Introduction
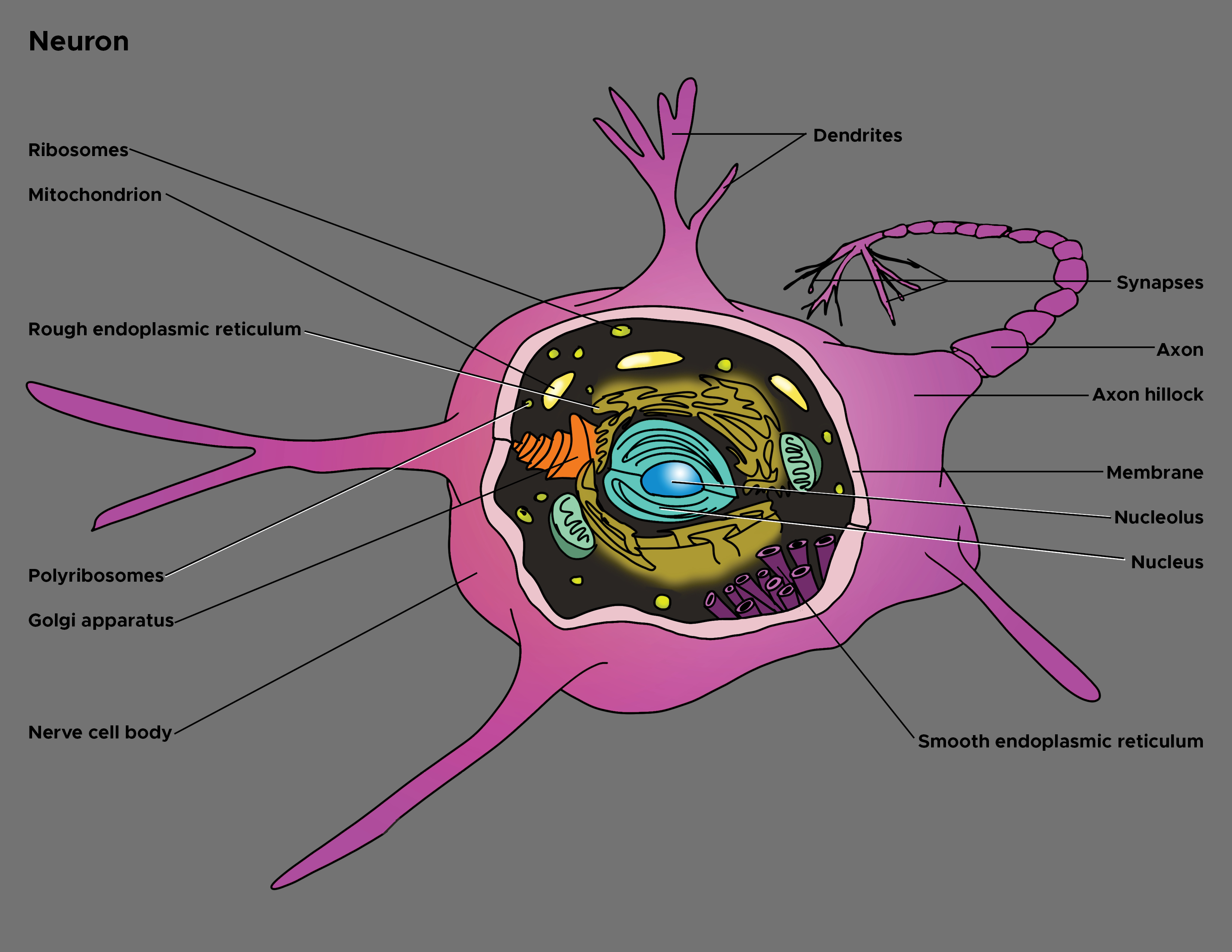
In 1860, the German anatomist Otto Friedrich Karl Deiters (1834-1863) described the basic structure of the nerve cell and identified two different protoplasmatic protrusions of the cell body that he termed as "axis cylinder," and "protoplasmatic processes," respectively axons and dendrites (see Illustration. Illustration of Axon).[1] Axons are the elongated portion of the neuron located in the center of the cell between the soma and axon terminals. In size, the axon may represent over 95% of the total volume of the neuron. Functionally, it carries electrical impulses and projects to synapses with dendrites or cell bodies of other neurons or with non-neuronal targets such as muscle fibers.
Concerning length, the length of axons varies according to the function of the neuron. Considering the functional distinction between projection neurons and interneurons, cortical projection neurons (CPNs), also termed as pyramidal neurons and spinal cord projection neurons (dorsal horn neurons), usually have long axons (from several mm and up to 1 m). In contrast, interneurons, that work within local circuits, have a short axonal terminal (up to several mm). The longest axons of the human body are those that make up the sciatic nerve where the length can exceed one meter. Furthermore, compared to projecting neurons, interneurons usually have smaller soma, fewer organelles, and a reduced amount of cytoplasm (axoplasm).
