Introduction
Atrioventricular reciprocating tachycardia (AVRT) is a type of supraventricular tachycardia (SVT) that requires specific electrophysiologic and electroanatomic characteristics. AVRT uses a circuit that consists of at least 2 different pathways with different electrical properties, including conduction velocity, refractory periods, and directionality. The anatomical substrate to sustain an AVRT can vary because AVRT may involve different configurations as follows:
- Two accessory pathways (APs) that enable communication between the atria and the ventricles.
- A single accessory pathway, in addition to the regular conduction system through the atrioventricular node, completes the circuit.
- Multiple accessory pathways through which the SVT can be perpetuated.[1]
AVRT commonly starts with an ectopic atrial or ventricular beat that travels through one of the circuit's limbs due to the distinct electrical properties of the tissues involved. These properties allow the initiation and persistence of an SVT at a heart rate ranging from 150 to 250 bpm.[2][3]
AVRT is the most common type of arrhythmia associated with Wolff-Parkinson-White (WPW) syndrome. In this condition, antegrade conduction at rest occurs through an accessory pathway (or accessory pathways) that manifests on the surface electrocardiogram (ECG). The ECG shows a shortened PR interval followed by a delta wave or slurring of the initial portion of the QRS complex, which results in a widened QRS complex. AVRT can use an antegrade and non-decremental accessory pathway in these patients, and retrograde conduction can occur through the atrioventricular node or another accessory pathway. These patients can also develop accelerated conduction of atrial arrhythmias through the accessory pathway or accessory pathways that bypass the atrioventricular node. These preexcited atrial arrhythmias, especially atrial fibrillation, can be lethal due to rapid conduction into the ventricles.[4]
Permanent junctional reciprocating tachycardia is a rare type of AVRT, which uses the atrioventricular node as its antegrade limb and a decrementally conducting accessory pathway as its retrograde limb. This type of tachycardia typically has a slower heart rate, between 130 and 150 bpm, and is commonly refractory to medical management.[5][6] Other unusual forms of AVRT use atrioventricular or nodoventricular accessory pathways, known as Mahaim pathways. These are decrementally conducting anomalous connections between the right atrium or the atrioventricular node and the right ventricle.[7][8]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Typically, the fibrous annuli of the tricuspid and mitral valves provide electrical insulation between the atria and ventricles, except at the level of the atrioventricular node. The accessory pathways required to sustain an AVRT consist of myocardial muscle bundles that connect the atria to the ventricles, thus bypassing the insulation provided by the fibrous annuli of tricuspid and mitral valves. Accessory pathways can conduct impulses in an anterograde, retrograde, or bidirectional manner.[2] Patients with Ebstein anomaly of the tricuspid valve commonly have more than one myocardial bundle or accessory pathway, which makes treatment of the AVRT challenging.[1]
The electrophysiologic properties of the accessory pathways typically resemble those of the Purkinje fibers, exhibiting rapid conduction, lack of decremental conduction, and often resistance to adenosine.[2] However, some accessory pathways can exhibit decremental conduction. These are known as Mahaim accessory pathways and are characterized by their sensitivity to adenosine.[7][9]
Epidemiology
AVRT is the fourth most common type of SVT in patients older than 20, after atrial fibrillation, atrial flutter, and atrioventricular nodal reentrant tachycardia (AVNRT). However, it is the most common type of SVT in the pediatric population.[2] In children, the highest incidence of SVT occurs during infancy, typically ranging from 1 month to 1 year (12 months), with spontaneous resolution in 90% of patients by 12 months. Of these, approximately 30% will have a recurrence during early childhood, between the ages of 6 and 9.[5] Multiple accessory pathways can be found in 4% to 10% of patients, especially those with Ebstein anomaly. Assessing the presence of other accessory pathways during an electrophysiologic study is crucial. Detecting and eliminating any additional accessory pathways with radiofrequency ablation or cryoablation will prevent AVRT recurrence.[10]
Pathophysiology
AVRT can be orthodromic, antidromic, or junctional reciprocating tachycardia.[11][12][2] These AVRTs have certain electrophysiologic features that can differentiate one from the other.
