Introduction
Pudendal neuralgia caused by pudendal nerve entrapment (PNE) is a chronic and often severely disabling neuropathic pain syndrome.[1] It presents in the sensory distribution region of the pudendal nerve and affects both males and females. The most characteristic symptom, found in over 50% of patients, is perineal pain exacerbated by sitting, which is relieved by standing or lying.[2] It is frequently misdiagnosed or underdiagnosed and inappropriately treated, initially causing a significant delay in proper management and severely negatively impacting the quality of life.
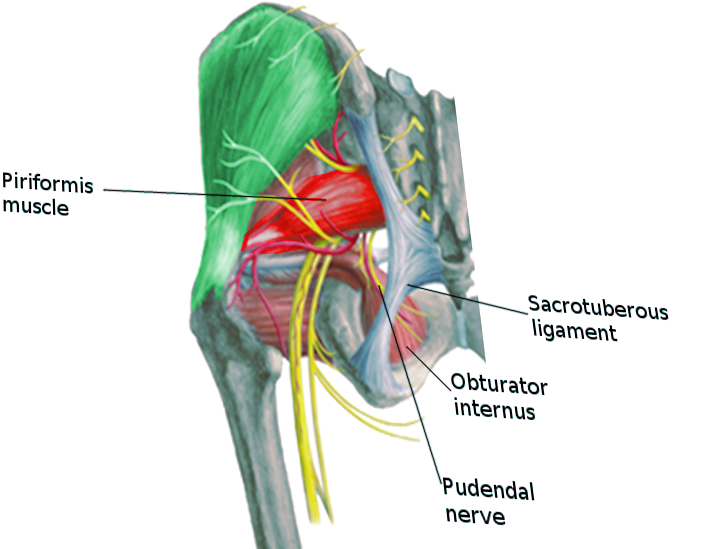
Anatomy of the Pudendal Nerve
The pudendal nerve emerges from the S2, S3, and S4 roots' ventral rami of the sacral plexus. It carries sensory, motor, and autonomic fibers; however, an injury to the pudendal nerve causes more sensory effects than motor. It initially courses between two muscles, the piriformis and coccygeus muscles, then departs the pelvic cavity through the greater sciatic foramen ventral to the sacrotuberous ligament.[3] It passes medial to and under the sacrospinous ligament at the level of the ischial spine to re-enter the pelvic cavity through the greater sciatic foramen. The pudendal nerve then courses in the pudendal canal, also called the Alcock canal. The three last branches of the pudendal nerve terminate in the ischioanal fossa. These are the inferior rectal branch, perineal branch, and dorsal sensory nerve of the penis or clitoris. However, there are case reports which have shown variability in the anatomy of the pudendal nerve.[4][5] (See our companion article on Anatomy, Abdomen and Pelvis, Pudendal Nerve)[3]
Pudendal Nerve Compression Based on Anatomy
The pudendal nerve entrapment syndromes are subdivided into four types based on the location of the compression.[2][6]
- Type I - Entrapment below the piriformis muscle as the pudendal nerve exits the greater sciatic notch.
- Type II - Entrapment between sacrospinous and sacrotuberous ligaments is the most common site of pudendal nerve entrapment.
- Type III - Entrapment in the Alcock canal.
- Type IV - Entrapment of terminal branches.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Pudendal neuralgia can arise from mechanical or non-mechanical injuries. The mechanical injury can be due to compression, transaction, or stretching. Amongst the mechanical causes, compression caused by pudendal nerve entrapment is the most common etiology. Non-mechanical causes of pudendal neuralgia include viral infections (herpes zoster, HIV), multiple sclerosis, radiation therapy, and diabetes mellitus, among others.[7]
The first reported case of pudendal neuralgia was due to cycling, which resulted from continuous pressure on Alcock's canal.[8]
Causes of Pudendal Neuralgia
- Pelvic surgery - The surgery for repair of prolapse of pelvic organs is reportedly the most common cause of pudendal neuralgia. The incidence increases if a mesh is used. Chronic persistent pain may require mesh removal in some cases.[9][10] It can also develop after mid-urethral sling surgery, hysterectomy, and anterior colporrhaphy.
- Direct trauma to the buttocks or back can result in pudendal neuralgia.[11]
- Childbirth - Vaginal delivery causes a significant stretching of pelvic floor muscles by the fetal head, which sometimes results in pudendal nerve damage.[12][13]
- Chronic constipation
- Excessive cycling - The condition is presumably because of chronic perineal microtrauma, which causes fibrosis in the pudendal canal as well as the sacrospinous and sacrotuberous ligaments.[7]
- Prolonged sitting can also contribute to this condition.
Epidemiology
Pudendal nerve entrapment is a rare syndrome, and its true prevalence is unknown. As estimated by the International Pudendal Neuropathy Foundation, the incidence of this condition is 1 per 100,000, but the actual prevalence is believed to be substantially higher than reported.[14] Pudendal nerve entrapment syndrome may affect 1% of the general population and accounts for about 4% of all patient consultations for pain control, with women affected more than twice as often as men.[15][16]
Pathophysiology
Direct compression of the pudendal nerve is often visible to the surgeon at the time of decompressive surgery. While repetitive actions such as sitting and cycling are primarily responsible, nerve compression may be congenital or the result of scarring after pelvic surgeries. Sports enthusiasts who regularly have significant and repetitive falls, such as skiers and snowboarders, may develop similar pelvic scarring due to frequent pelvic bruising and hematoma formation.
History and Physical
History
The classic symptom is perineal pain which is exacerbated on sitting and relieved on standing or sitting on a toilet.[7] Other presenting features of pudendal nerve entrapment are discussed below.[6] These include:
- It causes pain, numbness, and dysfunction in the distribution of the pudendal nerve. (This includes the genitalia, perineum, rectum, and lower urinary tract.)