Histological observation of axon shows a cylindrical structure, but recent 3D electron microscopy studies demonstrated that probably axon has not the shape of a perfect cylinder.[2] The diameter is variable as it ranges between 1 and 25 micrometers. In squid, it reaches a diameter of 1 mm. The variation of the diameter has important functional implications since the speed of propagation of the impulse (i.e., action potential), besides being dependent on the presence of the myelin sheath, is directly proportional to the diameter of the axon. Moreover, they have demonstrated significant changes in the diameter along the single axon.[2]
The axon is one of two types of protoplasmic protrusions of the neuronal soma. The other protrusion is the dendrites. Axons are distinguished from dendrites by several characteristics including:
- Shape. Dendrites are usually thin while axons typically maintain a constant radius
- Length. Dendrites are limited to a small region around the cell body while axons can be much longer
- Structure. Substantial structural differences exist between dendrites and axons. For example, only dendrites contain rough endoplasmic reticulum and ribosomes, and the structure of the cytoskeleton is different. Differences also affect the membrane as it contains mostly voltage-gated ion channels in axons, whereas ligand-gated ion channels are present, especially in dendrites.
- Functions. Dendrites usually receive signals, while axons typically transmit them. However, all these rules have exceptions. Furthermore, axons generate and transmit all-or-none action potential, whereas dendrites produce depolarizing (below the threshold of the action potential) or hyperpolarizing (lowering the resting membrane potential) graded potentials.
Of note, although each neuron has only one axon, bifurcations that are branches of the main axon can be present. A collateral branch is an axonal protrusion over10 micrometers in length.[3] These collaterals provide modulation and regulation of the cell firing pattern and represent a feedback system for the neuronal activity. The terminal part of the axon and collaterals tapers progressively. These parts are called telodendron and continue with the synapse (synaptic knob or button) which represents the specialized structure that comes into contact with another neuron (soma, axon or dendrite), or muscle fiber. Axon extension and growth of new telodendrons (and synapses) are guided by several factors, including the nerve growth factor (NGF). The branching processes, in turn, play a role of fundamental importance in neuroplasticity, for instance, in cognitive processes such as memory and learning.
Anatomically and based on the appearance of the protoplasmatic protrusions, neurons are classified into three groups:
- Multipolar neurons. They are the most common neurons; Shape: a single axon and many dendrites extending from the cell body. Localization: central nervous system (CNS)
- Unipolar (or pseudounipolar) neurons. Shape: a single short process that extends from the cell body and then splits into two branches in opposite directions; one branch travels to the peripheral nervous system (PNS) for the sensory reception, and the other to the CNS (central process). These neurons have no dendrites as the branched axon serving both functions. Localization: dorsal root ganglion and sensory ganglia of cranes nerves, and some mesencephalic nucleus
- Bipolar neurons. Shape: one axon and one dendrite that extend from the cell body in opposite directions. Localization: retinal cells and olfactory system
Two notable features distinguish the axon from the soma (also referred to as perikaryon). First, no rough endoplasmic reticulum extends into the axon; secondly, the composition of the axonic membrane (axolemma) is fundamentally different from that of the somatic membrane. These structural differences translate into functional distinctions. In fact, since the absence of ribosomes does not allow protein synthesis, all axon proteins originate in the soma. Furthermore, the particular structure of the membrane due to the presence of specific protein channels allows information to travel along the course of the axon. Again, depending on the location within the body, these structures can be covered in sheaths of an insulating material known as myelin. Based on the presence or absence of the myelin sheath, axons are distinguishable into myelinated and non-myelinated axons.
Myelin sheath
Myelin forms by the concentric wraps of the plasma membrane of neuroglia cells around the axon. These cells are the Schwann cells (or neurolemmocytes) in the PNS and oligodendrocytes in the CNS. As a general rule, oligodendrocytes myelinate multiple adjacent axons, while Schwann cells myelinate only one axon. In structural terms, the myelin sheath wraps the axons discontinuously as it is interrupted at regular intervals called Ranvier nodes (also termed as myelin sheath gaps), which represent the space between two consecutive Schwann cells and at which the axon is devoid of the sheath. In this way, employing the jump mechanism from one Ranvier node to the next, the propagation of the electrical signal is much faster than in the myelin sheathed axons. The cell membrane of Schwann cells is arranged around the axon, forming a double membrane structure (mesaxon), which elongates and wraps itself in a spiral, in concentric layers, around the axon itself. During this winding process, the cytoplasm of the Schwann cell is pushed towards the outside, while the surfaces of the contact membranes end up condensing, forming the lamellae of the myelin sheath. When the myelin sheath wraps around the axon, the mesaxon disappears by fusion of the cytoplasmic membranes in contact, except in correspondence with the innermost gyrus (internal mesaxon) and the outermost gyrus (external mesaxon or neurilemma) where there is a turn outermost rich in the cytoplasm. When the myelin sheath forms by oligodendrocytes (in PNS), the outermost gyrus reduces to a tongue and, in turn, although there is the internal mesaxon, the external one is not recognizable. Functionally, myelin represents an electrical insulator, allowing an increased speed of conduction along with an axon. It facilitates electrical transmission via saltatory conduction. Structurally, myelin is composed of approximately 80% of lipids (mostly cholesterol and variable amounts of cerebrosides and phospholipids) and 20% of proteins. However, depending on its location, myelin has a different composition as CNS myelin has more glycolipid and less phospholipid than PNS myelin.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
A significant issue of concern with the histology of axons is the staining. In addition to the presence of myelin (in myelinated axons) and to the particular membrane structure, another feature distinguishes the axon from the soma. The axon is devoid of a rough endoplasmic reticulum. As a result, traditional cellular stains such as the Nissl staining method, which identifies the so-called Nissl bodies (granules of the rough endoplasmic reticulum) is only able to stain the soma and dendrites but not the axon or axon hillock. That said, newer methods of staining involving luminescence approaches have produced enlightening results. Retrograde stained tracers using fluorescent dyes have shown promise with higher resolution photo data.
