaz-Lobo M, Vazquez M, Sanahuja RM, Gambús G, Ponsati B. First Generic Teriparatide: Structural and Biological Sameness to Its Reference Medicinal Product. Pharmaceutics. 2024 Apr 13:16(4):. doi: 10.3390/pharmaceutics16040537. Epub 2024 Apr 13 [PubMed PMID: 38675198]
Lee HC, Breitbart H, Berman M, Forte JG. Potassium-stimulated ATPase activity and hydrogen transport in gastric microsomal vesicles. Biochimica et biophysica acta. 1979 May 3:553(1):107-31 [PubMed PMID: 36910]
Geusens P, Marin F, Kendler DL, Russo LA, Zerbini CA, Minisola S, Body JJ, Lespessailles E, Greenspan SL, Bagur A, Stepan JJ, Lakatos P, Casado E, Moericke R, López-Romero P, Fahrleitner-Pammer A. Effects of Teriparatide Compared with Risedronate on the Risk of Fractures in Subgroups of Postmenopausal Women with Severe Osteoporosis: The VERO Trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018 May:33(5):783-794. doi: 10.1002/jbmr.3384. Epub 2018 Feb 9 [PubMed PMID: 29329484]
Nakamura T, Sugimoto T, Nakano T, Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H, Nishizawa Y, Fujita T, Shiraki M. Randomized Teriparatide [human parathyroid hormone (PTH) 1-34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. The Journal of clinical endocrinology and metabolism. 2012 Sep:97(9):3097-106. doi: 10.1210/jc.2011-3479. Epub 2012 Jun 20 [PubMed PMID: 22723322]
Level 1 (high-level) evidenceEastell R, Nickelsen T, Marin F, Barker C, Hadji P, Farrerons J, Audran M, Boonen S, Brixen K, Gomes JM, Obermayer-Pietsch B, Avramidis A, Sigurdsson G, Glüer CC. Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European Study of Forsteo (EUROFORS). Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009 Apr:24(4):726-36. doi: 10.1359/jbmr.081215. Epub [PubMed PMID: 19049337]
Level 1 (high-level) evidenceSilverman S, Miller P, Sebba A, Weitz M, Wan X, Alam J, Masica D, Taylor KA, Ruff VA, Krohn K. The Direct Assessment of Nonvertebral Fractures in Community Experience (DANCE) study: 2-year nonvertebral fragility fracture results. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013 Aug:24(8):2309-17. doi: 10.1007/s00198-013-2284-y. Epub 2013 Feb 12 [PubMed PMID: 23404615]
Shirley M. Abaloparatide: First Global Approval. Drugs. 2017 Aug:77(12):1363-1368. doi: 10.1007/s40265-017-0780-7. Epub [PubMed PMID: 28624872]
Tella SH, Kommalapati A, Correa R. Profile of Abaloparatide and Its Potential in the Treatment of Postmenopausal Osteoporosis. Cureus. 2017 May 31:9(5):e1300. doi: 10.7759/cureus.1300. Epub 2017 May 31 [PubMed PMID: 28680788]
Akel M, Patel P, Parmar M. Abaloparatide. StatPearls. 2025 Jan:(): [PubMed PMID: 36512663]
Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, Alexandersen P, Zerbini CA, Hu MY, Harris AG, Fitzpatrick LA, Cosman F, Christiansen C, ACTIVE Study Investigators. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA. 2016 Aug 16:316(7):722-33. doi: 10.1001/jama.2016.11136. Epub [PubMed PMID: 27533157]
Level 1 (high-level) evidenceBone HG, Cosman F, Miller PD, Williams GC, Hattersley G, Hu MY, Fitzpatrick LA, Mitlak B, Papapoulos S, Rizzoli R, Dore RK, Bilezikian JP, Saag KG. ACTIVExtend: 24 Months of Alendronate After 18 Months of Abaloparatide or Placebo for Postmenopausal Osteoporosis. The Journal of clinical endocrinology and metabolism. 2018 Aug 1:103(8):2949-2957. doi: 10.1210/jc.2018-00163. Epub [PubMed PMID: 29800372]
Matsumoto T, Sone T, Soen S, Tanaka S, Yamashita A, Inoue T. Abaloparatide Increases Lumbar Spine and Hip BMD in Japanese Patients With Osteoporosis: The Phase 3 ACTIVE-J Study. The Journal of clinical endocrinology and metabolism. 2022 Sep 28:107(10):e4222-e4231. doi: 10.1210/clinem/dgac486. Epub [PubMed PMID: 35977548]