- Urinary issues such as urgency, frequency, and painful ejaculations in men.[17]
- Sexual dysfunction, including persistent arousal dysfunction, dyspareunia, vulvodynia, and male erectile disorders.[17]
- Sphincteric dysfunction presents as constipation, dysuria, fecal incontinence, and urinary hesitancy.[18]
- There may be a foreign body sensation in the anus, rectum, urethra, or vagina.
Physical Examination
The physical examination in patients with pudendal nerve entrapment is relatively normal, except for pain reproduction.[19] The symptoms depend on the precise site and severity of the entrapment. If the nerve is compromised at the ischial spine or the sacrospinous ligament, it causes pain medial to the ischium—similarly, tenderness over the greater sciatic notch results when the nerve gets entrapped at that location. Entrapment at the piriformis leads to spasms and tenderness of the piriformis muscle. Lastly, entrapment in Alcock's canal results in tenderness and spasms of the obturator internus muscle.
Rectal and vaginal examinations are suggested to exclude other diagnoses such as prostatitis and to identify any intrapelvic entrapment.
Evaluation
Pudendal nerve entrapment is a potentially challenging condition to diagnose because there are no specific diagnostic tests. The clinician needs to realize that it is exceedingly mandatory to get a thorough history and perform a detailed physical examination to reach a diagnosis.[20] Dr. Roger Robert published the "Nantes" criteria to diagnose pudendal nerve entrapment, which appears in detail below.[20] This criterion has been validated by many European physicians who have substantial experience treating similar conditions.
If the patient fulfills all the "Nantes" criteria, no further investigation is generally needed to make the diagnosis. However, if the patient lacks any of the criteria, further evaluation should be pursued.
The "Nantes" Criteria
Inclusion criteria:
- Pain co-relates with the anatomical distribution of the pudendal nerve. (The pudendal nerve supplies the external genitalia and perineum. The pain can be superficial or deep in the vulvovaginal and anorectal areas as well as in the distal urethra.)
- Pain is predominantly in the sitting position. This symptom favors nerve compression because a decrease in nerve mobility makes it vulnerable to compressive trauma when pressed against hard ligamentous structures. This aspect of pain is dynamic as the pain results from compression and not from a sitting position alone.
- The patient does not get up at night due to pain, although many patients may experience difficulty going to sleep because of pain.
- There is no identifiable sensory loss. The presence of superficial perineal sensory impairment indicates a sacral root-lesion rather than pudendal nerve entrapment.
- Relief of pain occurs with a pudendal nerve block. This essential criterion is not specific, as any perineal disease other than entrapment can cause pain in the anatomic region of the pudendal nerve. A negative block also doesn't necessarily exclude the diagnosis of pudendal nerve entrapment if the block is placed incorrectly or performed too distally.
Complementary Diagnostic Criteria
- Pain is described as burning, shooting, or stabbing in nature and is associated with numbness
- Allodynia or hyperpathia
- Foreign body sensation or heaviness in the rectum or vagina
- The pain progressively increases and peaks in the evening but stops when the patient lies down and sleeps
- Pain is more on one side
- Pain is more prominent posteriorly and is triggered minutes or hours after defecation
- Tenderness felt around the ischial spine during a digital vaginal or rectal examination
- An abnormal result on neurophysiological tests
Exclusion Criteria
- Pain exclusively in the territory not served by the pudendal nerve, such as the hypogastrium, coccyx, pubis, or gluteus areas
- Pain is associated with pruritus (more suggestive of a skin lesion)
- Pain that is entirely paroxysmal in nature
- An imaging abnormality that identifies the cause of the pain
Associated Signs
- Pain in the buttock
- Referred sciatic pain
- Pain in the medial thigh (indicates obturator nerve involvement)
- Pain in the suprapubic region
- Increased frequency of urination or pain with a full bladder
- Pain after ejaculation
- Pain worsens hours after sexual intercourse
- Erectile dysfunction
- A normal result on electrophysiological tests
Diagnostic Tests
The following tests can help in the diagnosis:
- Quantitative warm sensory threshold testing works on the principle that compressed nerves cannot efficiently detect and transmit changes in vibration and temperature sensation. Thus patients with nerve injuries are unable to detect gradual temperature changes.[21] The most commonly used test of this type is the warm sensory threshold detection test. Many patients cannot detect changes in probe temperature until it is hot enough to cause a painful burning sensation.[22] The test can easily be performed in a physician's office or clinic. Biothesiometry, which tests vibratory sensation, can also be used similarly to identify any loss of vibrational sensitivity.
- Pudendal nerve terminal motor latency testing is a neurophysiological examination that measures the time it takes for a nerve signal to travel from the ischial spine to the anal sphincter. It is more invasive, challenging to perform, and uncomfortable for the patient than warm sensory threshold testing.[23]
- High-frequency ultrasonography is helpful in the detection of the site of compression. Compressed nerves and associated veins appear flat, whereas inflamed nerves appear edematous.[24]
- Doppler ultrasound has a role in the diagnosis of pudendal nerve entrapment. As the pudendal nerve and vessels course together in a neurovascular bundle, it can be assumed that if there is nerve compression, venous compression will also occur, which is diagnosable with a doppler ultrasound.[24]
- MRI (magnetic resonance imaging) of the pelvis is recommended as it can help rule out other causes of chronic pain. The advancement of MRI techniques in evaluating peripheral nerves provides a detailed description of the anatomy, fascicular details, the blood supply of the nerve, and detailed 3-D anatomy.[25] It also helps in localizing the exact site of entrapment. MRI testing is strongly recommended prior to any surgery for pudendal entrapment.[26]
- Functional MRI assesses nerve integrity based on its biological properties. Currently, it is considered experimental and not considered definitive.[27] There are no specific and consistent radiological findings in patients with pudendal nerve entrapment, and further research is necessary.