Issues of concern of clinical interest are manifold as a large number of functional and/or structural alterations of the axon are at the basis of neurological disorders that can involve the CNS or PNS. On these bases, the study of the structure and functioning of the axon (i.e., axonology) is a field of research of considerable interest. As a consequence, immunocytochemical and immuno-ultrastructural techniques have illustrated the structure of channels, exchangers, and pumps that are involved in the operative mechanisms of axons. Moreover, the development of targeted strategies against axonal damage, or focused on axon regeneration, are of fundamental importance for the majority of all neurological diseases.
Structure
Axon connects to the soma at a cone-like part of the structure known as the axon hillock. This part of the axon has considerable functional importance since action potential originates here. In other words, this region of the neurolemma processes the incoming signals from other neurons. It represents a trigger zone where the summation of incoming graded excitatory (excitatory postsynaptic potentials, EPSPs) and inhibitory (inhibitory postsynaptic potentials, IPSPs) potentials are realized and, in turn, the action potential is realized or not. If the summation overcomes the threshold limit of the axon hillock, potential starts, and it will be transmitted, in a continuous or saltatory mode, along the axon toward the synapse. In structural terms, the axon hillock may contain fragments of Nissl substance.
The axon hillock continues with the initial segment of the axone, located about 30 to 40 micrometers from the perikaryon and close to the first myelinated element. Structurally, in this axonal segment, the various axoplasmic elements begin to align longitudinally. Neurofilaments and mitochondria are present. Microtubules are also present, arranged into fascicles interconnected by sidearms. Furthermore, residual Nissl substance can persist. Functionally, in this region, axo-axonic synapses may occur. Of note, the axolemma of the axonal region where action potential originates and starts its run shows a dense granular layer similar to that demonstrated at the nodes of Ranvier. Recent studies proved that action potentials could originate not only at the axon hillock but also in this initial axonal segment. In some neurons, the action potential may even originate from the first Ranvier node.
Axons are both structurally supportive of a neuron and the facilitators of communication both intra- and interneuronally. The diameter of a single axon is incredibly small and, therefore, commonly measured in micrometers with the average diameter of an axon being about 1 micrometer. A cluster of axons together forms a nerve. The axonal membrane is a phospholipid bilayer that has proteins embedded inside it. These voltage-gated ion channels facilitate the movement of ions in and out of the membrane and critical to the neuronal transmission. Many neurons are myelinated, meaning they contain sectioned covers wrapping around the axon known as the myelin sheath.[4] Axons that are thicker and more densely myelinated will be faster and useful for reflex-based circuits and other somatic nervous system functions. Contrarily, pain, and hunger receptors from the autonomic nervous system have lightly or unmyelinated slender neurons and produce slower transmissions.
An important axonal structural element is the architecture of the cytoskeleton. Interestingly, this architecture is partly different in composition and organization from that of the dendrite. Although the cytoskeletal proteins are mainly synthesized in the cell body and then transported to their various cellular sites, the dendritic microtubule-associated protein 2 (MAP2) is synthesized directly in the dendritic compartment. On the contrary, due to the lack of a protein synthetic apparatus, the axonal microtubules are synthesized and organized in the soma (by the microtubule-organizing center, MTOC) and then to the axon compartment. Furthermore, MAP2 is not present in the axonal compartment. Another difference concerns the microtubule polarity. While dendritic microtubules show a mixed polarity with both plus and minus ends distal to the cell body, in the axon microtubules, have the plus end distal to the cell body. Again, research has demonstrated that axonal and dendritic microtubules differ in the different quantity of tau proteins and the different degrees of phosphorylation. Finally, although neurofilaments are abundant in the axons, they are scarce in the dendritic site.
The cytoskeleton plays a fundamental role in the processes of axon growth and guidance. Since these processes are the basis of the formation of neural networks, there is considerable interest in this aspect of neuroscience. Alterations during the constituent phases of the circuits, indeed, are linked to the pathogenesis of neurodevelopmental disorders such as intellectual disability and autism spectrum disorder.[5] On the other hand, in neurodegenerative pathologies, there is progressive destruction of these circuits. Axon growth and guidance are regulated by genes and originate from the so-called growth cone, a region described for the first time by the Spanish histologist Santiago Ramón y Cajal (1852-1934), in 1890. The composition of the environment around the axon guides the growth process of the nerve terminal and the achievement of its target. Environmental attractively or repulsively acting molecules interact with receptors located on the growth of cone and induce, in turn, modifications of the cytoskeleton. In particular, the cytoskeleton produces fine extensions like microspikes called filopodia that contain bundles of actin filaments (F-actin). Interposed between these secondary cytoplasmic projections are flat regions of dense actin. These regions that have a veil-like" appearance are termed as lamellopodia. In short, after the interaction between environmental factors (e.g., netrin, ephrins, and semaphorins), external to the axon and also present at a distance, with receptors located on the growth cone, microtubules polymerize into the growth cone and guide the elongation process through filopodia and lamellopodia.[6] The development of axonal collaterals, the mechanisms of axonal regeneration after injury, and the growth of dendrites also provide activation of the growth cone and processes involving filopodia and lamellopodia.