Orthodromic Atrioventricular Reentry Tachycardia
This form comprises 80% to 87% of AVRTs and can be initiated with atrial premature beats or ventricular premature beats. An atrial premature beat that initiates the AVRT is typically blocked in the accessory pathway. Subsequently, it conducts to the ventricles in an antegrade manner through the atrioventricular node and the His-Purkinje system. However, before reaching the His-Prkinje system, the electrical impulse travels retrogradely through the accessory pathway, establishing a reentry circuit that uses the accessory pathway and the atrioventricular node.[3] A ventricular premature beat initiating the AVRT conducts to the atria through the accessory pathway in a retrograde manner. The impulse travels back to the ventricles through the atrioventricular node to complete the circuit. In both cases, the impulse will travel through the atrioventricular node and the His-Purkinje system in an antegrade fashion and through the accessory pathway (or accessory pathways) in a retrograde manner.[13]
Antidromic Atrioventricular Reentry Tachycardia
This mechanism occurs in 5% to 10% of AVRTs. As with orthodromic AVRT, the tachyarrhythmia is initiated by either an atrial premature beat or a ventricular premature beat. When initiated by an atrial premature beat, the impulse is blocked in the atrioventricular node. Subsequently, it conducts to the ventricles through a manifest accessory pathway in an anterograde manner, preexciting the ventricles. The impulse travels back to the atria through the His-Purkinje system and atrioventricular node or another accessory pathway in a retrograde manner. If a ventricular premature beat initiates the reentrant tachycardia, the impulse conducts from the ventricles to the atria in a retrograde manner either through the His-Purkinje system and the atrioventricular node or through an accessory pathway. Subsequently, it returns to the ventricles in an antegrade manner through another accessory pathway, also displaying preexcitation. In both cases, the impulse will travel through the His-Purkinje system and the atrioventricular node or an accessory pathway in a retrograde manner and through another accessory pathway in an antegrade manner, which produces preexcitation with a wide QRS.[13]
Junctional Reciprocating Tachycardia
Junctional reciprocating tachycardia is a type of AVRT that occurs predominantly in infants and children, accounting for 1% of the SVTs in this age group. If left untreated, it can evolve into an incessant tachycardia, resulting in tachycardia-induced cardiomyopathy. During junctional reciprocating tachycardia, the atrioventricular node acts as the antegrade limb, whereas an accessory pathway with physiologic characteristics similar to the AVN, such as slow and decremental conduction, functions as the retrograde limb. The similar conduction characteristics shared between this type of accessory pathway and the atrioventricular node enable the formation of a stable, and thus persistent, reentrant circuit, resulting in a narrow-QRS tachycardia with heart rates ranging between 200 and 300 bpm in infancy and around 250 bpm in early childhood. If present during adulthood, heart rates can be slower, typically in the range of 120 bpm. Inverted P waves can typically be observed in inferior leads II, III, and aVF. This type of AVRT does not require a premature atrial contraction or a premature ventricular contraction for initiation. Typically, it exhibits a temporary response to vagal maneuvers or adenosine, resulting in slowing before termination. However, recurrence often follows shortly afterward.[12]
History and Physical
Similar to other SVTs, the clinical presentation of AVRT depends on the associated comorbidities. The most commonly reported symptom is palpitations. However, dizziness, chest pain, and syncope can occur.[13]
Evaluation
Electrocardiogram
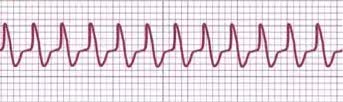
An experienced electrophysiologist can use a 12-lead ECG during SVT to differentiate between different mechanisms, such as an orthodromic AVRT using a concealed accessory pathway as the retrograde limb of the tachycardia, AVNRT, antidromic AVRT using the accessory pathway as the antegrade limb of the tachycardia (presenting as wide QRS tachycardia), and ventricular tachycardia. Artificial intelligence shows promising results in differentiating SVT mechanisms, offering comparable performance to experienced electrophysiologists.[14] See Image. AVRT ECG strip.