Miller SA, St Onge EL, Whalen KL. Romosozumab: A Novel Agent in the Treatment for Postmenopausal Osteoporosis. The Journal of pharmacy technology : jPT : official publication of the Association of Pharmacy Technicians. 2021 Feb:37(1):45-52. doi: 10.1177/8755122520967632. Epub 2020 Oct 23 [PubMed PMID: 34752536]
Krupa KN, Parmar M, Delo LF. Romosozumab. StatPearls. 2025 Jan:(): [PubMed PMID: 36256789]
Brown JP, Engelke K, Keaveny TM, Chines A, Chapurlat R, Foldes AJ, Nogues X, Civitelli R, De Villiers T, Massari F, Zerbini CAF, Wang Z, Oates MK, Recknor C, Libanati C. Romosozumab improves lumbar spine bone mass and bone strength parameters relative to alendronate in postmenopausal women: results from the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH) trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2021 Nov:36(11):2139-2152. doi: 10.1002/jbmr.4409. Epub 2021 Aug 10 [PubMed PMID: 34190361]
Cosman F, Crittenden DB, Ferrari S, Khan A, Lane NE, Lippuner K, Matsumoto T, Milmont CE, Libanati C, Grauer A. FRAME Study: The Foundation Effect of Building Bone With 1 Year of Romosozumab Leads to Continued Lower Fracture Risk After Transition to Denosumab. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018 Jul:33(7):1219-1226. doi: 10.1002/jbmr.3427. Epub 2018 May 17 [PubMed PMID: 29573473]
Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP, Daizadeh NS, Dokoupilova E, Engelke K, Finkelstein JS, Genant HK, Goemaere S, Hyldstrup L, Jodar-Gimeno E, Keaveny TM, Kendler D, Lakatos P, Maddox J, Malouf J, Massari FE, Molina JF, Ulla MR, Grauer A. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet (London, England). 2017 Sep 30:390(10102):1585-1594. doi: 10.1016/S0140-6736(17)31613-6. Epub 2017 Jul 26 [PubMed PMID: 28755782]
Level 1 (high-level) evidenceRobinson Y, Tschöke SK, Stahel PF, Kayser R, Heyde CE. Complications and safety aspects of kyphoplasty for osteoporotic vertebral fractures: a prospective follow-up study in 102 consecutive patients. Patient safety in surgery. 2008 Jan 15:2():2. doi: 10.1186/1754-9493-2-2. Epub 2008 Jan 15 [PubMed PMID: 18271950]
Gregson CL, Compston JE. New national osteoporosis guidance-implications for geriatricians. Age and ageing. 2022 Apr 1:51(4):. doi: 10.1093/ageing/afac044. Epub [PubMed PMID: 35403198]
Cosman F, Eriksen EF, Recknor C, Miller PD, Guañabens N, Kasperk C, Papanastasiou P, Readie A, Rao H, Gasser JA, Bucci-Rechtweg C, Boonen S. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011 Mar:26(3):503-11. doi: 10.1002/jbmr.238. Epub [PubMed PMID: 20814967]
Tsai JN, Uihlein AV, Lee H, Kumbhani R, Siwila-Sackman E, McKay EA, Burnett-Bowie SA, Neer RM, Leder BZ. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet (London, England). 2013 Jul 6:382(9886):50-6. doi: 10.1016/S0140-6736(13)60856-9. Epub 2013 May 15 [PubMed PMID: 23683600]
Level 1 (high-level) evidenceBuckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, Morrison L, Rao M, Robinson AB, Saha S, Wolver S, Bannuru RR, Vaysbrot E, Osani M, Turgunbaev M, Miller AS, McAlindon T. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis & rheumatology (Hoboken, N.J.). 2017 Aug:69(8):1521-1537. doi: 10.1002/art.40137. Epub 2017 Jun 6 [PubMed PMID: 28585373]
Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgström F, Cooper C, Diez Perez A, Eastell R, Hofbauer LC, Kanis JA, Langdahl BL, Lesnyak O, Lorenc R, McCloskey E, Messina OD, Napoli N, Obermayer-Pietsch B, Ralston SH, Sambrook PN, Silverman S, Sosa M, Stepan J, Suppan G, Wahl DA, Compston JE, Joint IOF-ECTS GIO Guidelines Working Group. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012 Sep:23(9):2257-76. doi: 10.1007/s00198-012-1958-1. Epub 2012 Mar 21 [PubMed PMID: 22434203]
Bauer D, Krege J, Lane N, Leary E, Libanati C, Miller P, Myers G, Silverman S, Vesper HW, Lee D, Payette M, Randall S. National Bone Health Alliance Bone Turnover Marker Project: current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012 Oct:23(10):2425-33. doi: 10.1007/s00198-012-2049-z. Epub 2012 Jul 14 [PubMed PMID: 22797491]