Function
Conduction of electrical impulses
From the broadest perspective, the function of axons is to carry electrical impulses that are the means of communication within the brain and between the brain and the rest of the body. These electrical impulses are known as action potentials and are a result of the manipulation of the electrochemical gradient inside and surrounding an axon. There are two primary excitable domains in an axon, with the proximal one being the initial axon segment and the comparatively distal one being the nodes of Ranvier. The initial axon segment is the point of origin for action potentials. The nodes of Ranvier, also indicated as myelin-sheath gaps, lie in between sheaths of myelin and are highly concentrated in voltage-gated ion channels that assist the progress of ions moving into and out of the axon, consequently triggering depolarization, which will initiate an action potential if it attains the threshold voltage. The nodes of Ranvier also allow the action potential to progress rapidly across the axon through saltatory conduction.[4] Action potentials are an all or nothing phenomenon with stronger stimulation correlating to a higher frequency of action potentials rather than a stringer current in a single action potential.
Axonal transport
The axonal transport is of fundamental importance for the development, maintenance, survival, and functioning of neurons. Since the axon is devoid of the apparatus for protein synthesis and therefore is under the control of the cell body, this metabolic dependence of the axon involves a continuous movement from the cell body along the axon to its endings, of proteins, enzymes, chemical transmitters, synaptic vesicles, monomeric subunits of neurofilaments, and membrane components. In addition to the continuous exchange of the structural constituents of the axon, axonic flow is necessary to continuously supply the synapses of neurotransmitters and enzymes for their synthesis and degradation. Approximately, a volume of cytoplasmic constituents equal to three times the volume of the cell body is transported daily along the axon.There are two types of axonal transport, a centrifugal transport from the soma to the synaptic terminal and a retrograde transport that specializes in carrying molecules from the nerve terminal to the soma. Centrifugal axonal transport takes place in different ways, which are characterized by different speeds. At least as a schematic, there would be a slow transport (1 to 10 mm per day) and a transport that takes place at a higher speed (1000 mm per day). The difference is related to the different functioning of the microtubule system. In fast transport, the material to be transported is conveyed into vesicles and transported by proteins of the kinesin family that flow along the axis of the microtubules. This process involves high ATP consumption.
While the antegrade transport aims at the vehicle of substances for the functioning of the synapse and the neurotransmission, the transport in the opposite direction has a pivotal role in neuronal survival, axon guidance, and growth.[7] Retrograde flow, indeed, leads to the body cell materials destined to be eliminated or reused, such as the degradation products of proteins and other cytoplasmic constituents, as well as substances that are taken by endocytosis from extracellular spaces, at the axonic terminations. The driver of this type of transport (signaling endosomes) is dyneins and involves several pathways such as the mitogen-activated protein kinase (MAPK), the phosphoinositide 3-kinase pathways as well as the brain-derived neurotrophic factor (BDNF), and the activated TrkB that provides anti-apoptotic effects.[8]
Tissue Preparation
Per standard procedure for tissue preparations, the nerve tissue is placed in a 4% paraformaldehyde solution for five hours at a controlled temperature of about 4 degrees Celsius.[9] This tissue is then washed in a phosphate buffer like PTX, which is 1% Triton x-100 in phosphate-buffered saline.[9][10][9] If the epineurium is present in the tissue sample, it requires removal to allow better penetration of antibodies. Incubate with blocking solution overnight and then transfer into PTX containing the primary antibodies and 10% FBS. Post this incubation with gentle agitation, and the nerves are washed again with PTX. The secondary antibodies are now introduced, and the nerves incubated in a solution of these diluted by PTX with 10% FBS. After a few more rounds of being washed in PTX, the nerves are stained with water-soluble fluorescent red dye. More often, carbocyanine dyes like DiI, DiA, and DiO are used with their fluorescence posing a visual advantage.[11] Other dyes used for axon tracing include but are not limited to other lipophilic dyes, NeuroVue dyes, lucifer yellow, and horseradish peroxidase.
Histochemistry and Cytochemistry
Cytochemical observation of myelinated fibers shows a variety of spatial differentiation in protein concentrations. This variation correlates with function, location, and genetic activity (epigenetic phosphorylation leading to increased tetanic stimulation). The highest concentration of voltage-gated sodium, potassium ion channels, and Na/K pumps is the axon hillock or initiating site of excitatory postsynaptic potentials (EPSPs). The strength and efficiency of neurons in the brain are dynamic and influenced by both long term potentiation and long term depression, causing morphological changes in wiring and size of neurons. The activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA), glutamate receptive gated ion channels for Na and Ca respectively, is responsible for long term morphological changes. Upon a large enough glutamate initiated current of Calcium, the cellular initiating signal, kinases like CaMKII are activated and can phosphorylate proteins that stimulate transcription in the nucleus to produce scaffolding proteins, voltage-gated receptors, neurotransmitter gated receptors, and other proteins necessary to morphological dynamics of a neuron.
Microscopy, Light
Nerve cells can be studied histologically under light microscopy through several methods:
- Methods that highlight the cell body, the nucleus, and any organelles present in the soma but do not allow the precise study of the axon and branching.
- Methods of impregnating the nerve tissue through different heavy metal salts such as gold and silver - these approaches can allow the demonstration of the axon, despite less data on the structure of the soma are provides.
- Methods that are useful for the study of white matter (myelin sheaths)
- Immunohistochemical methods that allow highlighting neurons from glial cells but above all to identify neuronal subpopulations using antibodies directed towards specific cellular components (e.g., vesicular structures).
Methods can also divide into myelin (e.g., Weigert and Pal-Weigert) and cell (e.g., Nissl) approaches. Interestingly, the myelin-stained material can be viewed as a 'negative' image of the soma-stained section. Thus, on the myelin-stained section, soma structures appear paler, whereas myelinated regions appear darker than a corresponding soma-stained section.