Narrow QRS Tachycardia: Differential diagnosis between Orthodromic AVRT and AVNRT
In patients without intraventricular conduction delay or a bundle branch block, an orthodromic AVRT will exhibit narrow QRS complexes, with a heart rate ranging from 150 to 250 bpm. When the duration of the RP interval is relatively long (>70 msec), retrograde P waves can be observed following the QRS complex because the activation of the atria is reversed. Clinically, carotid palpitation can be an indicator of AVNRT.[15]
Typical or slow-fast AVNRT usually has a short RP interval (<70 msec) with the retrograde P wave buried within the preceding QRS complex. Therefore, the P waves are not visible on the surface ECG. Occasionally, a pseudo r' deflection in V1 or QRS alternans will favor the diagnosis of AVNRT over AVRT. Orthodromic AVRT and atypical AVNRT, such as fast-slow and slow-slow AVNRT, may show retrograde P waves or pseudo repolarization changes after the QRS complexes during the tachycardia, which may be visible on the surface ECG. If a bundle branch block occurs ipsilaterally to the accessory pathway during the tachycardia with lengthening of the R-R interval (that is, lengthening of the tachycardia cycle length), orthodromic AVRT is the probable diagnosis.[16] This phenomenon is described in Coumel's law, which states that when a wide QRS tachycardia abruptly becomes a narrow QRS tachycardia with a simultaneous increase in heart rate, orthodromic AVRT using an accessory pathway ipsilateral to the bundle branch block is the probable diagnosis.[17]
Wide QRS Tachycardia: Differential diagnosis between Antidromic AVRT, AVNRT with a bundle branch block, and ventricular tachycardia
In patients with WPW syndrome, the resting ECG shows a delta wave with a short PR interval, resulting from the early depolarization of the ventricles through the accessory pathway.[18] During antidromic AVRT, the QRS complexes are wide as the impulse travels to the ventricles through an accessory pathway that bypasses the atrioventricular node. This type of AVRT might be difficult to differentiate from ventricular tachycardia (VT). Wide QRS tachycardia can also be observed in the setting of AVNRT with a bundle branch block, as described above. In this case, the heart rate during SVT typically falls within the range of 150 to 250 bpm, and retrograde P waves may be seen following the QRS complexes. Generally, ventricular tachycardia is more likely when the duration of the QRS complex is greater than 140 msec.[19][20]
Electrophysiologic Study
An electrophysiologic study involves inserting intracardiac catheters with electrodes to track and differentiate the arrhythmias. In addition, an electrophysiologic study allows radiofrequency ablation or cryoablation of an accessory pathway or tachycardia focus, which can cure the arrhythmia. Traditionally, catheter ablations have used fluoroscopy, which exposes the patient to radiation that is not insignificant, as such procedures can last several hours. However, several centers are achieving a decrease and even elimination of fluoroscopy by using intracardiac electrograms, electroanatomic mapping, and intracardiac echocardiography.[21]
During an electrophysiologic study, AVRT can be diagnosed when a bundle branch block occurs simultaneously with an increase in the VA interval (time interval between the earliest QRS deflection on the surface ECG and the earliest atrial deflection in the His bundle intracardiac electrogram) greater than 20 msec. As with the standard surface ECG, this intracardiac physiologic phenomenon helps narrow the localization of the accessory pathway during an electrophysiologic study. A sudden bundle branch block with prolongation in the VA interval occurs on the side where the functional block is occurring, that is, on the side of the accessory pathway. Another technique used during an electrophysiologic study involves producing a His-synchronous ventricular premature beat delivered either during His depolarization or within 40 msec of His depolarization. This technique is performed to determine whether the ventricular premature beat resulted in an advancement of atrial depolarization. If the subsequent atrial signal is advanced by the ventricular premature beat (that is, arrived earlier than expected), it indicates the presence of an accessory pathway. When the SVT terminates without conducting to the atrium, this shows not only the presence of an accessory pathway but also a pathway necessary for the arrhythmia circuit and not just a possible bystander accessory pathway. Differential ventricular pacing performed at the base and the apex of the right ventricle can also help detect an accessory pathway. In the absence of an accessory pathway, the VA interval is shorter when pacing is performed from the apex compared to the base of the ventricle, as it retrogradely conducts through the His-Purkinje system and atrioventricular node to the right atrium. However, in the presence of an accessory pathway, ipsilateral stimulation (to the accessory pathway) renders the same VA interval, whether pacing occurs from the apex or the base of the ventricle.[22]
Treatment / Management
The management of AVRT seeks the termination of the acute arrhythmia and the prevention of its recurrence. If the patient is hemodynamically unstable, has altered mental status, or is suspected to have ischemic chest pain, immediate electrical cardioversion is recommended.[23]
Acute Termination of Orthodromic AVRT