Taxel P, Kenny A. Differential diagnosis and secondary causes of osteoporosis. Clinical cornerstone. 2000:2(6):11-21 [PubMed PMID: 10938988]
Stumpf U, Hesse E, Böcker W, Kammerlander C, Neuerburg C, Schmidmaier R. [Differential diagnoses of osteoporosis]. Zeitschrift fur Gerontologie und Geriatrie. 2019 Aug:52(5):414-420. doi: 10.1007/s00391-019-01571-x. Epub 2019 Jul 11 [PubMed PMID: 31297588]
Coughlan T, Dockery F. Osteoporosis and fracture risk in older people. Clinical medicine (London, England). 2014 Apr:14(2):187-91. doi: 10.7861/clinmedicine.14-2-187. Epub [PubMed PMID: 24715132]
Balasubramanian A, Zhang J, Chen L, Wenkert D, Daigle SG, Grauer A, Curtis JR. Risk of subsequent fracture after prior fracture among older women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2019 Jan:30(1):79-92. doi: 10.1007/s00198-018-4732-1. Epub 2018 Nov 19 [PubMed PMID: 30456571]
Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2000:11(7):556-61 [PubMed PMID: 11069188]
Lau E, Ong K, Kurtz S, Schmier J, Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. The Journal of bone and joint surgery. American volume. 2008 Jul:90(7):1479-86. doi: 10.2106/JBJS.G.00675. Epub [PubMed PMID: 18594096]
Level 2 (mid-level) evidenceMarottoli RA, Berkman LF, Cooney LM Jr. Decline in physical function following hip fracture. Journal of the American Geriatrics Society. 1992 Sep:40(9):861-6 [PubMed PMID: 1512379]
Bubbear JS. Atypical Femur Fractures in Patients Treated with Bisphosphonates: Identification, Management, and Prevention. Rambam Maimonides medical journal. 2016 Oct 31:7(4):. doi: 10.5041/RMMJ.10259. Epub 2016 Oct 31 [PubMed PMID: 27824547]
Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, Ebeling PR, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O'Keefe R, Papapoulos S, Howe TS, van der Meulen MC, Weinstein RS, Whyte MP. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014 Jan:29(1):1-23. doi: 10.1002/jbmr.1998. Epub 2013 Oct 1 [PubMed PMID: 23712442]
Khan A. Bisphosphonate-associated osteonecrosis of the jaw. Canadian family physician Medecin de famille canadien. 2008 Jul:54(7):1019-21 [PubMed PMID: 18625828]
Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O'Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S, Watts NB, Brandi ML, Peters E, Guise T, Eastell R, Cheung AM, Morin SN, Masri B, Cooper C, Morgan SL, Obermayer-Pietsch B, Langdahl BL, Al Dabagh R, Davison KS, Kendler DL, Sándor GK, Josse RG, Bhandari M, El Rabbany M, Pierroz DD, Sulimani R, Saunders DP, Brown JP, Compston J, International Task Force on Osteonecrosis of the Jaw. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015 Jan:30(1):3-23. doi: 10.1002/jbmr.2405. Epub [PubMed PMID: 25414052]
Level 1 (high-level) evidenceChang J, Hakam AE, McCauley LK. Current Understanding of the Pathophysiology of Osteonecrosis of the Jaw. Current osteoporosis reports. 2018 Oct:16(5):584-595. doi: 10.1007/s11914-018-0474-4. Epub [PubMed PMID: 30155844]
Level 3 (low-level) evidenceTofé VI, Bagán L, Bagán JV. Osteonecrosis of the jaws associated with denosumab: Study of clinical and radiographic characteristics in a series of clinical cases. Journal of clinical and experimental dentistry. 2020 Jul:12(7):e676-e681. doi: 10.4317/jced.57019. Epub 2020 Jul 1 [PubMed PMID: 32904934]
Level 3 (low-level) evidenceSim IW, Borromeo GL, Tsao C, Hardiman R, Hofman MS, Papatziamos Hjelle C, Siddique M, Cook GJR, Seymour JF, Ebeling PR. Teriparatide Promotes Bone Healing in Medication-Related Osteonecrosis of the Jaw: A Placebo-Controlled, Randomized Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Sep 10:38(26):2971-2980. doi: 10.1200/JCO.19.02192. Epub 2020 Jul 2 [PubMed PMID: 32614699]
Level 1 (high-level) evidence