There are essentially four most common methods. Of note, each of them takes the name of its inventor: the Golgi method, the Nissl method, the Weigert method, and the Cajal method. Over time, there have been proposals for several changes for each staining.
In 1873, the Italian Nobel Prize winner Camillo Golgi (1843-1926) developed the dichromate-silver impregnation technique, also known as the Golgi method.[12] This coloring determines a characteristic black coloring of the neuron and its organelles. Subsequently, the method was revised, replacing the silver precipitate with gold, and immersing the sample in gold chloride and then in oxalic acid. In short, the method involves immersing the brain tissue fixed with formaldehyde for two days in a 2% aqueous solution of chromium. After the tissue dries with filter paper, it is again immersed in a 2% silver nitrate aqueous solution for another two days. Subsequently, the tissue is cut into sections (20 to 100 micrometers), and in turn, quickly dehydrated in ethanol. The Golgi silver method can be challenging to perform, and many times may present unsolvable problems. A well-performed stain shows the nerve cells stand out in intense black, on a tobacco-colored background. Several variants, such as the Golgi-Bubenaite, the Golgi-Cox, and the Golgi-Kopsch method that uses formaldehyde and glutaraldehyde, have been proposed. These variants are less influenced by the age of the specimen, temperature of the laboratory, and duration of the procedure. Particulars on the Golgi method are obtainable on the portal SynapseWeb of the National Institutes of Health (NIH).
For the study of neurons, the technic proposed by the German researcher Franz Alexander Nissl (1860-1919) is another option. Through basic aniline dyes such as cresyl violet, it intensely colors the cell body and dendrites as they contain granular endoplasmic reticulum and many free ribosomes. Due to the lack of such structures, axons are not are recognizable by Nissl's method. Despite these limitations, Nissl staining has allowed the acquisition of many notions of neurohistology. For example, the anatomist Korbinian Brodmann (1868-1918) carried out his studies on the structure of the mammalian cortex based on this type of staining. The method, thus, is still used for studying the structure of cortical layers.
Finally, the Cajal method, also termed as double impregnation technique, developed in 1891, allows the study of neurofibrils and shows fine details of the terminal branches. This method allowed for the first time to identify dendritic structures as authentic and not simply artifacts. Apart from this staining that represents a fast variant of the Golgi method, Cajal developed other methods for studying the nerve tissue, including the reduced silver nitrate method to stain neurofibrils, and the gold-sublimated staining that can be useful for staining astrocytes.[13]
A classic method used for the study of white matter is the Weigert method for the myelin sheaths of axons, described by the German pathologist Karl Weigert (1845-1904), in 1882. Several variations, such as the Pal-Weigert method and approaches for myelin staining, have been proposed. The Pal-Weigert method, for instance, is used for overcoming several difficulties of the original Weigert's technic such as the difficulty of observing the extent of the reaction occurring in the permanganate solution. Again, the osmium tetroxide stain colors the lipids of the myelin sheath in black but does not color the axons. Therefore, through this technic will be observed black rings corresponding to the myelin sheath of fiber the axon not colored (white) inside. A modification of the Pal-Weigert technic was proposed by Tolivia, in 1988.[14] Through this approach, conducted on paraffin sections by using pyronine, myelinated fibers appear dark/blue, and the nerve cell bodies stain red.
Other methods are the Ruffini stain and the Bielchowsky method. The former is used for the coloring of nerve endings and large peripheral fibers and requires fresh tissue, without preventive treatments such as washing in distilled water. It involves coloring with gold chloride and maceration in glycerin for at least six months. The nerve endings appear colored in intense black on a violet background. The Bielchowsky method is a complicated and difficult approach used above all for the peripheral nervous system even if it allows a good vision of the nerve terminals. Its basis is the affinity that ammonia silver has for nerve fibers when it reduces from formaldehyde to metallic silver. If the coloring is well-performed, the nerve fibers appear intensely colored in black on a more or less intense gray background. Because of the non-rectilinear trend of the fibers, the sections must be at least 12 to 14 micrometers thick.
Although impregnation techniques, especially if well-executed, offer fascinating images of the axonal structure, these approaches have been largely replaced by immunohistochemical methods as they allow to obtain better information on neuronal connections and in vivo demonstration of the axonal functioning. These techniques use:
- antegrade tracers such as phytohemagglutinin-L (PHA-L), and biotinylated dextran amines (BDA) tracers;
- tracers for retrograde axonal transport such as the horseradish peroxidase (HRP), wheat germ agglutinin conjugated to HRP (WGA-HRP), and cholera toxin subunit B conjugated to HRP (CTB-HRP);
- mixed tracers, antegrade, and retrograde (e.g., CTB).
Moreover, recent experiments have found fluorescence microscopy to be exceptionally useful. Super-resolution fluorescence microscopy has revealed the structure of an unmyelinated axon to be made up of actin filaments that have adducin capping them at one end that form a ring-like structure around the circumference of the axon. These rings of actin connect via spectrin tetramers that orient longitudinally along the direction of the axon. It extends this cytoskeletal structure across the entire axon.[15] The structure of an axon is telling in that it's mechanical properties will probably vary from those of the soma and dendrites. Among fluorescent dyes, we cite 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI). They are lipophilic dyes consisting of a fluorophore compost (carbocyanine) conjugated to two long aliphatic tails. These dyes are incorporated into membranes and follow axonal transport in both anterograde and retrograde direction, allowing detailed neuronal labeling and pathway tracing.