If the patient is hemodynamically stable, vagal maneuvers can be attempted first. A Valsalva maneuver involves instructing the patient to bear down against a closed glottis for 10 to 30 seconds. Carotid massage can be performed by applying steady pressure over the left and right carotid sinuses, one at a time. If vagal maneuvers are unsuccessful, the subsequent intervention aimed at terminating tachycardia is the intravenous administration of adenosine at a dose of 6 mg (0.1 mg/kg for children with a maximum dose of 6 mg). Adenosine has been reported to be 91% effective in terminating paroxysmal SVT with minimal and brief adverse effects. Adenosine can be increased to 12 mg if the initial dose is ineffective. Verapamil can also be effective in terminating AVRT when administered intravenously at a dosage of 5 mg every 2 to 3 minutes, with a maximum dose of 15 mg. Verapamil should be used with caution in patients with heart failure and reduced ejection fraction. The intravenous administration of procainamide or beta-blockers can also be attempted. Procainamide can be administered intravenously at 20 to 50 mg per minute until the arrhythmia stops, hypotension occurs, or the QRS complex widens by more than 50% (with a maximum dose of 17 mg/kg). Metoprolol can be administered intravenously at a dose of 5 mg through a slow push over 2 minutes and may be repeated every 10 minutes, up to a total dosage of 15 mg.[24](A1)
Acute Termination of Antidromic AVRT
In practice, confirming the diagnosis of antidromic AVRT, which presents as a wide QRS complex tachycardia, can be challenging in the acute setting. If antidromic AVRT is confirmed, atrioventricular node blockade agents should be avoided, and intravenous procainamide administration can be considered an alternative option.[24](A1)
Treatment to Prevent Recurrence of the Arrhythmia
In symptomatic individuals and select asymptomatic individuals, particularly younger patients, radiofrequency ablation of the accessory pathway is the preferred treatment to prevent arrhythmia recurrence. For individuals experiencing arrhythmia recurrence after radiofrequency ablation and refusing subsequent procedures, for those who refuse to undergo RFA, in cases involving very young and very small patients where radiofrequency ablation can be challenging, or when arrhythmia is expected to subside with age, antiarrhythmic medications are considered as an option.[19] Prophylactic antiarrhythmic medications are often successfully used as a single medication to prevent SVT. Beta-blockers such as propranolol are commonly used as the first line of therapy, especially in infants, due to their effectiveness in preventing arrhythmia recurrence.[25] Sodium channel blockers such as propafenone can also be used with caution due to QRS prolongation. Amiodarone is a broad-spectrum antiarrhythmic medication primarily blocking potassium channels, with additional blocking effects on sodium and calcium channels, and alpha- and beta-adrenergic receptors. While amiodarone is typically quite effective, its long-term use may lead to adverse effects and toxicity, including thyroid toxicity, interstitial lung disease, hepatotoxicity, and others. Therefore, amiodarone is used when other antiarrhythmic medications have failed to control the arrhythmia. Antiarrhythmic therapy can be discontinued in infants after 12 months, as approximately 70% are expected to become free of tachycardia by that age. However, infants diagnosed with SVT in utero appear to face a significantly higher risk of persistent tachycardia. In infants, radiofrequency ablation is limited to those with refractory SVT. In children weighing greater than 15 kg with persistent SVT, radiofrequency ablation has shown favorable results with a lower risk of complications.[26]
Differential Diagnosis
The differential diagnosis of an orthodromic AVRT includes all regular, narrow, complex tachycardias such as sinus tachycardia, atrial flutter, and AVNRT. The differential diagnosis of an antidromic AVRT includes regular, wide QRS tachycardias such as ventricular tachycardia.[3][27]
Prognosis
Patients with AVRT generally have a favorable prognosis as the arrhythmia can typically be terminated using interventions such as vagal maneuvers, adenosine, or through treatments such as radiofrequency ablation or cryoablation. When AVRT occurs in a patient with WPW syndrome with an antegrade-conducting accessory pathway, the occurrence of atrial fibrillation can lead to sudden cardiac death if atrial fibrillation is conducted rapidly into the ventricles. The prevalence of ventricular fibrillation in these patients can be up to 0.3%, and the occurrence of life-threatening events can be 70 times higher compared to the general population.[28]
Complications
Complications from AVRT can include dyspnea, chest pain, pre-syncope, or syncope.[5] Although rare, sudden cardiac death can occur as the initial presentation of an antidromic AVRT in a patient with atrial fibrillation who has an accessory pathway or accessory pathways with a short RR interval, indicating rapid conduction through the accessory pathway.[29]
Complications from radiofrequency ablation can occur in 2% to 4% of cases. Information gathered from a national registry involving 3357 patients reported cardiac tamponade, acute myocardial infarction, femoral artery pseudoaneurysms, atrioventricular block, pneumothorax, and pericarditis. Complications have been reduced with the advent of cryoablation and modern electrophysiologic mapping systems.[18]
Deterrence and Patient Education
Patient education plays a significant role in management. Patients should be cognizant of the signs and symptoms of a tachyarrhythmia, the risks and benefits of RFA, and the long-term effects of antiarrhythmic drugs when appropriate.