- Diagnostic nerve blocks: In females, an unguided block can be performed vaginally, and in males, transperineally. If there is pain relief immediately following the procedure, it indicates that pudendal nerve pathology is the likely cause of pain. The absence of pain relief doesn't necessarily mean that the patient doesn't have pudendal entrapment, as there may be a technical or operative error as well. Image guidance (using fluoroscopy, ultrasound, or CT scan imaging) significantly increases the reliability of the nerve blocks.[28]
- Pudendal nerve block injections with a local anesthetic have been recommended to help confirm the diagnosis of pudendal nerve entrapment, especially if the injection is done directly into Alcock's canal using image guidance. This is often used as an indicator of which patients are most likely to benefit from decompressive surgery.[26][29] About 20% of pudendal nerve blocks fail for various reasons, often due to physician error, inexperience, or lack of adequate training.[30][31]
Treatment / Management
Conservative: Avoidance of painful stimuli is one of the most important components of treatment. For instance, if cycling causes pain, the patient should use proper padding or cease the activity. Other activities to avoid might be hip flexion exercises, jogging, rowing, gymnastics, skiing, and snowboarding. Similarly, patients who present with pain on prolonged sitting should adopt lifestyle modifications to minimize that activity, such as using a standing workstation. Roughly 20% to 30% of patients will see relief from conservative measures alone.
Physical Therapy: Pelvic floor physical therapy works best for patients in whom pain results from muscle spasms such as levator ani syndrome and similar myofascial disorders. Physical therapy helps in the relaxation of pelvic floor muscles by releasing spasms and muscle lengthening. A course of 6 to 12 weeks is commonly recommended. Adding transcutaneous electrical nerve stimulation (TENS) to physical therapy appears to be helpful.[32][33][34] TENS is low risk, relatively inexpensive, readily available in most locations, and non-invasive.(A1)
Cognitive Behavioral Therapy: Behavioral therapy has been useful for various types of chronic pelvic pain syndromes, even though it has not been specifically tested for pudendal nerve entrapment.[35][36] It is generally recommended as an adjunctive treatment when there is evidence of psychological issues such as anxiety, depression, hopelessness, emotional instability, etc.[26][37] Frustration and depression are particularly common in patients with chronic neuropathic pain who have not enjoyed any relief from earlier treatments.(A1)
Pharmacologic therapy: The drugs used are analgesics, muscle relaxants, and anticonvulsants. There are no randomized trials to study and evaluate the efficacy of these drugs or which combinations might be most effective. Often, several medications from different drug classes are used. A typical combination would be a tricyclic antidepressant (amitriptyline), an SSNRI (duloxetine), and a neurotransmitter analog (gabapentin and/or pregabalin). Opioids are avoided as much as possible. Commonly used medications include:[38]
- Amitriptyline, starting at 10 mg HS and gradually increasing to 50 mg.
- Duloxetine (a selective serotonin-norepinephrine reuptake inhibitor) starting at 30 mg daily for seven days, then increasing to 60 mg daily. No benefit is seen from further dosage increases
- Gabapentin (with or without pregabalin), starting at 300 mg TID and gradually increasing up to a maximum of 900 mg TID
- Pregabalin (with or without gabapentin), starting at 75 mg BID and gradually increasing up to 300 mg BID
- Clonidine 0.1 mg HS not only helps with pain but is useful as a sleep aid
Pudendal Nerve Block: Infiltration with a local anesthetic or steroid in an area encircling the pudendal nerve is a mainstay of pudendal nerve pain. The block can be given unguided or with the aid of ultrasonography, fluoroscopy, or computed tomography (CT). The most consistently reported technique is the use of a CT scan.[39] Image guidance is suggested for better and more reliable results. While no standard medication or combination is used, one frequently used mixture includes 1% lidocaine, 0.25% bupivacaine, and a corticosteroid such as triamcinolone. The short-acting anesthetic starts working in 20 minutes or less, the bupivacaine can last much longer, and the steroid effect begins at about 3 to 5 days and can last about a month. At that time, another injection can be given if needed. About 25% of patients report pain relief lasting more than one month following pudendal nerve blocks.[26] While this can be effective, there is evidence that ongoing therapeutic pudendal blocks may lose efficacy after two years.[40] For patients receiving ongoing injections, ultrasonography is suggested to minimize costs and reduce patient exposure to ionizing radiation over time. (See our companion article on Pudendal Nerve Block for more details on the procedure.)[41](B3)
Surgical Decompression: Surgery to directly free the pudendal nerve in Alcock's canal is considered the most effective long-term treatment and potential cure for pudendal nerve entrapment. The four different approaches are transperineal, transgluteal, transischiorectal, and laparoscopic.[42] All methods destroy some nerve fibers but help equivalently by removing the underlying cause of the compressive neuropathy.[43][44] Overall success with surgical decompression is about 70% (60% to 80%).[43] The goal of decompressive surgery is to completely free the nerve from entrapment and compression while allowing it complete mobility.(B2)
Erdogru described a new modification (the Istanbul technique) of laparoscopy using an omental flap in 27 patients. The outcome measurement was defined in terms of pain scores and quality of life. Approximately 80% of patients reported more than an 80% reduction in pain after six months which is quite impressive.[42] Laparoscopy has the advantage of a better visual surgical field with built-in magnification. It allows for the option of leaving a neuromodulation electrode in place as a backup, but it has a steep learning curve.[42][45] Placing a neuromodulation electrode at the time of decompression surgery seems reasonable in selected complicated or severe cases, as the electrode can always be removed later if it's not needed.[42](B2)