Microscopy, Electron
Serial block face-scanning electron microscopy (SBF SEM) with super voxel-based segmentation is a method electron microscopy that generates high-resolution 3D images. This approach was used from the beginning to obtain information about the ultrastructure and the connectivity of the nervous tissue. Indeed, it offers detailed pieces of information on axonal diameter, fiber diameter, myelin thickness, and organelles.[16] Interestingly, ultrastructural analysis can be combined with in vivo imaging data from light microscopy immunohistochemical and traces approaches, especially in cells cultured but also in tissue sections. For the preparation of tissue section useful for electron microscopy after a light microscopy investigation, Hua's approach is the most used method.[17]
Moreover, electron microscopy offers significant information about the axonal structure and its alterations in the course of pathological processes. In the case of axonal degenerative alterations, for example, subversions of the layered structure of the myelin, alterations of the compactness, and lipid aggregates that are the expression of the myelin in decay can be found. Again, electron microscopy offers important experimental data in the evaluation of regenerative processes; therefore, in experimental models, it is useful to verify the effective efficacy of potential neuroprotective and neuroregenerative treatments.[18]
Pathophysiology
Demyelination
Demyelination is a destructive removal of myelin that induces a paramount impairment in impulse transmission. A demyelinated axon, indeed, transmits impulses up to 10 times slower than normal myelinated, whereas a complete stop of the transmission is also possible. Myelin and axonal degradation are observed in multiple sclerosis (MS). In this immune-mediated inflammatory disease, the inflammatory and demyelinating plaques are accompanied by perivenular macrophage, lymphocyte infiltration, and oligodendrocyte degradation. The observable clinical symptoms associated with the latter depend on the location of the affected neurons. The exponential downward trend of neurodegeneration development is complicated and could be related to the dysfunction of ion channels, calcium overload, synaptopathy, activation of apoptotic pathways, or glutamate-related excitotoxicity.[19]
Other pathologies may induce immune-mediated myelin damage. Multifocal motor neuropathy (MMN), also known as multifocal motor neuropathy with conduction block (MMNCB), is a rare, acquired, motor neuropathy featuring progressive asymmetric weakness without sensory problems. It typically involves upper limbs more than the lower limbs. Concerning pathophysiology, pieces of evidence suggest that anti-ganglioside antibodies (anti-GM1) may be responsible for sodium and potassium channel dysfunction at the node of Ranvier of myelinated motor axons ('node-paranodopathy').[20]
Axonal acute damage
Apart from MS, research focuses on understanding the mechanisms of axonal damage in acute and chronic pathologies. In the mid-nineteenth century, the British physiologist Augustus Volney Waller (1816-1870) showed that a full-thickness section of a nerve induces degeneration and reabsorption of its distal segment. The leading cause of this degeneration (Wallerian degeneration) is the interruption of axonal flow and the lack of ribosomes in the axon. The post-injury degenerative process has been subject to further dissection. In the case of a focal traumatic axonal lesion (e.g., due to spinal cord injury, SCI), the adjacent 400 to 600 micrometers of the axon on both sides of the lesion is involved in a rapid degenerative process (acute axonal degeneration). The molecular mechanisms comprise a cascade of events involving a rapid calcium influx into the axon, activation of calcium-dependent proteases (e.g., calpain and calcineurin), mitochondrial damage with the production of reactive oxygen species, alterations of neurofilaments with the fragmentation of microtubules, and impairment of axonal transport. The cascade culminates in the activation of autophagy. After 24 to 72 hours, the distal part of the axon is subject to Wallerian degeneration, which is mediated by the activation of nicotinamide mononucleotide adenylyltransferase (neuroprotective under physiological conditions) and is directed along the axon.[21]
The traumatic axonal injury (TAI) is severe axonal mechanical damage due to the high rotational acceleration of the brain.[22] Although its pathophysiology is complex, it seems that the damage is firstly produced by mechanically break involving axonal microtubules. This mechanical stretch reverberates on the axonal structure (undulations and breaks) and induces direct membrane mechanoporation with calcium influx that activates several injurious pathways such as the caspase-mediated proteolysis and the cytokine-mediated microglia recruitment. The effect is the impairment of axonal transport and the accumulation of transported proteins in varicose swellings.[23]
Chemotherapy-induced peripheral neuropathy
The neurotoxicity, due to the anticancer drugs, also referred to as chemotherapy-induced peripheral neuropathy (CIPN), has multiple mechanisms involving axon and other neuronal compartments.[24] For example, proteasome inhibitors (e.g., bortezomib) provoke pathological changes in Schwann cells and myelin and, in turn, axonal degeneration.[25] Again, microtubule alterations and impairment of axonal transport are primarily involved in dose-dependent taxane neurotoxicity, whereas calcium-related neurotoxicity has involvement in the pathogenesis of oxaliplatin-induced CIPN.[26]