Pearls and Other Issues
SVT in Sports
Athletes with frequent SVTs should be evaluated for an underlying cardiac or thyroid disease and the use of stimulants or performance-enhancing drugs. If SVT, such as AVRT, occurs during exercise with very rapid heart rates, it may cause hemodynamic impairment; thus, radiofrequency ablation is recommended as a treatment option. For athletes with sporadic SVT that is not associated with exercise and is well-tolerated, the sport does not pose a high risk of loss of consciousness during the SVT (in contrast to activities such as diving, piloting, and horse riding). In cases where radiofrequency ablation has been unsuccessful, these athletes may still be eligible to compete. Certain antiarrhythmic drugs are discouraged in athletes. Sodium channel blockers have a limited ability to prevent SVT recurrence during exercise. Beta-blockers reduce athletic performance and are banned by the World Anti-Doping Agency in certain sports requiring precision and stillness, such as golf, shooting, and archery.[19][30]
WPW syndrome in Athletes and Risk for AVRT and Sudden Cardiac Death
Although many young athletes with WPW syndrome remain asymptomatic, AVRT remains a possibility. Managing ventricular preexcitation in affected athletes is challenging due to the small but ever-present risk of sudden cardiac death, which appears to be higher in individuals younger than 40, especially males.[4] The current guidelines recommend performing an electrophysiologic study to risk-stratify the accessory pathway, irrespective of symptoms, especially in competitive athletes (and individuals with high-risk occupations such as airline pilots). Radiofrequency ablation or cryoablation is recommended if the accessory pathway is considered as high risk based on factors such as a shortest preexcited R-R interval or SPERRI of ≤250 msec, an effective refractory period or ERP of ≤250 msec, the presence of multiple accessory pathways, and the presence of an inducible accessory pathway-mediated tachycardia. Asymptomatic individuals stratified as having a low-risk accessory pathway can be allowed to participate in competitive sports, as AVRT over a concealed accessory pathway is not listed as a cause of sudden cardiac death during exercise in those with normal cardiac anatomy and function.[19] It appears that anteroseptal accessory pathways are more likely to be linked with the development of atrial fibrillation.[31]
SVT and Driving
Although the contribution of arrhythmia to motor vehicle accidents is unknown, it is believed to be small. The European Society of Cardiology (ESC) has established specific guidelines that prohibit driving for individuals who have experienced syncope during SVT until the SVT has been adequately treated. Driving can be continued in the absence of syncope or troublesome symptoms such as palpitations with dizziness. However, if the patient has WPW syndrome, driving is allowed only after an electrophysiologist evaluates their condition.[19]
Artificial Intelligence
Machine learning, or artificial intelligence, is now used to identify SVT mechanisms that may appear visually imperceptible. Adequate algorithms have successfully differentiated AVRT from AVNRT and atrial tachycardia with sensitivities similar to those of experienced cardiac electrophysiologists. Interestingly, with time, artificial intelligence can improve its diagnostic accuracy as it feeds from further ECG data.[32]
Fetal SVT
Fetal tachyarrhythmias occur in less than 0.1% of pregnancies, with orthodromic AVRT and atrial flutter being the most common types. When persistent, they can result in fetal cardiac failure, followed by hydrops and demise. Preterm delivery can be lifesaving in viable fetuses with hydrops. However, extreme prematurity still places these patients at a high morbidity and mortality risk. Thus, maternal antiarrhythmic therapy is considered the first line of treatment, which appears to be effective and well-tolerated in most cases. Due to the lack of large controlled studies, the choice of antiarrhythmic drugs varies based on institutional protocols and physician preferences. However, it is not uncommon to administer digoxin intravenously or orally to the mother in the absence of fetal hydrops. If digoxin fails to control fetal tachyarrhythmia after 3 days of treatment, it can be replaced by sotalol. Sotalol dosage can be increased; however, if it proves ineffective, flecainide can be added. An echocardiographic assessment of fetal heart rate and rhythm is used to evaluate treatment effectiveness.[33] Some practitioners might use beta-blockers. In this case, metoprolol or propranolol is favored over atenolol due to its reported association with small infants for gestational age. If calcium channel blockers are used, verapamil is recommended over diltiazem due to its reported teratogenicity in animal studies.[19]
Tachycardia-Induced Cardiomyopathy
The diagnosis and treatment of tachycardia-induced cardiomyopathy are crucial, as it is a reversible cause of cardiac failure. If left unrecognized, it can progress to the extent of requiring a cardiac transplant or could lead to death. The incidence of tachycardia-induced cardiomyopathy is unknown, but it has been reported in various age groups, ranging from fetuses to older adults. Any chronic arrhythmia can cause tachycardia-induced cardiomyopathy, such as incessant AVRT due to a septal accessory pathway, permanent junctional reciprocating tachycardia, rapid AF, idiopathic ventricular tachycardia, and focal atrial tachycardia. Cardiac function typically improves after 3 months of sinus rhythm restoration through radiofrequency ablation of the tachycardia focus and using a beta-blocker and angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker.[19]
Enhancing Healthcare Team Outcomes
Recognizing an AVRT and seeking the appropriate specialist are crucial steps in managing this condition. A cardiologist, especially specialized in electrophysiology, is best suited to diagnose patients and assess whether radiofrequency ablation of an accessory pathway is warranted. This evaluation involves weighing the benefits and risks of undergoing such a procedure, which can provide a definitive cure compared to long-term antiarrhythmic medication. Effective communication between the specialist and the primary care provider is crucial for a comprehensive approach to patient care. This collaboration should consider factors such as the patient's age, any underlying heart disease, comorbidities, and the individual's social support.