Sacral Neuromodulation: This minimally invasive treatment includes using a peripheral nerve stimulator, which causes neural regulation of the pudendal nerve in the ischioanal fossa. The first case report of this technique by Valovska mentioned the successful management of a patient with pudendal neuralgia with minimally invasive transforaminal sacral neurostimulation.[46] A prospective trial of 27 patients with refractory pudendal neuralgia showed promising results by neuromodulation of the conus medullaris, in which twenty out of twenty-seven patients had a positive response (defined as at least a 5-% reduction in pain).[47] Further, of those twenty patients, all had long-term relief.[47] (B3)
Sacral neuromodulation has often been used as a treatment of last resort when patients have failed all other treatments, including surgical decompression. In such cases, about two-thirds will respond favorably to neuromodulation.[47][48] Optimal settings and standards have not been established for this therapy, but there is some evidence that higher frequencies (>20 Hz) may provide better results. Sacral neuromodulation is safe, effective, minimally invasive, widely available, and generally underutilized for pudendal neuralgia. While surgical decompression is generally the preferred long-term curative therapy for most patients with pudendal nerve entrapment, sacral neuromodulation should be considered for those individuals who are not surgical candidates or where the decompressive procedure has failed.(B3)
Pulsed Radiofrequency Ablation: Pulsed radiofrequency ablation is a relatively new method of pudendal neuromodulation. It is considered safer than continuous radiofrequency treatment by reducing heat-related complications. It uses pulsed electromagnetic radiation to cause neuromodulation and appears to be potentially useful for chronic refractory neuropathic pudendal neuralgia.[49] When compared to pudendal nerve blocks, pulsed radiofrequency ablation showed equivalent pain relief, but the benefit extended substantially longer, out to three months.[50] Several recent studies showed persistent pain relief in 89% of the 90 pudendal neuralgia patients followed for six months after pulsed radiofrequency ablation therapy.[51][52] While promising, these studies are not yet sufficient to justify the widespread use of this treatment modality.(B2)
Cryotherapy: Early reports using cryotherapy in small series appear promising, which would be expected based on results from its use in other neuropathies.[53] However, there is currently insufficient evidence of efficacy and safety to justify its routine use outside of a clinical trial. (B2)
Lipofilling: This is a relatively new experimental treatment of pudendal neuralgia. Venturi first described this technique in fifteen female patients. It requires an autologous injection of adipose tissue along with stem cells into Alcock's canal. Ten patients showed significantly decreased pain and a better quality of life at the end of six months.[54] Since this was a single study with a very small sample size without a control group, further research into this therapy is needed before it can be recommended outside a clinical trial.(B2)
(For a more detailed and comprehensive description of all the various therapies, medications, and procedures for pudendal nerve entrapment, please see our companion article on Pudendal Neuralgia.)[38]
Differential Diagnosis
Since there is no confirmatory diagnostic test, pudendal neuralgia is a diagnosis of exclusion. Other conditions merit consideration before making a final diagnosis.[55]
- Compression by an external source, including a benign or malignant tumor or metastasis
- Superficial infections of the skin in the dermatomes covered by the pudendal nerve
- Neuropathy of the sacral region is caused by damage to the sacral nerve plexus
- Childbirth trauma that causes a stretching of the perineum
- Complex regional pain syndrome is a chronic pain condition that causes pain in one of the limbs and usually occurs after an injury
- Chronic prostatitis
- Prostatodynia
- Vulvodynia
- Vulvar vestibulitis
- Chronic pelvic pain syndrome
- Coccygodynia
- Sacroiliac joint dysfunction
- Piriformis syndrome
- Ischia bursitis
- Interstitial cystitis
Prognosis
Pudendal neuralgia due to pudendal nerve entrapment can immensely affect the quality of life, but it does not affect life expectancy. The overall response to pudendal nerve blocks in properly selected patients is roughly 80%, but the relief typically lasts only about 30 days in most patients. A repeat injection or an alternative treatment would then need to be utilized. The best long-term cures are from decompressive surgery, where the response rates are 60% to 80%.[43] Patients who fail decompressive surgery, possibly up to 80%, can still obtain relief from sacral neuromodulation.[15][48]
Complications
The complications associated with pudendal nerve blocks are rare. These complications include:
- Laceration of the vaginal mucosa
- Accidental intravascular injection of a local anesthetic can cause cardiovascular and CNS toxicity. The patient can present with palpitations, hypotension, bradycardia, dysarthria, tinnitus, drowsiness, confusion, loss of consciousness, and convulsions.
- Hematoma from injury to the pudendal artery or surrounding vessels
- Infection and/or pain at the injection site
- Abscess formation
Pudendal decompression surgery may also produce complications, although these are also uncommon. There can be an injury to a small branch of the nerve during surgery. A microsurgical repair of the injured nerve can be performed if necessary. An injury or transection of the sacrotuberous ligament can occur during surgery. This would cause an unstable pelvis. (Using a midline vertical incision would help avoid this rare complication.)
Relief from surgery is rarely immediate. Rather, the pain slowly decreases over a period of months. This is not a complication of the surgery but rather just the expected healing process.
Deterrence and Patient Education
Patients should be educated to avoid painful stimuli and actively participate in physiotherapy. Lifestyle modifications, such as avoiding aggravating activities and using a proper seating pad, are essential elements of the overall treatment plan.
Pearls and Other Issues
Patients with chronic pelvic pain and diagnosed with pelvic pathology who do not respond to standard therapy should be re-evaluated for possible pudendal nerve entrapment.
It is preferable to utilize minimally invasive therapies first, such as conservative measures with lifestyle changes, physical therapy, TENS, and pudendal nerve blocks.
The successful use of a diagnostic pudendal nerve block in Alcock's canal may strongly suggest pudendal nerve entrapment and the reasonable expectation of a good result from decompressive surgery.
Avoid using opioid medications if possible to minimize dependency.