Neurodegenerative processes
In neurodegenerative processes such as Parkinson disease (PD) and amyotrophic lateral sclerosis (ALS), the axonal compartment degenerates more slowly over longer time-periods. Research has also described the features of focal axonal degeneration. They manifest themselves as district morphological alterations due to damage of axonal transport, in turn, secondary to harmful processes affecting mitochondria and other support structures. Interestingly, these processes, within certain limits, can be reversible. Furthermore, several studies have focused on alterations of bi-directional axonal transport and the pathogenetic role of microtubule alterations in degenerative diseases.[27]
Clinical Significance
Axon density, the degree of myelination, and regional size distribution are of fundamental importance for neural functioning. Structural and functional axonal alterations underlie neurological symptoms in a wide range of disorders such as stroke, traumatic brain, and SCI, peripheral neuropathies (e.g., diabetic neuropathy, CIPN), and chronic neurodegenerative (and neuroinflammatory) diseases such as PD, MS, ALS, and Alzheimer disease. For example, in PD and ALS, the axon and the presynaptic terminals are the first neuronal compartments to be involved. Yet, the TAI is the most common and severe expression of brain injury following a head injury.[28]
One of the most studied diseases expressing axonal damage is the MS, although primary pathological immune response concerns glia cells and myelin sheath, and then axon. MS is an autoimmune disease associated with demyelination of CNS nerve cells. The disease results in areas of demyelination or lesions that, in turn, produce axonal loss with varying inflammation. Histologically these lesions may be classified as inactive, active, and remyelinated. The inactive form indicates a patient with a progressive form of the disease. In particular, MS correlates with more pervasive demyelination, loss of oligodendrocytes, axonal depletion, and generally lacks active inflammation.[29] Active areas of demyelination imply relapsing-remitting MS and are identifiable by the myelin degradation with corresponding preservation of axons, operative perivascular inflammation, reactive astrocytes, and macrophage infiltration. Remyelinated lesions are often present on the edges of an active lesion (sometimes inside) and characteristically demonstrate thinly myelinated axons oligodendrocyte precursor cells may appear in higher concentration around remyelinated lesions. Because MS primarily affects the spinal cord, optic nerves, and white matter of the brain, symptoms reflect sensory and motor issues associated with those areas. These issues may include poor balance and coordination, fatigue, pain in neck and back muscles, vision problems, sexual dysfunction, paresthesia, and more. The etiology of MS is inconclusive, but it is theorized to be caused by a combination of genetic and environmental factors.[30]
Media
References
Deiters VS, Guillery RW. Otto Friedrich Karl Deiters (1834-1863). The Journal of comparative neurology. 2013 Jun 15:521(9):1929-53. doi: 10.1002/cne.23316. Epub [PubMed PMID: 23436306]
Level 2 (mid-level) evidenceLee HH, Yaros K, Veraart J, Pathan JL, Liang FX, Kim SG, Novikov DS, Fieremans E. Along-axon diameter variation and axonal orientation dispersion revealed with 3D electron microscopy: implications for quantifying brain white matter microstructure with histology and diffusion MRI. Brain structure & function. 2019 May:224(4):1469-1488. doi: 10.1007/s00429-019-01844-6. Epub 2019 Feb 21 [PubMed PMID: 30790073]
Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, Lanier L, Svitkina T, Gallo G. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Developmental neurobiology. 2011 Sep:71(9):747-58. doi: 10.1002/dneu.20907. Epub [PubMed PMID: 21557512]
Level 3 (low-level) evidenceNelson AD, Jenkins PM. Axonal Membranes and Their Domains: Assembly and Function of the Axon Initial Segment and Node of Ranvier. Frontiers in cellular neuroscience. 2017:11():136. doi: 10.3389/fncel.2017.00136. Epub 2017 May 9 [PubMed PMID: 28536506]
Lee K, Cascella M, Marwaha R. Intellectual Disability. StatPearls. 2025 Jan:(): [PubMed PMID: 31613434]
McCormick LE, Gupton SL. Mechanistic advances in axon pathfinding. Current opinion in cell biology. 2020 Apr:63():11-19. doi: 10.1016/j.ceb.2019.12.003. Epub 2020 Jan 8 [PubMed PMID: 31927278]
Level 3 (low-level) evidenceWang T, Martin S, Nguyen TH, Harper CB, Gormal RS, Martínez-Mármol R, Karunanithi S, Coulson EJ, Glass NR, Cooper-White JJ, van Swinderen B, Meunier FA. Flux of signalling endosomes undergoing axonal retrograde transport is encoded by presynaptic activity and TrkB. Nature communications. 2016 Sep 30:7():12976. doi: 10.1038/ncomms12976. Epub 2016 Sep 30 [PubMed PMID: 27687129]
Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nature neuroscience. 2004 Jun:7(6):596-604 [PubMed PMID: 15122257]
Level 3 (low-level) evidenceDun XP, Parkinson DB. Visualizing peripheral nerve regeneration by whole mount staining. PloS one. 2015:10(3):e0119168. doi: 10.1371/journal.pone.0119168. Epub 2015 Mar 4 [PubMed PMID: 25738874]