Media
References
Zachariah JP, Walsh EP, Triedman JK, Berul CI, Cecchin F, Alexander ME, Bevilacqua LM. Multiple accessory pathways in the young: the impact of structural heart disease. American heart journal. 2013 Jan:165(1):87-92. doi: 10.1016/j.ahj.2012.10.025. Epub 2012 Nov 20 [PubMed PMID: 23237138]
Level 2 (mid-level) evidenceLink MS. Clinical practice. Evaluation and initial treatment of supraventricular tachycardia. The New England journal of medicine. 2012 Oct 11:367(15):1438-48. doi: 10.1056/NEJMcp1111259. Epub [PubMed PMID: 23050527]
Colucci RA, Silver MJ, Shubrook J. Common types of supraventricular tachycardia: diagnosis and management. American family physician. 2010 Oct 15:82(8):942-52 [PubMed PMID: 20949888]
Leung LWM, Gallagher MM. Review paper on WPW and athletes: Let sleeping dogs lie? Clinical cardiology. 2020 Aug:43(8):897-905. doi: 10.1002/clc.23399. Epub 2020 Jun 27 [PubMed PMID: 32592213]
Salerno JC, Seslar SP. Supraventricular tachycardia. Archives of pediatrics & adolescent medicine. 2009 Mar:163(3):268-74. doi: 10.1001/archpediatrics.2008.547. Epub [PubMed PMID: 19255396]
Rodríguez-Mañero M, Fernández-López XA, González-Melchor L, García-Seara J, Martínez-Sande JL, González-Juanatey JR. Permanent junctional reciprocating tachycardia in a patient with an atypically located accessory pathway in the left lateral mitral annulus. Revista portuguesa de cardiologia : orgao oficial da Sociedade Portuguesa de Cardiologia = Portuguese journal of cardiology : an official journal of the Portuguese Society of Cardiology. 2016 Jan:35(1):59.e1-5. doi: 10.1016/j.repc.2015.07.012. Epub 2015 Dec 31 [PubMed PMID: 26749575]
Katritsis DG, Wellens HJ, Josephson ME. Mahaim Accessory Pathways. Arrhythmia & electrophysiology review. 2017 Apr:6(1):29-32. doi: 10.15420/aer.2016:35:1. Epub [PubMed PMID: 28507744]
Kanzaki Y, Morishima I, Furui K, Yamauchi R. The spatial and temporal visualization of the entire atriofascicular fiber conduction during antidromic reciprocating tachycardia. HeartRhythm case reports. 2021 Mar:7(3):150-152. doi: 10.1016/j.hrcr.2020.11.023. Epub 2020 Dec 3 [PubMed PMID: 33786309]
Level 3 (low-level) evidenceLerman BB, Markowitz SM, Cheung JW, Liu CF, Thomas G, Ip JE. Supraventricular Tachycardia: Mechanistic Insights Deduced From Adenosine. Circulation. Arrhythmia and electrophysiology. 2018 Dec:11(12):e006953. doi: 10.1161/CIRCEP.118.006953. Epub [PubMed PMID: 30562103]
Gonzalez JE, Zipse MM, Nguyen DT, Sauer WH. Antidromic Atrioventricular Reciprocating Tachycardia Using a Concealed Retrograde Conducting Left Lateral Accessory Pathway. Cardiac electrophysiology clinics. 2016 Mar:8(1):37-43. doi: 10.1016/j.ccep.2015.10.001. Epub 2016 Jan 11 [PubMed PMID: 26920167]
Ko JK, Deal BJ, Strasburger JF, Benson DW Jr. Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. The American journal of cardiology. 1992 Apr 15:69(12):1028-32 [PubMed PMID: 1561973]
Kylat RI, Samson RA. Permanent junctional reciprocating tachycardia in infants and Children. Journal of arrhythmia. 2019 Jun:35(3):494-498. doi: 10.1002/joa3.12193. Epub 2019 May 15 [PubMed PMID: 31293698]
Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, Estes NA 3rd, Field ME, Goldberger ZD, Hammill SC, Indik JH, Lindsay BD, Olshansky B, Russo AM, Shen WK, Tracy CM, Al-Khatib SM, Evidence Review Committee Chair‡. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016 Apr 5:133(14):e471-505. doi: 10.1161/CIR.0000000000000310. Epub 2015 Sep 23 [PubMed PMID: 26399662]