The use of image guidance is suggested to make the injections more reliable.
For repeated injections, ultrasound is preferred over ionizing radiation for imaging guidance.
While there is no definitive analgesic or anesthetic medication, dosage, or mixture for pudendal nerve block injections, using a short and long-acting local anesthetic with a corticosteroid seems reasonable for maximum relief.
If repeated pudendal nerve blocks are used for therapy, be aware that they may lose efficacy after two years.
It is important to involve psychology and pain management early in treatment planning.
Patients should be well informed about diagnostic testing and realistic treatment options based on local experience and resources.
In the treatment planning of patients with chronic pelvic pain, it is crucial to understand that all pudendal neuralgias are not the result of nerve entrapment.
The pudendal nerve can get trapped at different locations; therefore, all patients will not benefit from the same therapy.
Patients with chronic pain syndromes tend to get frustrated with multiple failed treatments and can be clinically depressed as well.[56] Suicides have been reported in pudendal neuralgia patients.
A study was conducted by Raynor et al. on 1024 patients to study the prevalence of depression in patients with chronic pain and its impact on health care costs. They categorized 60.8% of patients with chronic pain into probable depression and 33.8% into severe depression based on a questionnaire survey. They also reported higher health care costs amongst patients with depression (p=0.001).[57] Similar results can be seen in the data analysis by the medical expenditure panel survey of 26,671 patients from 2008 to 2011. The research found that different levels of pain interference increase the total health care cost.[58]
Chronic pain poses a substantial mental and economic burden on the patient. These aspects should be considered when providing care to patients.
Enhancing Healthcare Team Outcomes
Pudendal neuralgia due to pudendal nerve entrapment is a rare neuropathic condition. It causes a significant impairment of quality of life, and the pain can become disabling. It often is not correctly diagnosed initially, so most patients get treated for other conditions, which are usually unsuccessful. The "Nantes" diagnostic criteria were established and validated by an interprofessional team to aid in the early diagnosis and allow earlier treatment of patients with pudendal entrapment syndrome. No further investigation is usually required if the patient fulfills all the "Nantes" criteria. However, the patient should be further evaluated if any of the criteria are not present. An MRI is generally recommended to rule out other treatable causes of chronic pain. Individualized treatment is necessary. It typically requires permanent lifestyle changes and physical therapy. The primary treatment options include conservative measures, physical therapy with or without TENS, pharmacological therapy, ultrasound or CT-guided nerve blocks, nerve decompression surgery, and neuromodulation.
Pudendal nerve entrapment and neuropathy are relatively unknown and unstudied conditions. There are few good prospective studies and virtually none that are large, comparative, include a proper control group, have standardized inclusion criteria, a uniform definition of "success), and long-term follow-up. The information provided in this review is therefore based on the best available data as well as consensus opinions by experienced experts. Better prospective studies are urgently needed to determine better treatment paradigms.
A well-coordinated interprofessional healthcare team comprised of pain management physicians, surgeons, anesthesiologists, nurses, radiologists, psychologists, gynecologists, urologists, and physiotherapists to help in physical rehabilitation is necessary to optimally treat this challenging neuropathic syndrome. These disciplines must collaborate across interprofessional boundaries to optimize care and outcomes. [Level 5]
Media
(Click Image to Enlarge)
References
Ploteau S, Labat JJ, Riant T, Levesque A, Robert R, Nizard J. New concepts on functional chronic pelvic and perineal pain: pathophysiology and multidisciplinary management. Discovery medicine... 2015 Mar:19(104):185-92 [PubMed PMID: 25828522]
Robert R, Prat-Pradal D, Labat JJ, Bensignor M, Raoul S, Rebai R, Leborgne J. Anatomic basis of chronic perineal pain: role of the pudendal nerve. Surgical and radiologic anatomy : SRA. 1998:20(2):93-8 [PubMed PMID: 9658526]
Kinter KJ, Newton BW. Anatomy, Abdomen and Pelvis, Pudendal Nerve. StatPearls. 2025 Jan:(): [PubMed PMID: 32134612]
Maldonado PA, Chin K, Garcia AA, Corton MM. Anatomic variations of pudendal nerve within pelvis and pudendal canal: clinical applications. American journal of obstetrics and gynecology. 2015 Nov:213(5):727.e1-6. doi: 10.1016/j.ajog.2015.06.009. Epub 2015 Jun 10 [PubMed PMID: 26070708]