Level 3 (low-level) evidenceJensen-Smith H, Gray B, Muirhead K, Ohlsson-Wilhelm B, Fritzsch B. Long-distance three-color neuronal tracing in fixed tissue using NeuroVue dyes. Immunological investigations. 2007:36(5-6):763-89 [PubMed PMID: 18161528]
Level 3 (low-level) evidenceHeilingoetter CL, Jensen MB. Histological methods for ex vivo axon tracing: A systematic review. Neurological research. 2016 Jul:38(7):561-9. doi: 10.1080/01616412.2016.1153820. Epub 2016 Apr 21 [PubMed PMID: 27098542]
Level 1 (high-level) evidenceBentivoglio M, Cotrufo T, Ferrari S, Tesoriero C, Mariotto S, Bertini G, Berzero A, Mazzarello P. The Original Histological Slides of Camillo Golgi and His Discoveries on Neuronal Structure. Frontiers in neuroanatomy. 2019:13():3. doi: 10.3389/fnana.2019.00003. Epub 2019 Feb 18 [PubMed PMID: 30833889]
Garcia-Lopez P, Garcia-Marin V, Freire M. The histological slides and drawings of cajal. Frontiers in neuroanatomy. 2010:4():9. doi: 10.3389/neuro.05.009.2010. Epub 2010 Mar 10 [PubMed PMID: 20339483]
Tolivia J, Tolivia D, Navarro A. New technique for differential staining of myelinated fibers and nerve cells on paraffin sections. The Anatomical record. 1988 Dec:222(4):437-40 [PubMed PMID: 2465706]
Level 3 (low-level) evidenceZhang Y, Abiraman K, Li H, Pierce DM, Tzingounis AV, Lykotrafitis G. Modeling of the axon membrane skeleton structure and implications for its mechanical properties. PLoS computational biology. 2017 Feb:13(2):e1005407. doi: 10.1371/journal.pcbi.1005407. Epub 2017 Feb 27 [PubMed PMID: 28241082]
Mukherjee K, Clark HR, Chavan V, Benson EK, Kidd GJ, Srivastava S. Analysis of Brain Mitochondria Using Serial Block-Face Scanning Electron Microscopy. Journal of visualized experiments : JoVE. 2016 Jul 9:(113):. doi: 10.3791/54214. Epub 2016 Jul 9 [PubMed PMID: 27501303]
Hua Y, Laserstein P, Helmstaedter M. Large-volume en-bloc staining for electron microscopy-based connectomics. Nature communications. 2015 Aug 3:6():7923. doi: 10.1038/ncomms8923. Epub 2015 Aug 3 [PubMed PMID: 26235643]
Hilton BJ, Blanquie O, Tedeschi A, Bradke F. High-resolution 3D imaging and analysis of axon regeneration in unsectioned spinal cord with or without tissue clearing. Nature protocols. 2019 Apr:14(4):1235-1260. doi: 10.1038/s41596-019-0140-z. Epub 2019 Mar 22 [PubMed PMID: 30903109]
Adamczyk B, Adamczyk-Sowa M. New Insights into the Role of Oxidative Stress Mechanisms in the Pathophysiology and Treatment of Multiple Sclerosis. Oxidative medicine and cellular longevity. 2016:2016():1973834 [PubMed PMID: 27829982]
Shibuya K, Tsuneyama A, Misawa S, Sekiguchi Y, Beppu M, Suichi T, Suzuki YI, Nakamura K, Kano H, Kuwabara S. Different distribution of demyelination in chronic inflammatory demyelinating polyneuropathy subtypes. Journal of neuroimmunology. 2020 Apr 15:341():577170. doi: 10.1016/j.jneuroim.2020.577170. Epub 2020 Jan 24 [PubMed PMID: 32006783]
Conforti L, Gilley J, Coleman MP. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nature reviews. Neuroscience. 2014 Jun:15(6):394-409. doi: 10.1038/nrn3680. Epub [PubMed PMID: 24840802]
Level 3 (low-level) evidenceDollé JP, Jaye A, Anderson SA, Ahmadzadeh H, Shenoy VB, Smith DH. Newfound sex differences in axonal structure underlie differential outcomes from in vitro traumatic axonal injury. Experimental neurology. 2018 Feb:300():121-134. doi: 10.1016/j.expneurol.2017.11.001. Epub 2017 Nov 22 [PubMed PMID: 29104114]
Hill CS, Coleman MP, Menon DK. Traumatic Axonal Injury: Mechanisms and Translational Opportunities. Trends in neurosciences. 2016 May:39(5):311-324. doi: 10.1016/j.tins.2016.03.002. Epub 2016 Mar 31 [PubMed PMID: 27040729]
Cascella M. Chemotherapy-induced peripheral neuropathy: limitations in current prophylactic strategies and directions for future research. Current medical research and opinion. 2017 Jun:33(6):981-984. doi: 10.1080/03007995.2017.1284051. Epub 2017 Feb 15 [PubMed PMID: 28097895]
Level 3 (low-level) evidenceCavaletti G, Cornblath DR. Chemotherapy-induced peripheral neurotoxicity: Facts, needs and future directions. Journal of the peripheral nervous system : JPNS. 2019 Oct:24 Suppl 2():S86-S87. doi: 10.1111/jns.12332. Epub [PubMed PMID: 31647156]
Level 3 (low-level) evidenceMarmiroli P, Cavaletti G, Carozzi V, Riva B, Lim D, Genazzani AA. Calcium-related neurotoxicity of oxaliplatin: understanding the mechanisms to drive therapy. Current medicinal chemistry. 2015:22(32):3682-94 [PubMed PMID: 26423088]
Level 3 (low-level) evidenceSainath R, Gallo G. The dynein inhibitor Ciliobrevin D inhibits the bidirectional transport of organelles along sensory axons and impairs NGF-mediated regulation of growth cones and axon branches. Developmental neurobiology. 2015 Jul:75(7):757-77. doi: 10.1002/dneu.22246. Epub 2014 Nov 20 [PubMed PMID: 25404503]
Level 3 (low-level) evidencePovlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. The Journal of head trauma rehabilitation. 2005 Jan-Feb:20(1):76-94 [PubMed PMID: 15668572]
Garg N, Smith TW. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain and behavior. 2015 Sep:5(9):e00362. doi: 10.1002/brb3.362. Epub 2015 Aug 3 [PubMed PMID: 26445701]
Kamm CP, Uitdehaag BM, Polman CH. Multiple sclerosis: current knowledge and future outlook. European neurology. 2014:72(3-4):132-41. doi: 10.1159/000360528. Epub 2014 Jul 30 [PubMed PMID: 25095894]