Level 1 (high-level) evidenceHiguchi S, Li R, Gerstenfeld EP, Liem LB, Im SI, Kalantarian S, Ansari M, Abreau S, Barrios J, Scheinman MM, Tison GH. Identification of supraventricular tachycardia mechanisms with surface electrocardiograms using a convolutional neural network. Heart rhythm O2. 2023 Aug:4(8):491-499. doi: 10.1016/j.hroo.2023.07.004. Epub 2023 Jul 13 [PubMed PMID: 37645266]
González-Torrecilla E, Almendral J, Arenal A, Atienza F, Atea LF, del Castillo S, Fernández-Avilés F. Combined evaluation of bedside clinical variables and the electrocardiogram for the differential diagnosis of paroxysmal atrioventricular reciprocating tachycardias in patients without pre-excitation. Journal of the American College of Cardiology. 2009 Jun 23:53(25):2353-8. doi: 10.1016/j.jacc.2009.02.059. Epub [PubMed PMID: 19539146]
González-Torrecilla E, Arenal A, Atienza F, Datino T, Atea LF, Calvo D, Pachón M, Miracle A, Fernández-Avilés F. EGC diagnosis of paroxysmal supraventricular tachycardias in patients without preexcitation. Annals of noninvasive electrocardiology : the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc. 2011 Jan:16(1):85-95. doi: 10.1111/j.1542-474X.2010.00399.x. Epub [PubMed PMID: 21251139]
Sasmita BR, Luo S, Huang B. Wide and Narrow QRS Complex Tachycardia With Cycle Length Alternans: What Is the Mechanism? Circulation. 2021 Nov 30:144(22):1824-1826. doi: 10.1161/CIRCULATIONAHA.121.057578. Epub 2021 Nov 29 [PubMed PMID: 34843393]
Pediatric and Congenital Electrophysiology Society (PACES), Heart Rhythm Society (HRS), American College of Cardiology Foundation (ACCF), American Heart Association (AHA), American Academy of Pediatrics (AAP), Canadian Heart Rhythm Society (CHRS), Cohen MI, Triedman JK, Cannon BC, Davis AM, Drago F, Janousek J, Klein GJ, Law IH, Morady FJ, Paul T, Perry JC, Sanatani S, Tanel RE. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart rhythm. 2012 Jun:9(6):1006-24. doi: 10.1016/j.hrthm.2012.03.050. Epub 2012 May 10 [PubMed PMID: 22579340]
Level 3 (low-level) evidenceBrugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist C, Calkins H, Corrado D, Deftereos SG, Diller GP, Gomez-Doblas JJ, Gorenek B, Grace A, Ho SY, Kaski JC, Kuck KH, Lambiase PD, Sacher F, Sarquella-Brugada G, Suwalski P, Zaza A, ESC Scientific Document Group. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). European heart journal. 2020 Feb 1:41(5):655-720. doi: 10.1093/eurheartj/ehz467. Epub [PubMed PMID: 31504425]
Brugada P, Brugada J, Mont L, Smeets J, Andries EW. A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation. 1991 May:83(5):1649-59 [PubMed PMID: 2022022]
Razminia M, Willoughby MC, Demo H, Keshmiri H, Wang T, D'Silva OJ, Zheutlin TA, Jibawi H, Okhumale P, Kehoe RF. Fluoroless Catheter Ablation of Cardiac Arrhythmias: A 5-Year Experience. Pacing and clinical electrophysiology : PACE. 2017 Apr:40(4):425-433. doi: 10.1111/pace.13038. Epub 2017 Mar 3 [PubMed PMID: 28160298]
Abisse S, Adelstein E, Jain S, Saba S. Choice and Utility of Pacing Maneuver in Establishing the Mechanism of Supraventricular Tachycardia: A Single Center Experience. Cardiology research. 2012 Feb:3(1):28-33. doi: 10.4021/cr135w. Epub 2012 Jan 20 [PubMed PMID: 28357021]
Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, Passman RS, White RD, Hess EP, Tang W, Davis D, Sinz E, Morrison LJ. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010 Nov 2:122(18 Suppl 3):S729-67. doi: 10.1161/CIRCULATIONAHA.110.970988. Epub [PubMed PMID: 20956224]
Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, Estes NA 3rd, Field ME, Goldberger ZD, Hammill SC, Indik JH, Lindsay BD, Olshansky B, Russo AM, Shen WK, Tracy CM, Al-Khatib SM, Evidence Review Committee Chair‡. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016 Apr 5:133(14):e506-74. doi: 10.1161/CIR.0000000000000311. Epub 2015 Sep 23 [PubMed PMID: 26399663]
Level 1 (high-level) evidenceMecklin M, Linnanmäki A, Hiippala A, Leino T, Arola A, Leskinen M, Ruotsalainen H, Happonen JM, Poutanen T. Multicenter cohort study on duration of antiarrhythmic medication for supraventricular tachycardia in infants. European journal of pediatrics. 2023 Mar:182(3):1089-1097. doi: 10.1007/s00431-022-04757-5. Epub 2022 Dec 28 [PubMed PMID: 36576576]
Bruder D, Weber R, Gass M, Balmer C, Cavigelli-Brunner A. Antiarrhythmic Medication in Neonates and Infants with Supraventricular Tachycardia. Pediatric cardiology. 2022 Aug:43(6):1311-1318. doi: 10.1007/s00246-022-02853-9. Epub 2022 Mar 8 [PubMed PMID: 35258638]
Helton MR. Diagnosis and Management of Common Types of Supraventricular Tachycardia. American family physician. 2015 Nov 1:92(9):793-800 [PubMed PMID: 26554472]
Janson CM, Millenson ME, Okunowo O, Dai D, Christmyer Z, Tan RB, Ramesh Iyer V, Shah MJ, O'Byrne ML. Incidence of life-threatening events in children with Wolff-Parkinson-White syndrome: Analysis of a large claims database. Heart rhythm. 2022 Apr:19(4):642-647. doi: 10.1016/j.hrthm.2021.12.009. Epub 2021 Dec 10 [PubMed PMID: 34902591]
Skanes AC, Obeyesekere M, Klein GJ. Electrophysiology testing and catheter ablation are helpful when evaluating asymptomatic patients with Wolff-Parkinson-White pattern: the con perspective. Cardiac electrophysiology clinics. 2015 Sep:7(3):377-83. doi: 10.1016/j.ccep.2015.05.002. Epub 2015 Jul 7 [PubMed PMID: 26304516]
Level 3 (low-level) evidenceCáceres C, Del Pilar Garcia Morgado M, Bozo FC, Piletsky S, Moczko E. Rapid Selective Detection and Quantification of β-Blockers Used in Doping Based on Molecularly Imprinted Nanoparticles (NanoMIPs). Polymers. 2022 Dec 11:14(24):. doi: 10.3390/polym14245420. Epub 2022 Dec 11 [PubMed PMID: 36559787]
Borregaard R, Lukac P, Gerdes C, Møller D, Mortensen PT, Pedersen L, Nielsen JC, Jensen HK. Radiofrequency ablation of accessory pathways in patients with the Wolff-Parkinson-White syndrome: the long-term mortality and risk of atrial fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2015 Jan:17(1):117-22. doi: 10.1093/europace/euu176. Epub 2014 Jul 10 [PubMed PMID: 25013013]
Level 2 (mid-level) evidenceKsiążczyk TM, Pietrzak R, Werner B. Management of Young Athletes with Asymptomatic Preexcitation-A Review of the Literature. Diagnostics (Basel, Switzerland). 2020 Oct 15:10(10):. doi: 10.3390/diagnostics10100824. Epub 2020 Oct 15 [PubMed PMID: 33076240]
Miyoshi T, Maeno Y, Hamasaki T, Inamura N, Yasukochi S, Kawataki M, Horigome H, Yoda H, Taketazu M, Nii M, Hagiwara A, Kato H, Shimizu W, Shiraishi I, Sakaguchi H, Ueda K, Katsuragi S, Yamamoto H, Sago H, Ikeda T, Japan Fetal Arrhythmia Group. Antenatal Therapy for Fetal Supraventricular Tachyarrhythmias: Multicenter Trial. Journal of the American College of Cardiology. 2019 Aug 20:74(7):874-885. doi: 10.1016/j.jacc.2019.06.024. Epub [PubMed PMID: 31416531]
Level 1 (high-level) evidence