Montoya TI, Calver L, Carrick KS, Prats J, Corton MM. Anatomic relationships of the pudendal nerve branches. American journal of obstetrics and gynecology. 2011 Nov:205(5):504.e1-5. doi: 10.1016/j.ajog.2011.07.014. Epub 2011 Jul 20 [PubMed PMID: 21889763]
Filler AG. Diagnosis and treatment of pudendal nerve entrapment syndrome subtypes: imaging, injections, and minimal access surgery. Neurosurgical focus. 2009 Feb:26(2):E9. doi: 10.3171/FOC.2009.26.2.E9. Epub [PubMed PMID: 19323602]
Ramsden CE, McDaniel MC, Harmon RL, Renney KM, Faure A. Pudendal nerve entrapment as source of intractable perineal pain. American journal of physical medicine & rehabilitation. 2003 Jun:82(6):479-84 [PubMed PMID: 12820792]
Level 3 (low-level) evidenceLeibovitch I, Mor Y. The vicious cycling: bicycling related urogenital disorders. European urology. 2005 Mar:47(3):277-86; discussion 286-7 [PubMed PMID: 15716187]
Marcus-Braun N, Bourret A, von Theobald P. Persistent pelvic pain following transvaginal mesh surgery: a cause for mesh removal. European journal of obstetrics, gynecology, and reproductive biology. 2012 Jun:162(2):224-8. doi: 10.1016/j.ejogrb.2012.03.002. Epub 2012 Mar 30 [PubMed PMID: 22464208]
Level 2 (mid-level) evidenceSancak EB, Avci E, Erdogru T. Pudendal neuralgia after pelvic surgery using mesh: Case reports and laparoscopic pudendal nerve decompression. International journal of urology : official journal of the Japanese Urological Association. 2016 Sep:23(9):797-800. doi: 10.1111/iju.13136. Epub 2016 Jun 2 [PubMed PMID: 27250921]
Level 3 (low-level) evidenceHeinze K, Nehiba M, van Ophoven A. [Neuralgia of the pudendal nerve following violent trauma: analgesia by pudendal neuromodulation]. Der Urologe. Ausg. A. 2012 Aug:51(8):1106-8. doi: 10.1007/s00120-012-2949-8. Epub [PubMed PMID: 22751935]
Level 3 (low-level) evidenceLien KC, Morgan DM, Delancey JO, Ashton-Miller JA. Pudendal nerve stretch during vaginal birth: a 3D computer simulation. American journal of obstetrics and gynecology. 2005 May:192(5):1669-76 [PubMed PMID: 15902175]
Sultan AH, Kamm MA, Hudson CN. Pudendal nerve damage during labour: prospective study before and after childbirth. British journal of obstetrics and gynaecology. 1994 Jan:101(1):22-8 [PubMed PMID: 8297863]
Hibner M, Desai N, Robertson LJ, Nour M. Pudendal neuralgia. Journal of minimally invasive gynecology. 2010 Mar-Apr:17(2):148-53. doi: 10.1016/j.jmig.2009.11.003. Epub 2010 Jan 12 [PubMed PMID: 20071246]
Guo KK, Wang L, Liu F, Niu JJ, Wang C, You SH, Feng ZG, Lu GJ. Sacral Nerve Stimulation in Patients With Refractory Pudendal Neuralgia. Pain physician. 2022 Jul:25(4):E619-E627 [PubMed PMID: 35793186]
Spinosa JP, de Bisschop E, Laurençon J, Kuhn G, Dubuisson JB, Riederer BM. [Sacral staged reflexes to localize the pudendal compression: an anatomical validation of the concept]. Revue medicale suisse. 2006 Oct 25:2(84):2416-8, 2420-1 [PubMed PMID: 17121249]
Level 1 (high-level) evidenceWaldinger MD, Venema PL, van Gils AP, Schweitzer DH. New insights into restless genital syndrome: static mechanical hyperesthesia and neuropathy of the nervus dorsalis clitoridis. The journal of sexual medicine. 2009 Oct:6(10):2778-87. doi: 10.1111/j.1743-6109.2009.01435.x. Epub 2009 Aug 28 [PubMed PMID: 19732313]
Shafik A, El Sibai O, Shafik IA, Shafik AA. Role of sacral ligament clamp in the pudendal neuropathy (pudendal canal syndrome): results of clamp release. International surgery. 2007 Jan-Feb:92(1):54-9 [PubMed PMID: 17390916]
Martin R, Martin HD, Kivlan BR. NERVE ENTRAPMENT IN THE HIP REGION: CURRENT CONCEPTS REVIEW. International journal of sports physical therapy. 2017 Dec:12(7):1163-1173 [PubMed PMID: 29234567]
Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurourology and urodynamics. 2008:27(4):306-10 [PubMed PMID: 17828787]
Walk D, Sehgal N, Moeller-Bertram T, Edwards RR, Wasan A, Wallace M, Irving G, Argoff C, Backonja MM. Quantitative sensory testing and mapping: a review of nonautomated quantitative methods for examination of the patient with neuropathic pain. The Clinical journal of pain. 2009 Sep:25(7):632-40. doi: 10.1097/AJP.0b013e3181a68c64. Epub [PubMed PMID: 19692806]
Beco J, Seidel L, Albert A. Normative values of skin temperature and thermal sensory thresholds in the pudendal nerve territory. Neurourology and urodynamics. 2015 Aug:34(6):571-7. doi: 10.1002/nau.22614. Epub 2014 Apr 30 [PubMed PMID: 24782126]
Tetzschner T, Sørensen M, Lose G, Christiansen J. Pudendal nerve function during pregnancy and after delivery. International urogynecology journal and pelvic floor dysfunction. 1997:8(2):66-8 [PubMed PMID: 9297593]
Mollo M, Bautrant E, Rossi-Seignert AK, Collet S, Boyer R, Thiers-Bautrant D. Evaluation of diagnostic accuracy of Colour Duplex Scanning, compared to electroneuromyography, diagnostic score and surgical outcomes, in Pudendal Neuralgia by entrapment: a prospective study on 96 patients. Pain. 2009 Mar:142(1-2):159-63. doi: 10.1016/j.pain.2009.01.019. Epub 2009 Feb 4 [PubMed PMID: 19195783]
Level 2 (mid-level) evidenceWadhwa V, Hamid AS, Kumar Y, Scott KM, Chhabra A. Pudendal nerve and branch neuropathy: magnetic resonance neurography evaluation. Acta radiologica (Stockholm, Sweden : 1987). 2017 Jun:58(6):726-733. doi: 10.1177/0284185116668213. Epub 2016 Sep 23 [PubMed PMID: 27664277]
Levesque A, Bautrant E, Quistrebert V, Valancogne G, Riant T, Beer Gabel M, Leroi AM, Jottard K, Bruyninx L, Amarenco G, Quintas L, Picard P, Vancaillie T, Leveque C, Mohy F, Rioult B, Ploteau S, Labat JJ, Guinet-Lacoste A, Quinio B, Cosson M, Haddad R, Deffieux X, Perrouin-Verbe MA, Garreau C, Robert R. Recommendations on the management of pudendal nerve entrapment syndrome: A formalised expert consensus. European journal of pain (London, England). 2022 Jan:26(1):7-17. doi: 10.1002/ejp.1861. Epub 2021 Oct 13 [PubMed PMID: 34643963]
Level 3 (low-level) evidenceFiller AG, Haynes J, Jordan SE, Prager J, Villablanca JP, Farahani K, McBride DQ, Tsuruda JS, Morisoli B, Batzdorf U, Johnson JP. Sciatica of nondisc origin and piriformis syndrome: diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment. Journal of neurosurgery. Spine. 2005 Feb:2(2):99-115 [PubMed PMID: 15739520]
Choi SS, Lee PB, Kim YC, Kim HJ, Lee SC. C-arm-guided pudendal nerve block: a new technique. International journal of clinical practice. 2006 May:60(5):553-6 [PubMed PMID: 16700853]
Engeler DS, Baranowski AP, Dinis-Oliveira P, Elneil S, Hughes J, Messelink EJ, van Ophoven A, Williams AC, European Association of Urology. The 2013 EAU guidelines on chronic pelvic pain: is management of chronic pelvic pain a habit, a philosophy, or a science? 10 years of development. European urology. 2013 Sep:64(3):431-9. doi: 10.1016/j.eururo.2013.04.035. Epub 2013 Apr 28 [PubMed PMID: 23684447]
Level 1 (high-level) evidenceFord JM, Owen DJ, Coughlin LB, Byrd LM. A critique of current practice of transvaginal pudendal nerve blocks: a prospective audit of understanding and clinical practice. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2013 Jul:33(5):463-5. doi: 10.3109/01443615.2013.771155. Epub [PubMed PMID: 23815197]
Level 3 (low-level) evidenceAntolak S Jr, Antolak C, Lendway L. Measuring the Quality of Pudendal Nerve Perineural Injections. Pain physician. 2016 May:19(4):299-306 [PubMed PMID: 27228517]
Level 2 (mid-level) evidenceEid MM, Rawash MF, Sharaf MA, Eladl HM. Effectiveness of transcutaneous electrical nerve stimulation as an adjunct to selected physical therapy exercise program on male patients with pudendal neuralgia: A randomized controlled trial. Clinical rehabilitation. 2021 Aug:35(8):1142-1150. doi: 10.1177/0269215521995338. Epub 2021 Feb 22 [PubMed PMID: 33611923]
Level 1 (high-level) evidenceVallinga MS, Spoelstra SK, Hemel IL, van de Wiel HB, Weijmar Schultz WC. Transcutaneous electrical nerve stimulation as an additional treatment for women suffering from therapy-resistant provoked vestibulodynia: a feasibility study. The journal of sexual medicine. 2015 Jan:12(1):228-37. doi: 10.1111/jsm.12740. Epub 2014 Nov 12 [PubMed PMID: 25388372]
Level 2 (mid-level) evidenceSikiru L, Shmaila H, Muhammed SA. Transcutaneous electrical nerve stimulation (TENS) in the symptomatic management of chronic prostatitis/chronic pelvic pain syndrome: a placebo-control randomized trial. International braz j urol : official journal of the Brazilian Society of Urology. 2008 Nov-Dec:34(6):708-13; discussion 714 [PubMed PMID: 19111075]
Level 1 (high-level) evidenceMasheb RM, Kerns RD, Lozano C, Minkin MJ, Richman S. A randomized clinical trial for women with vulvodynia: Cognitive-behavioral therapy vs. supportive psychotherapy. Pain. 2009 Jan:141(1-2):31-40. doi: 10.1016/j.pain.2008.09.031. Epub 2008 Nov 20 [PubMed PMID: 19022580]
Level 2 (mid-level) evidenceDesrochers G, Bergeron S, Khalifé S, Dupuis MJ, Jodoin M. Provoked vestibulodynia: psychological predictors of topical and cognitive-behavioral treatment outcome. Behaviour research and therapy. 2010 Feb:48(2):106-15. doi: 10.1016/j.brat.2009.09.014. Epub 2009 Oct 7 [PubMed PMID: 19879555]
Level 1 (high-level) evidenceBeerten SG, Calabrò RS. Pudendal Neuralgia: The Need for a Holistic Approach-Lessons From a Case Report. Innovations in clinical neuroscience. 2021 Apr-Jun:18(4-6):8-10 [PubMed PMID: 34980976]
Level 3 (low-level) evidenceLeslie SW, Antolak S, Feloney MP, Soon-Sutton TL. Pudendal Neuralgia. StatPearls. 2025 Jan:(): [PubMed PMID: 32965917]
Fanucci E, Manenti G, Ursone A, Fusco N, Mylonakou I, D'Urso S, Simonetti G. Role of interventional radiology in pudendal neuralgia: a description of techniques and review of the literature. La Radiologia medica. 2009 Apr:114(3):425-36. doi: 10.1007/s11547-009-0371-0. Epub 2009 Mar 10 [PubMed PMID: 19277838]
Basol G, Kale A, Gurbuz H, Gundogdu EC, Baydilli KN, Usta T. Transvaginal pudendal nerve blocks in patients with pudendal neuralgia: 2-year follow-up results. Archives of gynecology and obstetrics. 2022 Oct:306(4):1107-1116. doi: 10.1007/s00404-022-06621-1. Epub 2022 May 28 [PubMed PMID: 35633372]
Ghanavatian S, Leslie SW, Derian A. Pudendal Nerve Block. StatPearls. 2025 Jan:(): [PubMed PMID: 31855362]
Erdogru T, Avci E, Akand M. Laparoscopic pudendal nerve decompression and transposition combined with omental flap protection of the nerve (Istanbul technique): technical description and feasibility analysis. Surgical endoscopy. 2014 Mar:28(3):925-32. doi: 10.1007/s00464-013-3248-1. Epub 2013 Oct 23 [PubMed PMID: 24149853]
Level 2 (mid-level) evidenceWaxweiler C, Dobos S, Thill V, Bruyninx L. Selection criteria for surgical treatment of pudendal neuralgia. Neurourology and urodynamics. 2017 Mar:36(3):663-666. doi: 10.1002/nau.22988. Epub 2016 Mar 21 [PubMed PMID: 26999519]
Beco J, Climov D, Bex M. Pudendal nerve decompression in perineology: a case series. BMC surgery. 2004 Oct 30:4():15 [PubMed PMID: 15516268]
Level 2 (mid-level) evidenceMoncada E, de San Ildefonso A, Flores E, Garrido L, Cano-Valderrama O, Vigorita V, Sánchez-Santos R. Right laparoscopic pudendal release + neurostimulator prosthesis (LION procedure) in pudendal neuralgia. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2022 Oct:24(10):1243-1244. doi: 10.1111/codi.16190. Epub 2022 Jun 27 [PubMed PMID: 35575432]
Valovska A, Peccora CD, Philip CN, Kaye AD, Urman RD. Sacral neuromodulation as a treatment for pudendal neuralgia. Pain physician. 2014 Sep-Oct:17(5):E645-50 [PubMed PMID: 25247915]
Level 3 (low-level) evidenceBuffenoir K, Rioult B, Hamel O, Labat JJ, Riant T, Robert R. Spinal cord stimulation of the conus medullaris for refractory pudendal neuralgia: a prospective study of 27 consecutive cases. Neurourology and urodynamics. 2015 Feb:34(2):177-82. doi: 10.1002/nau.22525. Epub 2013 Nov 19 [PubMed PMID: 24249588]
Level 3 (low-level) evidenceMeier KM, Vecellio PM, Killinger KA, Boura JA, Peters KM. Pudendal Neuromodulation is Feasible and Effective After Pudendal Nerve Entrapment Surgery. The journal of sexual medicine. 2022 Jun:19(6):995-1001. doi: 10.1016/j.jsxm.2022.03.219. Epub 2022 Apr 19 [PubMed PMID: 35459633]
Frank CE, Flaxman T, Goddard Y, Chen I, Zhu C, Singh SS. The Use of Pulsed Radiofrequency for the Treatment of Pudendal Neuralgia: A Case Series. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2019 Nov:41(11):1558-1563. doi: 10.1016/j.jogc.2019.01.019. Epub 2019 Mar 23 [PubMed PMID: 30910339]
Level 2 (mid-level) evidenceZhu D, Fan Z, Cheng F, Li Y, Huo X, Cui J. The Efficacy of an Ultrasound-Guided Improved Puncture Path Technique of Nerve Block/Pulsed Radiofrequency for Pudendal Neuralgia: A Retrospective Study. Brain sciences. 2022 Apr 18:12(4):. doi: 10.3390/brainsci12040510. Epub 2022 Apr 18 [PubMed PMID: 35448041]
Level 2 (mid-level) evidenceWang CL, Song T. The Clinical Efficacy of High-Voltage Long-Duration Pulsed Radiofrequency Treatment in Pudendal Neuralgia: A Retrospective Study. Neuromodulation : journal of the International Neuromodulation Society. 2022 Dec:25(8):1372-1377. doi: 10.1111/ner.13401. Epub 2022 Feb 2 [PubMed PMID: 33945192]
Level 2 (mid-level) evidenceJi F, Zhou S, Li C, Zhang Y, Xu H. Therapeutic Efficacy of Ultrasound-Guided High-Voltage Long-Duration Pulsed Radiofrequency for Pudendal Neuralgia. Neural plasticity. 2021:2021():9961145. doi: 10.1155/2021/9961145. Epub 2021 Jul 30 [PubMed PMID: 34373690]
Prologo JD, Lin RC, Williams R, Corn D. Percutaneous CT-guided cryoablation for the treatment of refractory pudendal neuralgia. Skeletal radiology. 2015 May:44(5):709-14. doi: 10.1007/s00256-014-2075-3. Epub 2014 Dec 17 [PubMed PMID: 25511935]
Level 2 (mid-level) evidenceVenturi M, Boccasanta P, Lombardi B, Brambilla M, Contessini Avesani E, Vergani C. Pudendal Neuralgia: A New Option for Treatment? Preliminary Results on Feasibility and Efficacy. Pain medicine (Malden, Mass.). 2015 Aug:16(8):1475-81. doi: 10.1111/pme.12693. Epub 2015 Feb 12 [PubMed PMID: 25677417]
Level 2 (mid-level) evidenceLeone JE, Middleton S. Nontraumatic Testicular Pain due to Sacroiliac-Joint Dysfunction: A Case Report. Journal of athletic training. 2016 Aug:51(8):651-657 [PubMed PMID: 27626835]
Level 3 (low-level) evidenceSheng J, Liu S, Wang Y, Cui R, Zhang X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural plasticity. 2017:2017():9724371. doi: 10.1155/2017/9724371. Epub 2017 Jun 19 [PubMed PMID: 28706741]
Rayner L, Hotopf M, Petkova H, Matcham F, Simpson A, McCracken LM. Depression in patients with chronic pain attending a specialised pain treatment centre: prevalence and impact on health care costs. Pain. 2016 Jul:157(7):1472-9. doi: 10.1097/j.pain.0000000000000542. Epub [PubMed PMID: 26963849]
Stockbridge EL, Suzuki S, Pagán JA. Chronic pain and health care spending: an analysis of longitudinal data from the Medical Expenditure Panel Survey. Health services research. 2015 Jun:50(3):847-70. doi: 10.1111/1475-6773.12263. Epub 2014 Nov 25 [PubMed PMID: 25424348]
Level 3 (low-level) evidence